Abstract
Rationale
Ca2+-activated Cl channels (CaCCs) play pivotal roles in the cardiovascular system: they regulate vascular smooth muscle tone and participate in cardiac action potential repolarization in some species. CaCCs were recently discovered to be encoded by members of the Anoctamin (Ano, also called Tmem16) superfamily, but the mechanisms of Ano1 gating by Ca2+ remain enigmatic.
Objective
The objective was to identify regions of Ano1 involved in channel gating by Ca2+.
Methods and results
The Ca2+ sensitivity of Ano1 was estimated from rates of current activation and deactivation in excised patches rapidly switched between zero and high Ca2+ on the cytoplasmic side. Mutation of glutamates E702 and E705 dramatically altered Ca2+ sensitivity. E702 and E705 are predicted to be in an extracellular loop, but antigenic epitopes introduced into this loop are not accessible to extracellular antibodies, suggesting this loop is intracellular. Cytoplasmically-applied membrane-impermeant sulfhydryl reagents alter the Ca2+ sensitivity of Ano1 E702C and E705C, as expected if E702 and E705 are intracellular. Substituted cysteine accessibility mutagenesis of the putative re-entrant loop suggests that E702 and E705 are located adjacent to the Cl conduction pathway.
Conclusions
We propose an alternative model of Ano1 topology based on mutagenesis, epitope accessibility, and cysteine-scanning accessibility. These data contradict the popular re-entrant loop model by showing that the putative 4th extracellular loop (ECL 4) is intracellular and may contain a Ca2+ binding site. These studies provide new perspectives on regulation of Ano1 by Ca2+.
Keywords: chloride channel, calcium, ion channel, patch clamp, transmembrane topology
Introduction
Calcium-activated chloride channels (CaCCs) play vital roles in the cardiovascular system 1–4. In vascular smooth muscle, vasoconstrictor-stimulated mobilization of Ca2+ from intracellular stores opens CaCCs that serve in a positive feedback loop to sustain contraction by depolarizing the membrane and activating Ca2+ influx through voltage-gated Ca2+ channels. In portal vein smooth muscle, Ca2+ sparks generated from ryanodine-sensitive stores can activate CaCCs to generate spontaneous transient inward currents, which depolarize and activate voltage-gated Ca2+ channels. In cardiac myocytes of some species, including rabbit, pig, dog, and sheep, but probably not human, CaCC currents have been shown to play a role in cardiac action potential repolarization and to participate in arrhythmogenesis 5, 6. Recently, it has been proposed that the health benefits (including reduced risk of cardiovascular disease) of red wine and green tea may be explained by the direct effects of gallotannins on CaCCs 7.
Although the cardiovascular significance of CaCCs has long been apparent, understanding how these channels operate has been slow to develop because the molecular identity of CaCCs was not discovered until 2008, when three labs identified Tmem16A, now known as Ano1, as an essential subunit of CaCCs 8–10. Ano1 is a member of a 10-gene superfamily, of which two members, Ano1 and Ano2, have been clearly shown to encode CaCCs that participate in fluid and salt transport by epithelia, slow wave activity in the gut, regulation of smooth muscle contraction, and control of cellular excitability 11–13. Ano1 has been shown to encode CaCCs in vascular smooth muscles 14–16.
Understanding how Ano1 channels operate requires knowledge of how Ca2+ causes the channel to open. Even before CaCCs were identified at the molecular level, it was noticed that the biophyscial properties of CaCC currents depend on Ca2+ concentration: submicromolar Ca2+ activates a current that strongly outwardly rectifies and is time-dependent, whereas higher concentrations activate an instantaneous current with no rectification. This difference is physiologically relevant because it determines, for example, whether epithelial CaCCs function in a secretory or absorptive capacity or neuronal CaCCs carry outward or inward current 17, 18. However, it is enigmatic how voltage- and Ca2+-dependent gating comes about molecularly, largely because the location of the Ca2+ binding site(s) and the anion-selective pore remain unknown.
Studies on the structure-function of Ano1 to date have been guided by a topology model of eight transmembrane α-helices with a re-entrant loop between transmembrane helices 5 and 6. This model is based on hydropathy analysis 19 and experiments performed on Ano7 20. However, the validity of this model for Ano1 has not been experimentally established. This is especially critical because Ano7 has not yet been shown to be a Cl channel and because the amino acid sequences of Ano1 and Ano7 are only 32% identical 11, 21. The putative re-entrant loop has been a prime suspect for the channel pore in Ano1 because the R621E mutation was reported to drastically alter the anion-to-cation selectivity of the channel 8. Experiments described below raised questions in our mind about the location of the pore and stimulated us to re-examine the topology of Ano1 and potential Ca2+ binding sites. Our revised model moves the previously-designated 4th extracellular loop to an intracellular location and identifies a potential Ca2+-binding site in this domain. This model has the attractive feature that it places a Ca2+ binding site immediately adjacent to the pore and provides insights into Ano1 channel gating.
Materials and Methods
Methods have previously been described 18 and are presented in detail in the online supplement. The mAno1 a, c splice variant (Accession: Q8BHY3) was used. Mutations were made using PCR-based mutagenesis. mAno1 was transfected into HEK293 cells using Fugene-6; (Roche Molecular Biochemicals, Indianapolis, IN). Transfected HEK293 cells were patch clamped using conventional whole-cell and excised inside-out patches. The zero Ca2+ intracellular solution contained (mmol/L): 146 CsCl, 2 MgCl2, 5 EGTA, 10 HEPES, 10 sucrose, pH 7.3, adjusted with NMDG. High Ca2+ pipette solution contained 5 mmol/L Ca2+-EGTA, instead of EGTA (free Ca2+ 20 μM). The 126 μM and 2 mmol/L Ca2+ were made by adding 0.2 mmol/L and 2 mmol/L CaCl2 to high-Ca2+ solution. The standard extracellular solution contained (mmol/L): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 15 glucose, 10 HEPES, pH 7.4 with NaOH. Relative anion permeability was determined by measuring the shift in zero-current Erev after changing the bath solution from 151 mmol/L Cl− to 140 mmol/L substitute anion (X−) plus 11 mmol/L Cl−. The permeability (P) ratio was calculated by using the Goldman-Hodgkin-Katz equation. Permeability of Na+ or Cs+ relative to Cl− was determined by measuring changes in zero-current Erev when the concentration of extracellular NaCl or CsCl was changed (“dilution potential” method). The fast application of Ca2+ to excised inside-out patches was performed using a double-barreled theta tubing with a tip diameter of ~50 μm attached to a piezobimorph on a micromanipulator. One barrel was filled with standard zero-[Ca2+]i solution, and the other barrel was filled with intracellular solution containing the indicated free Ca2+. The time course of solution exchange across the laminar flow interface was estimated by liquid junction potential measurements to be 0.5 ms (10–90% rise time) for a 10-fold difference in ionic strength. mAno1 with inserted human influenza hemaglutinin (HA) epitopes at various positions were used to determine the topology by assessing the accessibility of the HA epitope to extracellularly applied antibody. Cells were fixed for 15 min at room temperature in 1% paraformaldehyde in 0.1mol/L phosphate buffer pH 7. Non-permeabilized cells were washed in 3 times in blocking buffer. Permeabilized cells were incubated in blocking buffer containing 0.15% - 0.3% Triton-X100. Cells were incubated with anti-HA antibody diluted 1:750 in blocking buffer for 2 h at room temperature, washed 3 times in blocking buffer, and then incubated in goat-anti-rabbit IgG conjugated with Dylight-549 (Jackson Immunochemicals).
Results
Topology of mAno1
We used 14 different web servers employing different strategies to predict mAno1 transmembrane domains (Online Table I). All algorithms consistently identified 7 segments corresponding to TMDs 1–4 and 6–8 in Fig. 1B (left panel). Two algorithms failed to identify TMD5 and five algorithms identified the proposed re-entrant loop as a TMD. In light of this ambiguity, we wanted to clarify Ano1 topology. We introduced HA epitopes into mAno1-EGFP at various positions (570, 614, 672, 700, or 824, Fig. 1A), expressed the constructs in HEK cells, and then evaluated the accessibility of the introduced epitopes to extracellularly-applied antibodies in permeabilized and non-permeabilized cells by confocal microscopy. We first performed patch clamp recording to verify that the introduction of the epitope did not destroy channel function. All of the constructs exhibited currents characteristic of Ano1 (Fig. 1A, right table) and mAno1-EGFP fluorescence at the membrane (Fig. 1A, left panels) indicating that the HA insertions did not significantly alter the tertiary structure of the channel. In non-permeabilized cells, HA epitopes were accessible to extracellular anti-HA antibody only at positions 614 and 824 (Fig. 1A, middle panels); all the other positions were inaccessible. The inaccessibility of the epitopes introduced at 672 and 700 is not consistent with the topology shown in Fig. 1B (left panel) and suggests that the putative extracellular loop 4 (amino acids 650 – 706) is oriented intracellularly.
Figure 1.
Immunofluorescent staining of mAno1 containing tandem HA epitopes inserted at various locations. (A) HA tags were inserted into mAno1-EGFP at amino acids 570, 614, 672, 700 and 824. After transient expression, non-permeabilzed intact cells were stained with antibody for HA epitope. Green: Ano1-GFP, Red: Anti-HA, and merged image. Duplicate cover slips were permeabilized prior to incubation with HA antibody (red: permeabilized, anti-HA). For each construct, images were acquired at the same gain and settings, but settings may differ between constructs that were imaged on different days. Raw images from the Zeiss Zen acquisition software from permeabilized and non-permeabilized cells were assembled in Adobe Photoshop CS5 and brightness and contrast adjusted for all 4 panels equally. Ano1 currents for each construct were recorded with 20 μmol/L Ca. Average peak amplitude at +100mV and the number of recorded cells are listed. (B) Topological models of mAno1. The locations of HA tags are indicated with red numbers. Left: re-entrant loop model. Right: revised model. The topology of the sequence depicted in grey remains in question.
E702 and E705 contribute to Ca2+ Gating
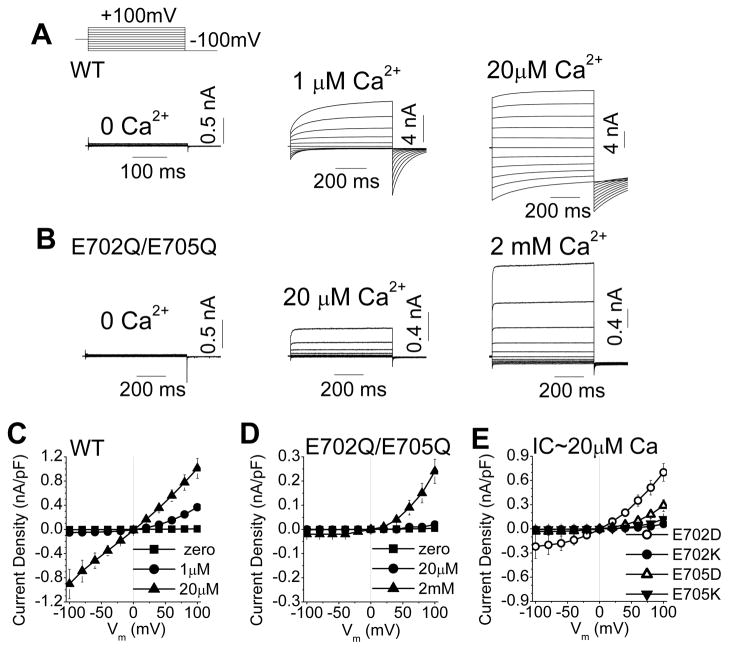
This revised Ano1 topology suggests new possibilities for the mechanism of channel gating by Ca2+. Amino acids 650–706, which were previously thought to form an extracellular loop, contain a sequence that is highly conserved among all members of the Anoctamin superfamily: [E/D]-[Y/F]-[M/L/Q]-E-[M/T/L/Q]. In Ano1 and Ano2, this sequence is invariably 702EYMEM. To test whether this region is involved in channel function, E702 and E705 in mAno1 were replaced with glutamines. This double mutation exhibited an apparent reduction in Ca2+ sensitivity (Fig. 2). The E702Q/E705Q mutant was activated only a small amount by 20 μM Ca2+, a concentration that maximally activated WT Ano1, but E702Q/E705Q was significantly activated by 100-fold higher Ca2+ concentrations (2mM Ca2+). This concentration of Ca2+ had no effect on Cl− currents in untransfected HEK cells (Online Fig. I).
Figure 2.
Mutation of two critical amino acids, E702 and E705, dramatically affects Ca2+-gated but not voltage-gated current of mAno1. (A, B) Representative whole-cell recordings of Ano1 current in transfected HEK293 cells at the indicated free [Ca2+]. Voltage protocol is shown above A. (A) WT-mAno1. (B) E702Q/E705Q mutant mANO1. (C, D) Steady-state current-voltage (I-V) relationships for (C) WT-mAno1and (D) E702Q/E705Q mANO1with different [Ca]i (N=5–9). (E) I–V relationships for E702D, E702K, E705D and E705K mutants with 20 μmol/L Ca2+ (N=5–9).
We have previously shown that Ano1 can be activated by high voltage in the absence of Ca2+ 18. High-voltage-activated currents for E702Q/E705Q and WT were similar in amplitude (WT: 46.1 ± 10.4 pA/pF, n=10; E702Q/E705Q: 45.0 ± 7.6 pA/pF, n=5 at +200 mV; p = 0.66), supporting the suggestion that this mutation mainly affects Ca2+-dependent gating while having little effect on voltage-dependent gating. Furthermore, the current activated by 500 μM Ca2+ was very strongly outwardly rectifying, which is characteristic of WT Ano1 currents that are activated by sub-maximal [Ca2+]. Voltage-dependent activation and deactivation of the current were accelerated, as expected if the apparent affinity of the channel for Ca2+ were decreased 18.
We then examined the effects of mutation of E702 and E705 individually. The conservative E702D substitution produced currents that were similar to WT but with slightly more outward rectification. In contrast, the charge-reversal E702K dramatically decreased Ca2+-activated current (Fig. 2E). Both the conservative E705D and the charge-reversal E705K mutations exhibited markedly reduced Ca2+-dependent activation (Fig. 2E). These results suggest that both E702 and E705 are important in Ca2+ sensing or gating.
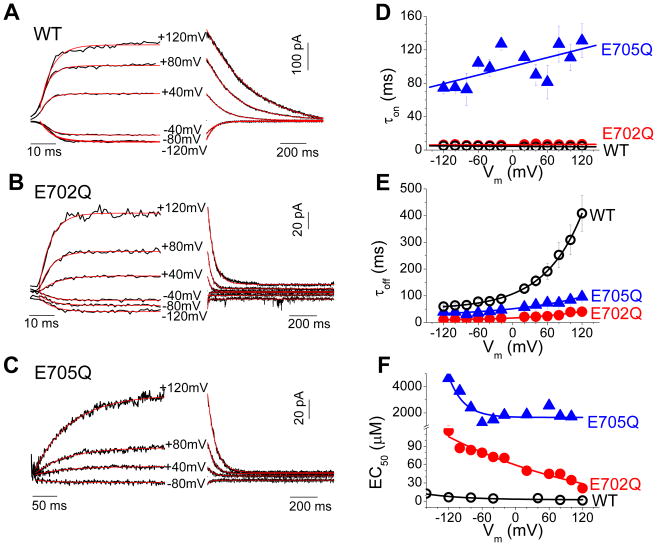
To quantify the effects of E702 and E705 substitutions on Ca2+-dependent gating, we performed experiments in which inside-out excised patches were rapidly switched between zero and high Ca2+ within several ms 18 (Fig. 3). Current decay was well-fit by a monoexponential equation. The time constant of deactivation (τoff), was Vm-dependent and was greatly accelerated by the E702Q and E705Q mutations (Fig. 3E). At +120 mV, τoff was 408.4 ± 67.3 ms for WT, 40.4 ± 4.1 ms for E702Q, and 96.3 ± 6.6 ms for E705Q.
Figure 3.
Activation and deactivation kinetics of Ano1 with rapid Ca2+ perfusion in inside-out excised patches. (A–C) Representative traces of Ano1 current in response to application (left) and washout (right) of Ca2+ at the indicated holding potentials. (A) WT-mAno1. (B) E702Q mANO1. (C) E705Q mANO1. (D-F) Vm dependence of τon, τoff, and EC50 for WT Ano1 (open circles), E702Q (filled circles), and E705Q (filled triangles).
In principle, the process of current deactivation upon switching to zero Ca2+ involves two steps: Ca2+ dissociation from its binding site followed by channel closure. However, the process is very likely dominated by Ca2+ dissociation because τoff is strongly dependent upon the ligand (Ba2+ or Ca2+) used to activate the channel 18 (see Discussion).
The time constant of activation (τon) of Ano1 current upon switching to Ca2+-containing solutions is dependent on [Ca2+], as expected if Ca2+ binding is a rate-limiting step in channel activation 17, 18. At +120 mV with 82 μmol/L [Ca2+], τon for WT current was 5.1 ± 1.1 ms, but was ~25-times slower for E705Q, 131.1 ± 20.2 ms (Fig. 3D). At very high [Ca2+] of 2 mM, τon for E705Q was still slower than wild type (35.9 ± 7.8 ms). The E702Q mutation had a smaller effect on τon than the E705Q mutation (Fig. 3D). τon of E702Q was indistinguishable from WT at 82 μmol/L Ca2+, but under these conditions τon is close to the switching time of the perfusion system and therefore not quantitatively reliable. However, lower Ca2+ concentrations could not be tested for E702Q because the current amplitude with low [Ca2+] was too small for accurate measurement.
The very slow τon observed with E705Q raised questions in our minds about possible artifacts of the fast perfusion system. To test the validity of this approach, we measured the activation of currents in response to photolysis of caged Ca2+ (NP-EGTA) under whole cell patch clamp. For the same intensity UV flash, WT currents activated much more quickly than E705Q currents (Online Fig. II). At the same flash intensity (90 mJ), E705Q activated with a time constant (τon = 80 msec) that was >20-times slower than WT (τon = 3.7 msec) (Online Fig. I). These values are somewhat faster than those observed with rapid perfusion using 82 μmol/L Ca2+ because the [Ca2+] released by photolysis was presumably greater than this. Activation of E702Q was consistently >20-times slower than WT at all equivalent flash intensities.
Assuming that the predominant rate-limiting steps in Ano1 current activation and deactivation in these experiments are Ca2+ binding and unbinding, the apparent EC50 for Ca2+ can be calculated as EC50 = α/β, where α = 1/τoff and β = {(1/τon)−α}/[Ca2+]. The calculations were performed with data utilizing different Ca2+ concentrations that provided reliable on-rates for wild type and mutant channels. The E705Q mutation increases the EC50 at all voltages (Fig. 3F), suggesting that it plays a key role in Ca2+ sensing. The EC50 for E702Q is likely underestimated as noted above.
Thiol reagents alter Ca2+ sensitivity of E702C and E705C
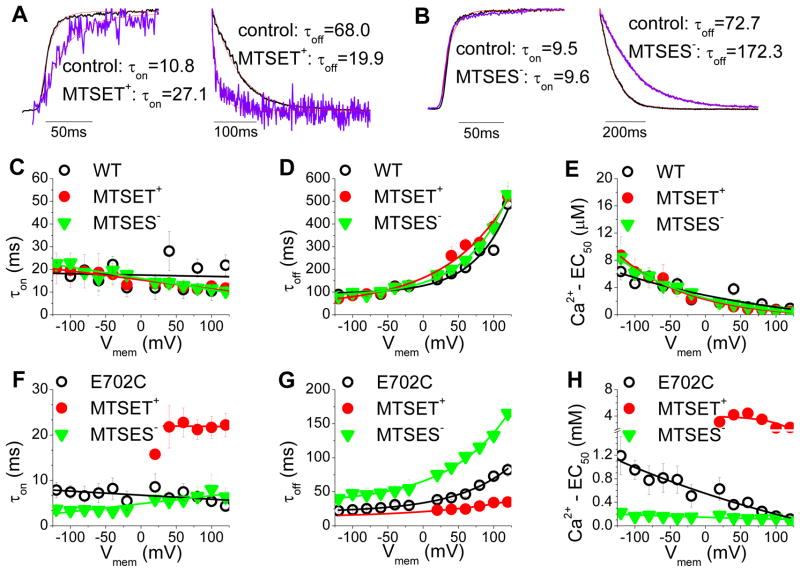
Alteration of the Ca2+ sensitivity of mAno1 by changing the charge at E702 and E705 by mutagenesis is consistent with the hypothesis that these amino acids contribute to a Ca2+ binding site. To test this hypothesis using a different approach, we asked whether modification of thiols introduced at these positions by charged MTS reagents would also alter Ca2+ sensitivity. E702 or E705 were replaced with cysteine and the effects of charged thiol reagents, MTSET+ and MTSES−, on channel gating by Ca2+ were measured in fast perfusion experiments. The MTS reagents were applied to the cytoplasmic face of inside-out excised patches. mAno1 has at least 5 cysteines that are predicted to be cytoplasmic, but neither MTSET+ nor MTSES− had any significant effect on the amplitude or kinetics of activation or deactivation of wild type mAno1 when applied to the cytoplasmic face of the patch (Fig. 4C–E). E702C (unmodified by MTS reagent) was much less sensitive to Ca2+ than wild type mAno1. At +120 mV, the EC50 for E702C was 114 μmol/L, compared to 0.94 μmol/L for WT and 21 μmol/L for E702Q. The larger EC50 for E702C compared to E702Q might be explained if the side chain oxygen of glutamine participates in Ca2+ coordination. MTSET+ increased the EC50 of E702C ~30-fold (from 114 μmol/L to ~ 3 mmol/L at 120 mV) by slowing τon and accelerating τoff (Fig. 4F–H). The decrease in Ca2+ affinity was accompanied by a decrease in current amplitude (56.4 ± 10.8%), as expected. MTSES− had the opposite effect on EC50 (Fig. 4F–H) and the uncharged MTSEH had no effect (Online Fig. IIIA). At 0 mV, the EC50’s were estimated to be 550 μmol/L for unmodified E702C, ~3890 μmol/L (extrapolated) for MTSET+-modified, and 144 μmol/L for MTSES−-modified currents. The results with E705C exhibited a similar trend but were decidedly less dramatic, possibly because of reduced accessibility of MTS reagents to this residue which is surrounded by very hydrophobic amino acids.
Figure 4.
Effects of sulfhydryl modification on activation and deactivation kinetics of Ano1 in excised inside-out patches with rapid Ca2+ perfusion. Excised patches were switched between zero and high Ca2+. They were then exposed to MTS reagent in the presence of zero Ca2+ for 10 sec, MTS reagent was washed away, and the patch was switched from zero Ca2+ to high Ca2+ again. (A, B) Normalized current traces of E702C mAno1showing examples of changes in τon and τoff caused by MTSET+ (A) and MTSES− (B). Traces were normalized to the same maximal amplitude. As shown in Fig. S3, MTSET+ causes a decrease in current and MTSES− increases the current. The magnitude of the effect of MTS reagent on current amplitude depends where on the Ca2+dose-response curve the experiment is performed. (C–E) Lack of effect of MTS reagents on τon, τoff, and EC50 for WT Ano1. (F–H) Effects of MTS reagents on τon, τoff, and EC50 for E702C mAno1. Open circle: before application of MTS reagent. Filled circle: after MTSET+, triangle: after MTSES−. (N=3–6).
If E702 and E705 are located on the cytoplasmic side of the membrane, we would predict that MTS reagents applied from the extracellular side would have no effect on E702C or E705C currents. We tested this prediction using whole-cell recording because outside-out patches could be obtained with only very low success. We also tested several other cysteine-substituted amino acids nearby. In whole-cell recording, extracellular application of MTSET+ and MTSES− had no significant effect on E702C or E705C currents (MTSET+ increased E702C currents by 4.7 ± 6.6 % and decreased E705C by −3.1 ± 0.9 %). This compares to a decrease of 56.4 ± 10.8% for E702C in excised patches exposed to cytosolic MTSET+. MTSET+ had no significant effect on any of the currents generated by these cysteine-substituted mutants (Online Fig. IIIB).
Online Fig. IV shows the time courses of the current changes caused by cytoplasmic MTS reagents on E702C and WT in inside-out excised patches. Currents were activated by a low concentration of Ca2+ in order to maximize the change in current amplitude that was produced by a change in Ca2+ affinity. MTSET+ (1 mmol/L) decreased the current with a τ = 52 msec and a rate constant of ~2×104 M−1s−1. This rate is close to the rate of modification of mercaptoethanol by MTSET+ in solution (~105 M−1s−1), suggesting that these residues are freely accessible to the aqueous environment. The effect of MTSES− is slower than MTSET+, but because MTSES− reacts with mercaptoethanol in solution about 5-times slower than MTSET−, this result is also consistent with high aqueous accessibility.
Cysteine accessibility to probe residues near the pore
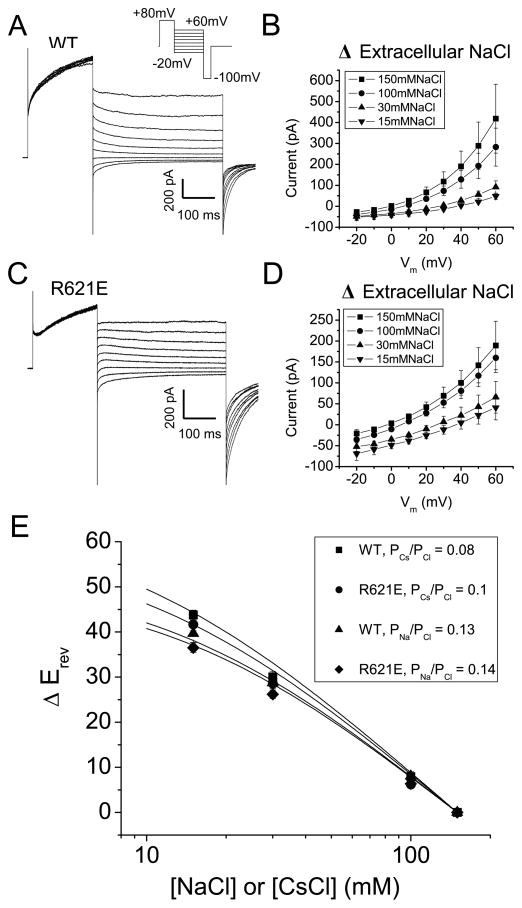
The region between F620 and N650 has been proposed to be important in forming the Ano1 pore because mutagenesis of positively charged amino acids (notably R621) in this region were reported to drastically alter ion selectivity 8. The R621E mutant was reported to exhibit a PNa/PK ratio of 0.87 compared to 0.03 for wild type. We have tried without success to confirm these observations using several different methods. In Fig. 5, PNa/PCl and PCs/PCl were determined by the dilution potential method 22. The ionic selectivity was found to be identical for WT (PNa/PCl = 0.13; PCs/PCl = 0.08) and R621E (PNa/PCl = 0.14; PCs/PCl = 0.1). The lack of effect of the R621E mutation on ion selectivity raised questions in our mind whether the pore of Ano1 was indeed located in this region.
Figure 5.
Effect of R621E mutation on anion:cation permeability of mAno1. (A, B) Whole-cell recordings of (A) WT mAno1 in symmetrical 150 mmol/L NaCl with 180 nmol/L Cai and (B) R621E mAno1 in symmetrical 150 mmol/L NaCl with 1.1 μmol/L Cai. The R621E mutation decreased the Ca2+ sensitivity of the channel, requiring a larger Ca2+ concentration to generate a measurable current. (C, D) Current-voltage relationships of (C) WT mAno1 and (D) R621E mAno1 with different extracellular [NaCl]. (E) Change in reversal potential (ΔErev) for different extracellular [NaCl] or [CsCl] determined from experiments like those in C and D. Lines are best fits to the Goldman-Hodgkin-Katz equation.
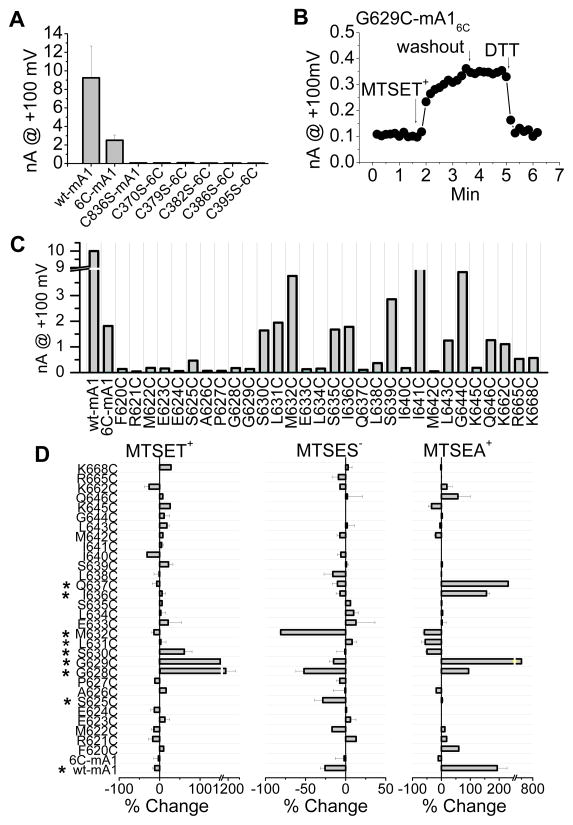
We used substituted cysteine accessibility mutagenesis 23 to glean more information about the topology of the putative re-entrant loop. mAno1 has 16 native cysteines which could possibly compromise the interpretation of the effects of thiol reagents on introduced cysteines. To determine which endogenous cysteines might be essential for Ano1 function, we first synthesized a cysteine-less mAno1 where all cysteines were replaced with serines. The cysteine-less mutant trafficked to the plasma membrane, as judged by the presence of a bright ring of fluorescence of the mAno1-EGFP tag at the cell surface, but it did not generate currents. To determine which cysteines were important for function, a series of mutants were generated with individual cysteines or groups of cysteines replaced with serine (Online Table II). Six cysteines (C370, C379, C383, C386, C395, or C836) were essential for Ano1 current. The essential cysteines are predicted to be extracellular: five are located in the first extracellular loop and one is located in the last extracellular loop. An Ano1 construct having the six essential cysteines intact and the remaining ten cysteines substituted with serine (mAno16C: C23S/C49S/C166S/C259S/C357S/C559S/C625S/C630S/C635S/C933S) exhibited identical biophysical properties to the wild type channel but had a reduced current amplitude. Replacing any one of the remaining six cysteines (C370, C379, C383, C386, C395, or C836) with serine in Ano16C completely eliminated the current (Fig. 6A). Because these six cysteines were essential for mAno1 function, the mAno16C construct was used as the template for cysteine scanning mutagenesis. Extracellular MTSET+, MTSES−, or MTSEA+ had little effect on Ano16C (Fig. 6C).
Figure 6.
Essential cysteines in mAno1 and scanning cysteine accessibility of the proposed new TMD6 (amino acids 620–646). (A) Six extracellular cysteines are required for mAno1 function. Amplitudes of whole-cell currents activated by 20μmol/L [Ca2+]i at +100mV from WT and cysteine-substituted mutants of mAno1 expressed in HEK293 cells. Inset shows location of cysteines (solid red circles: essential; open red circles: not-essential for channel function) in the old model of mAno1. 6C: mAno1 with all cysteines except C370, C379, C383, C386, C395 and C836 replaced with serines. C836S is wild type mAno1 with C836 only replaced with serine. The other constructs have each of the essential cysteines in mAno16C as indicated replaced with serine. (B) Example of change in current amplitude of G629C mANO16C. MTSET-induced increase in current is not reversed by washout of MTSET but is reversed by 5 mmol/L DTT. (C) Average Ano1 currents recorded from WT mAno1, mAno16C and cysteine-substituted mAno16C. (D) Effects of MTSET+ (left), MTSES− (middle) and MTSEA+ (right) on cysteine-substituted mAno16C. The percent change of current at +100mV within 2 min of exposure to MTS reagent was calculated by [100 (Iafter MTS − Ibefore MTS)/Ibefore MTS]. Cysteine-substituted amino acids labeled with an asterisk were significantly (p < 0.05) affected by MTS reagents (N= 3–6).
Each amino acid from F620 to Q646 in mAno16C was replaced with cysteine and extracellular MTS reagents were tested in whole cell recording (an example is shown in Fig. 6B). Cysteine substitution at positions 620–629, 633–634, and 637–638 significantly reduced current amplitude (Fig. 6C). MTSET+ significantly stimulated G628C, G629C and S630C* currents, while MTSES− decreased S625C*, G628C and M632C currents (Fig. 6D). (The asterisk denotes that this position is a native cysteine in wild type mAno1, but is mutated from S to C in mAno16C). These results support the idea that this region is important in channel function.
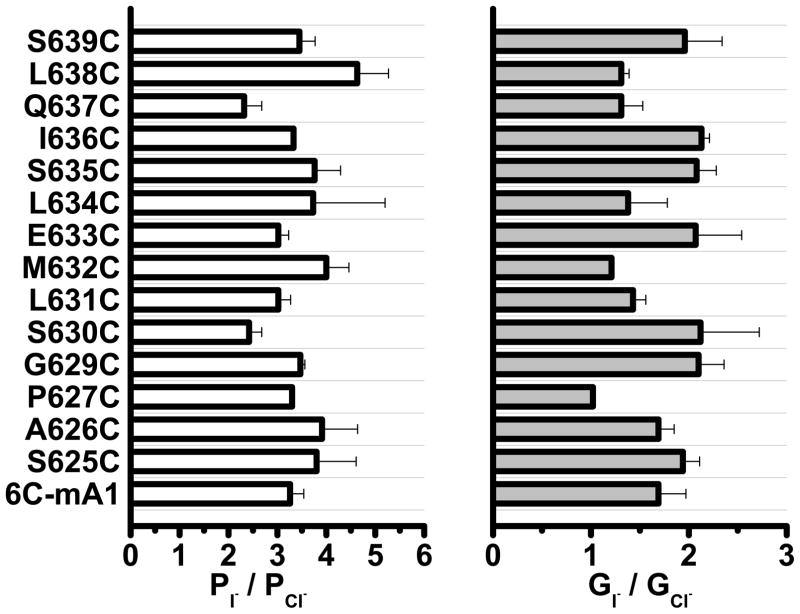
To locate amino acids that might be deeper in the pore, we tested the effect of MTSEA+, which is more membrane permeant than MTSET+ 24. MTSEA+ significantly increased I636C and Q637C currents, suggesting that these amino acids are located deeper in the membrane. In addition, MTSET+ increased K645C current extremely slowly over ~10 min (this is not reflected in summary data because the increase was so slow). Although our analysis cannot formally distinguish between a re-entrant loop and a membrane-spanning segment, these data are consistent with amino acids 625–630 contributing to an outer vestibule that is easily accessible to the extracellular fluid and amino acids beyond 635 being located deep in the pore. To determine whether these amino acids are located in the permeation pathway, we measured the relative iodide permeability (PI/PCl) and conductance (GI/GCl) of the cysteine-substituted mAno16C with the expectation that amino acids in the permeation pathway might affect ionic selectivity. However, none of the mutations significantly altered the ionic selectivity (Fig. 7). We also measured the relative Na+ permeability and conductance of selected mutants and did not detect any significant differences. These data suggest that this region of the protein may contribute to the permeation pathway but is not critical for forming the selectivity filter.
Figure 7.
Effects of replacement of amino acids in mANO16C with cysteine on I− permeability and conductance relative to Cl−. (A) Relative I− permeability was calculated from the shift in reversal potential after replacing extracellular Cl− with I−. (B) Relative I− conductance was measured as the ratio of slopes of the current-voltage relationship at the reversal potential after and before extracellular anion change (N= 3–10).
Discussion
The Ca2+ sensor of Ano1
One contender for the Ca2+ binding site is a stretch of 5 glutamic acids located in the first intracellular loop because of its resemblance to the “Ca-bowl” of the large-conductance K channel 18. Although a naturally-occurring splice variant (Δ448EAVK451) that deletes the 5th glutamic acid increases the EC50 for Ca2+ ~50-fold, neutralization of the first 4 glutamic acids (444EEEE447 changed to alanines) has little effect on Ca2+ sensitivity 18. Because Δ448EAVK451 and 444EEEE/AAAA447 also alter the voltage-dependent gating of the channel in the absence of Ca2+, we have suggested that the penta-glutamate region couples Ca2+ and voltage to channel opening, but is unlikely to be a major Ca2+ binding site itself. Other studies have implicated sites in the N-terminus as possible Ca2+ or CaM binding sites 25, 26, but there remains no consensus about the mechanisms of Ca2+ regulation.
The data presented here strongly implicate another region in Ca2+regulation. Surprisingly, this region was predicted to be an extracellular loop from hydropathy analysis and by analogy to Ano7, another member of the anoctamin superfamily 20. However, there is no convincing evidence that Ano7 is a Cl− channel. In our hands, Ano7 does not traffic to the plasma membrane or generate currents in transfected cells and is largely intracellular in adult human prostate where it is normally expressed 21. In a study by Schreiber et al. 27 it was suggested that Ano7 might function as a CaCC. However, in iodide flux assays, the ATP-stimulated flux in Ano7-transfected cells was similar to untransfected cells and <10% as large as in Ano1-transfected cells. The ionomycin-stimulated iodide flux in Ano7-transfected cells was actually less than in untransfected cells. Furthermore, the short form of Ano7 (Ano7S), which is a 179 amino acid protein with no predicted transmembrane domains, produced approximately the same iodide flux as the long form of Ano7. This supports the idea that Ano7 is not a plasma membrane CaCC and raises the possibility that the topology of Ano7 is different than that of Ano1.
A revised topology is required to explain our data
Our data show that HA tags introduced at positions 672 and 700 in Ano1 are not accessible to extracellular antibody. Furthermore, when E702 and E705 are replaced with cysteine, exposure of the cytosolic face of the membrane to membrane-impermeant MTS reagents causes a rapid change in current amplitude and/or Ca2+ sensitivity. This provides an independent piece of data that these residues are intracellular and not extracellular.
If this region is indeed intracellular, a transmembrane segment must exist somewhere between positions 614 where the introduced HA epitope is extracellular and 672 where the HA epitope is intracellular. Although computer algorithms for predicting transmembrane domains do not consistently identify a transmembrane domain between amino acids 614 and 672, between positions 631 and 648, 12 of the 18 amino acids are hydrophobic. Based on secondary structure predictions, all but one of the hydrophilic amino acids are located on one face of this segment. On this basis, we believe that residues 631–648 may form a transmembrane segment. The problem with this model is that the HA epitope introduced at position 824 is clearly extracellular, which means that there must be an even number of transmembrane segments between 614 and 824. Attempts to probe the topology of this region were unsuccessful because introduction of HA epitopes at several positions in this region produced channels that were non-functional or poorly expressed.
The first four and last two transmembrane segments of mAno1 are consistently identified by transmembrane prediction algorithms. These segments all exhibit a characteristic feature: tryptophan and basic residues are located at the predicted membrane interfaces. This is a common feature of transmembrane segments: tryptophan has a strong preference for lipid carbonyls and the hydrophobic aspects of the long side chains of lysine and arginine interact with the hydrophobic core of the membrane 28. However, in contrast to the six transmembrane segments that exhibit these characteristic features, the two putative transmembrane segments located between them lack these features. This suggests that the central part of the protein may have a more complex topology than previously predicted. A similar uncertainty existed regarding the transmembrane topology of the ClC transporters before they were crystallized 29. The uncertainty stemmed from the fact that the ClC transmembrane α-helices are variable in length and tilted at different angles relative to the plane of the membrane. Understanding the organization of mAno1 further will likely have to await direct structural studies.
Effects of thiol reagents support an intracellular location of E702 and E705
One observation that we believe strongly supports the hypothesis that E702 and E705 are intracellular is the finding that altering the charge on E702C and E705C with membrane-impermeant charged MTS reagents changes the Ca2+ affinity of the channel. MTSET+ renders the channel less sensitive to Ca2+ activation whereas MTSES− shifts the Ca2+ sensitivity of the channel closer to that of wild type. The size and charge of MTSES−-modified cysteine makes it an excellent surrogate for glutamic acid because MTSES−-modified cysteine is almost exactly the same volume as glutamic acid (142 Å3 for MTSES−-modified cysteine compared to 138 Å3 for glutamic acid) and the negative charge is relatively localized with an estimated charge of −0.79 on each oxygen (a preferred Ca2+ ligand) on the sulfonatoethyl group 30. In contrast, MTSET+ is larger (~173Å3) and its charge density is relatively diffuse. The +1 charge on MTSET− is distributed over ~17 atoms. This explains why addition of positive charge by cysteine modification by MTSET+ does not itself activate the channel, because its charge density is low compared to Ca2+, which has a +2 charge in a volume of ~20 Å3.
Separation of effects on Ca2+ binding and channel gating
Channel gating by Ca2+ involves at least 2 steps: Ca2+ binding and channel opening/closing. Because these steps are allosterically coupled, it is very difficult to unambiguously separate the effect of a mutation on Ca2+ binding from channel gating. However, we believe that the effects of the E702 and E705 mutations on the activation and deactivation of Ano1in response to Ca2+ jumps are most simply interpreted in terms of changes in Ca2+ binding. We have shown that the rates of Ano1 activation and deactivation depend upon the ligand used 18. For example, channel deactivation is more rapid and activation is slower using Ba2+ as ligand compared to Ca2+. The simplest explanation of these data is that ligand binding and unbinding are the predominant rate-limiting steps and that the probability of the channel being open in the absence of bound ligand is negligible. It is hard to imagine a mechanism that would change the open time that is dependent on the species of ligand (Ca, Ba) but is independent of ligand dissociation. Although one could argue that Ba2+ and Ca2+ place the channel into different conformations that relax with different kinetics upon ligand dissociation, there is no precedent supporting such an hypothesis.
Physiological significance of Ca2+ gating of Ano1
CaCCs participate in the transient outward current (Ito) that plays a role in repolarization of the action potential in some species. The best-studied component of Ito is a K+ current mediated by Kv4.x/Kv1.4 channels, but in some species, CaCC currents comprise a significant fraction of Ito 31. The contribution of CaCC current, because of its dependence on Ca2+, would be expected to increase under conditions of Ca2+ overload and could lead to arrhythmogenesis. CaCCs have been implicated in T-wave alternans, a prequel to malignant ventricular arrhythmias that occur during left ventricular hypertrophy 32, and delayed afterdepolarizations that may lead to arrhythmias 33, 34. Drugs targeting Ano1 could potentially provide new therapies for hypertension and/or cardiac arrhythmias.
Elucidating the molecular mechanisms of Ano1 gating is essential for developing new therapies targeting these channels. High-throughput screens have identified small molecule inhibitors and activators of Ano1 7, 35–38. However, evaluating these drugs requires a molecular understanding of how the Ano1 channel is gated. For example, drugs could activate or inhibit Ano1 channels by altering the affinity of the channel for Ca2+ or by affecting how the channel gates open in response to Ca2+ or to voltage. Drugs working by these different mechanisms are likely to have different pharmacological effects. For this reason, it is of paramount importance to understand how this channel operates. Another important question is that of possible side-effects of drugs that might occur because Ano1 is ubiquitously expressed in epithelia and smooth muscle of various types. However, Ano1 has multiple splice variants 25, 39, some of which affect the regions that we have identified as important in Ca2+ and voltage dependent gating. This not only raises the possibility that variant-specific drugs might be developed, but also emphasizes the importance of structure-function studies to establish the functions of various domains that are drug targets.
Supplementary Material
Novelty and Significance.
What is known?
The ion channel Ano1 regulates vascular smooth muscle tone and participates in cardiac action potential repolarization in some species (comprising the current known as Ito2).
Ano1 conducts chloride ions and is activated by increases in intracellular calcium.
What new information does this article contribute?
The study describes the structural determinants of calcium activation of Ano1.
The protein domain involved in Ano1 calcium regulation was formerly thought to be extracellular, hence, a new model for the orientation of the channel in the membrane is proposed.
The study provides insights that may be helpful in designing and evaluating new drugs that target Ano1.
Although chloride channels are known to contribute to cardiovascular regulation, relatively little is known at the molecular level about how they are governed. Here we study how the newly-discovered chloride ion channel Ano1 is turned on by intracellular calcium. We have identified two glutamic acid residues that drastically alter the sensitivity of the channel to intracellular calcium when they are mutated. Surprisingly, these amino acids were previously thought to be located in an extracellular loop. Using a variety of techniques, we suggest that this loop is instead located on the cytoplasmic side of the membrane and is involved in calcium sensing by the channel. These data contradict the popular reentrant loop model by showing that the putative 4th extracellular loop (ECL 4) is intracellular and may contain a Ca2+ binding site. Elucidating the molecular mechanisms of Ano1 regulation by calcium is essential for developing new therapies that target these channels. Drugs that target Ano1 could possibly have utility in treating hypertension or arrhythmias.
Acknowledgments
Sources of funding
Supported by grants from the NIH GM60448 (HCH), EY014852 (HCH), the Microscopy Core of the Emory Neuroscience NINDS Core Facilities Grant P30NS055077, and NEI Core Grant P30EY006360. C. Duran was supported by NEI training grant 5T32EY007092-25.
Non-standard abbreviations and acronyms
- Ano1
anoctamin-1, Tmem16a
- CaCC
calcium-activated chloride channel
- HA
human influenza hemaglutinin
- MTSET
[2-Trimethylammonioethyl]-methanethiosulfonate bromide
- MTSES
sodium [2-sulfonatoethyl]-methanethiosulfonate
- MTSEA
[2-aminoethyl]-methanethiosulfonate hydrobromide
- TMD
transmembrane domain
Footnotes
Disclosures
The authors state that they have no conflict of interest.
References
- 1.Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O’Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: A complex picture is emerging. Can J Physiol Pharmacol. 2005;83:541–556. doi: 10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 2.Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- 3.Duran C, Thompson CH, Xiao Q, Hartzell HC. Chloride channels: Often enigmatic, rarely predictable. Annu Rev Physiol. 2010;72:95–121. doi: 10.1146/annurev-physiol-021909-135811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan D. Phenomics of cardiac chloride channels: The systematic study of chloride channel function in the heart. J Physiol. 2009;587:2163–2177. doi: 10.1113/jphysiol.2008.165860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gwanyanya A, Macianskiene R, Bito V, Sipido KR, Vereecke J, Mubagwa K. Inhibition of the calcium-activated chloride current in cardiac ventricular myocytes by n-(p-amylcinnamoyl)anthranilic acid (ACA) Biochemical and Biophysical Research Communications. 2010;402:531–536. doi: 10.1016/j.bbrc.2010.10.069. [DOI] [PubMed] [Google Scholar]
- 6.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 7.Namkung W, Thiagarajah JR, Phuan PW, Verkman AS. Inhibition of Ca2+-activated Cl-channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 2010;24:4178–4186. doi: 10.1096/fj.10-160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. Tmem16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of Tmem16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJV. Tmem16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 11.Duran C, Hartzell HC. Physiological roles and diseases of Tmem16/Anoctamin proteins: Are they all chloride channels? Acta Pharmacol Sin. 2011;32:685–692. doi: 10.1038/aps.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrera L, Caputo A, Galietta LJ. Tmem16A protein: A new identity for Ca2+-dependent Cl channels. Physiology (Bethesda) 2010;25:357–363. doi: 10.1152/physiol.00030.2010. [DOI] [PubMed] [Google Scholar]
- 13.Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Thevenod F, Roussa E, Rock J, Schreiber R. Anoctamins. Pflugers Arch. 2011;462:195–208. doi: 10.1007/s00424-011-0975-9. [DOI] [PubMed] [Google Scholar]
- 14.Manoury B, Tamuleviciute A, Tammaro P. Tmem16A/Anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J Physiol. 2010;588:2305–2314. doi: 10.1113/jphysiol.2010.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis AJ, Forrest AS, Jepps TA, Valencik ML, Wiwchar M, Singer CA, Sones WR, Greenwood IA, Leblanc N. Expression profile and protein translation of Tmem16A in murine smooth muscle. Am J Physiol Cell Physiol. 2010;299:C948–959. doi: 10.1152/ajpcell.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas-Gatewood C, Neeb ZP, Bulley S, Adebiyi A, Bannister JP, Leo MD, Jaggar JH. Tmem16A channels generate Ca2+-activated Cl− currents in cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuruma A, Hartzell HC. Bimodal control of a Ca2+ -activated Cl− channel by different ca2+ signals. J Gen Physiol. 2000;115:59–80. doi: 10.1085/jgp.115.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Q, Yu K, Perez-Cornejo P, Cui Y, Arreola J, Hartzell HC. Voltage- and calcium-dependent gating of tmem16a/ano1 chloride channels are physically coupled by the first intracellular loop. Proceedings of the National Academy of Sciences. 2011;108:8891–8896. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milenkovic VM, Brockmann M, Stohr H, Weber BH, Strauss O. Evolution and functional divergence of the anoctamin family of membrane proteins. BMC Evol Biol. 2010;10:319. doi: 10.1186/1471-2148-10-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S, Hahn Y, Walker DA, Nagata S, Willingham MC, Peehl DM, Bera TK, Lee B, Pastan I. Topology of NGEP, a prostate-specific cell:Cell junction protein widely expressed in many cancers of different grade level. Cancer Res. 2008;68:6306–6312. doi: 10.1158/0008-5472.CAN-08-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duran C, Qu Z, Osunkoya AO, Cui Y, Hartzell HC. Anos 3–7 in the Anoctamin/Tmem16 Cl− channel family are intracellular proteins. Am J Physiol Cell Physiol. 2012;302:C482–493. doi: 10.1152/ajpcell.00140.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry P. The reliability of relative anion-cation permeabilities deduced from reversal (dilution) potential measurements in ion channel studies. Cell Biochemistry and Biophysics. 2006;46:143–154. doi: 10.1385/CBB:46:2:143. [DOI] [PubMed] [Google Scholar]
- 23.Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods in Enzymology. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 24.Holmgren M, Liu Y, Xu Y, Yellen G. On the use of thiol-modifying agents to determine channel topology. Neuropharmacology. 1996;35:797–804. doi: 10.1016/0028-3908(96)00129-3. [DOI] [PubMed] [Google Scholar]
- 25.Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, Pagani F, Galietta LJ. Regulation of tmem16a chloride channel properties by alternative splicing. J Biol Chem. 2009;284:33360–33368. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Y, Kongsuphol P, Hug M, Ousingsawat J, Witzgall R, Schreiber R, Kunzelmann K. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel tmem16a. FASEB J. 2010;25:1058–1068. doi: 10.1096/fj.10-166884. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber R, Uliyakina I, Kongsuphol P, Warth R, Mirza M, Martins JR, Kunzelmann K. Expression and function of epithelial anoctamins. J Biol Chem. 2010;285:7838–7845. doi: 10.1074/jbc.M109.065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killian JA, von Heijne G. How proteins adapt to a membrane-water interface. Trends Biochem Sci. 2000;25:429–434. doi: 10.1016/s0968-0004(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 29.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiological Reviews. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Kakhniashvili DA, Gremse DA, Wood DO, Mayor JA, Walters DE, Kaplan RS. The yeast mitochondrial citrate transport protein. Probing the roles of cysteines, arg(181), and arg(189) in transporter function. J Biol Chem. 2000;275:7117–7124. doi: 10.1074/jbc.275.10.7117. [DOI] [PubMed] [Google Scholar]
- 31.Li GR, Du XL, Siow YL, OK, Tse HF, Lau CP. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovasc Res. 2003;58:89–98. doi: 10.1016/s0008-6363(02)00859-3. [DOI] [PubMed] [Google Scholar]
- 32.Guo D, Young L, Patel C, Jiao Z, Wu Y, Liu T, Kowey PR, Yan GX. Calcium-activated chloride current contributes to action potential alternations in left ventricular hypertrophy rabbit. Am J Physiol Heart Circ Physiol. 2008;295:H97–H104. doi: 10.1152/ajpheart.01032.2007. [DOI] [PubMed] [Google Scholar]
- 33.Zygmunt AC, Goodrow RJ, Weigel CM. INaCa and ICl(Ca) contribute to isoproterenol-induced delayed after depolarizations in midmyocardial cells. American Journal of Physiology. 1998;275:H1979–H1992. doi: 10.1152/ajpheart.1998.275.6.H1979. [DOI] [PubMed] [Google Scholar]
- 34.Han X, Ferrier GR. Ionic mechanisms of transient inward current in the absence of Na+-Ca2+ exchange in rabbit cardiac purkinje fibres. J Physiol. 1992;456:19–38. doi: 10.1113/jphysiol.1992.sp019324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namkung W, Phuan PW, Verkman AS. Tmem16a inhibitors reveal Tmem16a as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Namkung W, Yao Z, Finkbeiner WE, Verkman AS. Small-molecule activators of tmem16a, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J. 2011 doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De La Fuente R, Namkung W, Mills A, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Molecular Pharmacology. 2008;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- 39.O’Driscoll KE, Pipe RA, Britton FC. Increased complexity of Tmem16a/Anoctamin 1 transcript alternative splicing. BMC Mol Biol. 2011;12:35. doi: 10.1186/1471-2199-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.