Abstract
Purpose.
To test the effect of pazopanib, a tyrosine kinase inhibitor that blocks VEGF and platelet-derived growth factor (PDGF) receptors and c-Kit, on vascular leakage and neovascularization (NV) in the retina.
Methods.
Pazopanib was tested to determine its effect on VEGF-induced vascular permeability via measurement of [3H]mannitol retina to lung (RLLR) and retina to renal leakage ratios (RRLR) and in rho/VEGF mice with subretinal NV. In rabbits, the effect of intravitreal, topical, and systemic pazopanib on VEGF-induced leakage was tested by vitreous fluorophotometry.
Results.
In mice, oral pazopanib (40 mg/kg twice a day [bid]) reduced RLLR (0.84 to 0.58, P = 0.0014) and RRLR (0.55 to 0.30, P = 0.0018) in VEGF-injected eyes. After intraocular injection of VEGF into both eyes, topical pazopanib (10 mg/mL three times a day [tid] for 14 days) reduced RLLR (0.85 vs. 0.56, P = 0.001), RRLR (0.44 vs. 0.28, P = 0.0075), and immunoreactive albumin in the retina compared to values in fellow eye controls. Treatment of one eye of rho/VEGF mice with 10 mg/mL, but not 5 mg/mL, pazopanib tid reduced the mean area of subretinal NV compared to that in fellow eyes (0.0055 vs. 0.0025 mm2, P = 0.020). In rabbits, intravitreal pazopanib suppressed VEGF-induced fluorescein leakage, but topical (10 mg/mL four times a day [qid] or 12 mg/mL bid) had no significant effect. Systemic administration of pazopanib by osmotic pump with or without 10 mg/mL drops tid also failed to suppress VEGF-induced leakage.
Conclusions.
Administration of pazopanib topically or systemically suppressed retinal vascular leakage in mice, but not rabbits. These data suggest differences in the blood–retinal barrier (BRB) of mice and rabbits and indicate that penetration through the outer BRB may be needed for topically administered drugs to exert effects in the retina.
Topical delivery to treat retinal disease is controversial. It is possible in rodents, and we previously found good agreement between results in mice and rabbits, but we now report that this is not the case for pazopanib. This suggests testing topical treatments for retinal disease in more than one species.
Introduction
Diabetic retinopathy is the most common cause of severe and moderate vision loss in working-aged Americans.1,2 Advanced retinopathy complicated by retinal neovascularization (NV) and traction retinal detachment is responsible for most severe loss of vision in diabetics, but diabetic macular edema (DME) is the most prevalent cause of moderate vision loss.
Poor glycemic control is associated with progression of retinopathy,3 indicating that hyperglycemia is a critical insult leading to hemorrhages, microaneurysms, and capillary closure. Capillary closure causes retinal hypoxia, which has long been known to be associated with retinal NV,4 but more recently it has also been linked to DME.5 Hypoxic retina produces high levels of vascular endothelial growth factor (VEGF), which can stimulate retinal NV,6,7 but also causes leakiness of retinal vessels when injected into the vitreous cavity of mice and macular edema when provided by sustained release in the vitreous of primates.8,9 This combination of observations in patients and animal models led to the hypothesis that VEGF plays an important role in the pathogenesis of DME.
An orally active nonselective blocker of VEGF receptors was found to significantly reduce DME, which recurred when the drug was stopped, providing the first suggestion that VEGF antagonists could potentially provide benefit in patients with DME.10 The development of selective antagonists of VEGF allowed for more definitive testing of the hypothesis. Ranibizumab is a Fab fragment of a humanized monoclonal antibody that binds all isoforms of VEGF-A with high affinity. In a small open-label study in DME patients, it was found that four intraocular injections of 0.5 mg ranibizumab over the span of 7 months resulted in a mean reduction in excess foveal thickening of 85% and an average improvement in visual acuity greater than two lines.11 This led to controlled clinical trials that have confirmed that intraocular injections of ranibizumab provide substantial benefit in patients with DME.12–14
A potential impediment to the use of ranibizumab is the chronic nature of DME, which requires frequent injections for many years. Antagonists of VEGF that can be delivered by routes other than intraocular injection would be a substantial advance. Pazopanib is a small-molecule kinase inhibitor that blocks VEGF, platelet-derived growth factor (PDGF), and c-Kit.15 Systemic administration of pazopanib causes strong suppression and regression of choroidal NV,16 and topical administration of 5 mg/mL eye drops also significantly suppressed choroidal NV.17 A 5 mg/mL solution of pazopanib was tested in patients with neovascular age-related macular degeneration (NVAMD) and showed evidence of activity, particularly in patients with a high-risk complement factor H allele (M. McLaughlin, GlaxoSmithKline, personal communication). In this study, we sought to determine if topical pazopanib can suppress VEGF-induced leakage or NV in the retina.
Materials and Methods
Animals
Wild-type 4- to 6-week-old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME), rho/VEGF transgenic mice, and 11- to 12-week old GD79b pigmented rabbits (Grimaud Frères, Roussay, France) were treated in accordance with the European Convention on Animal Protection for Scientific Experimentation and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice and rabbits were maintained in a 12-hour light/dark cycle.
Treatment of C57BL/6 Mice with Pazopanib
Female C57BL/6 mice weighing 15 to 20 g were given pazopanib (40 mg/mL) or vehicle gavage twice a day (bid) for 2 days before intraocular injection of 1 μL 10−6 M human recombinant VEGF165 (VEGF; R&D Systems, Minneapolis, MN) into one eye with a Harvard pump microinjection apparatus and pulled glass micropipettes as previously described.18 Alternatively, mice were dosed topically via delivery of a single 10 μL drop three times a day (tid) for 2 weeks. In some experiments, both eyes were treated with either 5 or 10 mg/mL pazopanib, and these mice were compared with littermates in which both eyes were treated with vehicle. In other experiments, one eye was treated with 5 or 10 mg/mL pazopanib and the fellow eye with vehicle, and 1 μL 10−6 M human VEGF was injected into both eyes.
Measurement of Blood–Retinal Barrier (BRB) Breakdown Using [3H]Mannitol as Tracer
Retinal vascular leakage was measured with [3H]mannitol by a previously described technique.19 The technique compares [3H]mannitol leakage into the retina to leakage into the lung, providing the retina to lung leakage ratio (RLLR), or into the kidney (retina to renal leakage ratio, RRLR), giving internal controls for the amount of [3H]mannitol injected into each mouse. Briefly, 6 hours after intraocular injection of VEGF, mice were given an intraperitoneal injection of 1 mCi/g body weight [3H]mannitol (Perkin Elmer, Waltham, MA). After 1 hour, mice were euthanized, eyes were removed, the cornea and lens were removed, and the entire retina was carefully dissected from the eye cup and placed in preweighed scintillation vials. The thoracic cavity was opened, and the left superior lobe of the lung was removed and placed in another preweighed scintillation vial. A left dorsal incision was made, and the retroperitoneal space was entered without entrance into the peritoneal cavity. The renal vessels were clamped with a forceps, and the left kidney was removed, cleaned of all fat, and placed into a preweighed scintillation vial. All liquid was removed from the vials, and remaining droplets were allowed to evaporate over 20 minutes. The vials were weighed and the tissue weights recorded. One milliliter NCSII solubilizing solution (GE Healthcare, Piscataway, NJ) was added to each vial, and the vials were incubated overnight in a 50°C water bath. The solubilized tissue was decolorized with 20% benzoyl peroxide in toluene, and 5 mL Scintiverse II (Fisher Scientific, Pittsburgh, PA) and 30 mL glacial acetic acid were added to each vial. The vials were stored for several hours in darkness at 4°C to eliminate chemiluminescence. Radioactivity was counted with a Wallac 1409 Liquid Scintillation Counter (model 1409; Wallac, Gaithersburg, MD).
Assessment of BRB Breakdown by Immunohistochemical Staining for Albumin
Immunohistochemical staining for albumin was done on 10 μm frozen sections from 4% paraformaldehyde-fixed eyes as previously described.9 Slides were rinsed in PBS for 10 minutes and blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) in PBS for 1 hour in a humidified chamber. Excess fluid was removed, and the sections were incubated overnight at 4°C with a 1:200 dilution of goat anti-mouse albumin antiserum (Bethyl Laboratories, Montgomery, TX) and 1% BSA in PBS. Sections were rinsed three times with PBS and subsequently incubated with a 1:500 dilution of FITC-GSA lectin (Vector Labs, Burlingame, CA), a 1:500 dilution of rabbit anti-goat Alexa Fluor 594 (Jackson ImmunoResearch Laboratories, West Grove, PA), and 1% BSA in PBS for 2 hours at room temperature. After three rinses with PBS, the sections were mounted with Vecta Shield (Vector Labs), examined with a Nikon microscope, and captured as digital files with the Nikon Digital Still Camera DXM1200 (Nikon Instruments, Inc., New York, NY).
Assessment of Effects of Pazopanib on VEGF-Induced Retinal NV
Hemizygous rhodopsin/VEGF transgenic mice that express VEGF in photoreceptors20,21 had topical application of 10 μL 5 or 10 mg/mL pazopanib drops in one eye and vehicle in the fellow eye tid between postnatal day (P)7 and P21. At P21, the mice were anesthetized and perfused with fluorescein-labeled dextran (2 × 106 average molecular weight; Sigma-Aldrich), and retinal flat mounts were examined by fluorescence microscopy (Axioskop 2 Plus; Zeiss, Thornwood, NY) at 400× magnification. This provides a narrow depth of field so that when NV along the outer edge of the retina is brought into focus, the remainder of the retinal vessels are out of focus, allowing easy delineation and quantification of the NV. Images were digitized with a three-color charge-coupled device video camera (CoolSNAP-Pro; Media Cybernetics, Silver Spring, MD) and a frame grabber. Image analysis software (Image-Pro Plus 5.0; Media Cybernetics) was set to recognize fluorescently stained NV and used to calculate the total area of NV per retina. The investigator performing image analysis was masked with respect to treatment group.
Treatment of Rabbits with Pazopanib
Rabbits were anesthetized with intramuscular ketamine/zylazine, and three 2ML1 Alzet osmotic pumps (Durect Corporation, Cupertino, CA) containing a 10 mg/mL pazopanib formulation (GlaxoSmithKline, Upper Providence, PA) were implanted subcutaneously between the scapulae with the flow moderator pointing away from the skin incision. Other rabbits were anesthetized; pupils were dilated; the conjunctiva was cleaned with povidone-iodine; and 50 μL pazopanib, vehicle, or 4% triamcinolone acetonide (Bristol-Myers Squibb, New York, NY) was injected into the center of the vitreous cavity with a 30-gauge (pazopanib and vehicle) or 27-gauge (triamcinolone) needle. For topical administration, a micropipette was used to deliver 30 μL pazopanib or vehicle to the eye.
Induction and Measurement of Retinal Vascular Leakage in Rabbits
Retinal vascular leakage was induced by intravitreal injection of 500 ng human recombinant VEGF165 in 50 μL PBS with carrier protein. The left eye served as untreated control. After 48 hours, vitreous fluorophotometry was done on both eyes with an FM-2 Fluorotron Master (OcuMetrics, Mountain View, CA). One hour prior to vitreous fluorophotometry, 50 mg/kg sodium fluorescein was injected intravenously. Rabbits were anesthetized, pupils were dilated, and a series of scans was performed; these consisted of measurements of fluorescence every 0.25 mm from the surface of the retina to the cornea along the visual axis. Quantification of vascular leakage with the Fluorotron (OcuMetrics) was accomplished with a 148-step series of scans (0.25 mm step size) from the retina to the cornea along the optical axis. Each complete scan revealed two distinct peaks for cornea/aqueous humor and retina/vitreous separated by a distinct valley (lens). The area under the curve (AUC) of the entire posterior peak (vitreoretinal compartment) was determined using the trapezoidal rule (Excel macro function; Microsoft, Redmond, WA) and expressed in arbitrary units of fluorescence times the number of scan steps (distance). The ratio of AUCs between the VEGF-injected right eye and the uninjected left eye was used to control for background fluorescence. Statistical analysis was carried out using analysis of covariance (ANCOVA); right eye log10(AUC) was analyzed with left eye log10(AUC) serving as a continuous covariate.
Measurement of Pazopanib in Plasma
At the appropriate time point, approximately 2 mL blood was sampled from the jugular vein using K2EDTA as the anticoagulant. Plasma was obtained from blood via centrifugation and stored at −80°C or on dry ice prior to analysis. Pazopanib was extracted from 50 mL plasma by protein precipitation and analyzed using high-performance liquid chromatography tandem mass spectrometry (LC/MS/MS) with a TurboIonspray interface and positive-ion, multiple-reaction monitoring (AB Sciex, Foster City, CA). The lower limit of quantitation for pazopanib was 1.0 ng/ml.
Results
Systemic Pazopanib Suppresses VEGF-Induced BRB Breakdown in Mice
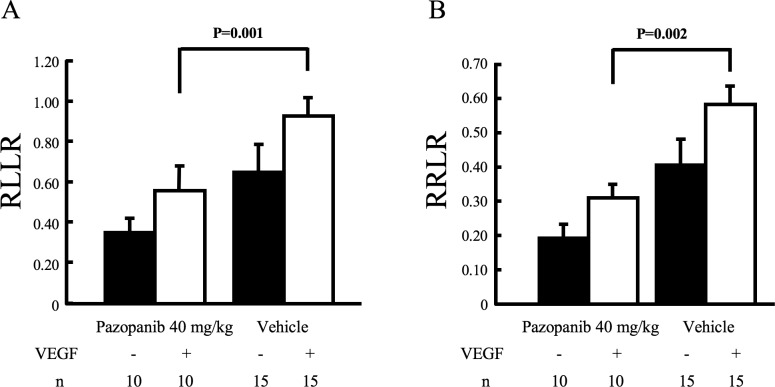
Intraocular injection of VEGF causes retinal vascular leakage like that seen in DME, and the amount of leakage can be quantified by comparing how much [3H]mannitol leaks into the retina versus the lung (RLLR) or the kidney (RRLR).19 In eyes injected with VEGF, oral administration of 40 mg/kg pazopanib bid for 2 days caused a significant reduction in the RLLR and RRLR, to 0.58 and 0.31 compared to 0.90 and 0.55 in eyes injected with vehicle (Fig. 1; P = 0.001 and P = 0.002 respectively).
Figure 1. .
Effect of orally administered pazopanib on VEGF-induced retinal vascular leakage. Mice were treated bid by gavage with vehicle or vehicle containing 40 mg/kg pazopanib bid for 2 days and then given an intravitreous injection of 1 μL 10−6 M VEGF in one eye. Six hours after injection, an intraperitoneal injection of 1 mCi/g body weight [3H]mannitol was given; after 1 hour, the RLLR and RRLR were measured as described in Methods. Eyes injected with VEGF from mice treated with pazopanib had a significant reduction (unpaired t-test) in RLLR (A) and RRLR (B).
Topical Pazopanib (10 mg/ml) Tid Suppresses VEGF-Induced BRB Breakdown in Mice
Compared to eyes of control mice that were injected with VEGF and treated with vehicle eye drops, eyes of mice injected with VEGF and given topical administration of 5 mg/mL pazopanib tid had no significant difference in RLLR or RRLR (Figs. 2A, 2B). However, eyes injected with VEGF and treated tid with 10 mg/mL pazopanib for 14 days had a significant reduction in RLLR and RRLR compared to their corresponding control groups (Figs. 2C, 2D). These data indicate that eye drops containing 10 mg/mL pazopanib are able to suppress VEGF-induced leakage, but do not indicate whether the effect occurs from local penetration into the eye or systemic absorption and delivery through the circulation. To distinguish between these possibilities, intraocular injections of VEGF were given in both eyes; 10 mg/mL drops of pazopanib were given tid in one eye, and vehicle drops were given in the fellow eye. Compared to fellow eyes, pazopanib-treated eyes had a significant reduction in RLLR (Fig. 2E, 0.88 vs. 0.55, P = 0.001) and RRLR (Fig. 2F, 0.48 vs. 0.28, P = 0.008).
Figure 2. .
Effect of topical administration of pazopanib on VEGF-induced retinal vascular leakage. Mice were given 10 μL drops of 5 mg/mL (A, B) or 10 mg/mL (C, D) pazopanib tid for 2 weeks or vehicle in both eyes, and then 1 μL 10−6 M VEGF was injected into one eye. In another group of mice, one eye was treated with 10 mg/mL pazopanib, the fellow eye was treated with vehicle, and 1 μL 10−6 M VEGF was injected into both eyes (E, F). Six hours after injections, the RLLR and RRLR were measured as described in Methods. In eyes injected with VEGF, those treated with 5 mg/mL pazopanib showed no significant difference in RLLR (A) or RRLR (B), but those treated with 10 mg/mL pazopanib had significant reductions in RLLR (C) and RRLR (D). In mice given bilateral injections of VEGF, eyes treated with 10 mg/mL pazopanib had a significant reduction in RLLR (E) and RRLR (F) compared to fellow eyes treated with vehicle. Statistical comparisons by unpaired t-test.
This quantitative analysis clearly shows that pazopanib drops can penetrate into the mouse eye and suppress VEGF-induced leakage provided that the frequency of administration and dose are sufficient. Immunohistochemical staining for serum albumin is a useful adjunctive technique because it allows assessment of leakage of a larger molecule (50 kD) than mannitol and can allow visual assessment of the effect on leakage.22,23 In eyes given an intraocular injection of vehicle, there was mild staining for albumin particularly adjacent to superficial blood vessels (Fig. 3A). In eyes given an intraocular injection of 1 μL 10−6 VEGF, there was a marked increase in albumin staining particularly adjacent to blood vessels throughout the retina (Fig. 3B). The staining was eliminated when the primary antibody was left out of the staining procedure (Fig. 3C). In mice given bilateral intraocular injections of VEGF, eyes treated tid with 10 mg/mL pazopanib drops (Fig. 3D) showed less immunoreactive albumin in the retina compared to fellow eyes treated with vehicle drops (Fig. 3E).
Figure 3. .
Topical pazopanib reduces immunoreactive albumin in the retina of eyes injected with VEGF. Adult C57BL/6 mice were given no injection (A) or a 1 μL intraocular injection of 10−6 M VEGF (B, C), and after 6 hours they were perfused with PBS. Frozen ocular sections were stained with Griffonia simplicifolia (GSA) lectin, which stains blood vessels (green), and immunohistochemically stained for albumin (orange). A section from an uninjected eye shows some staining for albumin in the choroid, but none in the retina (A), while a section from a VEGF-injected eye shows albumin staining in the choroid and in the retina adjacent to retinal vessels (B). There was no staining when the primary antibody was eliminated (C). Another group of mice were given 10 mg/mL pazopanib eye drops in one eye and vehicle eye drops in the other eye tid for 14 days, and then given an intraocular injection of 1 μL 10−6 M VEGF in each eye. Six hours after injection, sections from pazopanib-treated eyes showed staining for albumin in the choroid, but none in the retina (D), while vehicle-treated eyes showed staining for albumin around retinal blood vessels (E). High magnification shows albumin in the wall of vessels and in surrounding retina (F).
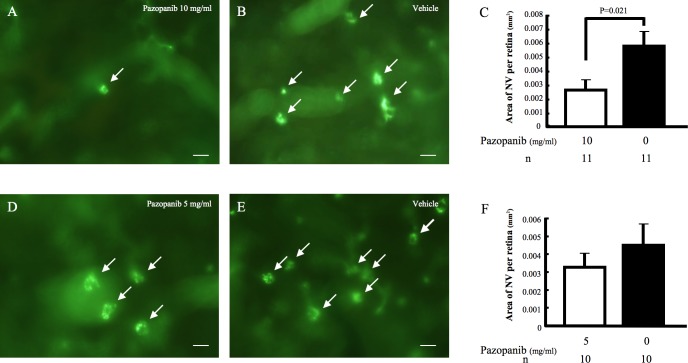
Topical Pazopanib (10 mg/ml) Tid Suppresses VEGF-Induced Subretinal NV in rho/VEGF Transgenic Mice
Rho/VEGF transgenic mice have sustained expression of VEGF within the retina.20 Compared to fellow eyes treated with vehicle drops, those treated tid with 10 mg/mL pazopanib drops had a significant reduction in the mean area of subretinal NV (P = 0.0205, Figs. 4A–C). Treatment with 5 mg/mL pazopanib drops tid did not cause a significant reduction in the area of subretinal NV.
Figure 4. .
Topical pazopanib suppresses VEGF-induced subretinal neovascularization (NV) in rhodopsin promoter/VEGF (rho/VEGF) transgenic mice. Hemizygous rho/VEGF mice were given 10 mg/mL or 5 mg/mL pazopanib eye drops tid in one eye and vehicle in the fellow eye between postnatal day (P)7 and P21. At P21, the mice were perfused with fluorescein-labeled dextran, and retinal flat mounts were examined by fluorescence microscopy. Retinas from eyes treated with 10 mg/mL pazopanib drops (A) showed many fewer tufts of NV than fellow eyes (B). Retinas from eyes treated with 5 mg/mL (C) also appeared to show some reduction in number of NV lesions compared to fellow eyes (D). Image analysis with the investigator masked with respect to treatment group showed that in comparison to fellow eyes, the mean (±SEM) area of NV per retina was significantly less for eyes treated with 10 mg/mL pazopanib but not for those treated with 5 mg/mL (E). Statistical comparisons were made by unpaired t-test.
Intravitreal Injection of Pazopanib Suppresses VEGF-Induced BRB Breakdown in Rabbits
We sought to compare the findings in mice with those in rabbits, which have an eye much closer in size to human eyes. Rabbits were given a 50 μL intravitreal injection of 250 μg pazopanib, 200 μg triamcinolone acetonide, or vehicle into the right eye. At 10, 31, or 87 days after the original injection, both eyes were given a 50 μL intravitreal injection of 500 ng VEGF, and vitreous fluorophotometry was done 48 hours later. The mean ratio of fluorescein leakage into pazopanib-treated versus untreated eyes was significantly less than the mean ratio of fluorescein leakage into vehicle-injected versus untreated eyes at 10 days, but not at 31 or 87 days after pazopanib injection (Fig. 5). The mean ratio of fluorescein leakage into triamcinolone-treated versus untreated eyes was significantly less than the mean ratio of fluorescein leakage into vehicle-injected versus untreated eyes at all three time points.
Figure 5. .
Intravitreous pazopanib suppresses VEGF-induced retinal vascular leakage in rabbits. Rabbits (n = 5–6 per group except for ranibizumab, for which n = 10) were given an intravitreous injection of 250 μg pazopanib, 2 mg triamcinolone acetonide, or vehicle followed in 8, 29, or 85 days by an intravitreous injection of 500 ng VEGF. Ranibizumab was administered intravitreously at a dose of 167 μg (human clinical dose scaled to the smaller rabbit eye) followed by intravitreous human VEGF 8 days later. Fluorescein angiograms were obtained (except for ranibizumab) just prior to vitreous fluorophotometry, which was performed 48 hours after injection of VEGF (day 10, 31, or 87). The bars (A) represent the mean (±SEM) ratio of fluorescein content (area under the curve; AUC) in the vitreoretinal compartment of the VEGF-injected right eye to that in the VEGF-naïve left eye. (B) Representative fluorescein angiograms from each group except ranibizumab (not done). *P < 0.05 versus vehicle; **P < 0.01 versus vehicle; #P < 0.05 versus triamcinolone; ##P < 0.01 versus triamcinolone by ANCOVA.
Topical Pazopanib Does Not Suppress VEGF-Induced BRB Breakdown in Rabbits
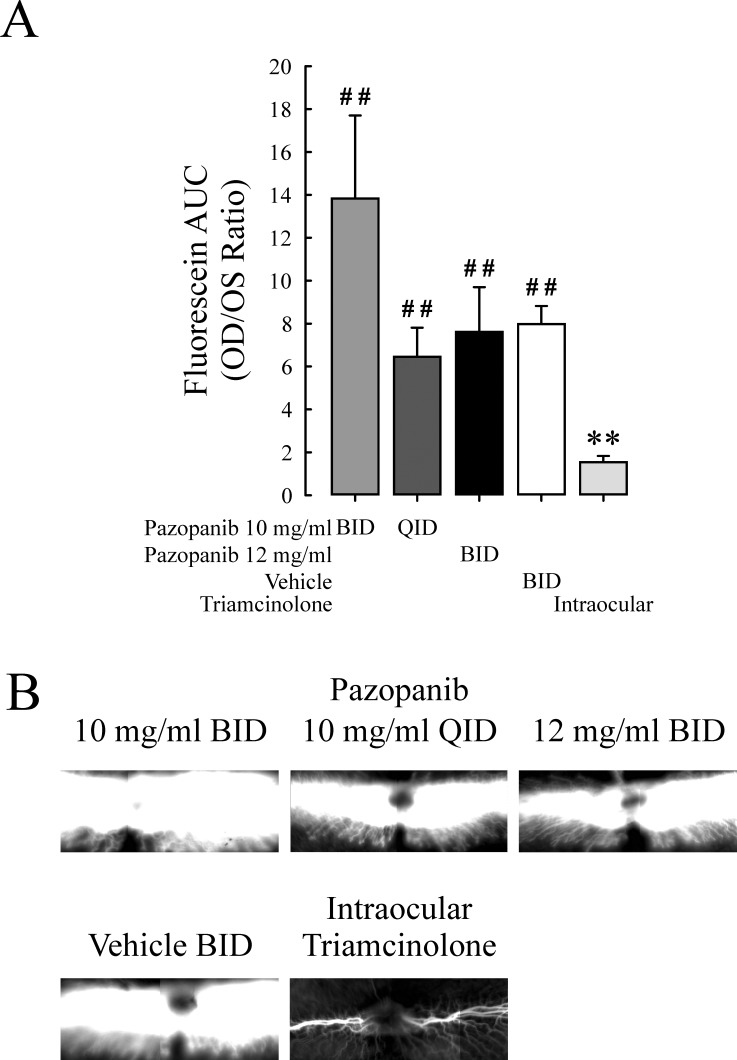
Rabbits were given topical administration of pazopanib according to one of three regimens (10 mg/mL bid, 10 mg/mL four times a day [qid], or 12 mg/mL bid) or vehicle in one eye for 28 days, and then both eyes were given a 50 μL intravitreal injection of 500 ng VEGF. The topical treatment was continued, and 48 hours later vitreous fluorophotometry was done. Another group of rabbits was given an intraocular injection of 200 μg triamcinolone acetonide 28 days prior to injection of VEGF. Compared to eyes treated topically with vehicle, there was no significant difference in fluorescein leakage in eyes treated topically with pazopanib with any of the three regimens (Fig. 6). There was a significant reduction in fluorescein leakage in triamcinolone-injected eyes compared to all the other groups.
Figure 6. .
Topical pazopanib does not suppress VEGF-induced retinal vascular leakage in rabbits. Rabbits (n = 7–8 per group) were treated bid with topical administration of 10 or 12 mg/mL pazopanib in one eye and vehicle in the fellow eye, or given an intraocular injection of 2 mg triamcinolone acetonide. After 26 days, an intravitreous injection of 500 ng of VEGF was given in each eye. Fluorescence angiograms were obtained just prior to vitreous fluorophotometry, which was performed 48 hours after injection of VEGF, that is, on day 28. The bars (A) represent the mean (±SEM) ratio of fluorescein content (area under the curve; AUC) in the vitreoretinal compartment of the VEGF-injected right eye to that of the VEGF-naïve left eye. (B) Representative fluorescein angiograms from each group. *P < 0.05 versus vehicle; **P < 0.01 versus vehicle; #P < 0.05 versus triamcinolone; ##P < 0.01 versus triamcinolone by ANCOVA.
Sustained Systemic Delivery of Pazopanib with or without Topical Pazopanib Does Not Suppress VEGF-Induced Leakage in Rabbits
Rabbits (n = 20) had implantation of osmotic pumps containing 10 mg/mL; half of them were also treated topically in one eye with 10 mg/mL pazopanib tid for 13 days prior to intraocular injection of VEGF followed by vitreous fluorophotometry 48 hours later. One control group was implanted with an osmotic pump containing vehicle and was given vehicle eye drops tid, and another control group was given an intraocular injection of 200 μg triamcinolone acetonide in one eye. On day 15, plasma concentration of pazopanib was 50.6 ± 8.77 ng in the pazopanib pump/pazopanib eye drops group and 38.0 ± 6.05 ng in the pazopanib pump group, but neither group had a significant reduction in fluorescein leakage compared to the vehicle pump/vehicle eye drops group (Fig. 7). Rabbits that had received an intraocular injection of triamcinolone had significantly less fluorescein leakage than each of the other groups.
Figure 7. .
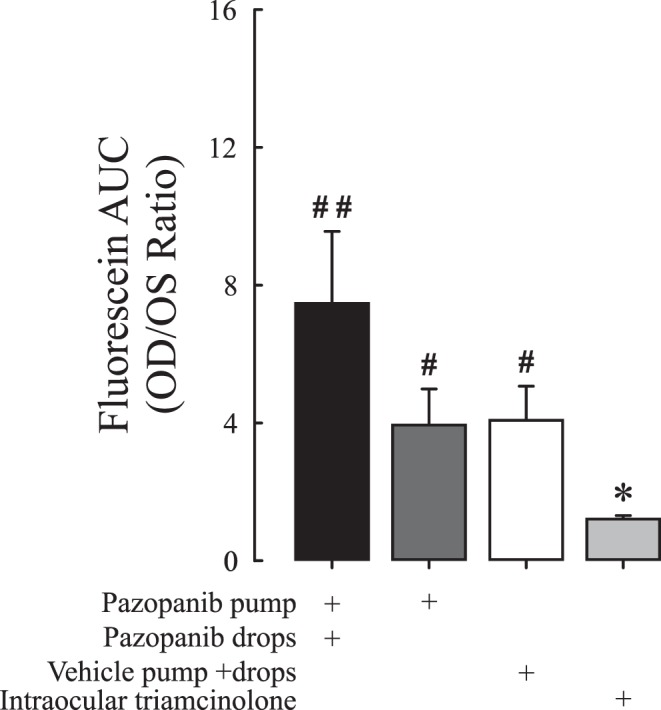
Systemic administration of pazopanib by osmotic pump with or without topical pazopanib does not suppress VEGF-induced retinal vascular leakage in rabbits. Rabbits (n = 8–10 per group) were given continuous delivery of pazopanib by osmotic pump alone or combined with topical administration of 10 mg/mL pazopanib tid. Control rabbits had implantation of a pump filled with vehicle and were given vehicle eye drops tid. Positive controls were given an intraocular injection of 2 mg triamcinolone acetonide. After 11 days, an intravitreous injection of 500 ng VEGF was given in each eye. Vitreous fluorophotometry was done 48 hours after injection of VEGF, that is, on day 13. No fluorescence angiograms were collected in this study. The bars represent the mean (±SEM) ratio of fluorescein content (area under the curve; AUC) in the vitreoretinal compartment of the VEGF-injected right eye to that of the VEGF-naïve left eye. *P < 0.05 versus vehicle; **P < 0.01 versus vehicle; #P < 0.05 versus triamcinolone; ##P < 0.01 versus triamcinolone by ANCOVA.
Discussion
Pazopanib is a small-molecule inhibitor of VEGF signaling that also blocks PDGF and c-Kit.15 An oral formulation of pazopanib has been approved for treatment of renal cell carcinoma and soft tissue sarcoma. Systemic pazopanib suppresses and causes regression of choroidal NV and could be considered in patients with NVAMD16; however, the tolerability of side effects is less for nonlethal ocular diseases compared to cancer. Pazopanib eye drops carry much less risk of systemic side effects, and an early-phase clinical trial suggested effects from 5 mg/mL pazopanib eye drops in patients with NVAMD (M. McLaughlin, GlaxoSmithKline, personal communication). After topical administration of pazopanib in normal animals, drug levels in the choroid are substantially higher than those in the retina (unpublished data), suggesting that the route of entry into the eye involves penetration through the sclera into the choroid and then into the retina. This has raised questions on the potential of utilizing topical pazopanib for retinal diseases such as DME. In this study, we found that 10 mg/mL pazopanib eye drops, but not 5 mg/mL drops, significantly reduced VEGF-induced retinal vascular leakage and VEGF-induced subretinal NV in mice. Studies identical to those reported here, examining retinal leak and NV after dosing of topical pazopanib for 28 days, confirm the findings of the shorter studies (data not shown) indicating that only the 10 mg/mL concentration of pazopanib is able to suppress both vascular leak and NV in the mouse retina. Topically administered pazopanib was also found to reduce leukostasis and retinal vascular leakage in diabetic rats.24 These data provided support for topical administration of pazopanib for treatment of retinal diseases, but before proceeding, studies in the larger rabbit eye were performed. These studies showed that topical administration of 10 mg/mL qid or 12 mg/mL bid pazopanib failed to suppress retinal vascular leakage induced by intravitreal injection of 500 ng VEGF into the rabbit eye. Even when combined with systemic delivery of pazopanib by osmotic pump, topical administration of 10 mg/mL tid had no significant effect on leakage. The lack of effect of systemic pazopanib in rabbits raises additional questions. Is the lack of effect of topical pazopanib in rabbits, despite its effect in mice, due to the larger size of the rabbit eye or to a difference in the BRB between rabbits and mice? Rabbit RPE may prevent pazopanib given topically or systemically from accessing the retina, while mouse RPE does not. Also, intraocular injection of 500 ng VEGF into a rabbit eye may not provide an appropriate model of human DME, since it results in intraocular levels of VEGF that are substantially higher; but lower doses of VEGF in rabbit eyes result in too much variability to allow assessment of the efficacy of an antagonist.
It has generally been felt that it is not possible to achieve adequate retinal drug levels after topical administration to treat retinal diseases. However, recent studies have suggested otherwise. Nepafenac, a prodrug for the nonsteroidal anti-inflammatory agent amfenac, reduces prostaglandin synthesis in the choroid by 50% at 4 hours after a single drop applied topically to the eyes of rabbits.25,26 In mice, compared to fellow eye controls, topical application of 0.1% or 0.3% nepafenac drops significantly reduced expression of VEGF and decreased ischemia-induced retinal NV and choroidal NV at laser-induced rupture sites in Bruch's membrane.27 These results have been confirmed by demonstration that topical nepafenac suppresses retinal NV in a rat model of ischemic retinopathy28 and microvascular changes in diabetic rats.29 There is also a case series suggesting possible benefit from topical nepafenac in patients with DME.30 A clinical trial testing the effect of topical nepafenac in patients with foveal-sparing DME is ongoing.
Systemic delivery of 4-chloro-3-{5-methyl-3-[4-(2-pyrrolidin-1-yl-ethoxy)phenylamino]benzo[1,2,4]triazin-7-yl}phenol (TG100572), a kinase inhibitor that blocks the receptors of VEGF, PDGF, FGF and several members of the Src kinase family,31 caused strong suppression of choroidal NV in mice.32 Although modest levels were achieved in the retina and choroid after topical delivery, penetration was improved using a prodrug, TG100801, which generates TG100572 by de-esterification. After topical administration in rabbits, radiolabeled TG100801 was identified in the posterior sclera, and pharmacokinetic studies showed a tissue gradient with sclera > choroid > retina, suggesting trans-conjunctival and trans-scleral penetration into the choroid and retina. Topically administered TG100801 suppressed laser-induced choroidal NV in mice and reduced retinal edema measured by optical coherence tomography in a rat model of retinal vein occlusion.32 The effectiveness of topical TG100801 was confirmed in other mouse models.33 Mecamylamine is a nicotinic acetylcholine receptor blocker that suppresses choroidal NV after topical administration in mice and has been shown to have biological effects in patients with DME.34,35
Thus, pazopanib joins a growing list of drugs that have shown efficacy in mouse models of choroidal NV and retinal vascular leakage after topical administration. However, whereas the other drugs showed good agreement between mouse efficacy studies and rabbit pharmacokinetic or efficacy studies, this was not true for pazopanib. Neither topical nor systemic administrations were able to reduce VEGF-induced leakage in rabbits; thus it appears that pazopanib, whether given topically or systemically, has difficulty crossing the BRB in rabbits. This suggests that penetration through the outer BRB may be a requirement for access to the retina, and testing in multiple animal models may be needed to determine if a drug has this capability.
Footnotes
Supported by grants from the National Eye Institute (EY012609 [PAC]) the George S. and Dolores Dore Eccles Professor of Ophthalmology.
Disclosure: T. Iwase, None; B.C. Oveson, None; N. Hashida, None; R. Lima e Silva, None; J. Shen, None; A.H. Krauss, GlaxoSmithKline (E); D.C. Gale, GlaxoSmithKline (E); P. Adamson, GlaxoSmithKline (E); P.A. Campochiaro, GlaxoSmithKline (F)
References
- 1. Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984; 102: 520–526 [DOI] [PubMed] [Google Scholar]
- 2. Klein R, Klein B. Vision disorders in diabetes. In: National Diabetes Data Group, ed. Diabetes in America. Washington, DC: National Institutes of Health; 1995: 293–330 [Google Scholar]
- 3. The Diabetes Control and Complications Trial Research Group Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmology. 1995; 102: 647–661 [DOI] [PubMed] [Google Scholar]
- 4. Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981; 88: 601–612 [DOI] [PubMed] [Google Scholar]
- 5. Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, Campochiaro PA. Supplemental inspired oxygen improves diabetic macular edema; a pilot study. Invest Ophthalmol Vis Sci. 2003; 45: 617–624 [DOI] [PubMed] [Google Scholar]
- 6. Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995; 92: 905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ozaki H, Seo M-S, Ozaki K, et al. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000; 156: 679–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozaki H, Hayashi H, Vinores SA, Moromizato Y, Campochiaro PA, Oshima K. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997; 64: 505–517 [DOI] [PubMed] [Google Scholar]
- 9. Saishin Y, Saishin Y, Takahashi K, Melia M, Vinores SA, Campochiaro PA. Inhibition of protein kinase C decreases prostaglandin-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003; 195: 210–219 [DOI] [PubMed] [Google Scholar]
- 10. Campochiaro PA; C99-PKC412-003 Study Group. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci. 2004; 45: 922–931 [DOI] [PubMed] [Google Scholar]
- 11. Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006; 142: 961–969 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen QD, Shah SM, Heier JS, et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology. 2009; 116: 2175–2181 [DOI] [PubMed] [Google Scholar]
- 13. Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology. 2010; 117: 2146–2151 [DOI] [PubMed] [Google Scholar]
- 14. The Diabetic Retinopathy Clinical Research Network Randomized trial evaluating ranibzumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmolgy. 2010; 117: 1064–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007; 6: 2012–2021 [DOI] [PubMed] [Google Scholar]
- 16. Takahashi K, Saishin Y, Saishin Y, King A, Levin R, Campochiaro PA. The multi-targeted kinase inhibitor pazopanib causes suppression and regression of choroidal neovascularization. Arch Ophthalmol. 2009; 127: 494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yafai Y, Yang XM, Niemeyer M. Anti-angiogenic effects of the receptor tyrosine kinase inhibitor, pazopanib, on choroidal neovascularization in rats. Eur J Pharmacol. 2011; 666: 12–18 [DOI] [PubMed] [Google Scholar]
- 18. Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001; 188: 253–263 [DOI] [PubMed] [Google Scholar]
- 19. Derevjanik NL, Vinores SA, Xiao W-H, et al. Quantitative assessment of the integrity of the blood-retinal barrier in mice. Invest Ophthalmol Vis Sci. 2002; 43: 2462–2467 [PubMed] [Google Scholar]
- 20. Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997; 151: 281–291 [PMC free article] [PubMed] [Google Scholar]
- 21. Tobe T, Okamoto N, Vinores MA, et al. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci. 1998; 39: 180–188 [PubMed] [Google Scholar]
- 22. Vinores SA, Gadegbeku C, Campochiaro PA, Green WR. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am J Pathol. 1989; 134: 231–235 [PMC free article] [PubMed] [Google Scholar]
- 23. Vinores SA, Campochiaro PA, Lee A, McGehee R, Gadegbeku C, Green WR. Localization of blood-retinal barrier breakdown in human pathologic specimens by immunohistochemical staining for albumin. Lab Invest. 1990; 62: 742–750 [PubMed] [Google Scholar]
- 24. Thakur A, Scheinman RL, Rao VR, Kompella UB. Pazopanib, a multitargeted tyrosine kinase inhibitor, reduces diabetic retinal vascular leukostasis and leakage. Microvasc Res. 2011; 82: 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000; 24: 357–370 [DOI] [PubMed] [Google Scholar]
- 26. Ke T-L, Graff G, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation. 2000; 24: 371–384 [DOI] [PubMed] [Google Scholar]
- 27. Takahashi K, Saishin Y, Saishin Y, et al. Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci. 2003; 44: 409–415 [DOI] [PubMed] [Google Scholar]
- 28. Yanni SE, Clark ML, Yang R, Bingaman DP, Penn JS. The effects of nepafenac and amfenac on retinal angiogenesis. Brain Res Bull. 2010; 81: 310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kern TS, Miller CM, Du Y, et al. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes. 2007; 56: 373–379 [DOI] [PubMed] [Google Scholar]
- 30. Callanan D, Williams P. Topical nepafenac in the treatment of diabetic macular edema. Clin Ophthalmol. 2008; 2: 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palanki MSS, Akiyama H, Campochiaro P, et al. Development of prodrug 4-chloro-3-(5-methly-3{[4-(2-pyrrolidin-1-ylethoxy)phenyl]amino}-1,2,4-benzotriazin-7-yl)phenyl benzoate (TG100801): a topically administered therapeutic candidate in clinical trials for the treatment of age-related macular degeneration. J Med Chem. 2008; 51: 1546–1559 [DOI] [PubMed] [Google Scholar]
- 32. Doukas J, Mahesh S, Umeda N, et al. Topical administration of a multi-targeted kinase inhibitor suppresses choroidal neovascularization and retinal edema. J Cell Physiol. 2008; 216: 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scheppke L, Aguilar E, Gariano RF, et al. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J Clin Invest. 2008; 118: 2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiuchi K, Matsuoka M, Wu JC, et al. Mecamylamine suppresses basal and nicotine-stimulated choroidal neovascularization. Invest Ophthalmol Vis Sci. 2008; 49: 1705–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Campochiaro PA, Shah SM, Hafiz G, et al. Topical mecamylamine for diabetic macular edema. Am J Ophthalmol. 2010; 149: 839–851 [DOI] [PMC free article] [PubMed] [Google Scholar]