Abstract
Purpose
This study sought to identify and characterize major patterns of functional aging based on activities of daily living (ADL).
Methods
754 community-living adults 70 years or older were followed monthly for ADLs, instrumental ADLs (IADLs), hospitalization and restricted activity over ten years. A generalized growth mixture model was used to identify trajectories of ADL disability across seven 18-month intervals. Cumulative burdens of disability and morbidity from different trajectories were examined using a generalized estimating equation Poisson model.
Results
Five distinct trajectories emerged. The predominant trajectory maintained ADL independence, with membership probability being 61.6%. The remaining trajectories either stayed at low (1 or 2 ADLs, 13.6%) or high (3 or 4 ADLs, 7.0%) levels of disability or declined gradually towards low (11.2%) or high (6.5%) disability. The independent trajectory was associated with the lowest burdens of disability and morbidity and a decreasing time trend of restricted activity; whereas the high disability trajectory demonstrated opposite trends. About 31% of the cohort remained in the same trajectory throughout the follow-up period.
Conclusion
The course of functional aging is heterogeneous and dynamic. While most older adults maintain functional autonomy, some may experience persistent disability or progress towards severe disability with substantial morbidity.
Keywords: Latent class growth mixture model, functional aging, activities of daily living, hospitalizations, restricted activities
INTRODUCTION
The last half century witnessed a rapid expansion of the oldest sector of the population, along with a substantial increase in life expectancy (1). However, it is debatable whether older adults experience improved quality of life or a reduced burden of disability and morbidity as they live longer. While some prior studies suggest that the onset of both severe disabilities and severe morbidities have been “compressed” to very later age (2, 3), others show increasing prevalence and/or longer duration of chronic, nonfatal diseases in older adults (4, 5). In addition, trends in active and disabled life expectancy appear to vary over time and across demographic subpopulations. The longevity of women is associated with more years of active life and of disabled life (6–8), whereas the increase in overall active life expectancy documented in the 1980s was limited to those with high educational attainments (9). It has yet to be clarified whether these discrepancies resulted from differences in the measures of disability or of morbidity and life expectancy across studies, or reflected the de facto heterogeneity of functional aging process (10). Due to lack of adequate measures, predictions of population trends of functional aging based on vital statistics or other aggregated data often omit important risk factors of the disablement process, such as depression, cognitive impairment and physical frailty (8, 11–13), and hence, may not be easily generalizable to clinical practice.
A number of population-based cohort studies have attempted to address the heterogeneity of functional aging. Liang et al followed over 18,000 Americans aged 50 years or older for ADL and IADL disabilities over 11 years and identified three stable and two declining trajectories using a group-based trajectory model (14). A similar finding of five trajectories (or course types) was reported by Deeg and colleagues in a cohort of older adults aged 55–85 years using cluster analyses (15). However, these studies only assessed disability 3 to 5 times at 2- to 3-year intervals, which may not capture the inherent dynamics of functional aging; in addition, trajectory model built upon spars outcome data may be sensitive to population heterogeneity or unmeasured confounders. Indeed, after accounting for time-varying covariates, including health conditions and depression, only three trajectories were retained in the Liang study (14).
The objective of the current study is to examine the dynamics of functional aging that may underlie the conflicting research findings in the literature. Specifically, we sought: 1) to identify latent-class trajectories of functional aging based on longitudinal data of activities of daily living (ADL) while accounting for important risk factors of functional disability, including but not limited to, depression, cognitive impairment, physical frailty and chronic health conditions (8, 11–15); and 2) to characterize the identified trajectory classes based on measures of cumulative burdens of disability and morbidity beyond the ADL domain, including IADL, hospitalizations and restricted activities. Our hypothesis is that functional aging is a multifactorial process, involving not merely ADLs but also IADLs, and interacts with morbidity and health conditions. Accordingly, we expect that the identified latent classes for functional aging should not only follow distinct ADL trajectories, but also differentiate one another in the total burdens of disability and morbidity beyond the boundary of ADL domain. It is such empirical evidence that signifies the applicability and usefulness of statistically derived functional trajectories to clinical and public health practice.
METHODS
Participants
This study used data from the Precipitating Events Project, a longitudinal cohort of 754 community-living persons aged 70 years or older, assembled between March 1998 and October 1999. All participants were functionally independent at baseline, requiring no personal assistance with four essential ADL tasks —bathing, dressing, walking inside the house, and transferring from a chair (11–12). Complete details about the eligibility criteria and recruitment process have been described previously (11–12). The study protocol was approved by the Yale Human Investigation Committee. By December 31 2009, the telephone interviews were conducted monthly for up to 141 months, and the comprehensive, home-based assessments were completed approximately every 18 months for up to 108 months.
Measurements
Functional disabilities
Participants were assessed for disability during monthly telephone intervews (11–12). For the four essential ADL tasks (i.e., bathing, dressing, walking and transferring), participants were asked, “At the present time, do you need help from another person to (complete the task)?” Those who needed help with any of the tasks were considered to be disabled. Disability in instrumental activities of daily living (IADL) was assessed in a similar fashion based on five tasks: shopping, doing housework, preparing meal, taking medications and managing finance. The reliability of the disability assessment was high, with kappa ranging from 0.75 to 1.0 (11–12).
Hospitalizations and restricted activities
Episodes of hospitalizations were obtained during the monthly telephone interviews based on a question asking whether a participant had stayed at least overnight in a hospital during the past month. The accuracy of these self-reports was high, with a kappa of 0.94 based on an independent review of hospital records among a subgroup of 94 participants (12). Restricted activity was ascertained using a standardized protocol with high reliability (Kappa = 0.90), i.e., stayed in bed for at least half a day and/or cut down on usual activities due to an illness, injury, or other problem. Participants who answered yes to one or both of these questions and who did not report a hospital stay during the same month were considered to have restricted activity (11–12). Hospitalizations and restricted activities were used to measure morbidity, because the former typically results from serious and/or acute diseases while the latter can be sequela of either minor or subacute ailments or severe medical conditions.
Predictors of ADL trajectories
Seven established risk factors of functional disability were considered as predictors of ADL trajectory (11–15), including older age (≥85 years), female gender, living alone, frailty as defined by slow gait (12, 13), depression as defined by a score≥20 on the short-form of the Center for Epidemiologic Studies-Depression scale (CES-D) (16), cognitive impairment as defined by a score<24 on the Mini-Mental State Examination (MMSE) (17) and having 2 or more chronic conditions. These factors were measured during the comprehensive, in-home assessments at baseline and then approximately every 18 months.
Statistical Analyses
The characteristics of the study population were summarized using frequency (percentages) for categorical and means (± standard deviations) for continuous variables.
To identify trajectory classes of functional aging, we used a generalized growth mixture model with random effects to model ADL disability as a discrete outcome (18, 19). The outcome had 3 states based on the four essential ADL items, no disability, mild disability (in 1–2 items) and severe disability (in 3–4 items), which can change from one state to another every month. The growth curve of the ADL disability over an 18-month interval for trajectory class k is modeled using the following generalized mixed effect model:
where µimsk is the probability individual i being in ADL state s at month m if the individual belongs to trajectory class k, β0k., β1k. and β2k. are respectively the coefficients for the intercept, linear and quadratic terms for the month, and bisk. is the individual i specific random effect given the individual’s ADL state s and assigned trajectory class k (19).
To reveal the inherent dynamics of functional trajectories over an extended follow-up period of more than ten years, we allowed participants to be assigned to a different class from one 18-month interval to the next, conditioning on the seven a priori selected, time-dependent predictors updated at month 1 of each interval (as defined in Predictors of ADL Trajectory), plus age by cognitive impairment and age by depression interactions. This was achieved by restarting the ”clock” at each subsequent 18-month follow-up, such that at any given month of the composite 18-month timewindow participants could enter a disability state despite all were independent at study entry. The conditional probability for a membership in trajectory class k for individual i in interval t, πitk, is modeled using polytomous logistic regression as:
where αk, is a vector of the regression coefficients for trajectory class k with αk1 = 0 since the label of k = 1 serves as the reference class, and Xit is a vector of the predictors for individual i in interval t. The class membership of each participant during an 18-month interval was assigned based on the maximum value of πitk among all the trajectory classes in that interval. The overall posterior probability of class membership was calculated as the total number of person-intervals assigned to each specific trajectory class among the total number of observed person-intervals throughout the follow-up period.
We fit respective growth mixture models with the number of trajectory classes varying from 1 to 6, and chose the best-fitting model based on the lowest BIC value (18, 20, 21). The adequacy of the final model was further evaluated using the average posterior probabilities of assignement, with a value of above 0.70 indicating a good fit (21).
To examine the validity of the estimated ADL trajectories and to facilitate their clinical and public health implications, we quantified the cumulative burden of disability and morbidity over successive 18-month intervals for each trajectory class. Specifically, the incidence density rates per 1000-person months were estimated for three measures of disability burden and two measures of morbidity burden using a generalized estimating equation (GEE) Poisson model (22). The three disability burdens were defined as with 1) any ADL disability (at least one disability out of the 4 essential ADL tasks), 2) any IADL disability (at least one disability out of the 5 IADL tasks), and 3) concurrent ADL and IADL disability (having both ADL and IADL disability in the same month). The two morbidity burdens were defined by 1) a hospitalization and 2) restricted activity. Participants who died or dropped out of the study during an interval were censored at the month of death or withdrawal. Apart from the enhanced interpretability, these “cumulative” measures directly accounted for the varied numbers of person-months under observation across individuals and trajectory classes, and therefore may compensate “over-fitting” of the growth mixture model from “noise” contained in the intensive, monthly varying ADL data. In this regard, the rescaled, cumulative ADL burden also serves as a criterion for the internal validity of the growth mixture model that was derived from the 3-state, discrete ADL outcome.
We fitted separate GEE Poisson models for each outcome, with the five trajectory classes being represented as time-dependent indicator variables. First, incidence-density rates of each outcome measure were estimated for each trajectory class over successive 18-month intervals, using robust standard error estimators for 95% confidence intervals (CI). Next, the incidence-density rates were compared among the five trajectory classes while adjusting for age, sex and education. To examine the hypothesized disability “compression” or “expansion” towards later age in the literatures (2–6), we explored potential time trends of incidence-density rates over successive 18-month intervals with including the interactions between trajectory classes and years of follow-up in the GEE Poisson models. Proportional increment/decrement of the incidence density rates per annum and their 95% CI were estimated for each trajectory class, with adjustment for three important predictors of population aging, gender, age and years of education (7–10, 23).
To provide further insight into the dynamics of the five identified ADL trajectories, we tracked each person through seven successive follow-up intervals until the end of follow-up, death, or drop-out of the study. The prevalence of trajectory class among those who never switched class membership, and the prevalence of those who switched to a higher disability class and/or recovered to a lower disability class, are estimated. To assess the impact of potentially “informative” cohort attrition due to death on the observed association, we repeated the estimation of the trajectory-specific disability and morbidity burdens and their time trend by excluding the last 18-month intervals of each decedent.
All the statistical analyses were conducted using SAS software version 9.2 (SAS Institute, Cary NC 2008), with the mixture model being fitted using PROC NLMIXED (24). The hypotheses were tested at a two-sided significance level of α =0.05.
RESULTS
Study population at baseline
The baseline characteristics of the 754 participants are shown in Table 1. The average age was nearly 80 years old, with the majority being female, white, living with others, having a high school or higher education, and two or more chronic conditions. Over more than ten years of follow-up, 433 (57.4%) participants died after a median follow-up of 56.5 months, while 35 (4.6%) dropped out of the study after a median follow-up of 23.5 months. Data were otherwise available for 98.7% of the 70500 monthly telephone interviews.
Table 1.
Characteristic of the Study Cohort at Baseline (N=754)
| Characteristic | n (%) |
|---|---|
| Age (yr), 85 or older | 102 (13.5) |
| —Mean ± SD | 78.4 ± 5.3 |
| Female | 487 (64.6) |
| Non-white race | 72 (9.5) |
| Lives alone | 298 (39.5) |
| Education (yr), less than 12 | 249 (33.0) |
| — Mean ± SD | 12.0 ± 2.9 |
| Chronic conditions,* ≥ 2 | 405 (53.7) |
| — Mean ± SD | 1.8 ± 1.2 |
| Cognitive impairment † | 86 (11.4) |
| Depression‡ | 100 (13.3) |
| Physical frailty‖ | 322 (42.7) |
Abbreviations: SD, standard deviation.
Based on 9 self-reported, physician-diagnosed, including hypertension, myocardial infarction, heart failure, stroke, diabetes mellitus, arthritis, hip fracture, chronic lung disease, and cancer (other than minor skin cancers).
Based on a score of < 24 on the Mini-Mental State Examination (MMSE).
Based on a score ≥20 on the Center for Epidemiologic Studies Depression scale (CES-D).
Based on a score > 10 seconds on rapid gait test.
Estimated class trajectories based on generalized growth mixture model
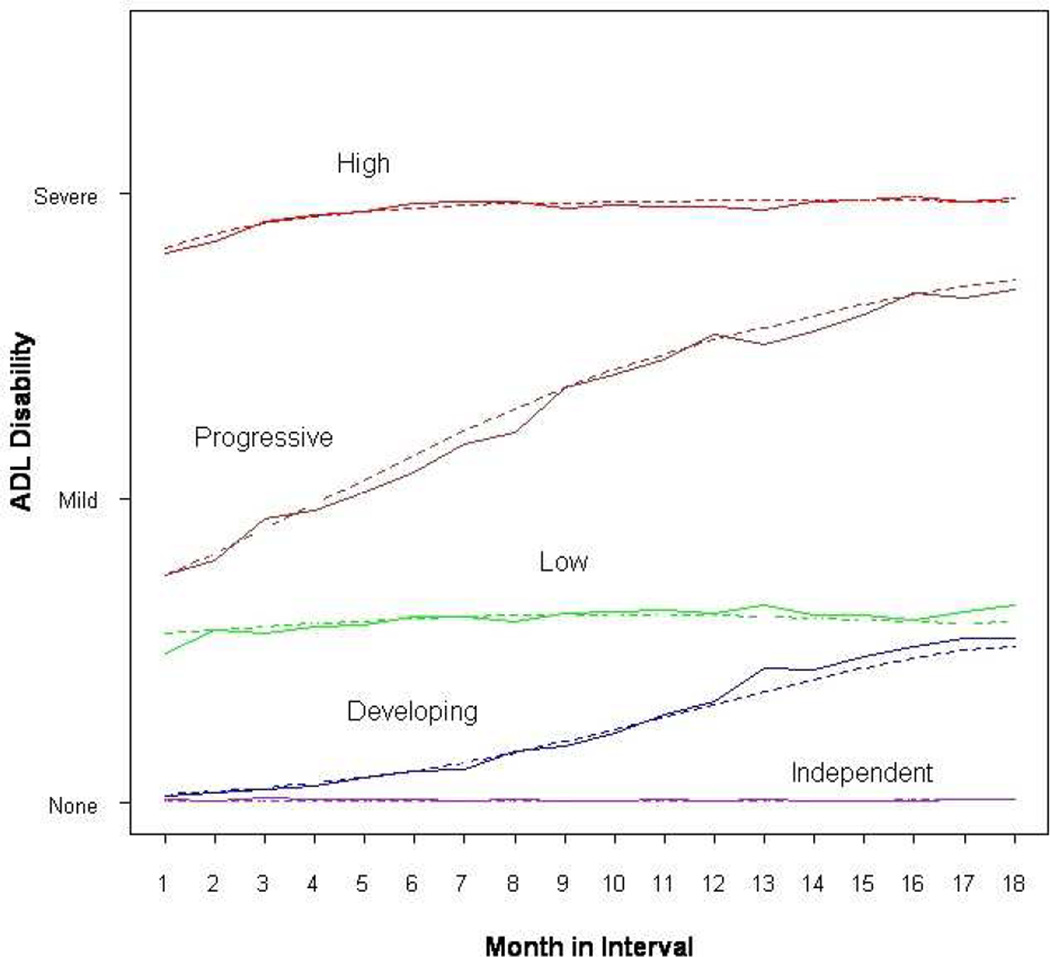
Among the growth mixture models we evaluated, a five-class solution provided best fit based on BIC (36,081), relative to the six-trajectory (36,109) and four-trajectory (39,268) solutions. The average membership probabilities of the five classes ranged from 0.86 to 0.94. The mean profiles of the observed and model-predicted ADL disabilities of the five trajectories across seven 18-month intervals are presented in Figure 1. We operationally defined these trajectories as independent, developing, low, progressive and high disability.
Figure 1.
Latent Class Trajectories of Activities of Daily Living Across Seven 18-Month Follow-up Intervals Among 754 Community-living Older Persons Solid line: Observed ADL disability (None=no disability, Mild=1 or 2 disabilities, Severe=3 or 4 disabilities) at each month of an 18-month interval averaged across all seven intervals Dashed line: Predicted ADL disability status (None=No disability, Mild=1 or 2 disabilities, Severe=3 or 4 disabilities) at each month of an 18-month interval, calculated using the derived parameter estimates from the generalized growth mixture model
Three classes demonstrated relatively stable ADL trajectory over time, with. 61.6% of person-intervals in the independent, and 13.6% and 7.0% in low and high disability classes, respectively. The two remaining trajectories represented transitions between states of disability, with the developing class depicting movement from independent to low disability (11.2%) and the progressive class showing movement from low to high disability (6.6%).
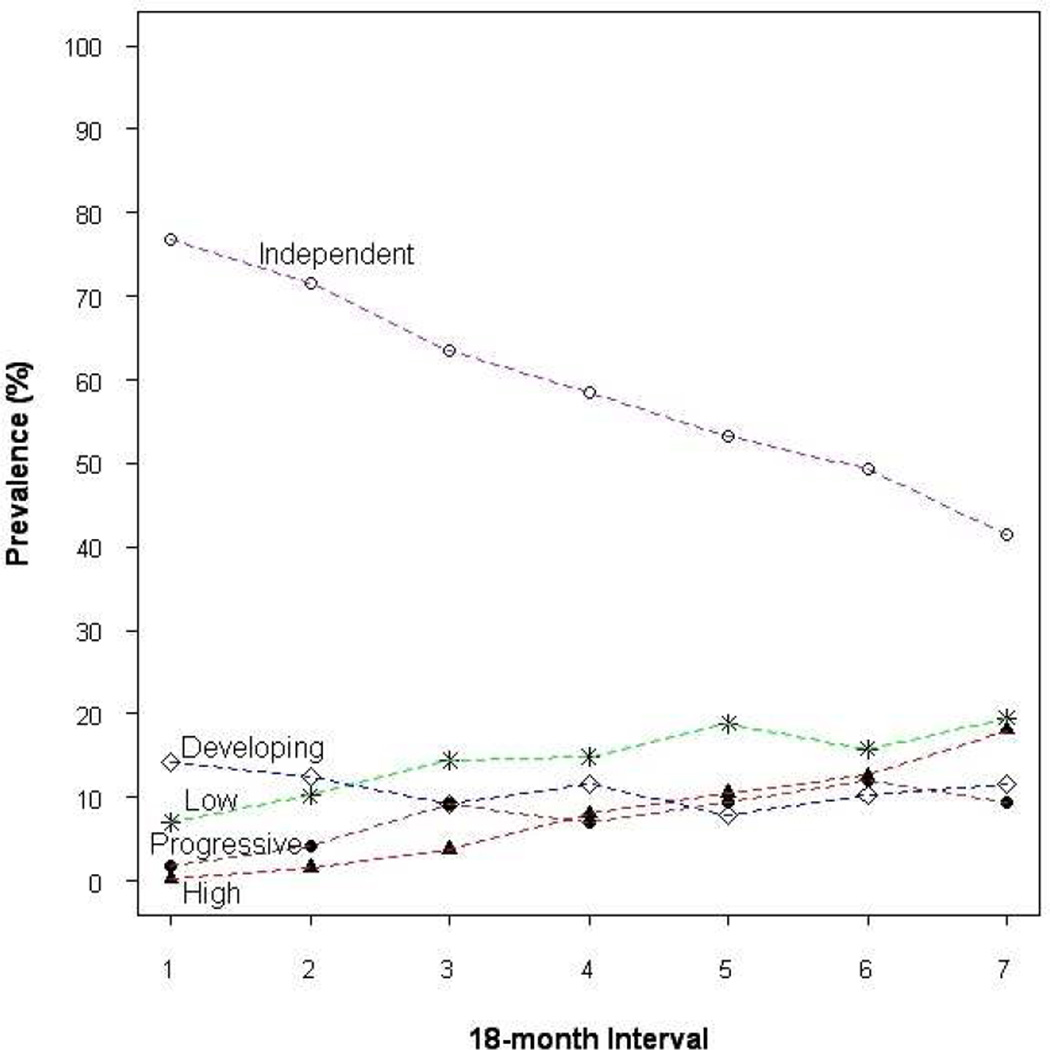
When each 18-month interval was examined separately, all five trajectory classes were retained and the shapes of the trajectory curves were strikingly similar. However, the prevalence of trajectory classes changed over time, most dramatically for the independent (decreased from 76.8% to 41.4%) and the high disability (increased from 0.3% to 18.0%) classes, as demonstrated in Figure 2.
Figure 2.
Prevalence of Latent Class Trajectories of Activities of Daily Living Over Seven Successive 18-Month Follow-up Interval
Dashed line: Prevalence (%) of Trajectory Classes Over Seven Followw-up Intervals: 0=Baseline to 18 months, 2=18–36 months, 3=36–54 months, 4=54–72 months, 5=72–90 months, 6=90–108 months
The profile of potential risk factors according to the five trajectory classes is presented in Table 2. All factors, except race, differed significantly across the five classes (p<0.05 to 0.001). The prevalence of each risk factor is the lowest in the independent class and the highest in the high disability class.
Table 2.
Risk Factor Profile* of Estimated Latent Class Trajectories of Activities of Daily Living
| Latent Class Trajectories† |
|||||
|---|---|---|---|---|---|
| Risk factor‡ | Independent | Developing | Low | Progressive | High |
| Number of observations (%) | 2477 (61.6) | 452 (11.2) | 547 (13.6) | 280 (7.0) | 265 (6.6) |
| Age, 85 yrs or older | 462 (18.7) | 167 (37.0) | 276 (50.5) | 149 (53.2) | 162 (61.1) |
| Female | 1579 (63.8) | 267 (59.1) | 443 (81.0) | 198 (70.7) | 188 (70.9) |
| Lives alone | 1024 (41.3) | 199 (44.0) | 249 (45.5) | 109 (38.9) | 15 (5.7) |
| Chronic conditions,‖≥ 2 | 1463 (59.1) | 312 (69.0) | 400 (73.1) | 210 (75.0) | 193 (72.8) |
| Cognitive impairment¶ | 209 (8.4) | 86 (19.0) | 164 (30.0) | 128 (45.7) | 207 (78.1) |
| Depression§ | 292 (11.8) | 119 (26.3) | 164 (30.0) | 94 (33.6) | 109 (41.1) |
| Physical frailty** | 748 (30.2) | 276 (61.1) | 486 (88.9) | 239 (85.4) | 263 (99.3) |
Values represent cumulative number (%) of person-intervals assigned to each trajectory class across seven 18-month follow-up intervals.
Estimated using a generalized growth mixture model of three discrete (no, mild and severe) disability states of activities of daily living over seven successive 18-month intervals.
The seven risk factors for functional decline are selected a priori and used as time-dependent predictors of class memberships in the generalized growth mixture model, whose values are updated every 18 months up to 108 months during the follow-up.
Based on 9 self-reported, physician-diagnosed, including hypertension, myocardial infarction, heart failure, stroke, diabetes mellitus, arthritis, hip fracture, chronic lung disease, and cancer (other than minor skin cancers).
Based on a score of < 24 on the Mini-Mental State Examination (MMSE).
Based on a score ≥ 20 on the Center for Epidemiologic Studies Depression scale (CES-D).
Based on a score > 10 seconds on rapid gait test.
Cumulative disability and morbidity burdens according to ADL trajectories
The predicted incidence-density rates for each trajectory are presented in Table 3. As expected, the independent class had the lowest burdens for all the outcome measures, while the high disability class had the highest burden in ADL and concurrent ADL/IADL disability. On the other hand, the progressive disability class had the highest burden of hospitalizations (123.6, 95% CI: 108.4–141.1) and IADL disability (958.3, 949.5–967.3). After accounting for the effects of age, gender and education, the other four trajectory classes had significantly higher incident density rates on all the five outcome measures than the independent class, with adjusted rate ratios ranged from 1.4 (95% CI: 1.2–1.7) for restricted activity in the progressive class to 93.3 (95% CI: 77.9–111.8) for concurrent ADL/IADL disabilities in the high disability class.
Table 3.
Estimated Incidence Density Rates* for Different Measures of Disability and Morbidity Burden According to Latent Class Trajectories of Activities of Daily Living
| Latent Class Trajectories† |
|||||
|---|---|---|---|---|---|
| Outcome Measure | Independent | Developing | Low | Progressive | High |
| ADL disability | 10.0 (8.5, 11.8) |
216.1 (198.3,235.7) |
567.0 (522.6,615.3) |
848.2 (824.8,872.2) |
954.6 (946.8, 962.3) |
| IADL disability | 471.8 (444.3,501.0) |
778.3 (747.9,809.9) |
909.7 (888.3,931.5) |
958.3 (949.5,967.3) |
957.5 (950.0,965.0) |
|
Concurrent ADL and IADL disabilities |
9.2 (7.8,10.9) |
210.5 (192.4,230.2) |
554.9 (510.4,603.5) |
846.5 (823.2,870.7) |
952.2 (944.3,960.1) |
| Hospitalizations | 20.2 (18.4,22.1) |
75.5 (68.1,83.8) |
61.1 (54.1,69.2) |
123.6 (108.4,141.1) |
84.1 (69.3,102.2) |
| Restricted activities | 116.6 (108.0,125.8) |
167.9 (152.0,185.5) |
188.2 (168.1,210.8) |
153.9 (131.7,179.9) |
185.1 (151.1,226.9) |
Abbreviations: ADL, Activities of daily living; IADL, Instrumental activities of daily living.
Values represent the predicted number of events (95% Confidence Intervals) per 1000-person months based on separate GEE Poisson models for each outcome, with empirical standard error accounting for repeated measures on the same persons over seven 18-month intervals.
In comparison to the Independent trajectory (reference), all other classes had significantly higher rates for all the 5 outcomes, with rate ratios (adjusted for age, gender and education) ranging from 1.4 to 93.3 (see text for details).
Based on a generalized growth mixture model of 3 discrete (no, mild and severe) disability states of activities of daily living over seven successive 18-month intervals (see text for details).
Time trend of disability and comorbidity burdens according to trajectory
Over the seven successive 18-month intervals, the independent class demonstrates an average of 4% to 20% increment per year in the estimated incidence density rates with ADL disability and concurrent ADL/IADL disability, and a 7% decrement with restricted activity. For the high class, incidence density rates for restricted activity increases about 20% per year. The other 3 classes only demonstrate a trivial increase in the IADL (low and progressive classes) or concurrent ADL/IADL (progressive class) disability (p<0.05 to 0.001). There was no significant linear trend of change over time in the incidence density rates for hospitalization (p>0.05) regardless of trajectory class.
When tracking each person through seven successive 18-month intervals, 230 (30.5%) of the 754 original cohort members never switched class membership. Among these 230 persons, the five trajectories emerged in the same hierarchy as the overall sample across persons-intervals, i.e., independent (n=188, 81.7%), developing (20, 8.7%), low (11, 4.8%), progressive (9, 3.9%) and high (2, 0.9%) classes. The remaining 524 (69.5%) participants switched class at least once, with the majority (361, 68.9%) having switched only to a class of higher disability and a minority only recovered to a lower disability class (15, 2.9%). Another 28.2% (148) had transitions to both higher and lower disability class. Removal of the last person-intervals for decedents did not materially change the estimated relationships between trajectory classes and burdens of disability or morbidity; nor did it alter the observed time trends associated with each trajectory group (data available upon request).
DISCUSSION
In this cohort of community-living older persons, we identified five distinct trajectory classes based on longitudinal ADL, with a predominant (62%) trajectory of no ADL decline and a minority (7%) having a trajectory of staying at a high level of disability. The independent class was characterized by the most favorable risk factor profile (e.g., younger age, higher education and lower comorbidities etc) and experienced the lowest burdens of IADL, concurrent ADL/IADL disability and morbidity, whereas the opposite was true for the high disability trajectory. The remaining three trajectories varied in terms of the initial level and slope of the estimated trajectories, as well as in the total disability or morbidity burdens.
The above findings are comparable to other studies that attempted to capture common patterns or latent-classes of disability progression (14, 15). For instance, in Liang study (14), five trajectory groups were identified based on the total count of ADL and IADL disabilities. Of the three stable trajectories, an excellent functional health group included majority (61%) of the cohort members, while two stable disability groups captured 3% (persistent severe disability) and 7% (stable high () disability), respectively. Similarly, Deeg and colleagues (15) classified survivors of an aging cohort in Amsterdam into five 5 course types, with three stable (no, mild and severe ADL limitation) and two unstable or declining trajectories based on cluster analysis. The consistent findings of common longitudinal trajectories of ADL disability seem to support the concept of “universal” latent classes of functional aging that may have clinical and public health implications. For instance, older persons classified into the high disability trajectory at any given time may be those with greatest need for future long-term care, while the independent trajectory may be a prototype for successful or exceptionally healthy aging, especially for the subset of people who remained in this favorable trajectory (14, 25). Likewise, the progressive trajectory may identify older adults entering a downward transition towards functional dependence, possibly due to the deleterious effects of intervening morbidity events, such as hospitalization and restricted activity (11–13). Prompt medical intervention may help halt further functional decline in these persons. Admittedly, the status of the developing and low trajectory classes in terms of morbidity is less clear.
On the other hand, our study distinguishes itself from previous ones with several methodological advances, including modeling ADL disability as a discrete, multinomial outcome, allowing class membership reassignment over time as a function of time-varying risk factors, and mapping of the estimated latent-class trajectories to clinically interpretable measures of disability and morbidity burden. Such advances helped to mitigate technical challenges in fitting complex statistical models of intensive longitudinal data, while attempting to answer clinically relevant questions regarding the common patterns of functional aging and their long-run probability of change. As observed in this study, even for the five seemingly “universal” latent-classes, the membership probability over long-run appears to be dynamic rather than static. The great majority of individuals switched to a different class, usually an unfavorable one, consistent with some previous reports (13, 26). These findings raise the possibility that the five latent-classes may reflect a “dynamic equilibrium” among different underlying forces of the functional aging process, which involves not only disability (especially, ADL and IADL) (27–29), but also morbidity and disease process, as well as the force of mortality (4, 5, 27–30). Further effort is needed to delineate the interrelationship between these different forces, and the potentially diverging paths of progression for morbidity and disability (29, 30).
This study has limitations. To avoid undue computational complexities, we have treated death as a non-informative censoring event. In addition, our measures of morbidity burden based merely on hospitalization and restricted activities may be less optimal for capturing all disease burdens. Finally, some potentially important components of functional aging, such as community mobility (31), were not investigated.
To conclude, the five trajectory classes identified here not only depict distinct paths of ADL disability, but also differentiate one another in terms of the important risk factors for functional decline and the magnitude and time trend of total burdens of disability and morbidity beyond the ADL domain. Therefore, they may serve as useful prototypes for planning clinical, public health and policy interventions. These findings justify the continued effort to delineate the sources of heterogeneity and dynamics of functional aging towards targeted interventions and resource allocation to reduce population burdens of disability in our rapidly aging societies. Future studies using more precise or alternative outcome measures of disability and morbidity (e.g., community mobility and diagnoses-specific hospitalizations etc.) and joint modeling of longitudinal functional outcomes and death are warranted (32).
Acknowledgement
We thank Evelyne A. Gahbauer, M.D., M.P.H. for assistance with data management; Linda Leo-Summers, M.P.H., for assistance with drafts of the figures; Denise Shepard, B.S.N., M.B.A., Andrea Benjamin, B.S.N., Paula Clark, R.N., Martha Oravetz, R.N., Shirley Hannan, R.N., Barbara Foster, Alice Van Wie, B.S.W., Patricia Fugal, B.S., Amy Shelton, M.P.H., Alice; Wanda Carr and Geraldine Hawthorne, B.S., for assistance with data collection or entry; Peter Charpentier, M.P.H., for development of the participant tracking system; Joanne McGloin, M.Div., M.B.A., for leadership and advice as the project director.
Funding sources: The study was conducted at the Yale Claude D. Pepper Older Americans Independent Center (P30AG21342) and supported by grants from the National Institute on Aging (Lin R01AG031850-01A1 and Gill R37AG17560). Dr. Gill is the recipient of a Midcareer Investigator Award in Patient-Oriented Research (K24AG021507) from the National Institute on Aging.
Abbreviations
- ADL
Activities of daily living
- BIC
Bayesian information criterion
- CES-D
Center for epidemiologic studies-depression scale
- CI
Confidence interval
- GEE
generalized estimating equation
- IADL
Instrumental activities of daily living
- Mini-Mental State Examination
Mini-Mental State Examination
- SD
Standard deviations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Center for Health Statistics. Health, United States, 2004. Hyattsville, MD: 2004. [PubMed] [Google Scholar]
- 2.Fries JF. Aging, natural death and the compression of morbidity. New Eng J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 3.Fries JF. Measuring and monitoring success in compressing morbidity. Ann Intern Med. 2003;139:455–459. doi: 10.7326/0003-4819-139-5_part_2-200309021-00015. [DOI] [PubMed] [Google Scholar]
- 4.Olshansky SJ, Rudberg MA, Carnes BA, Cassel BA, Brady JA. Trading off longer life for worsening health: The expansion of morbidity hypothesis. J Aging Heal. 1991;3:194–216. [Google Scholar]
- 5.Rothenberg R, Lentzner HR, Parker RA. Population aging patterns: The expansion of mortality. J Gerontol Soc Sci. 1991;46:S66–S70. doi: 10.1093/geronj/46.2.s66. [DOI] [PubMed] [Google Scholar]
- 6.Crimmins EM, Saito Y, Ingegneri D. Trends in disability-free life expectancy in the United States, 1970–90. Population Develop Review. 1997;23:555–572. [Google Scholar]
- 7.Newman AB, Brach JS. Gender gap in longevity and disability in older persons. Epidemiolo Rev. 2001;23:343–350. doi: 10.1093/oxfordjournals.epirev.a000810. [DOI] [PubMed] [Google Scholar]
- 8.Hardy SE, Allore GA, Guo Z, Gill TM. Explaining the effects of gender on functional transitions in older persons. Gerontol. 2008;54:79–86. doi: 10.1159/000115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crimmins EM, Saito Y. Trends in healthy life expectancy in the United States, 1970–1990: gender, racial and educational differences. Soc Sci Med. 2001;52:1629–1641. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 10.Nelson EA, Dannefer D. Aged heterogeneity: Fact or fiction? The fate of diversity in gerontological research. Gerontologist. 1992;32:17–23. doi: 10.1093/geront/32.1.17. [DOI] [PubMed] [Google Scholar]
- 11.Gill TM, Gahbauer EA, Han L, Allore H. Functional trajectories in the last year of life. N Engl J Med. 2010;362:1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 13.Gill TM, Gahbauer EA, Han L, Allore HG. Factors associated with recovery of prehospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol A Biol Sci Med Sci. 2009;64:1296–303. doi: 10.1093/gerona/glp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang J, Xu X, Bennett JM, Ye W, Quiñones AR. Ethnicity and changing functional health in middle and late life: a person-centered approach. J Gerontol Soc Sci. 2008;65:470–481. doi: 10.1093/geronb/gbp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeg DJH. Longitudinal characterization of course types of functional limitations. Disab Rehabilitation. 2005;27:253–261. doi: 10.1080/09638280400006507. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Muthén B. Latent variable analysis: growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newsciences. Newbury Park, CA: SAGE; 2004. [Google Scholar]
- 19.Hedeker D,Gibbons RD. Application of random-effect pattern-mixture models for missing data in longitudinal studies. Psychol Method. 1997;2:64–78. [Google Scholar]
- 20.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 21.Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 22.McCulloch CE, Searle SR. Generalized, linear and mixed models. New York NY: John Wiley & Sons, Inc; 2001. [Google Scholar]
- 23.Zimmer Z, House JS. Education, income, and functional limitation transitions among American adults; contrasting onset and progression. Int J Epidemiol. 2003;32:108–197. doi: 10.1093/ije/dyg254. [DOI] [PubMed] [Google Scholar]
- 24.SAS OnlineDoc® 9.2 Generalized linear model. Cary, NC, USA: SAS Institute Inc; 2007. [Google Scholar]
- 25.Kivimaki M, Ferrie JE. Epidemiology of healthy ageing and the idea of more refined outcome measures (Editorial) Int J Epidemiol. 2011;40:845–847. doi: 10.1093/ije/dyr114. [DOI] [PubMed] [Google Scholar]
- 26.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291:1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 27.Manton KG. Changing concepts of morbidity and mortality in the elderly population. Milbank Memorial Fund Quarterly/Health and Society. 1982;60:183–244. [PubMed] [Google Scholar]
- 28.Cai L, Lubitz J. Was there compression of disability for older Americans from 1992 to 2003? Demography. 2007;44:479–495. doi: 10.1353/dem.2007.0022. [DOI] [PubMed] [Google Scholar]
- 29.Klijsa B, Mackenbacha JP, Kunst AAE. Future disability projections could be improved by connecting to the theory of a dynamic equilibrium. J Clin Epidemiol. 2011:436–443. doi: 10.1016/j.jclinepi.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Terry DF, Sebastiani P, Andersen SL, Perls TT. Disentangling the roles of disability and morbidity in survival to exceptional old age. Arch Intern med. 2008;168:277–283. doi: 10.1001/archinternmed.2007.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: A cohort of old persons. Ann Intern Med. 2012;22:9–16. doi: 10.1059/0003-4819-156-2-201201170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy T, Han L, Allore HG, Peduzzi PN, Gill TM, Lin H. Treatment of death in the analysis of longitudinal studies of gerontological outcomes. J Gerontol A Biol Sci Med Sci. 2011;66A:109–114. doi: 10.1093/gerona/glq188. [DOI] [PMC free article] [PubMed] [Google Scholar]