Abstract
Males of the North American cicada Okanagana rimosa (Homoptera: Cicadidae, Tibicininae) emit loud airborne acoustic signals for intraspecific communication. Specialised vibratory signals could not be detected; however, the airborne signal induced substrate vibrations. Both auditory and vibratory spectra peak in the range from 7–10 kHz. Thus, the vibrations show similar frequency components to the sound spectrum within biologically relevant distances. These vibratory signals could be important as signals involved in mate localization and perhaps even as the context for the evolution of the ear in a group of parasitoid flies.
Keywords: auditory, acoustic communication, evolution, insect
Introduction
Insects communicate by means of most modalities known for animals. Often, different modalities are involved in intra- and interspecific communication in insects. In some cases, different modalities are coupled to each other, either successively or simultaneously. We focus here on sinusoidal compression wave signals produced by insects that are not incidental to other activities and which elicit predictable responses (i.e. they are communicative). Signals that have most of their energy in a fluid medium (air or water) are usually called acoustic or sound signals, particularly if they contain loud enough and low enough frequencies to be heard by humans, while those that have most of their energy propagated in a solid substrate are commonly called vibrational signals. Some species of Hemiptera and Orthoptera emit acoustic signals as well as vibratory signals (Moore, 1961; Keuper and Kühne, 1983; Gogala, 1985; Weidemann and Keuper, 1987). Both signals are usually distinct from each other in frequency content and temporal pattern and are produced by different mechanisms. Both signals may be important for localization of a conspecific mate, or in other contexts. In tettigoniids, substrate vibrations are an accessory signal in finding the precise place of the sound emitting conspecific, particularly within the complex three-dimensional environment of a woody plant (Latimer and Schatral, 1983; Stiedl and Kalmring, 1989). Other insect species communicate primarily with either airborne sound, like many grasshoppers (Elsner and Popov, 1978), or with vibratory signals, like ants (Markl, 1983). In the case of planthoppers and leafhoppers (Homoptera), acoustic sounds are of low intensity, and the most important signal seems to be substrate vibrations (Moore, 1961; Gogala et al., 1974; Claridge, 1985; Gogala, 1985; Strübing and Rollenhagen, 1988; Cocroft, 1996). Species of cicadas in the related taxon Homoptera are well known for their acoustic communication, but not for vibratory communication with the exception of some primitive species (Moore, 1973; Claridge et al., 1999). Male cicadas produce loud airborne signals which attract conspecific females, or males in a few cases (Alexander and Moore, 1958; Simmons et al., 1971; Moore, 1973; Popov, 1990; Moore, 1993).
The cicada Okanagana rimosa produces communicative sound primarily by a timbal mechanism (Moore and Sawyer, 1966; Moore, 1973). The calling song has a spectrum with a main energy peak at around 7–10kHz and a sound intensity of about 90dB SPL measured at a distance of 15cm (Huber et al., 1980; Lakes-Harlan et al., 2000). A singing male usually sits on woody plant parts such as branches, or the trunk of trees such as aspen and maple. Females perform phonotaxis to find the male within the complex tree and shrub habitat, and can acoustically locate the sound source during flight (unpublished observations), like other species of cicadas (Doolan and Young, 1989; Moore et al., 1993). The sound produced by males carries significant energy (Bennet-Clark, 1994) that is produced while standing on a woody plant, suggesting the possibility of simultaneous transmission or induction of signals in the plant substrate. Further, the contractions of the large timbal muscles even during periods of “silent singing” might be propagated to the substrate as signals useful in communication (Weber et al., 1988; Hennig et al., 1994). Thus, it might be possible that substrate vibrations are transmitted to the plant, which also might help the female to find the male cicada within the labyrinth of branches. Therefore, we set out to investigate if such vibratory communication might occur in large cicadas, as has recently been reported for a western Palaearctic cicada (Gogala et al., 1996).
Our search for the substrate vibrations emitted by cicadas was also stimulated by a second finding. Some parasitoid fly species detect singing cicadas by airborne sound (Soper et al., 1976; Lakes-Harlan et al., 1999; Robert et al., 1999). An hypothesis put forward to explain the evolution of the parasitoid sensory system suggests the possibility that the fly ear evolved from a vibration receiver (Lakes-Harlan et al., 1999). Therefore, this receiver might originally have served for detection of vibrations emitted by the host, and might still also be used for this purpose. Thus we wanted to study whether or not substrate vibrations occur in the communicatory repertoire of the modern host.
Materials and Methods
Investigations with Okanagana rimosa, (Say) (Homoptera, Cicadidae, Tibicininae) were performed at the Biological Station of the University of Michigan, Cheboygan Co., near Pellston, Michigan. Male cicadas were caught 25 km east of Grayling, Crawford County, MI, and were transferred to the Biological Station. Animals were kept in small cages with freshly cut twigs, and all experiments were performed within two days after capture. Care was taken to use only males which were in good condition and no differences were observed between males used immediately after capture and those after one or two days in captivity. Observations on singing males and courting males were performed at various places over several years in the field in the lower peninsula of Michigan.
As it is difficult to record spontaneous calling songs from captured males, we used electrical stimulation to elicit calling songs (n=169 songs from 10 males). For these experiments, a thin copper wire was inserted frontally into the supraesophageal ganglion (brain). A second wire was placed laterally inside the prothorax. Both wires were fixed to the cuticle with crazy glue and connected to a 12 V battery. The voltage could be adjusted with a potentiometer and was slowly increased until the cicada produced sounds (usually at about 6 V). Current was given for one to ten seconds, and sounds were only produced during these periods. Room temperature was 24°C-26°C, and the light intensity was 380–400 Lux.
A freshly cut branch of maple (one of the typical trees on which the cicadas were found in the field) was fixed with its thick end in a holder. A branch was approximately 1.40 m long and from 0.8 to 1.0 cm in diameter, with further distal small side branches bearing leaves. The accelerometer (Bruel & Kjaer 4369; flat frequency response 2 Hz-12 kHz) was screwed into the branch at a distance of about 70 cm from the holder. The male cicada was placed at different distances between the accelerometer and the holder, or distally on the smaller side branches. The vibratory signals were amplified (Nexus 2690) and recorded on DAT tape (Sony 5DJ A; 44.1kHz sampling rate). The airborne sound was recorded using a sound level meter (Bruel & Kjaer 2203) equipped with a 1/2″ microphone (Bruel & Kjaer 4165), and also stored on DAT tape. All signals were subsequently analysed with Fast-Fourier transformation using a spectral analyser (Hewlett-Packard 5327; 2048 lines; Hanning filter; 44.1 kHz sampling rate).
Experiments on transmission of broadcast airborne sound to the substrate were performed in a soundproof room at the Zoological Institute in Göttingen, Germany. A pine stick (1m in length, 12mm in diameter) was attached at one end to a holder (Fig. 1). A loudspeaker (Dynaudio D21 AF) was placed at a distance of 30 cm from and pointing toward the side of the stick, at a distance of 55 cm from the holder. Sound intensity was measured at the stick with a sound level meter (Bruel & Kjael 2203) equipped with a 1/2″ microphone (Bruel & Kjael 4165). The accelerometer, and additionally a solid small glass sphere (about 150 µm diameter; <0.2 µg; for improvement of signal-to-noise ratio of the laser measurements), were both attached to the stick at a distance of 90 cm from the holder. The beam of the laser vibrometer (Polytec OFV-2100) was focused on the sphere, and the vibrometer signal was analysed with a Hewlett-Packard spectral analyser (356XA). This arrangement allowed comparative measurements between the laser vibrometer and the accelerometer (which was used in the field experiments with the cicadas). Both methods revealed the same spectra, and the sensitivity of the accelerometer was in the range of the laser vibrometer. For stimulation, pure frequency tones (3–15 kHz; 60–90 dB SPL relative to P0=2*10−5 N/m2 at the site of the measurement; 1sec duration) were generated with the Hewlett-Packard 356XA analyser and broadcast from the loudspeaker.
Figure 1. A.
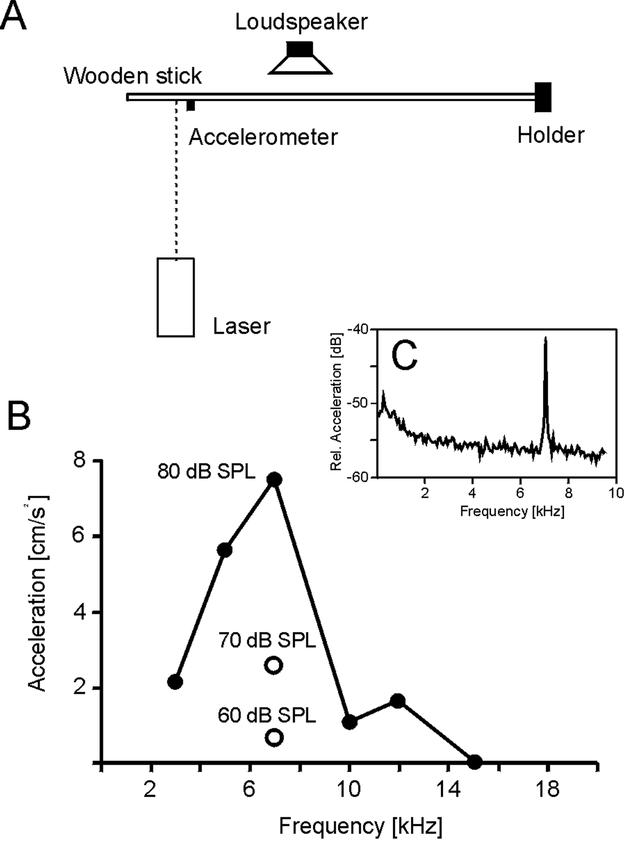
Diagram of experimental setup for investigation of airborne sound-induced substrate vibrations in a pine stick, not shown to scale. The accelerometer was screwed into the stick and the laser beam was focussed on a grass sphere nearby. B. Graph of the acceleration of induced substrate vibrations during broadcast stimulation of the stick with pure sine wave tones from a loudspeaker of 80 dB SPL intensity; at 7 kHz three sound intensities were tested. C. Graph of the vibration spectrum induced in the pine stick by a 7 kHz airborne sound broadcast at 80 dB SPL, as recorded by a laser vibrometer. Velocity: 20*log (X mms−1/90 mms−1) [dB]
Results
Singing Okanagana rimosa males usually sit on woody parts of a plant, including smaller branches. Field observations of calling and courting males did not reveal any special movements that likely would be involved in distinct vibratory signalling. Males produce the airborne calling song using their timbals, and it functions in long range signalling. Single low amplitude wing flips are sometimes delivered during calling song production, particularly near the beginning of the song, and also if another cicada or a human approaches slowly during singing (unpublished observations). The shorter courtship sound is similarly produced, but, in contrast, it increases in amplitude from beginning to end and is usually accompanied by one to three low amplitude wings flips, particularly between bursts of sound production (Moore unpublished observations). Females perform phonotaxis during flight and land near the male. Thereafter, females may approach the male more closely by further short flights, or by walking, particularly after visual contact is made. Walking seems to take place within close range of the male, perhaps 50 cm or less. During walking, there may be a premium on choosing the correct branch to approach the male in the shortest time, which potentially could be facilitated by vibratory signals as well as by acoustic and visual cues.
However, as specialised vibratory signalling from males could not be demonstrated readily, we asked if airborne sound signals might induce vibrations in the substrate. Therefore laboratory experiments were performed to investigate possible substrate vibration induced by airborne sound in the overall frequency range of the cicada's song (Figs. 1A, B). Acoustic stimulation of a pine stick with pure sine wave tones resulted in substrate vibrations with acceleration values in the cm/s2 range. The magnitude of vibration depended on frequency as well as on acoustic stimulus intensity (Fig. 1B). In the frequency range of the cicada song, even with broadcast levels as low as 60 dB SPL (at 7 kHz), peaks could be measured in the vibration spectrum by both the laser vibrometer and the accelerometer. Vibrations in response to airborne sound were detected up to 12 kHz (with 80 dB SPL stimulation intensity); stimulation with 15 kHz, even at 90 dB SPL, failed to evoke detectable substrate vibration.
Encouraged by this finding, we attempted to record vibratory signals of singing male cicadas. Caged or tethered males, however, did not sing after capture, either indoors or outdoors, despite seemingly good abiotic conditions (bright sunshine, temperature above 25°C). Therefore, experiments were performed with singing induced by electrical brain-stimulation. Electrically-stimulated O. rimosa males produced sound pulses, either as a continuous sequence (rather like calling songs) or grouped in irregular chirps (rather like disturbance squawks) (Fig. 2A and OR_airborne.wav [OR_airborne.wav is available for download at http://insectscience.org/2.2]). The sound pulses had a slower repetition rate (varying from 30 to 70 pulses per second) than the calling song recorded in the field (about 80 pulses per second). These induced airborne sounds had a spectral content which peaked around 7–10 kHz (Fig. 2B), similar to the spectrum of the natural calling song (Huber et al., 1980; Lakes-Harlan et al., 2000). Substrate vibrations could be registered from the branch on which the male sat. Spectral analysis of the vibrations showed that they contained high frequency components (Fig. 2C and OR_vibration.wav [OR_vibration.wav is available for download at http://insectsciecne.org/2.2]). The main peak seen was from 6 to 10 kHz, closely resembling that of the airborne sound, although the overall frequency distribution from the branch differed from natural songs. In the frequency range below 4 kHz, additional components in the vibratory frequency spectrum were found. The peak of acceleration was up to 0.7 m/s2. The vibrations were propagated through the branch over a distance of at least 60 cm. The peak amplitude seemed not to decrease within the tested distance of 20 to 60 cm of the male in proximal parts of the branch (diameter 10–12 mm; Fig. 2C). Vibrations could also be detected at the same distances from the male in the distal parts of the branches (diameter 6–8 mm, data not shown).
Figure 2. A.
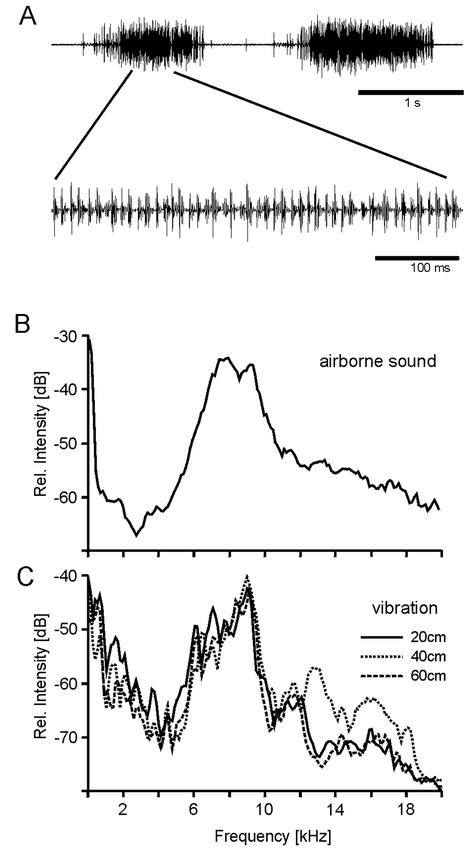
Oscillographic traces of the temporal pattern of sound elicited by electrical stimulation of a male Okanagana rimosa cicada. B. Graph of the frequency spectrum of the airborne sound produced by an electrically-stimulated cicada; overall sound pressure level about 80 dB SPL (rms) measured at a distance of approximately 10 cm. C. Graph of spectra of vibrations produced in a maple branch by the same electrically-stimulated singing male as in B, sitting at a distance of 20, 40 and 60 cm from the accelerometer; the overall peak acceleration was 50 cm/s2.
Further tests verified that the registered vibrations originated from the airborne sound produced by the cicada. If the animal were held so that it sat on the branch, vibration could be measured as described above (Fig. 3A, contact). If the animal was lifted until not in physical contact with the branch, the peaks of vibration were reduced in a distance-dependent manner (Fig. 3A), but still showed a similar frequency content. Further support for the vibrations being induced by airborne sound came from experiments in which the timbals had been destroyed by cutting the timbal ribs of both sides. These animals (N=3) contracted the timbal muscles during electrical stimulation; however, almost no noise was produced. Consequently, the high frequency components in the vibration spectrum were completely missing (Fig. 3B).
Figure 3. A.
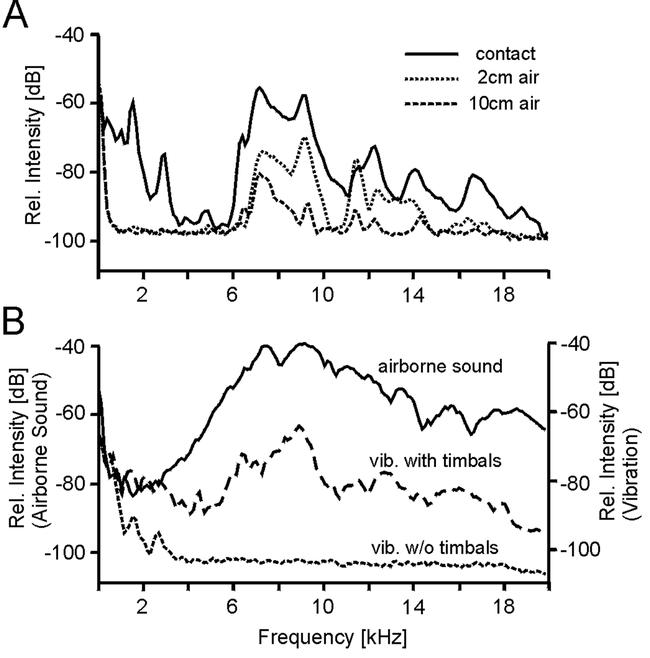
Graph of spectra of relative intensity of vibrations in a maple branch produced by an electrically stimulated singing male, placed at a distance of 10 cm from the accelerometer. Solid line (contact): animal sits on the stick (overall peak acceleration was 45 cm/s2); dashed lines: animal lifted 2 cm (2 cm air) and 10 cm (10 cm air) above and out of physical contact with the stick. B. Graph of spectra of relative airborne sound pressure level and of relative vibration acceleration in a maple branch produced by an electrically stimulated singing male, before and after destroying its timbals. For visualization of the differences all spectra have been similarly scaled, although absolute scales differ. Solid line: (airborne sound) timbals intact; dashed line (vib. with timbals): timbals intact; narrow dashed line (vib. w/o timbals): both timbals destroyed. Vibrations (vib.) recordings from an animal in contact with the branch in 10 cm distance to the accelerometer.
Discussion
Cicada calling song and vibration
The male calling song attracts conspecific females. The males sit on stems and branches of trees and bushes from about 1 m to more than 10 m in height while producing songs. Females of O. rimosa perform phonotaxis during flight similar to that of other species of cicadas (Lakes-Harlan and Moore unpublished; Doolan and Young, 1989; Moore et al., 1993; Daws et al., 1997). If a female lands nearby a calling male, she either sits, reorients and starts a further flight sequence, or walks to the male. During walking, substrate vibrational signals could provide important cues for locating a male in a dense three-dimensional labyrinth of branches, similar to the approach context and behavior of tettigoniids (Latimer and Schatral, 1983). However, no specialised cicada vibratory signalling was observed (although such signals occur in primitive cicada species of Tettigarcta that produce pure vibratory signals instead of acoustic signals; Claridge et al., 1999). Other possible sources of substrate vibratory signals are, therefore, vibrations induced by walking of the males or females, by wing flips of males or females, and by the high intensity airborne timbaling sounds of males. Wing flips are probably most important as visual and/or acoustic signals during courtship and disturbance situations in O. rimosa. They are known to be involved in acoustic (and probably substrate and visual as well) signalling in at least platypediine, tettigadine, and some other tibicinine cicadas (Moore, 1973; Sanborn and Phillips, 1999). Walking of males (and presumably of females) induces irregular low frequency vibrations (unpublished results), but their specificity and importance is unclear.
Therefore we focused on the high intensity airborne sound of males as a potential source for inducing substrate vibrations. The calling song of Okanagana rimosa lasts from about one second up to several seconds. Spectral analysis reveal that the song contains frequencies from 2 kHz to about 12 kHz, and that its energy peaks at around 7–10 kHz (Huber et al., 1980; Lakes-Harlan et al., 2000). Cicadas are able to discriminate different frequencies (Huber et al., 1980; Fonseca et al., 2000) which might be important for signal recognition, e.g. during phonotaxis (Lakes-Harlan et al., 2000). The intensity of the calling song of a single male is about 87–90 dB SPL (rms), measured 15 cm dorsally to the cicada. Such high intensity airborne sound is quite usual for cicadas, which can reach even higher intensities (Pringle, 1954; Simmons and Young, 1978; Bennet-Clark and Young, 1994; Sanborn and Phillips, 1995; Bennet-Clark, 1999). The energy of signals generated by the timbals of males, with their hollow abdomens acting as Helmholtz resonators, travels through the whole body of the insect, including the contralateral timbals and tympanal membranes (Young, 1990; Fonseca and Bennet-Clark, 1998; Bennet-Clark, 1999). This induces loud airborne sounds and can induce surprisingly strong vibrations in the substrate as well, as reported here.
Substrate vibration induced by airborne sound (Properties of the substrate)
The frequency characteristics of the airborne sound were also seen in the substrate vibrations. Controls with separation of the accelerometer from the substrate, separation of the singing insect from the substrate, and stimulation with sine wave acoustic signals via a nearby loudspeaker confirmed the observed transmission of sound energy to the substrate. Examples of airborne sound-induced vibrations are rare in insect physiology, despite a wealth of literature on vibratory communication. Induction of vibrations by airborne sound has been found in rice plants with tones of 72–76 dB SPL intensity and 150–1600 Hz frequency (Saxena and Kumar, 1980). To the best of our knowledge, the proposed disruption of communication by airborne sound in insect pest management has not been demonstrated to be effective and has not been followed further. Among tettigoniids, airborne sound-induced vibrations have been found in addition to pure vibratory signals. In Tettigonia cantans, the vibratory signal contains components below 5 kHz that are missing in playback experiments with a loudspeaker (Keuper and Kühne, 1983). Thus, these vibratory signals are coupled differently to plants than are those of cicadas.
Within plants, vibration waves have very complex patterns. For example, bending waves occur with complex reflection patterns whose amplitude may vary greatly (Michelsen et al., 1982; Keuper and Kühne, 1983; Cocroft et al., 2000). There is often no simple relationship between amplitude and distance from the signalling animal. Comparisons between the low intensity communication signals of small planthoppers and bugs showed that the frequency spectra of airborne sounds and vibrations had identical ranges, but relatively more energy was present in low frequencies in the vibrations (Gogala et al., 1974; Michelsen et al., 1982; Gogala et al., 1996). Our study showed similar frequency components in both systems. One should keep in mind that our study was not intended to analyze physical properties of the induction and transmission of vibratory signals within the substrate, but rather to show whether or not such vibrations were possible as a by-product of acoustic communication in cicadas.
Use of vibrations for phonotaxis
Vibratory signals are an important communication channel in many insects (Moore, 1961; Alexander et al., 1963; Markl, 1983; Claridge, 1985; Gogala, 1985; Kalmring, 1985). In particular, many small insects, like true bugs, produce vibratory signals in a variety of communicative contexts. Small Hemiptera and Homoptera may produce both mate-attracting and defensive vibratory signals (Moore, 1961; Gogala et al., 1974; Michelsen et al., 1982; Gogala, 1985; Strübing and Rollenhagen, 1988; Cocroft, 1996; Cocroft et al., 2000). Vibratory signals of many orthopterans are described as comprising a separate communication channel in addition to their acoustic signals. The vibratory information helps responding individuals find the sender in complex environments such as multiple-branched plants (Keuper and Kühne, 1983; Latimer and Schatral, 1983; Weidemann and Keuper, 1987; Stiedl and Kalmring, 1989). Cicadas are well known for their acoustic communication (Alexander and Moore, 1958; Moore, 1973; Claridge, 1985; Moore, 1993), although vibratory signalling is known from primitive cicadas (Moore, 1973; Claridge et al., 1999). A function of the newly described vibratory signals in O. rimosa, and from direct mechanical initiation in timbal-less platypediine cicadas is yet to be shown. It seems possible, at least in O. rimosa, that they might function as an additional information channel involved in mate finding. Substrate vibrations contain directional information either due to amplitude modulation or to complex transfer functions in the insect body (Latimer and Schatral, 1983; Cocroft et al., 2000). Substrate vibrations are perceived by various receptor organs that have been studied in Hemiptera, but not in cicadas (Cicadidae) (Michel et al., 1982; Cokl, 1983). In the primitive cicadas of Tettigarcta, potentially sensory structures at the tarsal empodium might act as vibration receivers (Moulds, 1990).
Vibratory signals are also used by many parasitoids to locate a host. These vibrations are typically associated with feeding and other movements (Meyhöfer and Casas, 1999). However, it cannot be excluded that specialized vibratory signals also are used. O. rimosa is host to a dipteran parasitoid (Emblemasoma auditrix) which locates male cicadas by their acoustic signals (Soper et al., 1976; Lakes-Harlan et al., 2000). The homologous organ in non-hearing flies is sensitive to substrate vibrations in a similarly high frequency range (Lakes-Harlan et al., 1999). An evolutionary transformation from a vibratory sense organ into an ear seems a plausible, though speculative, scenario. Thus, this hypothesis, which is derived from morphological and physiological evidence (Lakes-Harlan et al., 1999), is now further supported by the occurrence of substrate vibration production during acoustic signalling of hosts. Vibrations such as those shown in this study might also have been used by a hypothetical non-hearing ancestral species of this modern parasitoid to locate its host, most likely in addition to other cues.
Acknowledgments
We thank M. Hennig for advice on electrical stimulation of sound production. T. Weber and M. Gogala critically commented on an earlier version of the manuscript. We also thank the University of Michigan Biological Station (UMBS), Pellston, Michigan, for making laboratory and living facilities as well as field sites available. The work was supported by the Deutsche Forschungsgemeinschaft (La 741/4-1), by the Ammermann Fund of the University of Michigan Museum of Zoology, and by a UMBS grant to R. L.-H.
Glossary
| Abbreviation: | |
|---|---|
| dB | decibels |
| kHz | kilohertz |
| SPL | sound pressure level |
| rms | root mean square |
References
- Alexander RD, Moore TE. Studies on the acoustical behavior of seventeen-year cicadas (Homoptera: Cicadidae: Magicicada) Ohio Journal of Science. 1958;58:107–127. [Google Scholar]
- Alexander RD, Moore TE, Woodruff RE. The evolutionary differentiation of stridulatory signals in beetles (Insecta: Coleoptera) Animal Behaviour. 1963;11:111–115. [Google Scholar]
- Bennet-Clark HC. The world's noisiest insects - cicada song as a model of the biophysics of animal sound production. Verhandlungen der Deutschen Zoologischen Gesellschaft. 1994;87:165–176. [Google Scholar]
- Bennet-Clark HC. Resonators in insect sound production: How insects produce loud pure-tone songs. Journal of Experimental Biology. 1999;202:3347–3357. doi: 10.1242/jeb.202.23.3347. [DOI] [PubMed] [Google Scholar]
- Bennet-Clark HC, Young D. The scaling of song frequency in cicadas. Journal of Experimental Biology. 1994;191:291–294. doi: 10.1242/jeb.191.1.291. [DOI] [PubMed] [Google Scholar]
- Claridge MF. Acoustic Signals in the Homoptera: Behavior, Taxonomy, and Evolution. Annual Review of Entomology. 1985;30:297–317. [Google Scholar]
- Claridge MF, Morgan JC, Moulds MS. Substrate-transmitted acoustic signals of the primitive cicada, Tettigarcta crinita Distant (Hemiptera Cicadoidea, Tettigarctidae) Journal of Natural History. 1999;33:1831–1834. [Google Scholar]
- Cocroft RB. Insect vibrational defence signals. Nature. 1996;382:679–680. [Google Scholar]
- Cocroft RB, Tieu TD, Hoy RR, Miles RN. Directionality in the mechanical response to substrate vibration in a treehopper (Hemiptera: Membracidae: Umbonia crassicornis) Journal of Comparative Physiology A. 2000;186:695–705. doi: 10.1007/s003590000123. [DOI] [PubMed] [Google Scholar]
- Cokl A. Functional properties of vibroreceptors in the legs of Nezara viridula (L.) (Heteroptera, Pentatomidae) Journal of Comparative Physiology A. 1983;150:261–269. [Google Scholar]
- Daws AG, Hennig RM, Young D. Phonotaxis in cicadas Cystosoma saundersii and Cyclochila australasiae. Bioacoustics. 1997;7:173–188. [Google Scholar]
- Doolan JM, Young D. Relative importance of song parameters during flight phonotaxis and courtship in the bladder cicada Cystosoma saunderii. Journal of Experimental Biology. 1989;141:113–131. [Google Scholar]
- Elsner N, Popov AV. Neuroethology of acoustic communication. Advances in Insect Physiology. 1978;13:229–355. [Google Scholar]
- Fonseca PJ, Bennet-Clark HC. Asymmetry of tymbal action and structure in a cicada: a possible role in the production of complex songs. Journal of Experimental Biology. 1998;201:717–730. doi: 10.1242/jeb.201.5.717. [DOI] [PubMed] [Google Scholar]
- Fonseca PJ, Münch D, Hennig RM. How cicadas interpret acoustic signals. Nature. 2000;405:297–298. doi: 10.1038/35012696. [DOI] [PubMed] [Google Scholar]
- Gogala M. 1985 Vibrational communication in insects (Biophysical and behavioural aspects). In: Kalmring K, Elsner N, editors. Acoustic and vibrational communication in insects. 117–126.Berlin, Hamburg. Parey Verlag. [Google Scholar]
- Gogala M, Cokl A, Draslar K, Blazevic A. Substrate-borne sound communication in Cydnidae (Heteroptera) Journal of Comparative Physiology A. 1974;94:25–31. [Google Scholar]
- Gogala M, Popov AV, Ribaric D. Bioacoustics of singing cicadas of the western palaearctic: Cicadetta tibialis (Panzer)(Cicadoidea: Tibicinidae) Acta Entomologica Slovenica. 1996;4:45–62. [Google Scholar]
- Hennig RM, Weber T, Moore TE, Huber F, Kleindienst H-U, Popov AV. Function of the tensor muscle in the cicada Tibicen linnei. Journal of Experimental Biology. 1994;187:33–44. doi: 10.1242/jeb.187.1.33. [DOI] [PubMed] [Google Scholar]
- Huber F, Wohlers DW, Moore TE. Auditory nerve and interneurone responses to natural sounds in several species of cicadas. Physiological Entomology. 1980;5:25–45. [Google Scholar]
- Kalmring K. 1985 Vibrational communication in insects (Reception and integration of vibratory information). In: Kalmring K, Elsner N, editors. Acoustic and vibrational communication in insects. 127–134.Berlin Hamburg. Parey Verlag. [Google Scholar]
- Keuper A, Kühne R. The acoustic behaviour of the bushcricket Tettigonia cantans. Behavioral Processes. 1983;8:125–145. doi: 10.1016/0376-6357(83)90002-5. [DOI] [PubMed] [Google Scholar]
- Lakes-Harlan R, Stölting H, Moore TE. Phonotactic behavior of a parasitoid (Emblemasoma auditrix, Sarcophagidae, Diptera) in response to the calling song of the host (Okanagana rimosa, Cicada, Homoptera) Zoology. 2000;103:31–39. [Google Scholar]
- Lakes-Harlan R, Stölting H, Stumpner A. Convergent evolution of insect hearing organs from a preadaptive structure. Proceedings of the Royal Society London B. 1999;266:1161–1167. [Google Scholar]
- Latimer W, Schatral A. The acoustic behaviour of the bushcricket Tettigonia cantans. I. Behavioural responses to sound and vibration. Behavioral Processes. 1983;8:113–124. doi: 10.1016/0376-6357(83)90001-3. [DOI] [PubMed] [Google Scholar]
- Markl H. 1983 Vibrational communication. In: Huber F, Markl H, editors. Neuroethology and behavioral physiology. 332–353.Berlin. Springer. [Google Scholar]
- Meyhöfer R, Casas J. Vibratory stimuli in host location by parasitic wasps. Journal of Insect Physiology. 1999;45:967–971. doi: 10.1016/s0022-1910(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Michel K, Amon T, Cokl A. The morphology of the leg scolopidial organs in Nezara viridula (L.)(Heteroptera, Pentatomidae) Rev Can Biol Exp. 1982;42:139–150. [Google Scholar]
- Michelsen A, Fink F, Gogala M, Traue D. Plants as transmission channels for insect vibrational songs. Behaviour, Ecology and Sociobiology. 1982;11:269–281. [Google Scholar]
- Moore TE. Audiospectrographic analysis of sounds of Hemiptera and Homoptera. Annals of the Entomological Society of America. 1961;54:349–355. [Google Scholar]
- Moore TE. 1973 Acoustical behavior of insects. In: Tipton VJ, editors. Syllabus for an Introductory Entomology Course (Entomol Soc Am). 310–333.+slides & cassette. Provo, Utah. Brigham Young Univ Press. [Google Scholar]
- Moore TE. 1993 Acoustic signals and speciation in cicadas (Insecta: Homoptera: Cicadidae). In: Lees DR, Edwards D, editors. Evolutionary Patterns and Processes, Linnean Society Symposium No 14. 269–284.London. Academic Press. [Google Scholar]
- Moore TE, Huber F, Weber T, Klein U, Bock C. Interaction between visual and phonotactic orientation during flight in Magicicada cassini (Homoptera: Cicadidae) Great Lakes Entomologist. 1993;26:199–221. [Google Scholar]
- Moore TE, Sawyer RT. The mechanism of cicada timbal action (Insecta, Homoptera, Cicadidae) American Zoologist. 1966;6:509. [Google Scholar]
- Moulds MS. 1990 Australian Cicadas. Kensington. New South Wales University Press. [Google Scholar]
- Popov AV. 1990 Co-evolution of sound-production and hearing in insects. In: Gribakin FG, Wiese K, Popov AV, editors. Sensory systems and communication in arthropods. 301–304.Basel, Boston, Berlin. Birkhäuser Verlag. [Google Scholar]
- Pringle JWS. A physiological analysis of cicada song. Journal of Experimental Biology. 1954;31:525–560. [Google Scholar]
- Robert D, Miles RN, Hoy RR. Tympanal hearing in the sarcophagid parasitoid fly Emblemasoma sp.: the biomechanics of directional hearing. Journal of Experimental Biology. 1999;202:1865–1876. doi: 10.1242/jeb.202.14.1865. [DOI] [PubMed] [Google Scholar]
- Sanborn AF, Phillips PK. Scaling of sound pressure level and body size in cicadas (Homoptera: Cicadidae: Tibicinidae) Annals of the Entomological Society of America. 1995;88:479–484. [Google Scholar]
- Sanborn AF, Phillips PK. Analysis of acoustic signals produced by the cicada Platypedia putnami variety lutea (Homoptera: Tibicinidae) Annals of the Entomological Society of America. 1999;92:451–455. [Google Scholar]
- Saxena KN, Kumar H. Interruption of acoustic communication and mating in a leafhopper and a planthopper by aerial sound vibrations picked up by plants. Experientia. 1980;36:933–935. [Google Scholar]
- Simmons JA, Wever EG, Pylka JM. Periodical cicada: Sound production and hearing. Science. 1971;171:212–213. doi: 10.1126/science.171.3967.212. [DOI] [PubMed] [Google Scholar]
- Simmons P, Young D. The tymbal mechanism and song patterns of the bladder cicada, Cystosoma saunderii. Journal of Experimental Biology. 1978;76:27–45. [Google Scholar]
- Soper RS, Shewell GE, Tyrrell D. Colcondamyia auditrix nov. sp. (Diptera: Sarcophagidae), a parasite which is attracted by the mating song of its host, Okanagana rimosa (Homoptera: Cicardidae) Canadian Entomologist. 1976;108:61–68. [Google Scholar]
- Stiedl O, Kalmring K. The importance of song and vibratory signals in the behaviour of the bushcricket Ephippiger ephippiger Fiebig (Orthoptera, Tettigoniidae): taxis by females. Oecologica. 1989;80:142–144. doi: 10.1007/BF00789945. [DOI] [PubMed] [Google Scholar]
- Strübing H, Rollenhagen T. Ein neues Aufnehmersystem für Vibrationssignale und seine Anwendung auf Beispiele aus der Familie Delphacidae. Zoologisches Jahrbuch Physiologie. 1988;92:245–268. [Google Scholar]
- Weber T, Moore TE, Huber F, and Klein U. 1988 Sound production in periodical cicadas (Homoptera: Cicadidae: Magicicada septendecim, M. cassini). In: Vidano C, Arzone A, editors. Proceedings 6th Auchenorrhyncha Meeting. 329–336.Turin. University Turin. [Google Scholar]
- Weidemann S, Keuper A. Influence of vibratory signals on the phonotaxis of the gryllid Gryllus bimaculatus DeGeer (Ensifera: Gryllidae) Oecologia. 1987;74:316–318. doi: 10.1007/BF00379376. [DOI] [PubMed] [Google Scholar]
- Young D. Do cicadas radiate sound though their ear drums? Journal of Experimental Biology. 1990;151:41–56. [Google Scholar]


