Abstract
Highly accurate positioning is fundamental to the performance of vitreoretinal microsurgery. Of vitreoretinal procedures, membrane peeling is among the most prone to complications since extremely delicate manipulation of retinal tissue is required. Associated tool-to-tissue interaction forces are usually below the threshold of human perception, and the surgical tools are moved very slowly, within the 0.1–0.5 mm/s range. During the procedure, unintentional tool motion and excessive forces can easily give rise to vision loss or irreversible damage to the retina. A successful surgery includes two key features: controlled tremor-free tool motion and control of applied force. In this study, we present the potential benefits of a micro-force sensing robot in vitreoretinal surgery. Our main contribution is implementing fiber Bragg grating based force sensing in an active tremor canceling handheld micromanipulator, known as Micron, to measure tool-to-tissue interaction forces in real time. Implemented auditory sensory substitution assists in reducing and limiting forces. In order to test the functionality and performance, the force sensing Micron was evaluated in peeling experiments with adhesive bandages and with the inner shell membrane from chicken eggs. Our findings show that the combination of active tremor canceling together with auditory sensory substitution is the most promising aid that keeps peeling forces below 7 mN with a significant reduction in 2–20 Hz oscillations.
I. Introduction
Retinal microsurgery involves various micron scale maneuvers for which precise manual dexterity, fine visual-motor coordination, and application of forces that are well below human tactile sensation are highly required. Membrane peeling is a typical task that is performed to remove epiretinal or internal limiting membranes. During the procedure, the thin fibrous layer on the retina, which is typically ~60µm thick [1], is carefully delaminated off the retina surface by using either a bent blade or a hook. Since the retina is a very delicate tissue, the procedure is associated with high risks of irreversible retinal damage that may result in vision loss. Unintentional inaccurate tool positioning and excessive forces applied during the peeling procedure can easily give rise to serious complications such as iatrogenic retinal breaks [2,3,4], vitreous hemorrhage as well as subretinal hemorrhage [5]. Similarly, retinal veins and other tissues can be damaged during manipulation [6].
During membrane peeling, the instruments are moved very slowly, within a range of 0.1–0.5 mm/s, while the surgeon visually monitors local surface deformation simultaneously [29]. Depending on visual cues, the surgeon retracts the instrument and adjusts the peeling speed in order to prevent any excessive force application and trauma on the retina. This task depends highly on experience and is extremely challenging due to three main reasons: (1) inadequate spatial resolution and depth perception of microstructures to identify tissue planes, (2) imprecise movements during micromanipulation of tissue due to physiological tremor, and (3) lack of force sensing since the required forces for dissection are well below the surgeon’s sensory threshold. Hence for a successful operation, controlled tremor-free tool motion and limitation of force on the retina are two highly desired features.
In order to reduce physiological hand tremor, provide fine motion control, and consequently enhance microsurgical accuracy, several robotic systems have been developed by different researchers. Earliest examples include the RAMS (Robot-Assisted Micro-Surgery) system at JPL(Jet Propulsion Laboratory) [7,8] and the work of Hunter et al. [9,10]. These and many other tele-operated robots utilized remote control and motion scaling to reduce hand tremor [11,12,13,14]. Apart from these, JHU Steady-hand Eye Robot was developed based on a cooperative control scheme between the surgeon and a stiff non-backdrivable robot arm to combine manipulative transparency and immediacy of hand-held tools with precision and sensitivity of a machine [15,20,21]. Unlike any of these table-mounted systems, Micron was designed as a fully handheld instrument [16]. This micromanipulator robot can sense its own motion, estimate the undesired hand tremor component in real time, and moves the tool tip relative to the handle to counteract this component. Being a handheld instrument, Micron possesses some advantages compared to the aforementioned alternatives. First, it is mechanically a simpler robot with fewer degrees of freedom and smaller footprint, which reduces the cost significantly. Second, the intuitive feel of handheld tools is preserved and motion control of the main tool still remains in the surgeon's hands. This adds to the overall safety of the system since the surgeon can react quickly to manipulate the tool in case the patient moves and finish the procedure manually even if the robotic system fails. Despite these advantages in terms of positioning accuracy, safety, economy and ease of use, Micron has a major drawback: it does not provide any feedback or limitation on the applied forces during the surgery.
Focusing on force sensing for micromanipulation purposes, results in vitro have been presented for force-reflecting teleoperation by Salcudean et al. [17] and for reflected torque feedback by Tokuyasu and Kitamura [18]. For microassembly tasks, similar results were reported by Zhou et al [19]. However, these approaches were not appropriate for use on a handheld device due to size limitations. Using a single degree of freedom (DOF) force sensing retinal pick, axial tool shaft forces during retinal manipulation in porcine cadaver eyes were recorded by Gupta et al [22]. It was reported that 75% of all measured forces were below 7.5 mN in magnitude and only 19% of events at this force level could be sensed by the surgeon. Subsequently, a miniature 3-DOF force sensing microsurgical instrument with sub-mN resolution was developed by Berkelman et al. [23,24]. This strain gauge based force sensor was mounted on the handle of the first version of CMU Micron by Jagtap et al [25]. However, when forces exerted during several vitreoretinal surgery tasks were measured in-vivo in rabbits, they were considerably higher than the results presented in [22]. This could be attributed to the friction and other forces between the tool and the trocar, which can significantly attenuate or distort the propagation of the forces to the tissues inside of the eye. Therefore, a handle mounted force sensor is not practical for vitreoretinal surgery. This limitation can be overcome by incorporating force sensing elements along the distal portion of the instrument shaft, such that the active sensing part of the sensor is completely situated along the tool shaft inside the eye [26].
In this paper, we report the integration of force sensing capabilities on a hand-held tremor canceling micromanipulator, Micron, by using fiber Bragg grating (FBG) strain sensors in order to provide microsurgical aids and reduce complications during vitreoretinal surgery. In the following sections, we will first present the details of this micro-force sensing micromanipulator. This will be followed by the experiment results and performance assessment for membrane peeling on two types of phantoms. The paper concludes with discussion of the results.
II. Micro-Force Sensing MicroManipulator
A. Micron
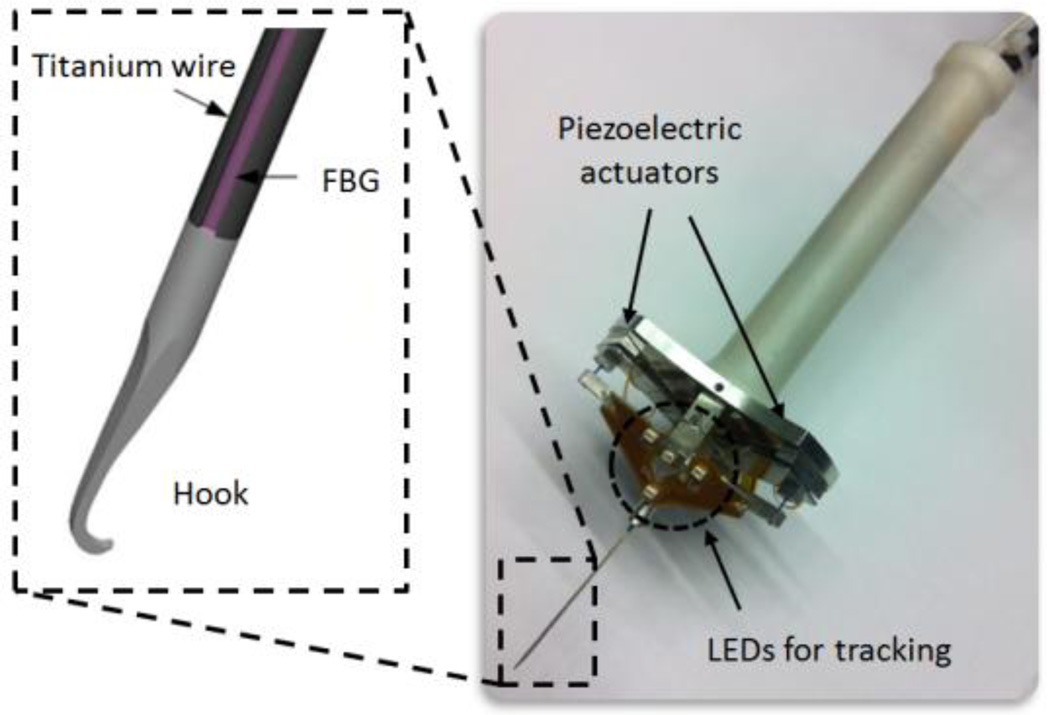
Highly accurate positioning is fundamental to the performance of microsurgery, which is hindered by tremor in many cases, including epiretinal membrane peeling. Micron is a handheld actively stabilized 3-DOF micromanipulator that is mainly designed to remove unintentional motion and enhance positional accuracy [16]. The device operates by activating three piezoelectric actuators based on the sensed motion of the handle. Each actuator has 400 µm range of motion. The position information is provided by ASAP optical sensors with a resolution of 4 µm at a rate of 2 kHz by tracking the LEDs mounted to Micron, which are shown in Fig. 1. After sensing its own motion, Micron separates it into involuntary components, such as hand tremor, and desired components. This step is achieved by either a first order low-pass Butterworth filter with a corner frequency of 1.5 Hz or a more advanced low-pass shelving filter depending on the application. Then Micron moves its tip to counteract the involuntary motion component. The workspace of Micron is specified as a 1×1×0.5 mm volume centered on the handle position. The device has about 1 N force capability, and bandwidth over 100 Hz.
Figure 1.
Micron and the integrated 2-DOF force sensing hook tool.
B. Integrated Tip Force Sensor
The forces associated with vitreoretinal microsurgery are routinely less than 7.5 mN [22]. Measuring these forces demand a sensing instrument with sub-mN accuracy. The size and weight of the sensing instrument are critical factors since the sensor will be mounted on a handheld device. Furthermore, the sensing instrument should be able to pass through a 25 Ga sclerotomy opening so that the sensor can be located close to the tool tip. Only in this way, can force measurements pertain solely to the instrument’s tip, with no contribution from the sclera.
Considering these limitations, a hook tool with 3 integrated FBG sensors was manufactured following the design of Iordachita et al. as shown in Fig. 1 [26]. FBGs are robust optical sensors capable of detecting changes in strain. By measuring the bending of the tool they allow for calculation of the force in the transverse plane with a sensitivity of 0.25 mN. The tool is a simple hook, and is mounted on the tip of Micron at a specific orientation. The calibration procedure was completed to get the following matrix:
The obtained calibration matrix (K) was used in the linear relationship given by (1) to calculate force (F) from FBG wavelength shift (ΔS) [26].
| (1) |
During the operation, the sensor data were collected and processed at 2 kHz and transmitted over TCP/IP.
C. Control Scheme
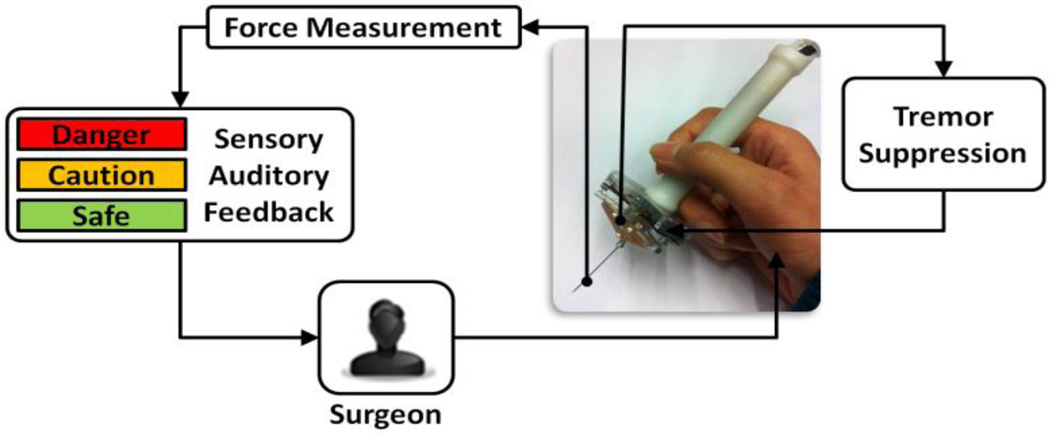
The developed control scheme aims at combining Micron with FBG force sensing in order to avoid unintentional hand motion, filter out physiological hand tremor, and keep applied forces below a certain safety limit. This requires two main control loops that manipulate the tool tip position and applied forces jointly. In current practice, control of force is based on visual cues only. During the operation, the surgeon visually monitors local surface deformation and changing light reflections to assess the stress applied to the tissue. However, with such a visual sensory substitution that highly relies on experience and concentration, the success is very limited. It was previously shown by Kitagawa et al. that continuous real-time auditory feedback representing applied force could be a clearer cue and thus significantly improve the performance in complex surgery [27]. For simultaneous control of tool position and force, we implemented auditory sensory substitution to work jointly with Micron's own tremor canceling control loop.
Our current control scheme is shown in Fig. 2. Accordingly, as the surgeon moves the tool, Micron device senses the hand-tremor and cancels it. Meanwhile, tool-to-tissue interaction forces are measured and sent to the workstation in real-time. Then, the integrated tip force sensor output is translated into auditory signals. Depending on the tool tip force level, the surgeon can hear 3 different tempos of audio “beeps” representing 3 safety zones. The borders of these zones were defined based on typical vitreoretinal operations. The audio remains silent until 1 mN or greater force is measured. Between 1 mN and 3.5 mN, a constant slow beeping is emitted, which is designated to be a “safe zone”. The second zone is between 3.5mN and 7 mN. A proportionally increasing tempo is generated to indicate this “caution zone”. Beyond 7 mN is a "danger zone" for potential retinal breaks and tears, which is represented by a constant high beeping.
Figure 2.
Force sensing Micron control scheme. The measured forces on the tool tip are translated into auditory signals to guide the surgeon while Micron supresses hand-tremor in another loop.
III. Experiments
A. Setup
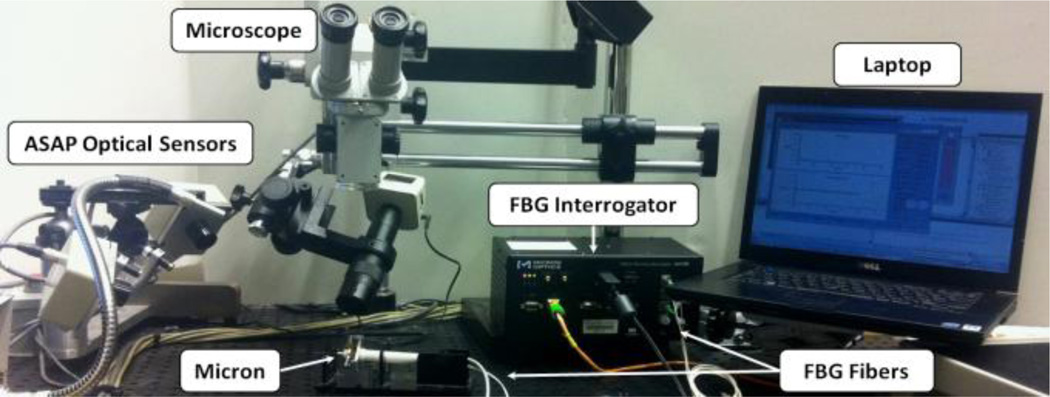
To simulate membrane peeling, a series of experiments have been performed on the setup shown in Fig. 3. The applied force and tool velocity were recorded for different cases and phantoms. The tool velocity was tracked by ASAP optical sensors. In order to monitor the integrated force sensor, an optical sensing interrogator, sm130–700 from Micron Optics Inc. (Atlanta GA), was used.
Figure 3.
Setup for epiretinal membrane peeling experiments.
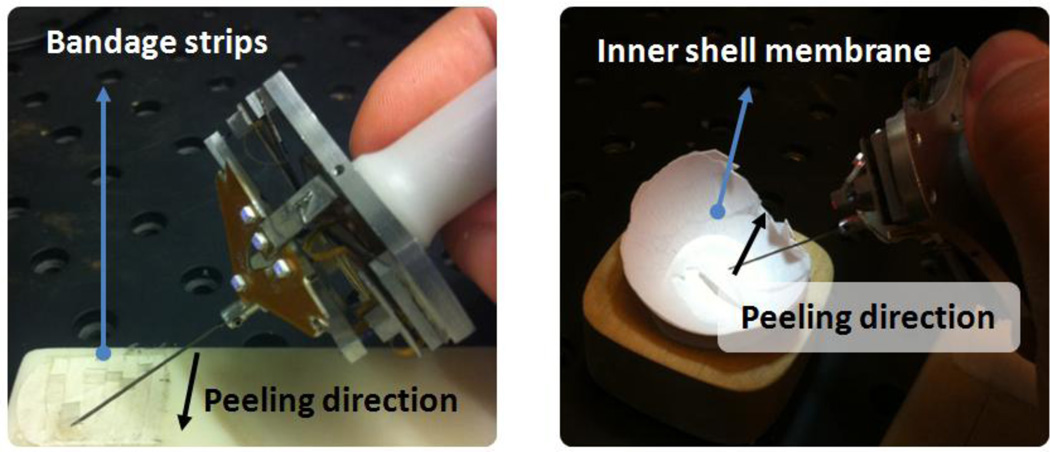
Tests were done on two types of phantoms, which have previously been reported and used in our laboratory as suitable surrogates for an epiretinal membrane: bandage phantom[29], inner shell membrane of raw chicken eggs[15].
B. Procedure
In this preliminary experiment, two sets of tests were performed. The performance of the same subject was analyzed for different cases. For each phantom type, an extensive training period (~3 hrs) was allowed before data collection.
Membrane Peeling on Bandage Phantom: Sticky tabs from 19 mm Clear Bandages (RiteAid brand) were sliced to produce strips 2 mm wide. The subject was asked to peel a 10 mm section of the strip steadily and without stopping while holding the Micron instrument almost perpendicular to the bandage surface in order to minimize the forces along the tool axis.
Membrane Peeling on Raw Chicken Eggs: The inner shell membrane of raw chicken eggs were peeled following a linear trajectory. Unlike the bandages, this phantom accounted for biological factors such as heterogeneous tissue structure and its effect on force. A single egg shell was used for each test, and it was assumed that the membrane structure did not vary significantly between eggs.
For each experiment set, four different cases were evaluated as shown in Table I. Five trials were performed in random order for each of these scenarios. The tool tip force, position, speed and video were recorded. Based on the video timestamp, starting and ending points of the peeling motion were identified in the acquired data. The assessment was based on the applied forces during delaminating motion.
TABLE I.
Membrane Peeling Performance Test Cases
| Case | Tremor Suppression |
Force Feedback |
|---|---|---|
| Completely unaided | No | No |
| Only Micron aided | Yes | No |
| Only with auditory feedback | No | Yes |
| Micron aided with auditory feedback | Yes | Yes |
IV. Results and Discussion
A. Membrane Peeling on Bandage Phantom
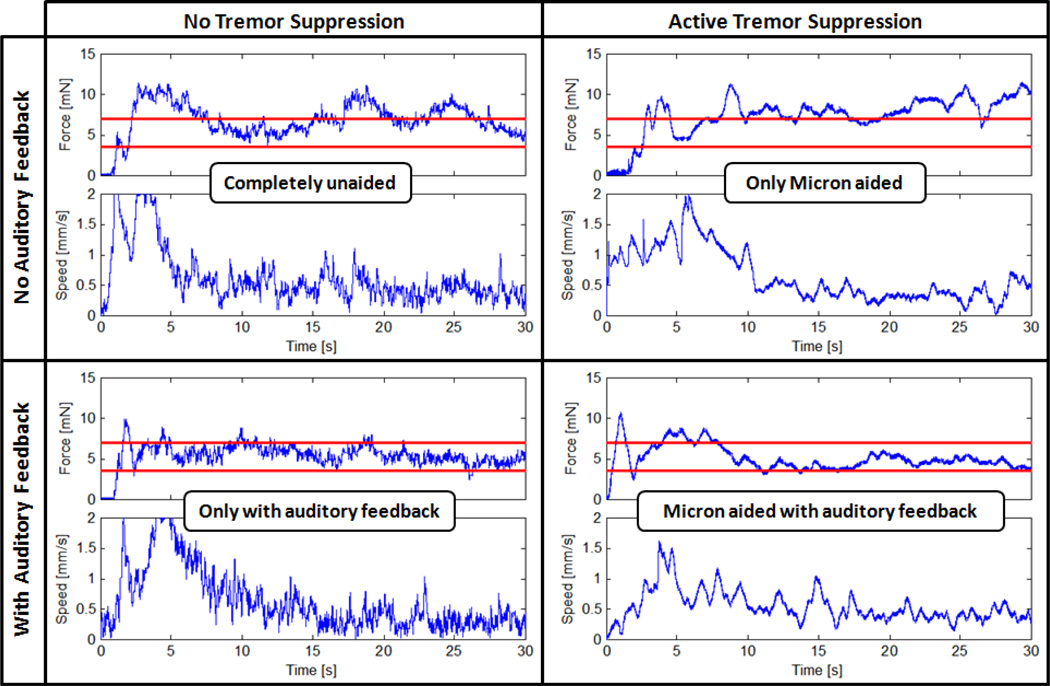
In Fig. 5, the results of a test set on bandage phantom are presented for all cases. Accordingly, free hand membrane peeling forces exhibited two main types of variations during the delaminating period: low frequency changes due varying peeling speed and high frequency oscillations due hand tremor. In the absence of auditory and tremor suppression aids, peeling forces exceeding 7 mN were recorded with high frequency oscillations. Upon activation of Micron, these oscillations were significantly reduced. However, still, forces in danger zone were observed due lack of force feedback. When auditory sensory substitution was used alone, almost all forces were kept below the safety threshold successfully, nevertheless with high frequency oscillations, which appeared both in speed and force plots. In the last case, combination of active tremor canceling together with auditory sensory substitution provided the best performance, where peeling forces were kept within the safe operation zone free of high frequency oscillations.
Figure 5.
Measured membrane peeling force samples for four different cases using bandage phantom [29]. Maximum allowable operation zone (3.5 mN – 7mN) is indicated with horizontal red lines. High frequency oscillations are canceled when Micron is activated. Auditory sensory substitution helps in keeping the peeling force below the safety threshold.
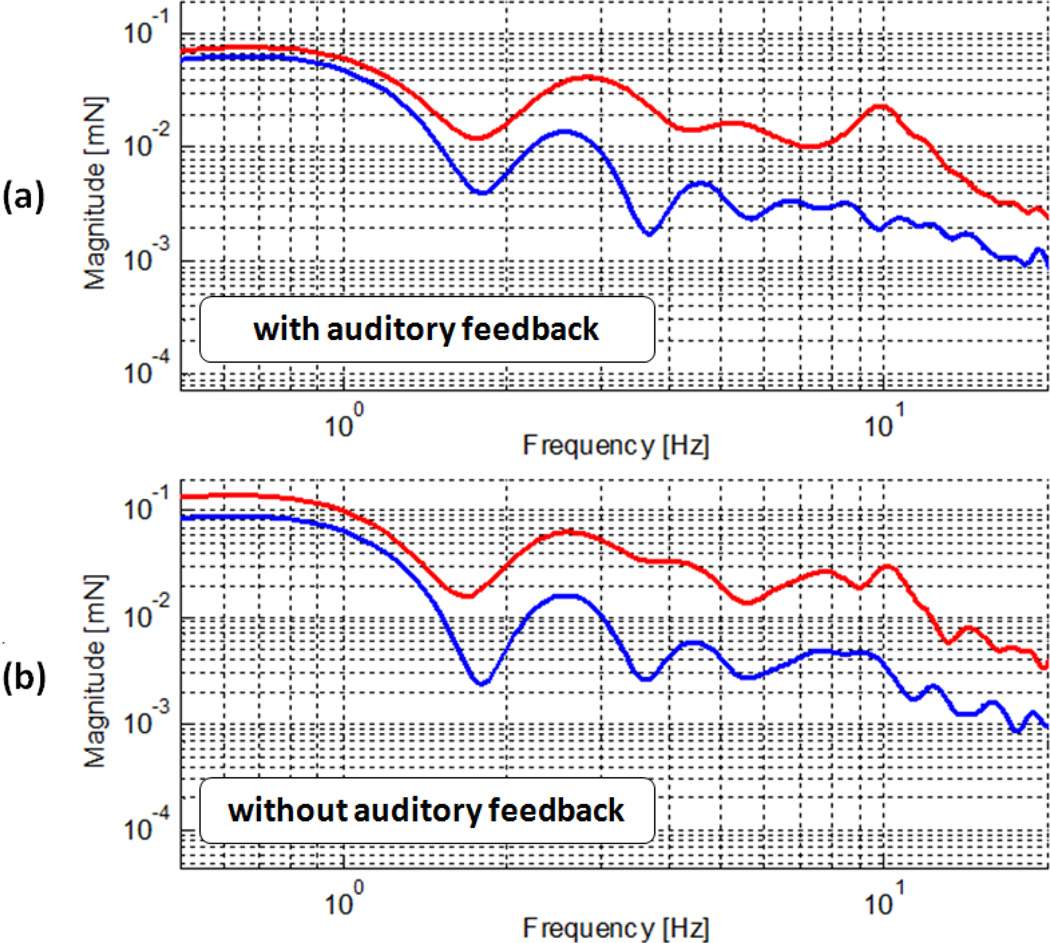
In order to assess the effect of Micron in our tests, we performed frequency analysis based on 5 trials per each case. The results are presented in Fig. 6 and Fig. 7. The bandwidth of human eye-hand feedback usually is from 0.5 Hz to 2 Hz [28]. Below 0.5 Hz, eye-hand feedback becomes effective, which explains the flat region below 0.5 Hz in Fig. 6.a and 6.b. The prominence of the 10 Hz peak in both figures primarily stems from postural hand tremor as it occurs at a frequency between about 8–10 Hz in normal humans[28].
Figure 6.
Frequency analysis of recorded forces for all 4 cases on bandage phantom: with auditory feedback (a), without auditory feedback (b), with tremor suppression (blue), and without tremor suppression (red).
Figure 7.
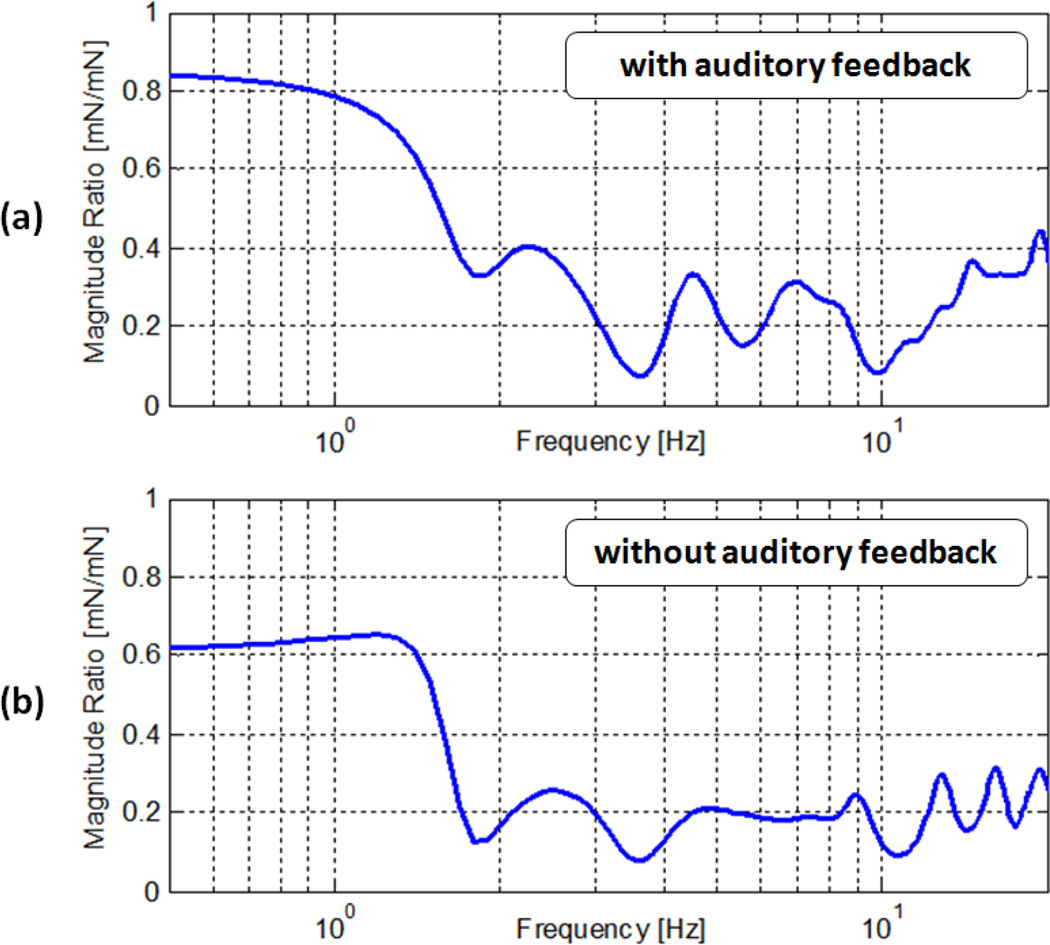
Micron aided to free-hand force magnitude ratio with respect to frequencies. Significant reduction in 2–20 Hz forces: (a) 60%–90% with auditory feedback, (b) 70%–90% without auditory feedback.
Beyond 2 Hz until 20 Hz, the magnitude of forces is greatly reduced in both the absence and presence of auditory force feedback. In this range, the recorded reduction is 60%–90% with auditory sensory substitution, and 70%–90% without auditory sensory substitution as shown in Fig. 7.a and 7.b respectively.
B. Membrane Peeling on Raw Chicken Eggs
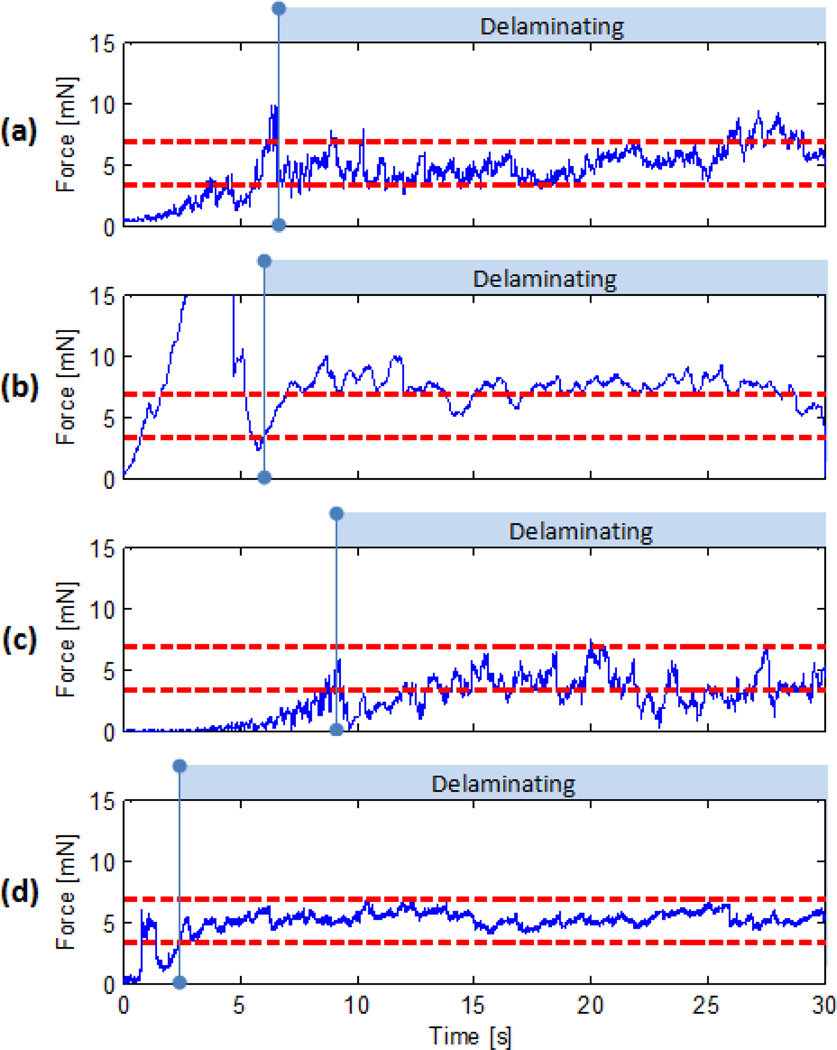
Although a different phantom type is used, the results are in great agreement with bandage phantom observations as shown in Fig. 8. The peeling forces exhibit very similar characteristics for each of the tested cases. In free hand tests (Fig. 8.a), high force variations with occasional non-safe forces were observed. Micron was successful in eliminating these high frequency oscillations (Fig. 8.b) while auditory feedback reduced overall forces below the safety threshold (Fig. 8.c).
Figure 8.
Membrane peeling forces on raw chicken eggs:(a) completely unaided, (b) only Micron aided, (c) only with auditory feedback, (d) Micron aided with auditory feedback. Oscillations in delaminating forces are highly reduced by Micron while auditory feedback could prevent application of forces above 7 mN.
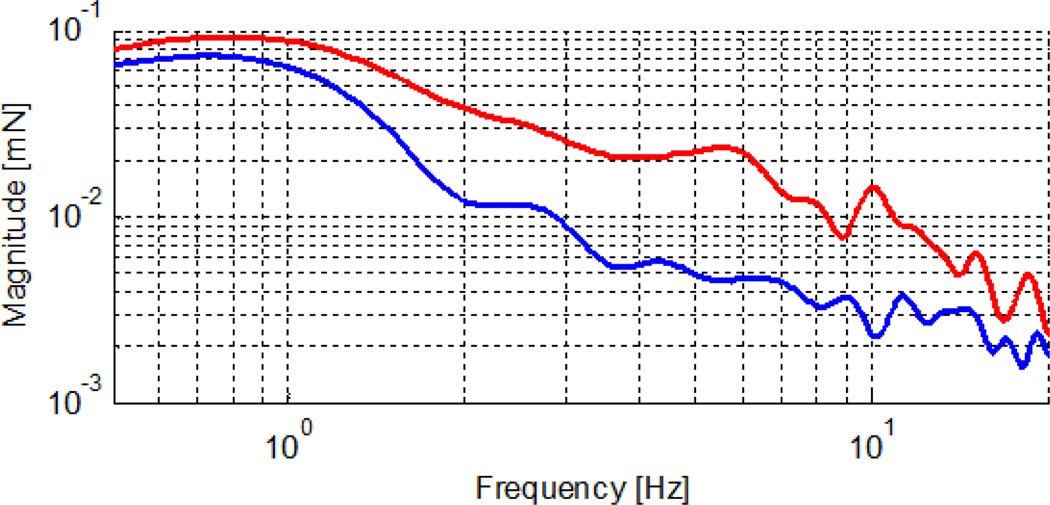
Frequency analysis of forces measured in Micron-activated and Micron-deactivated cases revealed a significant reduction between 2–10 Hz, which is shown in Fig.9. In free-hand peeling tests (red line in Fig. 9), a peak was observed again at 10 Hz, which disappeared upon activation of Micron (blue line in Fig. 9). Despite this improvement, the forces still could go beyond the safety limits when no auditory guidance was provided. When Micron was used together with auditory sensory substitution, the operation was accomplished within the desired zone successfully.
Figure 9.
Frequency analysis of recorded forces on raw chicken eggs: with tremor suppression (blue), and without tremor suppression (red).
V. Conclusion
This paper has reported the integration of a 2-DOF force sensing tool with a handheld robot, Micron, for superior performance in membrane peeling operations. Micron alone could provide tremor free and accurate positioning control as well as a great reduction in force oscillations between 2–20 Hz. On the other hand, FBG based force sensing instruments could directly inform the surgeon of the extremely delicate peeling forces with no adverse effect from tool-to-sclera interaction. By mapping this information into auditory signals in real time, the forces could be kept below a safety threshold throughout the operation. Providing these two advantages on a single surgical tool, micro-force sensing Micron is able to respond to two of the most critical requirements of vitreoretinal surgery. Our preliminary tests on two types of phantoms have shown that Micron, with integrated force sensing capabilities, is an attractive solution for vitreoretinal surgery.
In this study, we used a 2-DOF hook tool with Micron. A limitation of this approach is the inability to measure axial forces. In order to minimize axial components, the operations need to be performed while holding the Micron instrument almost perpendicular to the membrane surface, which is not always practical. This can be resolved by integrating a complete 3-DOF force sensing tool, which is currently in progress. Another limitation of Micron is its ASAP optical sensors, which can track the tool only when held in a certain orientation and position range. Resolving these limitations, more realistic circular trajectories can be followed better during membrane peeling operation.
Our future studies aim at conducting more experiments on chick chorioallantoic membrane (CAM). We are planning to perform these tests with multiple subjects to assess the performance of the surgical tool. Upon success, the experiments will be carried in a simulated clinical environment using rabbit eyes. With its position and force sensing capabilities and intuitive feedback, we believe that the presented micromanipulator can be a viable option not only for membrane peeling but also for many other microsurgical procedures where positioning accuracy and force limitation problems coexist and need to be addressed together. In order to extend the applications, we also plan to integrate a force sensing forceps tool with Micron and assess the performance similarly.
Figure 4.
Experiments were done on two types of phantoms: bandage phantom (left) and inner shell membrane of raw chicken eggs (right).
Acknowledgments
Research supported by in part by the National Institutes of Health under R01 EB007969, and R01 EB000526, and in part by Johns Hopkins internal funds.
Contributor Information
Berk Gonenc, Email: bgonenc1@jhu.edu, ERC for Computer Integrated Surgery at Johns Hopkins University, Baltimore, MD 21218 USA.
Marcin A. Balicki, Email: marcin@jhu.edu, ERC for Computer Integrated Surgery at Johns Hopkins University, Baltimore, MD 21218 USA.
James Handa, Email: jthanda@jhmi.edu.
Peter Gehlbach, Email: pgelbach@jhmi.edu.
Cameron N. Riviere, Email: camr@ri.cmu.edu, Robotics Institute at Carnegie Mellon University, Pittsburgh, PA 15213 USA.
Russell H. Taylor, Email: rht@jhu.edu, ERC for Computer Integrated Surgery at Johns Hopkins University, Baltimore, MD 21218 USA.
Iulian Iordachita, Email: iordachita@jhu.edu, ERC for Computer Integrated Surgery at Johns Hopkins University, Baltimore, MD 21218 USA.
REFERENCES
- 1.Wilkins JR, Puliafito CA, Hee MR, Duker JS, Reichel E, Coker JG, Schuman JS, Swanson EA, Fujimoto JG. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103(12):2142–2151. doi: 10.1016/s0161-6420(96)30377-1. [DOI] [PubMed] [Google Scholar]
- 2.Sjaarda RN, Glaser BM, Thompson JT, Murphy RP, Hanham A. Distribution of iatrogenic retinal breaks in macular hole surgery. Ophthalmology. 1995;102(9):1387–1392. doi: 10.1016/s0161-6420(95)30859-7. [DOI] [PubMed] [Google Scholar]
- 3.Carter JB, Michels RG, Glaser BM, DeBustros S. Iatrogenic retinal breaks complicating pars plana vitrectomy. Ophthalmology. 1990;97(7):848–853. doi: 10.1016/s0161-6420(90)32492-2. [DOI] [PubMed] [Google Scholar]
- 4.Grigorian RA, Castellarin A, Fegan R, Seery C, Del Priore LV, von Hagen S, Zarbin MA. Epiretinal membrane removal in diabetic eyes: Comparison of viscodissection with conventional methods of membrane peeling. Br J Ophthalmology. 2003;87:737–741. doi: 10.1136/bjo.87.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakata K, Ohji M, Ikuno Y, Kusaka S, Gomi F, Tano Y. Sub-retinal hemorrhage during internal limiting membrane peeling for a macular hole. Graefes Arch Clin Exp Ophthalmol. 2003;241:582–584. doi: 10.1007/s00417-003-0676-y. [DOI] [PubMed] [Google Scholar]
- 6.Tsilimbaris MK, Lit ES, D'Amico DJ. Retinal microvascular surgery: A feasibility study. Invest Ophthalmol Vis Sci. 2004;45(6):1963–1968. doi: 10.1167/iovs.03-0874. [DOI] [PubMed] [Google Scholar]
- 7.Das H, Zak H, Johnson J, Crouch J, Frambach D. Evaluation of a telerobotic system to assist surgeons in microsurgery. Comput Aided Surg. 1999;4:15–25. doi: 10.1002/(SICI)1097-0150(1999)4:1<15::AID-IGS2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Schenker PS, Barlow EC, Boswell CD, Das H, Lee S, Ohm TR, Paljug ED, Rodriguez G, Charles ST. Development of a telemanipulator for dexterity enhanced microsurgery. Proc 2nd Int Symp Med Rob Comput Asst Surg. 1995:81–88. [Google Scholar]
- 9.Hunter IW, Doukoglou TD, Lafontaine SR, Charette PG, Jones LA, Sagar MA, Mallinson GD, Hunter PJ. A teleoperated microsurgical robot and associated virtual environment for eye surgery. Presence. 1993;2:265–280. [Google Scholar]
- 10.Hunter IW, Jones LA, Sagar MA, Lafontaine SR, Hunter PJ. Opthalmic microsurgical robot and associated virtual environment. Comput Biol Med. 1995;25(2):173–182. doi: 10.1016/0010-4825(94)00042-o. [DOI] [PubMed] [Google Scholar]
- 11.Ueta T, Yamaguchi Y, Shirakawa Y, Nakano T, Ideta R, Noda Y, Morita A, Mochizuki R, Sugita N, Mituishi M, Tamaki Y. Robot-assisted vitreoretinal surgery: Development of a prototype and feasibility studies in an animal model. Ophthalmology. 2009;116:1538–1543. doi: 10.1016/j.ophtha.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Das H, Zak H, Johnson J, Crouch J, Frambach D. Evaluation of a telerobotic system to assist surgeons in microsurgery. Comput Aided Surg. 1999;4:15–25. doi: 10.1002/(SICI)1097-0150(1999)4:1<15::AID-IGS2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Jensen PS, Grace KW, Attariwala R, Colgate JE, Glucksberg MR. Toward robot-assisted vascular microsurgery in the retina. Graefes Arch Clin Exp Ophthalmol. 1997;235:696–701. doi: 10.1007/BF01880668. [DOI] [PubMed] [Google Scholar]
- 14.Mulgaonkar AP, Hubschman J-P, Bourges J-L, Jordan BL, Cham C, Wilson JT, Tsao T-C, Culjat MO. A prototype surgical manipulator for robotic intraocular micro surgery. Stud Health Technol Inform. 2009;142:215–217. [PubMed] [Google Scholar]
- 15.Uneri A, Balicki MA, Handa J, Gehlbach P, Taylor RH, Iordachita I. New steady-hand Eye Robot with micro-force sensing for vitreoretinal surgery. Biomedical Robotics and Biomechatronics (BioRob), 2010 3rd IEEE RAS and EMBS International Conference on. 2010 Sept.vol.(no.):814–819. doi: 10.1109/BIOROB.2010.5625991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLachlan RA, Becker BC, Cuevas Tabarés J, Podnar GW, Lobes LA, Riviere CN. Micron: an actively stabilized handheld tool for microsurgery. IEEE Trans Robot. 2012 Feb.vol.28(no. 1):195–212. doi: 10.1109/TRO.2011.2169634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salcudean SE, Ku S, Bell G. Performance measurement in scaled teleoperation for microsurgery. Proc. CVRMed- MRCAS'97, Lect. Notes Comput. Sci. 1997;vol. 1205:789–798. [Google Scholar]
- 18.Tokuyasu T, Kitamura T. A study on minute torque transmission for tele microsurgery; Proc. 2001 JSME Conf. Robotics and Mechatronics (ROBOMEC'01); Takamatsu, Japan. 2001. Jun, [Google Scholar]
- 19.Zhou Y, Nelson BJ, Vikramaditya B. Fusing force and vision feedback for micromanipulation. Proc. IEEE Int. Conf. Robot. Autom; Leuven; Belgium. 1998. May, pp. 1220–1225. [Google Scholar]
- 20.Taylor R, Jensen P, Whitcomb L, Barnes A, Kumar R, Stoianovici D, Gupta P, Wang Z, deJuan E, Kavoussi L. A steady-hand robotic system for microsurgical augmentation. MICCAI. 1999:1031–1041. [Google Scholar]
- 21.Mitchell B, Koo J, Iordachita M, Kazanzides P, Kapoor A, Handa J, Hager G, Taylor R. Development and application of a new steady-hand manipulator for retinal surgery. IEEE ICRA. 2007 Apr;:623–629. [Google Scholar]
- 22.Gupta PK, Jensen PS, de Juan E., Jr Surgical forces and tactile perception during retinal microsurgery. MICCAI’99. LNCS. 1999;1679:1218–1225. [Google Scholar]
- 23.Berkelman PJ, Whitcomb LL, Taylor RH, Jensen P. A miniature microsurgical instrument tip force sensor for enhanced force feedback during robot-assisted manipulation. IEEE Trans Robot Autom. 2003;19(5):917–921. [Google Scholar]
- 24.Berkelman PJ, Whitcomb LL, Taylor RH, Jensen P. Medical Image Computing and Computer-Assisted Interventions. Pittsburgh: 2000. A miniature Instrument Tip Force Sensor for Robot/Human Cooperative Microsurgical Manipulation with Enhanced Force Feedback; pp. 897–906. [Google Scholar]
- 25.Jagtap D, Riviere CN. Applied force during vitreoretinal microsurgery with handheld instruments; Proc 26th IEEE engineering in medicine and biology conference (EMBS); San Francisco. 2004. pp. 2771–2773. [DOI] [PubMed] [Google Scholar]
- 26.Iordachita I, Sun Z, Balicki M, Kang J, Phee S, Handa J, Gehlbach P, Taylor R. A sub-millimetric, 0.25 mn resolution fully integrated fiber-optic force-sensing tool for retinal microsurgery. International Journal of Computer Assisted Radiology and Surgery (IJCARS) 2009 Jun;vol. 4(no. 4):383–390. doi: 10.1007/s11548-009-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitagawa M, Dokko D, Okamura A, Yuh D. Effect of sensory substitution on suture manipulation forces for robotic surgical systems. JTCS. 2005;129(1):151–158. doi: 10.1016/j.jtcvs.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Stiles RN. Mechanical and neural feedback factors in postural hand tremor of normal subjects. J. Neurophysiol. 1980;vol. 44:40–59. doi: 10.1152/jn.1980.44.1.40. [DOI] [PubMed] [Google Scholar]
- 29.Balicki M, Uneri A, Iordachita I, Handa J, Gehlbach P, Taylor R. Micro-force Sensing in Robot Assisted Membrane Peeling for Vitreoretinal Surgery. Med Image Comput Comput Assist Interv. 2010;13(Pt 3):303–310. doi: 10.1007/978-3-642-15711-0_38. [DOI] [PMC free article] [PubMed] [Google Scholar]