SUMMARY
Drug addiction is driven, in part, by powerful drug-related memories. Deficits in social life, particularly during adolescence, increase addiction vulnerability. Social isolation in rodents has been used extensively to model the effects of deficient social experience, yet its impact on learning and memory processes underlying addiction remains elusive. Here we show that social isolation of rats during a critical period of adolescence (postnatal days 21–42) enhances long-term potentiation of NMDA receptor (NMDAR)-mediated glutamatergic transmission in the ventral tegmental area (VTA). This enhancement, which is caused by an increase in metabotropic glutamate receptor-dependent Ca2+ signaling, cannot be reversed by subsequent resocialization. Notably, memories of amphetamine- and ethanol-paired contextual stimuli are acquired faster and, once acquired, amphetamine-associated contextual memory is more resistant to extinction in socially isolated rats. We propose that NMDAR plasticity in the VTA may represent a neural substrate by which early life deficits in social experience increase addiction vulnerability.
INTRODUCTION
Defective social experiences such as isolation, abandonment, and neglect, especially during early stages of life, increase an individual’s risk of developing drug addiction and other psychiatric disorders both concurrently and in the future (Lu et al., 2003b; Meyer-Lindenberg and Tost, 2012; Sinha, 2008). Social isolation has been used extensively as an animal model to investigate the impact of early life social deficits on brain and behavior. Here, animals raised under isolated conditions, thus deprived of social stimuli, display a suite of cognitive and behavioral alterations, such as heightened anxiety and aggression, cognitive rigidity, and impaired spatial learning, together with changes in neuronal morphology and function, including reduced cortical and hippocampal synaptic plasticity (Fone and Porkess, 2008; Lukkes et al., 2009b; Robbins et al., 1996). There is a critical period during which social isolation has the most profound and often irreversible effects, which begins during the earliest stage of adolescence immediately after weaning (typically postnatal day 21 [P21]) and extends through mid-adolescence (~P40–50) in rats (Einon and Morgan, 1977).
The mesolimbic dopaminergic system that originates in the ventral tegmental area (VTA) plays an essential role in learning which environmental stimuli and behaviors lead to the receipt of rewards, including addictive drugs (Schultz, 2010). During repeated exposure to addictive drugs, strong synaptic plasticity develops in the VTA and its projection areas, forming powerful and enduring memories of drug-related experiences (Hyman et al., 2006; Kauer and Malenka, 2007; Stuber et al., 2010). Social isolation has been reported to result in altered responding to both natural rewards and drug rewards. For example, isolated animals display increases in food, sucrose and drug intake and in drug-induced hyperlocomotion, associated with elevated basal and drug-induced dopamine release in the nucleus accumbens, the major projection target of VTA dopamine neurons (Brenes and Fornaguera, 2008; Fabricius et al., 2011; Howes et al., 2000; Smith et al., 1997). However, how social isolation affects the reward learning processes underlying the development of addiction remains poorly understood.
During behavioral conditioning, a large population of dopamine neurons acquire phasic burst responses to environmental stimuli that signal the availability of rewards, thereby encoding increased motivational values of those stimuli (Schultz, 2010). Glutmatergic inputs activating NMDA receptors (NMDARs) play a major role in driving dopamine neuron burst firing (Deister et al., 2009; Wang et al., 2011; Zweifel et al., 2009). Repeated pairing of glutamatergic input stimulation with postsynaptic burst firing induces long-term potentiation (LTP) of NMDAR-mediated transmission (Harnett et al., 2009), which may contribute to the acquisition of burst responses to reward-predicting stimuli. Mechanistically, LTP induction requires amplification of action potential (AP)-evoked Ca2+ signals by preceding activation of metabotropic glutamate receptors (mGluRs; more specifically mGluR1). This amplification occurs via generation of the Ca2+-releasing intracellular messenger inositol 1,4,5-trisphosphate (IP3), causing increased Ca2+-induced Ca2+ release triggered by AP-induced Ca2+ influx (Cui et al., 2007). In this study, we asked how social isolation during early adolescence affects 1) the mGluR/IP3-dependent induction mechanism of NMDAR LTP in VTA dopamine neurons using ex vivo brain slice preparations and 2) the learning of environmental stimuli paired with addictive drugs (amphetamine and ethanol) using a conditioned place preference (CPP) paradigm in behaving animals.
RESULTS
Social Isolation during Early Adolescence Enhances mGluR-Induced Facilitation of AP-Evoked Ca2+ Signals
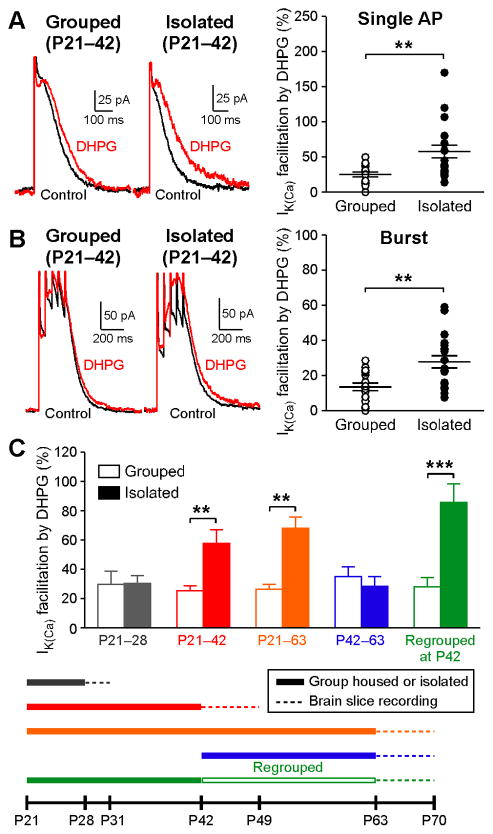
mGluR-induced facilitation of AP-evoked Ca2+ signals is critical for the induction of NMDAR LTP in dopamine neurons (Harnett et al., 2009). Thus, we first examined the effect of the mGluR agonist DHPG (1 μM; bath applied for 5 min) on AP-evoked Ca2+signals, assessed by the size of small-conductance, Ca2+-sensitive K+ (SK) currents (IK(Ca); see Methods), in VTA dopamine neurons from group housed and socially isolated rats. In these experiments, IK(Ca) was evoked with a single AP (Figure 1A) and a burst of 5 APs at 20 Hz (Figure 1B). The magnitude of DHPG-induced facilitation of IK(Ca) was significantly larger in animals isolated for 3–4 weeks from P21 (labeled P21–42) compared to group housed controls. We next varied the duration and the age of onset of isolation (Figure 1C). Extending the isolation period to 6–7 weeks (labeled P21–63) resulted in a comparable increase in the DHPG effect on IK(Ca)as 3–4 week isolation. However, there was no change in the DHPG effect when the isolation period was shortened to 7–10 d (labeled P21–28) or when the onset of isolation was delayed to P42 (labeled P42–63). Therefore, an increase in DHPG-induced facilitation of IK(Ca) is apparent only if isolation is prolonged (≥ 3 weeks) and commences in early adolescence close to weaning. The DHPG effect on IK(Ca) was further elevated after 3–4 weeks of resocialization (p < 0.05 vs. P21–42 isolation; Bonferroni post hoc test), indicating that the increased mGluR action after social isolation cannot be reversed, and may be further augmented, by subsequent social contact (Figure 1C). Group housed controls displayed similar DHPG effects on IK(Ca) regardless of the housing period (thus the age of animals), demonstrating no developmental influence. There was no difference in the basal size of IK(Ca) following different housing conditions/periods (Figure S1A), suggesting that voltage-gated Ca2+ channels mediating AP-induced Ca2+ influx were not affected. Furthermore, DHPG-induced inward currents, which are independent of intracellular Ca2+ signaling (Guatteo et al., 1999), were not altered by housing conditions (Figure S1B). These data demonstrate that prolonged deprivation of social interactions during early adolescence augments mGluR-mediated facilitation of AP-evoked Ca2+ signals via alterations in the signaling pathway downstream of mGluRs. Subsequent electrophysiology experiments were conducted using rats isolated for 3–4 weeks from P21 and their group housed controls.
Figure 1. Social Isolation during Early Adolescence Causes a Persistent Increase in mGluR-Dependent Facilitation of AP-Evoked Ca2+ Signals.
(A and B) Example traces and summary graph illustrating the effects of DHPG (1 μM) on IK(Ca) evoked by a single AP (A) and a burst (B) in VTA neurons from group housed and socially isolated rats (grouped: 17 cells from 8 rats, isolated: 19 cells from 10 rats; single AP: t34= 3.13, p < 0.01; burst: t34 = 2.98, p < 0.01; unpaired t test).
(C) Summary bar graph depicting the effects of DHPG on single AP-evoked IK(Ca) in rats isolated for the indicated periods and in their group housed controls. The housing schedule is illustrated at the bottom. Housing conditions were maintained until the day of recording. Each bar represents averaged data in 8–19 cells from 3–10 rats (housing condition: F1,109= 24.9, p < 0.0001; housing period: F4,109= 3.51, p < 0.01; housing condition × housing period: F4,109= 5.73, p < 0.001; two-way ANOVA). **p < 0.01, ***p < 0.001 (Bonferroni post hoc test). Error bars indicate SEM. See also Figure S1.
Adolescent Social Isolation Increases IP3 Sensitivity
mGluR-dependent facilitation of AP-evoked Ca2+-signals is mediated by generation of IP3 in the cytosol (Cui et al., 2007). To examine if IP3 signaling is altered by social isolation, we directly applied relatively low concentrations of IP3 (50–200 μM·μJ; see Methods) into the cytosol, using UV photolysis of caged IP3, and measured the resulting SK-mediated outward current (IIP3). In these experiments, IIP3 was significantly larger in isolated rats compared to group housed controls (Figure 2A). We next used larger concentrations of IP3 (175–250 μM·μJ) to evoke saturating IIP3 in each cell, then determined the IP3 concentration (EC25) that produced ~25% (group housed: 24.4 ± 1.0%, n = 9; isolated: 25.5 ± 0.7%, n = 14) of the maximal IIP3 amplitude. We found that the EC25 value thus determined was significantly smaller in isolated animals, while there was no difference in the maximal IIP3 amplitude between the two groups (Figures 2B and 3A). These data demonstrate that early life social isolation increases IP3 sensitivity in VTA neurons.
Figure 2. Social Isolation Increases IP3 Sensitivity.
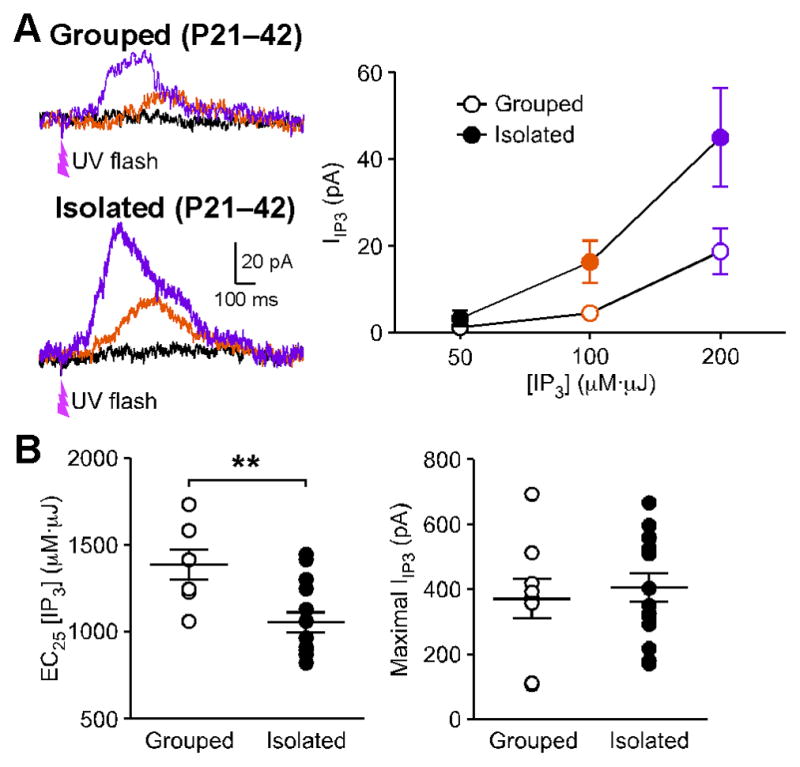
(A) Example traces and summary graph of IIP3 evoked by low concentrations of IP3 (black: 50 μM·μJ, orange: 100 μM·μJ, purple: 200 μM·μJ) in group housed and socially isolated rats (grouped: 12 cells from 8 rats, isolated: 14 cells from 9 rats; housing condition: F1,48 = 4.87, p < 0.05; IP3 concentration: F2,48 = 19.0, p < 0.0001; mixed two-way ANOVA). Cells were loaded with caged IP3(1 – 2 μM) in these experiments. UV flashes were applied at the time indicated to photolyze caged IP3.
(B) The IP3EC 25 value for evoking IIP3 was reduced in socially isolated rats (grouped: 9 cells from 6 rats, isolated: 14 cells from 11 rats; t21 = 3.34, p < 0.01; unpaired t test), while there was no change in the maximal IIP3 amplitude. A higher concentration of caged IP3 (100 μM) was used in these experiments. Error bars indicate SEM.
Figure 3. CRF Further Increases IP3 Sensitivity in Socially Isolated Animals.
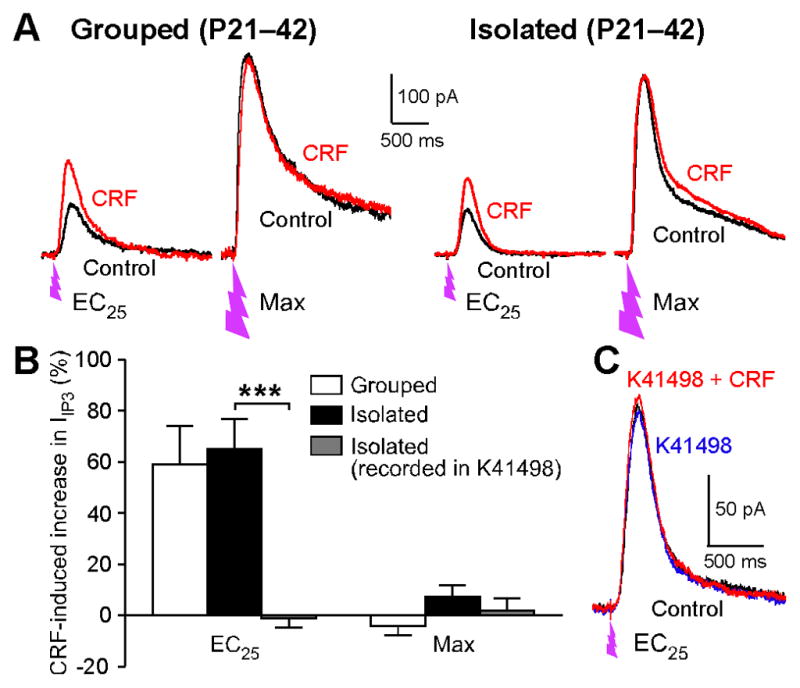
(A) Representative traces depicting the effects of CRF (300 nM) on IIP3 evoked by EC25 and saturating concentrations of IP3in VTA neurons from group housed and socially isolated rats. Cells were loaded with caged IP3 (100 μM). UV flashes were applied at the time indicated. Note that CRF increased IIP3-EC25 but not IIP3-max.
(B) Summary bar graph showing the effects of CRF on IIP3-EC25 and IIP3-max in group housed and socially isolated rats and the blockade of CRF effect by the CRF2 receptor antagonist K41498 (grouped: 9 cells from 6 rats, isolated: 9 cells from 8 rats, isolated/recorded in K41498: 5 cells from 3 rats; housing/recording condition: F2,20= 4.51, p < 0.05; IP3 concentration: F1,20= 34.0, p < 0.0001; housing/recording condition × IP3 concentration: F2,20 = 8.20, p < 0.01; mixed two-way ANOVA). ***p < 0.001 (Bonferroni post hoc test).
(C) Example traces of IIP3-EC25 recorded in control solution (black), in K41498 (blue), and in CRF and K41498 (red). Error bars indicate SEM.
Rats isolated during early adolescence have been shown to exhibit enhanced responsiveness to the stress-related neuropeptide corticotropin releasing factor (CRF), together with increased expression levels of CRF2 receptors in the dorsal raphe (Lukkes et al., 2009a; Serra et al., 2005). CRF facilitates mGluR/IP3-induced Ca2+ signaling by activation of CRF2 receptors in dopamine neurons (Bernier et al., 2011; Riegel and Williams, 2008), likely via protein kinase A (PKA)-dependent phosphorylation of IP3 receptors causing an increase in IP3 sensitivity (Wagner et al., 2008). Thus, we next asked if the CRF effect on IP3 signaling is altered following social isolation. IIP3 was evoked with an EC 25(IIP3-EC25) and a saturating concentration of IP3(IIP3-max) in each cell, as described above, to test if CRF application alters the potency of IP3 (i.e., IP3 sensitivity). In both group housed and socially isolated animals, bath application of CRF (300 nM) increased IIP3-EC25 amplitude, while having no significant effect on IIP3-max, consistent with CRF-induced increase in IP3 sensitivity. The CRF effect on IIP3-EC25 in socially isolated animals, which was comparable to that in group housed controls, was abolished by prior application of the CRF2 receptor antagonist K41498 (600 nM) (Figures 3B and 3C), confirming the involvement of CRF2 receptors. K41498 had no measurable effect on IIP3 by itself, indicating that the increased IP3 sensitivity observed in socially isolated animals is not due to increased CRF tone in VTA slices. These results suggest that social isolation does not alter CRF2 receptor expression/function in the VTA.
Social Isolation Enhances NMDAR Plasticity
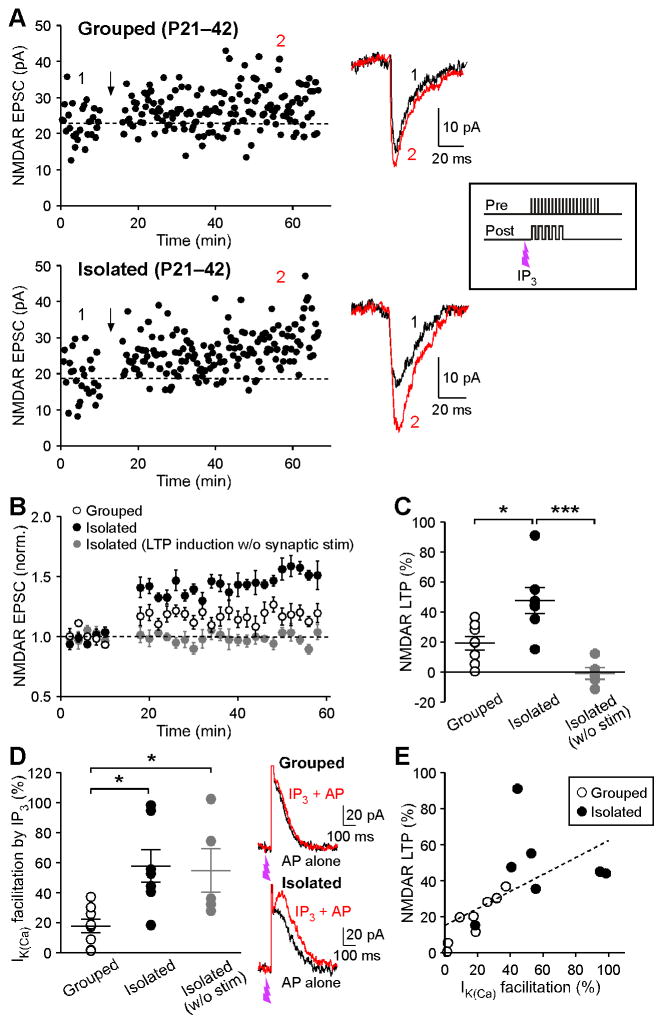
We next examined whether adolescent social isolation affects NMDAR LTP, which requires mGluR/IP3-mediated facilitation of burst-induced Ca2+ signals for its induction (Harnett et al., 2009). Application of low concentrations of IP3 preceding APs can effectively facilitate IK(Ca) (Ahn et al., 2010; Bernier et al., 2011; Cui et al., 2007). Thus, we used an LTP induction protocol comprising application of IP3 (100 μM·μJ), which produces very small responses by itself (Figure 2A), 50 ms prior to the pairing of synaptic stimulation (20 stimuli at 50 Hz) and a burst (5 APs at 20 Hz), where the onsets of synaptic stimulation and burst were simultaneous. It should be noted that this type of simultaneous synaptic stimulation-burst pairing by itself (without prior IP3 application) is ineffective at inducing NMDAR LTP (Harnett et al., 2009). The IP3-synaptic stimulation-burst combination was repeated 10 times every 20 s. While this induction protocol utilizing a low concentration of IP3 resulted in relatively small LTP of NMDAR EPSCs in group housed controls, socially isolated animals exhibited significantly larger magnitude of LTP (Figures 4A, 4B, and 4C). IP3-induced facilitation of single AP-evoked IK(Ca), which was assessed immediately before running the LTP induction protocol in each cell, was significantly increased in isolated rats (Figure 4D). Furthermore, the magnitude of NMDAR LTP was positively correlated with that of IP3-induced IK(Ca) facilitation across neurons from both group housed and isolated animals (r = 0.61, p < 0.05) (Figure 4E). These results strongly suggest that social isolation enhances the induction of NMDAR plasticity via an increase in IP3-induced facilitation of AP-evoked Ca2+ signals.
Figure 4. Social Isolation Results in Increased Susceptibility to the Induction of NMDAR LTP.
(A)Example experiments to induce NMDAR LTP in group housed and socially isolated rats. Time graphs of NMDAR EPSC amplitude are shown on the left. The LTP induction protocol, which consisted of IP3-synaptic stimulation-burst combination (illustrated in the right inset), was delivered at the time indicated by the arrow. Traces of NMDAR EPSCs at times indicated by numbers in the time graphs are also shown.
(B) Summary time graph of NMDAR LTP experiments (grouped: 7 cells from 6 rats, isolated: 8 cells from 8 rats, isolated/LTP induction without synaptic stimulation: 5 cells from 3 rats).
(C) Summary graph plotting the magnitude of NMDAR LTP (F2,17 = 13.2, p < 0.001; one-way ANOVA).
(D) Summary graph plotting the magnitude of IK(Ca) facilitation produced by preceding application of IP3 (100 μM·μJ) (F2,17= 5.86, p < 0.05; one-way ANOVA). Example traces depicting IP3-induced facilitation of IK(Ca) are shown on the right. IP3 was applied at the time indicated.
(E) The magnitude of NMDAR LTP is plotted versus the magnitude of IP3-induced facilitation of IK(Ca) in the experiments where LTP was induced with the IP3-synaptic stimulation-burst protocol. Dashed line is a linear fit to all data points from both group housed and socially isolated rats. *p < 0.05, ***p < 0.001 (Bonferroni post hoc test). Error bars indicate SEM.
We further attempted to induce NMDAR LTP by combining IP3 application with burst alone, i.e., without synaptic stimulation, in neurons from socially isolated rats. This IP3-burst protocol failed to induce measurable LTP, even if robust IP3-induced IK(Ca) facilitation was observed (Figures 4C and 4D). This observation is consistent with our previous study demonstrating that LTP induction also requires synaptic NMDAR activation at the time of postsynaptic burst, which likely accounts for the input specificity of LTP (e.g., via providing additional highly localized Ca2+ signals only at activated synapses during induction) (Harnett et al., 2009).
Social Isolation and Repeated Amphetamine Exposure Produce Similar Enhancement of mGluR-Dependent Ca2+ Signal Facilitation
Repeated in vivo exposure to amphetamine or ethanol enhances mGluR-induced facilitation of AP-evoked Ca2+ signals via a PKA-dependent increase in IP3 sensitivity (Ahn et al., 2010; Bernier et al., 2011). We next asked if amphetamine experience could further enhance mGluR/IP3 signaling following prolonged social isolation. In these experiments, once daily injections of amphetamine (5 mg/kg, i.p.) were made for 1 d or 3 d in group housed and socially isolated rats. In group housed animals, 3 d, but not 1 d, amphetamine exposure increased the effect of DHPG on IK(Ca) compared to naïve controls (Figure 5), in line with our previous study (Ahn et al., 2010). However, 1 d and 3 d amphetamine exposure failed to affect the DHPG effect on IK(Ca) in socially isolated animals. Importantly, DHPG effects became comparable between group housed and isolated rats after 3 d amphetamine exposure. Thus, adolescent social isolation enhances mGluR-dependent Ca2+ signal facilitation in a manner analogous to repeated amphetamine exposure.
Figure 5. Repeated Amphetamine Exposure Does Not Produce Further Enhancement of mGluR-Dependent Facilitation of AP-Evoked Ca2+ Signals in Socially Isolated Animals.
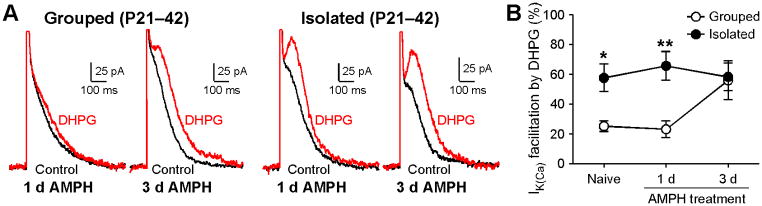
(A) Example traces illustrating DHPG-induced facilitation of IK(Ca) after 1 d or 3 d amphetamine (AMPH) treatment in group housed and socially isolated rats.
(B) Summary graph plotting DHPG effect on Ik(Ca) after amphetamine treatment in group housed and socially isolated rats (grouped/1 d AMPH: 13 cells from 6 rats, grouped/3 d AMPH: 14 cells from 5 rats, isolated/1 d AMPH: 13 cells from 5 rats, isolated/3 d AMPH: 14 cells from 7 rats). Data for naïve animals were from those shown in Fig 1c (housing condition: F1,84 = 12.5, p < 0.001; two-way ANOVA). *p < 0.05, **p < 0.01 versus grouped animals (Bonferroni post hoc test). Error bars indicate SEM.
Adolescent Social Isolation Promotes the Learning of Drug-Associated Contextual Stimuli
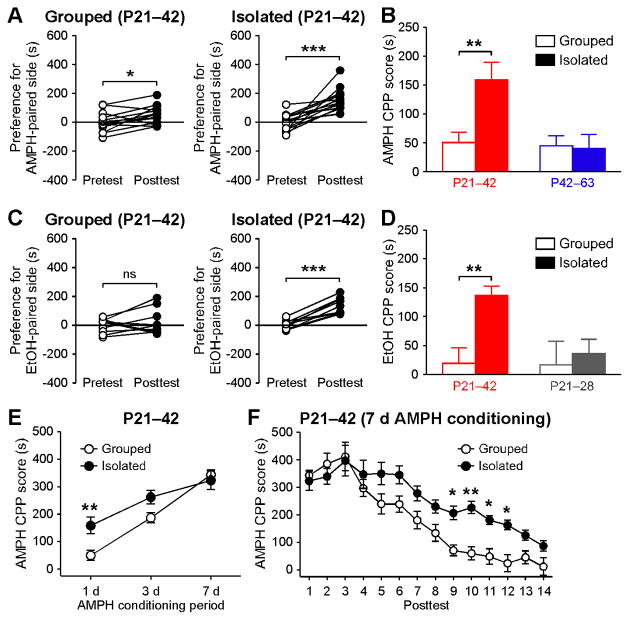
Enhancement of mGluR-dependent NMDAR plasticity in the VTA may facilitate the learning of environmental stimuli associated with rewards, including addictive drugs such as amphetamine and ethanol (Ahn et al., 2010; Bernier et al., 2011). Hence, we asked if early life social isolation promotes the learning of drug-associated cues using a CPP paradigm. In these experiments, rats were group housed or socially isolated for 3 weeks from P21 (P21–42), as in the electrophysiology experiments. Then, after initial preference for the two compartments, which had different wall colors and floor textures, was determined on the pretest day, rats were subjected to 1 d conditioning with amphetamine (5 mg/kg, i.p.). One day later, a posttest was performed to determine the change in preference for the amphetamine-paired compartment. Socially isolated rats displayed overall more robust and consistent increases in the preference for the amphetamine-paired compartment, resulting in significantly greater CPP magnitude (Figures 6A and 6B). Furthermore, we found that 1 d conditioning with ethanol (0.5 g/kg, i.p.) produced significant CPP only in socially isolated rats (Figures 6C and 6D). Three week isolation during a later adolescent period (P42–63; Figures 6B and S2A) or 1 week isolation from P21 (P21–28; Figures 6D and S2B) failed to enhance amphetamine or ethanol CPP with 1 d conditioning, respectively. The magnitude of amphetamine CPP increased as the conditioning period was extended (3–7 d) in both group housed and isolated animals (P21–42). However, the difference between the two types of animals became smaller, and group housed rats exhibited robust CPP comparable to isolated ones after 7 d amphetamine conditioning (Figures 6E and S2C). Together, these results demonstrate that prolonged isolation during early adolescence results in an increase in the rate of learning of drug-associated contextual stimuli.
Figure 6. Social Isolation during Early Adolescence Enhances Amphetamine and Ethanol CPP.
(A) Changes in the preference for the amphetamine-paired side after 1 d conditioning are shown for group housed and socially isolated rats (grouped: t12 = 2.80, p < 0.05; isolated: t12 = 5.23, p < 0.001; paired t test).
(B) Summary graph plotting the 1 d amphetamine CPP score in rats group housed or isolated during P21–42 or P42–63 (grouped/P21–42: 13 rats, isolated/P21–42: 13 rats, grouped/P42–63: 9 rats, isolated/P42–63: 9 rats; housing condition: F1,40= 4.43, p < 0.05; housing period: F1,40= 6.38, p < 0.05; housing condition × housing period: F1,40= 5.28, p < 0.05; two-way ANOVA).
(C) Changes in the preference for the ethanol-paired side after 1 d conditioning are shown for group housed and socially isolated rats (grouped: t9 = 0.72, p = 0.49; isolated: t8= 8.80, p < 0.0001; paired t test).
(D) Summary graph plotting the 1 d ethanol CPP score in rats group housed or isolated during P21–28 or P21–42 (grouped/P21–42: 10 rats, isolated/P21–42: 9 rats, grouped/P21–28: 7 rats, isolated/P21–28: 10 rats; housing condition: F1,32 = 6.36, p < 0.05; two-way ANOVA). Bars in (B) and (D) are color coded as in Figure 1C.
(E) Summary graph plotting the amphetamine CPP score with different conditioning periods (grouped/1 d: 13 rats, grouped/3 d: 9 rats, grouped/7 d: 10 rats, isolated/1 d: 13 rats, isolated/3 d: 9 rats, isolated/7 d: 8 rats; housing condition: F1,56= 6.93, p < 0.05, conditioning period: F2,56= 44.0, p < 0.0001, housing condition × housing period: F2,56= 3.49, p < 0.05; two-way ANOVA).
(F) Summary graph plotting amphetamine CPP score during 14 consecutive posttests following 7 d conditioning in group housed and socially isolated rats (housing condition: F1,208= 6.81, p < 0.05; posttest day: F13,208= 45.1, p < 0.0001; housing condition × posttest day: F13,208= 3.51, p < 0.0001; mixed two-way ANOVA). *p < 0.05, **p < 0.01 versus grouped animals (Bonferroni post hoc test). Error bars indicate SEM. See also Figure S2.
We further compared the rate of extinction of amphetamine CPP between group housed and socially isolated animals (P21–42). Following 7 d amphetamine conditioning, during which both types of rats acquired similar CPP (Figure 6E), animals were subjected to consecutive posttests (once daily for 14 d) to repeatedly expose them to the CPP compartments without amphetamine. During this 14 d extinction training, socially isolated animals displayed significantly slower decline in amphetamine CPP compared to group housed controls (Figure 6F). Notably, preference for the amphetamine-paired side was significantly elevated for 13 d in isolated animals but only for 8 d in group housed controls (Figure S2D). Therefore, contextual drug memory appears to be more resistant to extinction in animals raised in isolated conditions.
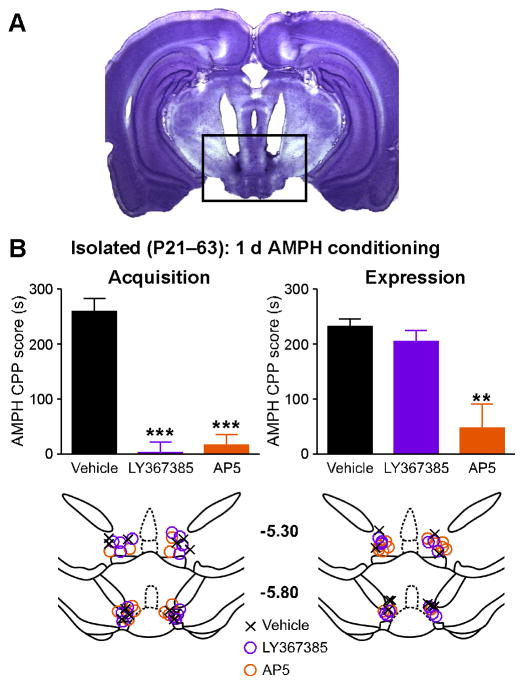
Finally, we tested the effects of intra-VTA injections of the mGluR1 antagonist LY367385 (0.6 nmol/side) and the NMDAR antagonist AP5 (0.6 nmol/side) on amphetamine CPP in rats isolated for 6 weeks (P21–63; bilateral guide cannulae implanted at P56). Robust amphetamine CPP with 1 d conditioning was consistently observed in these animals when the vehicle (PBS) was injected into the VTA before the amphetamine conditioning session or the posttest (Figures 7 and S3). CPP acquisition was suppressed by both LY367385 and AP5 when injected before the amphetamine conditioning session, while AP5, but not LY367385, attenuated CPP expression when injected before the posttest. Likewise, in group housed animals (group housed during P21–56, then isolated for 7 d after bilateral guide cannulae were implanted at P56), acquisition and expression of amphetamine CPP (3 d conditioning) were suppressed by intra-VTA injections of LY367385 and AP5, respectively (Figure S4). These results are consistent with the role of NMDAR LTP in this form of contextual learning. Here, inhibition of LTP induction via mGluR1 blockade would suppress CPP acquisition. On the other hand, blockade of NMDARs at the glutamatergic synapses activated by contextual stimuli of the CPP box would suppress both CPP acquisition, via inhibiting LTP induction, and expression, via blocking potentiated NMDAR-mediated excitation. Furthermore, AP5 may also interfere with DA neuron burst firing triggered by amphetamine, as it would for the bursts triggered by cocaine (Koulchitsky et al., 2012; Sombers et al., 2009), providing an additional mechanism to inhibit plasticity induction during amphetamine conditioning sessions.
Figure 7. Involvement of mGluR1 and NMDAR in the VTA in the Acquisition and Expression of Amphetamine CPP in Socially Isolated Rats (P21–63).
(A) Representative photomicrograph of a cresyl violet-stained section illustrating bilateral cannula placements. This section was obtained from a rat injected with LY367385 before the amphetamine conditioning session. The boxed area roughly corresponds to the areas schematized in (B).
(B) Left: both LY367385 and AP5 blocked the acquisition of amphetamine CPP (F2,17= 45.8, p < 0.0001; one-way ANOVA). In these experiments, vehicle, LY367385, or AP5 was injected into the VTA before the single amphetamine conditioning session during 1 d CPP training. Right: AP5, but not LY367385, attenuated the expression of amphetamine CPP (F2,15 = 9.81, p < 0.01; one-way ANOVA). Vehicle, LY367385, or AP5 was injected into the VTA before the posttest following 1 d amphetamine CPP conditioning. Approximate locations (mm from bregma) of cannula tips are depicted at the bottom. **p < 0.01, ***p < 0.001 versus vehicle control (Bonferroni post hoc test). Error bars indicate SEM. See also Figures S3.
DISCUSSION
Neuronal properties and wiring in the CNS are shaped by experience during critical periods in early life. This is best illustrated by the critical period plasticity in the primary sensory cortex, in which sensory deprivation during early postnatal development produces deficiencies in cortical structure and function (Feldman, 2009; Hubel and Wiesel, 1970; Maffei and Turrigiano, 2008). The present study demonstrates that deprivation of social stimuli during early adolescence in rats enhances synaptic plasticity of NMDAR-mediated glutamatergic transmission in the VTA, a subcortical area critically involved in reward-based learning and the development of addictive behaviors. This enhancement of synaptic plasticity, or metaplasticity, occurs via increased mGluR/IP3-mediated Ca2+ signaling that drives NMDAR plasticity induction. Importantly, socially isolated animals display an increase in the rate of learning of drug-associated contextual stimuli. Furthermore, once acquired, drug-associated memory is more resistant to extinction in isolated animals. Therefore, changes in this form of Ca2+ signaling and synaptic plasticity may represent a potential mechanism through which addiction vulnerability is regulated during an adolescent critical period.
Our data indicate that IP3 sensitivity is increased in socially isolated rats. Animals repeatedly exposed to amphetamine or ethanol also display similar increases in IP3 sensitivity, which is reversed by inhibition of PKA activity in brain slices prepared from these animals (Ahn et al., 2010; Bernier et al., 2011). This is consistent with PKA-mediated phosphorylation of IP3 receptors increasing their affinity for IP3 (Wagner et al., 2008), although there are other potential mechanisms by which PKA can enhance IP3-induced Ca2+ signaling (Bruce et al., 2003). We found that previous social isolation occludes the effect of repeated amphetamine exposure in increasing DHPG-induced facilitation of IK(Ca), suggesting the involvement of a common neuroadaptation. This occlusion is not due to saturation at the level of IP3 signaling, as IP3 sensitivity can be further elevated in socially isolated animals by acute stimulation of PKA activity with CRF, which activates adenylyl cyclase via CRF2 receptors, as has been reported in animals repeatedly treated with ethanol (Bernier et al., 2011). It is well established that chronic exposure to addictive drugs leads to increased expression levels of adenylyl cyclase and/or PKA proteins (Hyman et al., 2006; Nestler, 2001). Therefore, it is possible that long-term social isolation may produce similar upregulation of these proteins, causing an increase in basal IP3 sensitivity.
Only animals isolated for an extended period (≥ 3 weeks) during early adolescence (P21–42), but not during a later adolescent period (P42–63), display an increase in mGluR/IP3-dependent facilitation of AP-evoked Ca2+ signals, which cannot be reversed by resocialization for ≥ 3 weeks. Thus, our data reveal a critical period during which social isolation persistently affects this type of Ca2+ signaling. A similar critical period during which isolation produces profound and irreversible changes has been identified previously (Einon and Morgan, 1977; Fone and Porkess, 2008; Lukkes et al., 2009b). Here, long-term post-weaning isolation (“isolation rearing”) produces a number of behavioral abnormalities, such as heightened aggression and anxiety, which usually cannot be reversed by resocialization. In contrast, isolation starting at mid- to late adolescence or adulthood (“isolation housing”) exerts milder and frequently reversible effects (Cilia et al., 2001; Wolffgramm, 1990; Wright et al., 1991) [but see (Wallace et al., 2009)]. Interestingly, amphetamine exposure is capable of enhancing mGluR-induced Ca2+ signaling even at P42, an age where social isolation has no effect. Furthermore, increased mGluR-dependent Ca2+ signaling following repeated ethanol exposure during early adolescence can be reversed after a month of abstinence (Bernier et al., 2011), as opposed to the persistence of the social isolation effect after resocialization. These observations suggest that social isolation and drug exposure engage different mechanisms to achieve a common neuronal adaptation, as described above. Emerging evidence implicates epigenetic modifications of the genome in experience-dependent plasticity of developing brain that persists into adulthood, such as neocortical plasticity resulting from sensory deprivation during a postnatal critical period (Fagiolini et al., 2009). It is not known if similar mechanisms are involved in the persistent effects of social deprivation during early adolescence observed in the current and previous studies (Einon and Morgan, 1977; Fone and Porkess, 2008; Lukkes et al., 2009b).
VTA neurons from socially isolated rats exhibit increased magnitude of NMDAR LTP compared to group housed controls. In our previous studies, NMDAR LTP was induced by pairing sustained glutamatergic input stimulation with postsynaptic burst firing (Ahn et al., 2010; Bernier et al., 2011; Harnett et al., 2009). In this induction protocol, sustained glutamatergic input stimulation starting before the burst onset enables preceding mGluR activation causing sufficient elevation in cytosolic IP3 levels to facilitate burst-evoked Ca2+ signals, in addition to NMDAR activation at the stimulated synapses at the time of the burst. In the present study, glutamatergic input stimulation preceding the burst was replaced with photolytic application of a defined concentration of IP3 directly into recorded neurons. Thus, our results clearly demonstrate that increased IP3 sensitivity, not increased mGluR-dependent production of IP3, drives enhanced LTP induction.
NMDARs on dopamine neurons are critical for the development of drug and food CPP (Zweifel et al., 2009) [but also see (Engblom et al., 2008)]. We found that amphetamine and ethanol CPP after a single conditioning session are greatly enhanced in rats isolated during early adolescence (P21–42). In line with our findings on mGluR/IP3-dependent Ca2+ signaling in the VTA, enhancement of CPP is not observed if the isolation period is short (P21–28) or delayed (P42–63), indicating that these cellular and behavioral changes are not due to the current state of isolation but rather a consequence of long-term isolation during an adolescent critical period. The intra-VTA microinjection experiments support the idea that increased mGluR-dependent NMDAR LTP drives the enhancement of amphetamine CPP in isolated animals, although mGluR1 and NMDAR antagonists may affect other processes in the VTA as well (Luo et al., 2010; Mameli et al., 2009).
It is of note that group housed rats acquire robust amphetamine CPP comparable to isolated animals after extended conditioning (3–7 d). It is likely that enhancement of mGluR/IP3-dependent NMDAR plasticity with repeated daily amphetamine exposure allows group housed animals to approach the performance level of isolated animals faster during extended conditioning. Therefore, long-term social isolation increases the initial rate of learning of drug-associated contextual stimuli without affecting the maximal level of learning. This may, at least partially, account for the discrepancies in previous studies investigating the impact of post-weaning social isolation on drug CPP (Bowling and Bardo, 1994; Schenk et al., 1986; Zakharova et al., 2009). Post-weaning isolation has been shown to result in a similar increase in the rate of acquisition of conditioned approach to sucrose-associated cues without affecting the asymptotic level of performance (Harmer and Phillips, 1998), raising the possibility that isolation-induced enhancement of NMDAR plasticity might facilitate appetitive Pavlovian conditioning in general. Isolated animals also exhibit increased operant responding for food and addictive drugs, especially at low dosage (Howes et al., 2000; Smith et al., 1997); however, it is not clear if this is due to an enhancement of the behavioral learning processes underlying operant conditioning (Wickens et al., 2007).
Enhanced NMDAR plasticity in the VTA stands in contrast to dampened glutamatergic synaptic plasticity after post-weaning social isolation observed in other brain areas, such as the hippocampus and prefrontal cortex (Lu et al., 2003a; Quan et al., 2010). These studies have further shown impairments in learning and memory tasks that are dependent on these brain areas, such as spatial learning and reversal learning, as opposed to enhanced drug-induced contextual learning found in the present study. Enhancement of synaptic plasticity of NMDARs may be viewed as “homeostatic metaplasticity” in which dopamine neurons increase their capacity to undergo activity-dependent synaptic strengthening to compensate for the paucity of synaptic drive and lack of opportunities for activity-dependent plasticity, as would occur when animals are placed in an impoverished environment deprived of social stimuli. It remains to be determined how social isolation affects other forms of synaptic plasticity in the mesolimbic system that have been implicated in the development of addiction (Mameli et al., 2009; Nugent and Kauer, 2008; Saal et al., 2003; Wolf and Ferrario, 2010).
Enduring memories of drug-associated stimuli, which persist even when the drug becomes unavailable during abstinence or extinction, play a pivotal role in drug addiction (Hyman et al., 2006). Extinction is thought to involve both unlearning of previous conditioning and new inhibitory learning to suppress the expression of conditioned responses (Mauk and Ohyama, 2004; Myers and Davis, 2002; Pan et al., 2008). Socially isolated rats exhibit a slower rate of extinction of amphetamine CPP compared to group housed controls, even if the magnitude of CPP is comparable after the same 7 d conditioning. This is consistent with general resistance to extinction observed with different memory tasks in isolation reared animals, which is thought to reflect impairments in the new learning processes [e.g., synaptic plasticity in the prefrontal cortex (Myers and Davis, 2002; Quan et al., 2010)] during extinction training (Fone and Porkess, 2008; Robbins et al., 1996). However, our results suggest that robust potentiation of NMDAR-mediated transmission in the VTA developed during conditioning might make it more resistant to depotentiation, thus retarding the unlearning component of extinction. Therefore, enhanced NMDAR plasticity in the VTA resulting from early life social isolation may increase addiction vulnerability, not only at the time of isolation but also in the future, by promoting the acquisition of drug-associated memories and their endurance.
EXPERIMENTAL PROCEDURES
Animals and Housing Conditions
Male Sprague-Dawley rats (3 or 6 weeks old) were obtained from Harlan Laboratories. Upon arrival in the animal facility, they were housed either in groups of three or in isolation. Food and water were available ad libitum. Grouped and isolated rats were maintained in the same room to provide a similar environmental experience besides the presence or absence of social contact. However, isolated rats may experience lower body temperature during sleep, especially at younger ages when they have not gained enough body fat. Animals spent 1–7 weeks under these housing conditions before experiments. For resocialization experiments, animals were housed in isolation or in groups of three from P21–42. At P42, isolated animals were housed together in groups of three for 3–4 weeks. Grouped animals were shuffled into new groups of three at P42, where they remained for 3–4 weeks. Thus, none of the regrouped animals were previously housed together in the same cage. All animal procedures were approved by the University of Texas Institutional Animal Care and Use Committee.
In Vivo Amphetamine Treatment
Rats received i.p. injections of D-amphetamine sulfate (5 mg/kg) once per day for either 1 d or 3 d. Approximately 24 hr after the final injection, animals were sacrificed for use in electrophysiological experiments.
Electrophysiology
Rats were decapitated under isoflurane anesthesia, and horizontal midbrain slices (190–220 μm) were cut in ice-cold solution containing (in mM) 205 sucrose, 2.5 KCl, 1.25 NaH2PO4, 7.5 MgCl2, 0.5 CaCl2, 10 glucose, and 25 NaHCO3, saturated with 95% O2 and 5% CO2 (~300 mOsm/kg) and incubated >1 h at 35°C in a solution containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 11 glucose, and 25 NaHCO3, saturated with 95% O2 and 5% CO2 (pH 7.4, ~295 mOsm/kg). Recordings were made at 33–35 °C in the same solution perfused at 2–3 ml/min. Intracellular solution contained (in mM): 115 K-methylsulfate, 20 KCl, 1.5 MgCl2, 10 HEPES, 0.025 EGTA, 2 Mg-ATP, 0.2 Na2-GTP, and 10 Na2-phosphocreatine (pH 7.25, ~285 mOsm/kg).
Cells were visualized using an upright microscope (Olympus) with infrared/differential interference contrast or oblique illumination optics. Recordings were performed in the lateral VTA located 50–150 μm from the medial border of the medial terminal nucleus of the accessory optic tract. Putative DA neurons were identified by their spontaneous firing (1–5 Hz) with broad APs (>1.2 ms) recorded in cell-attached configuration and large Ih currents (>200 pA elicited by 1.5 s hyperpolarizing steps from −62 mV to −112 mV) in whole-cell configuration. Whole-cell voltage-clamp recordings were made at a holding potential of −62 mV, corrected for a liquid junction potential of −7 mV. Pipettes with tip resistance of 1.6–2.0 MΩ were used. Series and input resistances were monitored throughout experiments and recordings were excluded if the series resistance increased beyond 20 MΩ or the input resistance dropped below 200 MΩ. A Multiclamp 700B amplifier (Molecular Devices) and AxoGraph X software (AxoGraph Scientific) were used to record and collect data (filtered at 1–5 kHz and digitized at 2–10 kHz).
A 2 ms depolarizing pulse from −62 mV to −7 mV was used to elicit an unclamped AP. The time integral of the outward tail current, termed IK(Ca), was calculated between 20 ms and 500 ms after the depolarizing pulse (expressed in picocoulombs). We have shown previously that IK(Ca) thus measured is completely eliminated by TTX and also by apamin, a selective blocker of Ca2+-gated SK channels, and thus can be used as a readout of AP-induced Ca2+ transients(Cui et al., 2007). For measuring IK(Ca) of a burst (five APs at 20 Hz), a 22 ms window from the beginning of each 2 ms depolarizing pulse was removed, then the integral of the outward current was calculated till 500 ms after the fifth pulse.
Flash Photolysis
Cells were dialyzed with caged IP3(1 – 100 μM) through the whole-cell pipette for 15–20 min after break-in. A brief UV flash (~1 ms) was applied with a xenon arc lamp driven by a photolysis system (Cairn Research) to rapidly photolyze caged IP3. The UV flash was focused through a 60x objective onto a 350 μm area surrounding the recorded neuron. The Cairn system has the capacity to vary the intensity, but not the duration, of UV flash. Since the degree of photolysis of caged compounds is proportional to the UV flash intensity (McCray et al., 1980), the concentration of IP3 released into the cell is reported as the concentration of caged IP3 in the pipette (in μM) multiplied by the UV flash intensity measured at the tip of the objective (in μJ), thus expressed in μM·μJ (e.g., 1000 μM·μJ IP3 can be achieved by photolyzing 10 μM caged IP3 with 100 μJ UV flash or by photolyzing 100 μM caged IP3 with 10 μJ UV flash). The degree of photolysis of caged IP3 does not reach 100% with the range of UV flash intensity achieved with our system (up to ~300 μJ), since the IP3 response, i.e., IIP3, shows no sign of saturation when low concentrations of caged IP3(1 – 10 μM) are used. A high IP3 concentration (100 μM) was necessary for experiments evoking saturating IIP3.
LTP Experiments
Synaptic stimuli were applied every 20 s using a bipolar tungsten electrode (~100 μm tip separation) placed 50–150 μm rostral to the recorded neuron. To isolate NMDAR EPSCs, recordings were performed in the presence of DNQX (10 μM), picrotoxin (100 μM), and eticlopride (100 nM) to block AMPA, GABAA, and D2 DA receptors, and in low Mg2+ (0.5 mM) to reduce Mg2+ blockade of NMDARs. The LTP induction protocol consisted of photolytic application of IP3 (100 μM·μJ) 50 ms prior to the pairing of synaptic stimulation (20 stimuli at 50 Hz) and a burst (5 APs at 20 Hz), in which the onset of synaptic stimulation was coincident with the burst onset. This IP3 application-synaptic stimulation-burst combination was repeated 10 times every 20 s. Magnitude of LTP was determined by comparing the average EPSC amplitude over the 10 min baseline period with that during another 10 min window ~30–40 min post-induction.
Conditioned Place Preference (CPP)
A CPP box consisting of two distinct compartments, separated by a small middle chamber, was used for conditioning (Med Associates). One compartment had a mesh floor with white walls, while the other had a grid floor with black walls. Rats were first subjected to a pretest, in which they were allowed to freely explore the entire CPP box for 20 min. The percentage of time spent in each compartment was determined after excluding the time spent in the middle chamber. Any rats that displayed >60% initial preference for either compartment during the pretest were not used for conditioning. For amphetamine CPP, rats were subjected to 1 d, 3 d, or 7 d conditioning starting the next day, in which they were given a saline injection (1 ml/kg) and confined to one compartment for 20 min in the morning and received an injection of amphetamine (5 mg/kg, i.p.) and confined to the other compartment for 20 min in the afternoon on each day. Assignment was counterbalanced such that animals had, on average, ~50% initial preference for the amphetamine-paired side in the pretest. A 20 min posttest was performed 1 d after the last conditioning session. Preference for the amphetamine-paired side (expressed in seconds) was defined as the time spent in the amphetamine-paired compartment minus that in the saline-paired compartment. CPP score was determined by subtracting the preference for the amphetamine-paired side in the pretest from that in the posttest. In extinction experiments, animals were subjected to a 20 min posttest every day for 14 d following 7 d amphetamine conditioning. For ethanol CPP, rats were subjected to 1 d conditioning in which they were given saline (4.2 ml/kg; morning) or ethanol (0.5 g/kg, 15% v/v; afternoon) and confined to one compartment for 7 min. Housing conditions were maintained throughout the CPP experiments.
Intra-VTA Microinjections
Rats were housed in isolation or in groups of three for 5 weeks from P21 before surgery. At P56, they were anesthetized with a mixture of ketamine and xylazine (90 mg/kg and 10 mg/kg, i.p.) and implanted with bilateral chronic guide cannulae (22 gauge; Plastics One), with dummy cannulae (32 gauge) inside, aimed at 1 mm above the VTA (anteroposterior, −5.3; mediolateral, +2.2; dorsoventral, −7.5; 10° angle) (Paxinos and Watson, 1998). The guide cannulae were fixed to the skull with stainless steal screws and dental cement. After the surgery, rats remained singly housed for another 7 d before being subjected to 1 d or 3 d amphetamine CPP experiments. We noted that more robust amphetamine CPP was observed when the experiments were started at P63 compared to those started at P42 for both group housed and isolated animals (compare data in Figure 6E with vehicle data in Figures 7 and S4). The reason for this age dependence of CPP magnitude is not clear.
Intra-VTA microinjections were made via injection cannulae (28 gauge; Plastics One) that extended 1 mm beyond the tip of the guide cannulae. Injection cannulae were connected to 1 μl Hamilton syringes mounted on a microdrive pump (Harvard apparatus). Rats received bilateral infusions (0.3 μl/side, 0.15 μl/min) of vehicle (PBS), LY367385 (0.6 nmol), or AP5 (0.6 nmol) 5 min before the afternoon amphetamine conditioning session or the posttest to test their effects on CPP acquisition or expression, respectively. The injection cannulae were left in place for 60 s after the end of infusion. In a separate series of experiments, to test if LY367385 or AP5 cause CPP or conditioned place aversion by itself, systemic saline injections were paired with both compartments of the CPP box, and bilateral intra-VTA microinjections of LY367385 or AP5 were made 5 min before the afternoon conditioning session.
After the CPP posttest, rats were anesthetized with a mixture of ketamine and xylazine (90 mg/kg and 10 mg/kg, i.p.) and transcardially perfused with 4% paraformaldehyde. Brains were then carefully removed and stored in 4% paraformaldehyde. Coronal sections (100 μm) were cut using a vibratome and stained with cresyl violet for histological verification of injections sites. Data from rats with injection sites outside the VTA were excluded from the analysis.
Drugs
DHPG, CRF, K41498, picrotoxin, eticlopride, DNQX, and D-AP5 were obtained from Tocris Biosciences. Caged IP3 was a generous gift from Dr. Kamran Khodakhah at Albert Einstein College of Medicine. All other drugs were obtained from Sigma.
Data Analysis
Data are expressed as mean ± SEM. Statistical significance was determined by Student’s ttest or ANOVA followed by Bonferroni post hoc test. The difference was considered significant at p < 0.05.
Supplementary Material
HIGHLIGHTS.
Social isolation of rats enhances synaptic plasticity of NMDARs in the VTA
Enhanced NMDAR plasticity occurs via increased mGluR/IP3-dependent Ca2+ signaling
Social isolation promotes amphetamine and ethanol conditioned place preference
Social isolation is effective only during an adolescent critical period (P21–42)
Acknowledgments
We thank Dr. Kamran Khodakhah for the generous gift of caged IP3 made in his lab. We also thank Dr. Yavin Shaham for discussions regarding CPP data analysis, Drs. Mark Harnett and Nikolai Dembrow for comments on the manuscript, Claire Stelly for assistance with animal housing, and Kavita Thakkar for assistance with histological verification of intracranial injections sites. This work was funded by NIH grants DA015687 and AA015521.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn KC, Bernier BE, Harnett MT, Morikawa H. IP3 receptor sensitization during in vivo amphetamine experience enhances NMDA receptor plasticity in dopamine neurons of the ventral tegmental area. J Neurosci. 2010;30:6689–6699. doi: 10.1523/JNEUROSCI.4453-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier BE, Whitaker LR, Morikawa H. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J Neurosci. 2011;31:5205–5212. doi: 10.1523/JNEUROSCI.5282-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol Biochem Behav. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J. Effects of environmental enrichment and social isolation on sucrose consumption and preference: associations with depressive-like behavior and ventral striatum dopamine. Neurosci Lett. 2008;436:278–282. doi: 10.1016/j.neulet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Bruce JI, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Cilia J, Reavill C, Hagan JJ, Jones DN. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats. Psychopharmacology (Berl) 2001;156:327–337. doi: 10.1007/s002130100786. [DOI] [PubMed] [Google Scholar]
- Cui G, Bernier BE, Harnett MT, Morikawa H. Differential regulation of action potential- and metabotropic glutamate receptor-induced Ca2+ signals by inositol 1,4,5-trisphosphate in dopaminergic neurons. J Neurosci. 2007;27:4776–4785. doi: 10.1523/JNEUROSCI.0139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deister CA, Teagarden MA, Wilson CJ, Paladini CA. An intrinsic neuronal oscillator underlies dopaminergic neuron bursting. J Neurosci. 2009;29:15888–15897. doi: 10.1523/JNEUROSCI.4053-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Steiniger-Brach B, Helboe L, Fink-Jensen A, Wortwein G. Socially isolated rats exhibit changes in dopamine homeostasis pertinent to schizophrenia. Int J Dev Neurosci. 2011;29:347–350. doi: 10.1016/j.ijdevneu.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Guatteo E, Mercuri NB, Bernardi G, Knopfel T. Group I metabotropic glutamate receptors mediate an inward current in rat substantia nigra dopamine neurons that is independent from calcium mobilization. J Neurophysiol. 1999;82:1974–1981. doi: 10.1152/jn.1999.82.4.1974. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Isolation rearing enhances the rate of acquisition of a discriminative approach task but does not affect the efficacy of a conditioned reward. Physiol Behav. 1998;63:177–184. doi: 10.1016/s0031-9384(97)00417-4. [DOI] [PubMed] [Google Scholar]
- Harnett MT, Bernier BE, Ahn KC, Morikawa H. Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron. 2009;62:826–838. doi: 10.1016/j.neuron.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Koulchitsky S, De Backer B, Quertemont E, Charlier C, Seutin V. Differential Effects of Cocaine on Dopamine Neuron Firing in Awake and Anesthetized Rats. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003a;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003b;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009a;158:845–855. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009b;3:18. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Good CH, Diaz-Ruiz O, Zhang Y, Hoffman AF, Shan L, Kuang SY, Malik N, Chefer VI, Tomac AC, et al. NMDA receptors on non-dopaminergic neurons in the VTA support cocaine sensitization. PloS one. 2010;5:e12141. doi: 10.1371/journal.pone.0012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Ohyama T. Extinction as new learning versus unlearning: considerations from a computer simulation of the cerebellum. Learn Mem. 2004;11:566–571. doi: 10.1101/lm.83504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray JA, Herbette L, Kihara T, Trentham DR. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980;77:7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Kauer JA. LTP of GABAergic synapses in the ventral tegmental area and beyond. J Physiol. 2008;586:1487–1493. doi: 10.1113/jphysiol.2007.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Schmidt R, Wickens JR, Hyland BI. Tripartite mechanism of extinction suggested by dopamine neuron activity and temporal difference model. J Neurosci. 2008;28:9619–9631. doi: 10.1523/JNEUROSCI.0255-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Quan MN, Tian YT, Xu KH, Zhang T, Yang Z. Post weaning social isolation influences spatial cognition, prefrontal cortical synaptic plasticity and hippocampal potassium ion channels in Wistar rats. Neuroscience. 2010;169:214–222. doi: 10.1016/j.neuroscience.2010.04.048. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–570. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Jones GH, Wilkinson LS. Behavioural and neurochemical effects of early social deprivation in the rat. J Psychopharmacol. 1996;10:39–47. doi: 10.1177/026988119601000107. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, Amit Z. Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol Biochem Behav. 1986;24:1793–1796. doi: 10.1016/0091-3057(86)90523-x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behavioral and brain functions: BBF. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Floris I, Biggio G. Social isolation-induced changes in the hypothalamic-pituitary-adrenal axis in the rat. Stress. 2005;8:259–264. doi: 10.1080/10253890500495244. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berl) 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Current topics in behavioral neurosciences. 2010;3:3–27. doi: 10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- Wagner LE, 2nd, Joseph SK, Yule DI. Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation. J Physiol. 2008;586:3577–3596. doi: 10.1113/jphysiol.2008.152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Li F, Wang D, Xie K, Wang D, Shen X, Tsien JZ. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163:890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.