Abstract
Purpose.
To characterize the hemodynamic features and the association with structural damage in the optic nerve head (ONH) of idiopathic bilateral optic atrophy (BOA) in rhesus macaque monkeys.
Methods.
In five animals with BOA and nine healthy animals under general anesthesia (pentobarbital), intraocular pressure (IOP) was manometrically controlled. ONH blood flow was measured with a laser speckle flow graph device. Basal blood flow in global and quadrantal sectors was measured with IOP set at 10 mm Hg; autoregulation capacity was assessed by comparing blood flow changes before and after IOP was increased from 10 to 30 mm Hg. Spectral-domain optic coherence tomography was used to measure retinal nerve fiber layer thickness (RNFLT) by peripapillary circular scans.
Results.
Compared with control eyes, RNFLT in BOA eyes was significantly less in all sectors (P < 0.001) except the nasal (P = 0.25); the average global and sectoral blood flow in all quadrants was significantly lower (P < 0.001). These blood flow changes were significantly correlated with corresponding sectoral RNFLT (P < 0.01) except the nasal (P = 0.25). After IOP was increased to 30 mm Hg, global blood flow was significantly reduced (P < 0.001), but with no regional preferences despite prominent temporal RNFLT loss; no significant blood flow change was observed in control eyes (P = 0.24).
Conclusions.
Basal blood flow and autoregulation capacity in the ONH of BOA were significantly compromised, with a close correlation to structural changes. The hemodynamic changes showed no regional preference across the ONH, which was consistent with postmortem histological observations.
In idiopathic bilateral optic atrophy in the rhesus monkey, basal blood flow and autoregulation capacity in the optic nerve head were compromised and closely correlated with structural damage. The finding suggests that similar changes may develop in other diseases with optic nerve atrophy, such as glaucoma.
Introduction
Idiopathic bilateral optic atrophy (BOA) is a newly identified disease observed in rhesus macaque monkeys.1 A previous study has characterized the structural and functional abnormalities found in nine animals with the signs of this disease.1 Anatomical characteristics include loss of macular retinal ganglion cells, thinning of the retinal nerve fiber layer papillomacular bundles, temporal optic disc pallor, and temporal sector atrophy of the retrobulbar optic nerve. Results of electroretinography (ERG) and visually evoked cortical potential studies reveal loss of macular ganglion cell function consistent with the anatomical findings. It is not yet known, however, whether hemodynamic changes are associated with the affected tissues in BOA of the macaque.
In general, the basal blood flow to a tissue is maintained at, or slightly higher than, a tissue's minimal metabolic requirement.2 The basal blood flow in neural tissues, including ocular tissue such as the retina and optic nerve head (ONH), is intrinsically regulated under normal physiological conditions. This regulation maintains a close correlation between local neural activities and blood flow (known as neurovascular coupling) to allow appropriate oxygen and glucose delivery under fluctuating metabolic demand.3,4 Autoregulation mechanisms also maintain blood flow at a relatively constant level as perfusion pressure fluctuates.5 Thus, the efficiency of basal blood flow regulation is essential to maintain normal tissue perfusion and functions. Under pathological conditions, blood flow regulation may be disrupted.6 Studies in cerebral circulation have demonstrated that autoregulation may be modified or disturbed in several disease conditions resulting in hemodynamic changes and tissue damage.7,8 Impaired autoregulation in the ONH associated with altered blood flow was also demonstrated in experimental diabetes9 and hypercholesterolemia.10 In BOA eyes, although the specific cause of the disease remains unknown, the profound structural and functional damage in the retina and ONH may be associated with significantly reduced basal blood flow and perhaps also deteriorated autoregulation capacity.
Investigating these potential hemodynamic changes in the ONH of monkeys with BOA may help further our understanding of vascular pathology in diseases associated with optic nerve degeneration, such as glaucoma. For decades, autoregulation dysfunction has been proposed as one of the mechanisms of insufficient blood flow perfusion in glaucoma.11–15 Although a line of studies supports the existence of systemic vascular dysregulation, the evidence is mixed about whether local blood flow dysregulation in the ONH occurs as a preexisting condition or as a consequence of glaucomatous optic neuropathy. The current study was a cross-sectional comparison of blood flow in the ONH between five monkeys with BOA and a cohort of healthy controls using a laser speckle flowgraphy (LSFG) device. The LSFG measurement for ONH blood flow has been validated in a recent study.16 Autoregulation capacity was also investigated through comparison of blood flow changes in the ONH before and after the ocular perfusion pressure (OPP) was challenged by a controlled step increase of intraocular pressure (IOP). The results demonstrate significantly reduced basal blood flow and autoregulation capacity in the ONH of BOA monkeys.
Methods
Animals
All experimental methods adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the local Institutional Animal Care and Use Committee. In total, 28 eyes of 14 adult rhesus monkeys (Macaca mulatta) were studied. Nine animals (seven female and two male, 9.4 ± 3.1 years) served as controls for this study. It was determined that the other five animals (three female and two male, 9.2 ± 5.5 years) had BOA on the basis of initial screening by direct ophthalmoscopy at Oregon National Primate Research Center; this was subsequently confirmed by more extensive ophthalmoscopy and ERG studies in our laboratory. The clinical findings included bilateral, symmetric thinning of the retinal never fiber layer thickness (RNFLT), predominantly within the papillomacular bundle; corresponding temporal pallor of the optic disc; and reduced ERG components corresponding to macular retinal ganglion cell function.1 Three of the five BOA monkeys were among those reported in our previous communication for structural and functional characterization,1 whereas two others have not been reported previously.
Anesthesia
All procedures were performed with animals under general anesthesia. In all cases, anesthesia was induced with 15 mg/kg intramuscular ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and 1.5 mg/kg intramuscular xylazine (Phoenix Scientific Inc., St Joseph, MO), along with a single subcutaneous injection of atropine sulfate (0.05 mg/kg; Phoenix Scientific Inc.). Animals were intubated and breathed air. Heart rate and arterial oxygenation saturation were monitored continuously (Propaq Encore model 206EL; Protocol Systems, Inc., Beaverton, OR). Body temperature was maintained with a warm-water heating pad at 37°C. Pupils were fully dilated with 1.0% tropicamide (Alcon Laboratories Inc., Fort Worth, TX). One of the superficial branches of a tibial artery was cannulated with a 27-gauge needle, which was connected to a pressure transducer (BLPR2; World Precision Instruments, Manchester, NH) and a four-channel amplifier system (Lab-Trax-4/24T; World Precision Instruments). Systolic, diastolic, and mean blood pressure (BP) in the artery were recorded continuously. If the arterial cannulation could not be obtained, the BP was intermittently measured with an automated Propaq system sphygmometer. Anesthesia was maintained by continuous administration of pentobarbital (6–9 mg/kg/h, intravenous) using an infusion pump (Aladdin; World Science Instruments Inc., Sarasota, FL). Pentobarbital was selected because unlike the volatile gas anesthetics, it has minimal impact on autoregulation capacity.17–21
Measurement of RNFLT
A spectral-domain optical coherence tomography (SD-OCT) instrument (Spectralis HRA+OCT; Heidelberg Engineering, GmbH, Heidelberg, Germany) was used to measure the RNFLT. Standard RNFLT scans consisted of a peripapillary circular B-scan with 1536 A-scans and a standardized diameter of 12°. Nine individual B-scan sweeps were averaged in real time using the instrument's eye-tracking software to reduce speckle noise in the final recorded scan. Layer segmentations were evaluated and corrected manually if necessary (wherever the RNFL borders were incorrectly identified by the instrument's native segmentation algorithm).
Blood Flow Measurement with Laser Speckle Flowgraphy
A laser speckle flowgraphy device (LSFG; Softcare, Iizuka, Japan) was used to measure the blood flow in the ONH. The principles of the laser speckle technique and its application in our lab to measure ONH blood flow in nonhuman primates have been described in previous publications.22–25
First, a fundus camera within the device was used to define an area centered on the ONH, which has dimensions of approximately 3.8 mm × 3 mm (width × height). After switching on the laser (λ = 830 nm, maximum output power 1.2 mW), a speckle pattern appeared due to random interference of the scattered light from the illuminated area, which was continuously imaged by a charge-coupled device (700 × 480 pixels) at a frequency of 30 frames per second for 4 seconds at a time. Offline analysis software computed mean blur rate (MBR) of the speckle images. MBR is a squared ratio of mean intensity to the standard deviation of light intensity, which varies in time according to the velocity of blood cell movement and correlates well with capillary blood flow within the ONH.23,26 A composite MBR map representing blood flow arbitrarily distributed within the ONH was generated from each of the images within each 4-second series. After the area within the images corresponding to large blood vessels was eliminated, the capillary blood flow (AU) in the ONH was averaged. In addition, sectoral blood flow was determined for temporal, nasal, superior, and inferior quadrants of the ONH.25
Assessment of ONH Basal Blood Flow and Autoregulation by Stepped Increase in IOP
The animal was placed on a table in the prone position. Head position was fixed with an adjustable headrest and a bite bar to keep the face forward. Proparacaine HCl (0.5%) was administered topically, and a speculum was used to keep the eye open. The pupil was fully dilated with 1.0% tropicamide. Two 27-gauge needles were inserted into the anterior chamber via the temporal corneoscleral limbus in a direction parallel to the iris. One of the two needles was connected to a pressure transducer to register the IOP; the other needle was connected to one of either two sterile saline reservoirs, each set at a different height. The connection of the reservoirs to the anterior chamber was controlled by a solenoid valve (Valcor Engineering, Springfield, NJ), which allows one of the reservoirs to be opened at the instant the other is closed so that the IOP can be changed rapidly from one level to the other.
Under stabilized BP with IOP set manometrically at 10 mm Hg, a basal ONH blood flow measurement was acquired by LSFG. The IOP was then elevated from 10 to 30 mm Hg, by switching the solenoid valve, and maintained at the new level for at least 3 minutes. A second blood flow measurement was performed in the same manner as the first. Based on one of our previous studies,24 blood flow stabilizes at 3 minutes after IOP elevation. This measurement pair therefore represents a static phase of autoregulation, in contrast to dynamic-phase autoregulation as reported in previous studies.25 The blood flow difference between before and after the IOP elevation was calculated as a measure of autoregulation capacity in the ONH.
Histology
In one eye of four BOA and five normal control animals, retrobulbar optic nerves were sampled approximately 3 mm posterior to the globe (tissues from the other eyes were used for other histopathological studies). Transverse sections (0.5 mm thick, ∼2 mm behind the globe) were cut with a vibrotome for each nerve and processed for immunohistochemical labeling of capillaries and astrocytes. The sections were first permeablized with 3% Triton X-100 in 0.01 M PBS for 1 hour and then incubated with a mixture of 10% serum corresponding to the host species of secondary antibodies and 2% bovine serum albumin for 1 hour. Primary polyclonal rabbit anti-human glial fibrillary acidic protein (GFAP) (Z0334, 1:1000; DakoCytomation, Glostrup, Denmark) and monoclonal mouse anti-human CD31 (130 kDa, 1:400; Dako North America, Carpinteria, CA) were applied and incubated at 4°C for 4 days. After three 1-hour rinses in 0.01 M PBS, corresponding secondary antibodies (Alexa Fluor 555 goat anti-rabbit, 1:400, and Alexa Fluor 488 goat anti-mouse, 1:100; Invitrogen Corporation, Carlsbad, CA) were applied overnight at 4°C. The sections were washed in PBS (0.01 M) and mounted for microscopy. Negative controls for immunohistochemical stains were performed with omission of corresponding primary antibody from the solution.
Statistics
All blood flow data are reported as mean ± SD. Statistical analysis was performed using commercially available software (Statistica; StatSoft Inc., Tulsa, OK). The average ONH blood flow differences between BOA and control eyes were evaluated using analysis of variance (ANOVA). Bonferroni correction was employed to post hoc tests of group differences. The Mann-Whitney test was used to compare the median RNFLT thickness in each retinal region between BOA and normal eyes. Correlation between the RNFLT and blood flow in the ONH in each quadrant and globally in each eye was evaluated using Deming regression. A probability <0.05, unless otherwise specified, was considered the critical level for rejecting the null hypothesis.
Results
Global and Regional RNFLT, BP, and IOP
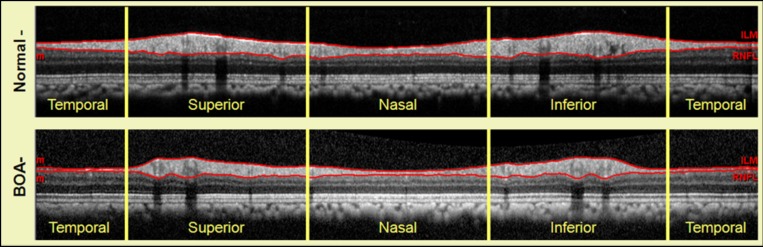
The global average RNFLT was 69.9 ± 7.6 μm in the BOA eyes, which was significantly thinner than the value of 98.1 ± 6.1 μm observed in the normal eyes (P < 0.001). A typical example is shown in Figure 1.
Figure 1. .
SD-OCT peripapillary RNFL scans obtained in a normal eye (top) and a BOA eye (bottom) using a 12° diameter circular scan centered on the optic disc. The two red lines delineate the anterior and posterior borders of RNFL along the scan path and show remarkable thinning in the temporal quadrant of the BOA eye.
The Table lists the RNFLT for each of the four quadrants. As expected, the most severe RNFL thinning was found in the temporal quadrant, but RNFL loss was somewhat surprisingly found in the other quadrants as well. The average mean arterial BP during blood flow measurements was 75.8 ± 11.2 mm Hg (ranging from 60 to 91 mm Hg) in monkeys with BOA and 79.4 ± 6.36 (ranging from 63 to 90 mm Hg) in normal controls (P = 0.30 between the two groups). The corresponding OPP was 15 mm Hg less than the BP at IOP of 10 mm Hg and was 35 mm Hg less at IOP of 30 mm Hg. The additional 5 mm Hg was to correct the height difference between the tested eye and the level at which BP was measured.
IOP measured with a handheld tonometer (Tonopen; Oculab, Inc., Glendale, CA) with monkeys under ketamine/xylazine anesthesia was 13.9 ± 2.1 and 13.6 ± 2.1 mm Hg for right and left eyes in normal monkeys. The IOP in BOA eyes was 15.8 ± 4.8 and 15.8 ± 5.6 mm Hg for right and left eyes, respectively. The mean difference between the normal and BOA eyes was not statistically significant (P = 0.12).
Table. .
Average Global Peripapillary RNFLT and in Quadrants
|
|
Peripapillary RNFLT (μm, Mean ± SD) |
BOA/Normal |
||
|
BOA (n
= 10) |
Normal (n
= 18) |
P
Value |
||
| Global | 69.9 ± 7.6 | 98.1 ± 6.1 | <0.001 | −29% |
| Superior | 90.8 ± 12.8 | 118.5 ± 7.2 | <0.001 | −23% |
| Temporal | 34.3 ± 15.2 | 71.0 ± 6.2 | <0.001 | −52% |
| Inferior | 99.5 ± 18.2 | 139.8 ± 12.7 | <0.001 | −29% |
| Nasal | 53.9 ± 11.1 | 62.6 ± 8.5 | = 0.25 | −14% |
Mean Blood Flow and Autoregulation Capacity in the ONH of BOA
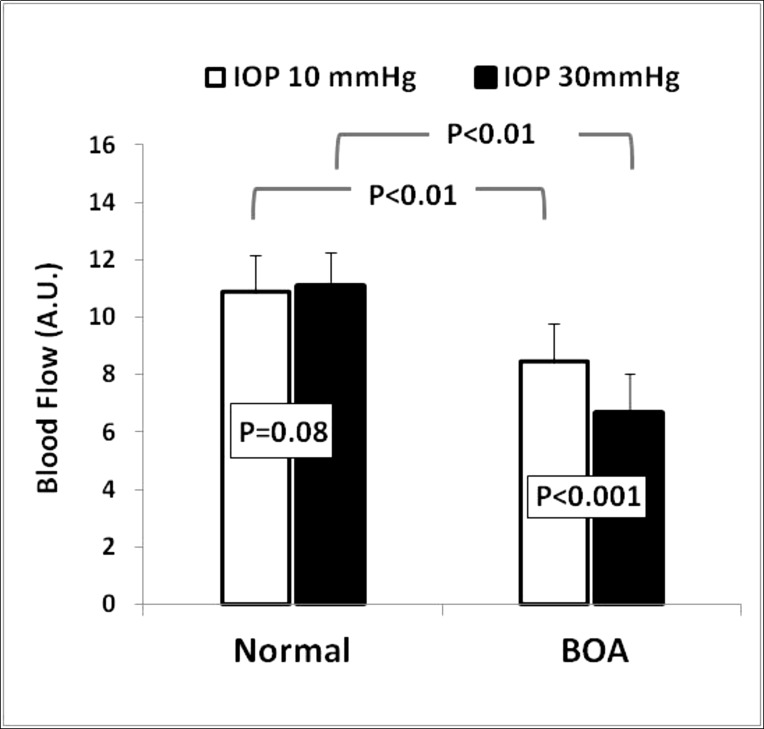
The average basal ONH blood flow measured at an IOP of 10 mm Hg was 8.44 ± 1.17 (AU) in BOA eyes, which was significantly lower than the value of 10.9 ± 1.28 (P < 0.001) measured in control eyes (Fig. 2). The corresponding mean OPP during basal blood flow measurements in BOA and normal eyes, respectively, was approximately 60 and 63.5 mm Hg.
Figure 2. .
The average ONH blood flow in normal eyes (left pair of columns) and BOA (right pair of columns) measured at IOP 10 mm Hg (left of each pair) and IOP 30 mm Hg (right of each pair). ONH blood flow was significantly reduced in BOA eyes under both IOP 10 and 30 mm Hg. After IOP was increased from 10 to 30 mm Hg, there was a significant further blood flow decrease in BOA eyes, but not in normal eyes.
Figure 2 shows the ONH blood flows measured at IOP 10 and 30 mm Hg in both normal and BOA eyes. Three minutes after the IOP was increased to 30 mm Hg, the average blood flow was reduced from 8.44 ± 1.17 to 6.65 ± 1.36 in BOA eyes (P < 0.001, ANOVA); however, there was little change in normal control eyes (10.9 ± 1.28 vs. 11.08 ± 1.36, P = 0.24). On average, the ONH blood flows were reduced by 21% in BOA eyes; they actually increased slightly, by 2%, in normal eyes after IOP was increased to 30 mm Hg. The corresponding OPP level during the IOP 30 mm Hg step challenge in BOA and normal eyes, respectively, was approximately 40 and 43.5 mm Hg.
Regional Blood Flow
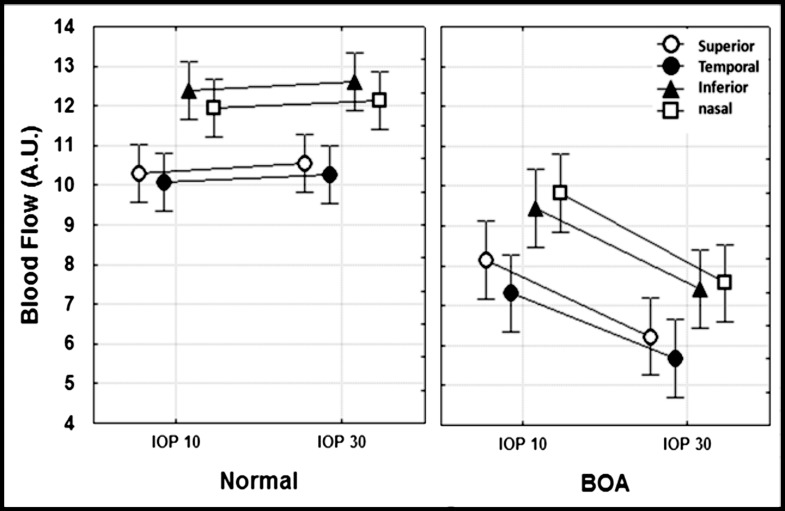
Regional ONH blood flow difference was also evaluated in four individual ONH sectors (superior, temporal, inferior, and nasal quadrants) to determine if the temporal sector in the BOA eyes had significantly greater blood flow decrease than the other ONH sectors due to significantly more RNFLT loss as shown in the Table (Fig. 3).
Figure 3. .
The figure shows blood flow in all four quadrants in BOA (left, n = 10) and normal eyes (right, n = 18). In BOA eyes, the blood flow measured at IOP 30 mm Hg was significantly lower compared with that measured at IOP 10 mm Hg (P < 0.001 for all regions). No regions in the normal eyes showed significant blood flow changes between IOP 10 and 30 mm Hg (P > 0.27 for all regions).
This same pattern of regional blood flow variation was observed in BOA eyes for both IOP levels as well, although blood flow in all four quadrants was significantly lower than in control eyes at IOP 30 mm Hg (P < 0.05 for all quadrants with Bonferroni correction). Two-way ANOVA showed that there was no significant interaction between IOP and sector (P = 0.70); thus it was concluded that the relative degree of autoregulation dysfunction is equivalent for all sectors/quadrants. Further, the results indicate that quadrant blood flow of BOA eyes was evenly reduced across ONH sectors, regardless of the actual IOP level (10 to 30 mm Hg).
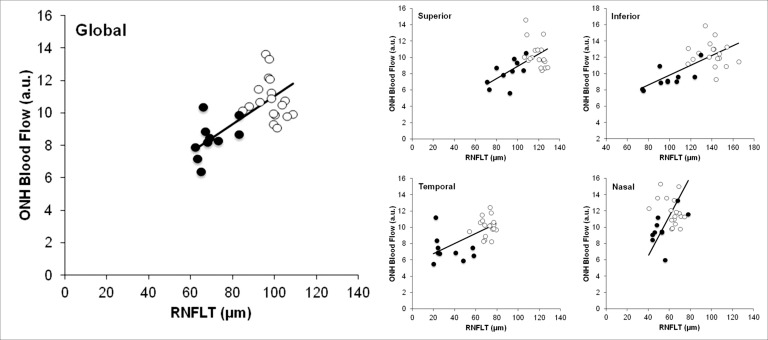
Correlation between the RNFLT and Blood Flow
The correlation between the basal blood flow at IOP 10 mm Hg and RNFLT was analyzed for global average values as well as individual quadrants. The global average ONH blood flow for each eye was closely correlated with global average RNFLT (P = 0.0003, Fig. 4, left panel). Similarly, ONH blood flow correlated significantly with corresponding sectoral RNFLT for three of the four quadrants (Fig. 4, right panels), with only the nasal sector showing no significant association. The P values of the correlation for the superior, temporal, inferior, and nasal quadrants were, respectively, 0.01, 0.001, 0.0002, and 0.25.
Figure 4. .
The relationship between RNFLT and blood flow was assessed for global average (left panel) and each of four sectors (right panels). The open circles represent the values in normal control eyes (n = 18); the solid circles represent values in BOA eyes (n = 10). The correlations were all statistically significant except for the nasal quadrant.
ONH Astrocytes and Capillaries in BOA and Normal Eyes
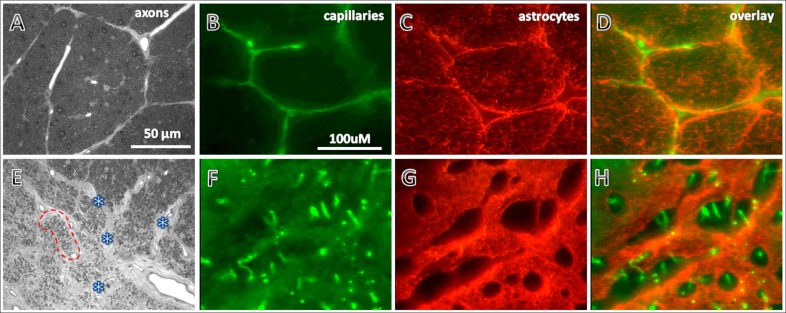
In all eyes for which histological studies were available (four BOA and five normal control animals), astrocytes labeled with GFAP and capillaries labeled with CD31 immunohistochemically could be observed across the transversely sectioned optic nerve (see Fig. 5). As expected, GFAP immunoreactivity in BOA eyes was significantly increased in areas with tissue damage compared with normal eyes, as demonstrated in our previous study.1 Surprisingly, the capillaries in the damaged regions of BOA eyes appeared to have a much higher density compared with normal eyes. This pattern of increased capillary density was observed in all four BOA eyes examined; Figure 5 shows one example.
Figure 5. .
Micrographs show histological observations in one representative BOA eye (bottom panels) compared with a representative control eye (top panels). Axons (A, E) were stained with toluidine blue. Capillaries (B, F) and astrocytes (C, G) were immunohistochemically stained with endothelium markers CD31 and GFAP, respectively, in 50 μm thick sections. Compared with those in the normal eye (A), axons in the damaged area of the BOA eye (E, lower left area) were so scarce that the corresponding fascicles were shrunken and collapsed (red dotted lines encircle one of the collapsed fascicles). The connective tissues in the septa became thickened (marked with asterisks). These changes in the BOA eye led to an appearance of higher capillary density (F) compared with normal eyes (B). In the same area, the GFAP immunoreactivity in the BOA eye was significantly increased (G) compared with the normal eye (C). Overlay of capillaries (D) and astrocytes (H) in the normal and BOA eyes, respectively. Scale bars: (A, E) 50 μm; other photographs 100 μm.
Discussion
The coupling between neural activity and blood flow in neural tissues has been demonstrated in studies mostly through enhancement of the neuronal activities.27 That is, a provoked neural activity leads to blood flow increase27 as demonstrated in human ONH by experimental visual stimuli.14,28–31 Following the same concept based on acute activity, one could suppose that chronically reduced neural activity may cause basal blood flow to decrease over the long term. In the normal ONH of nonhuman primates, metabolic rate and blood flow are both relatively high.32 In BOA eyes, RNFLT was 20% to 50% thinner compared to normal controls (see Table), with fewer axons passing through the ONH; these anatomical findings are associated with significant functional loss assessed by in vivo electrophysiology.1 Thus, neuronal activities in the ONH of BOA eyes were expected to be low; so were the energy demands and blood supply. In agreement with this premise, blood flow in the ONH of BOA eyes was on average 24% lower and closely correlated with the extent of RNFL thinning of the ONH. Thus, the blood flow in the ONH is coupled not only with increasing but also with decreasing neuronal activities. This notion may have some clinical importance with regard to the interpretation of blood flow changes observed under chronic disease conditions such as glaucoma.
Previous studies have shown a close relationship between ONH blood flow and RNFLT in human glaucomatous eyes.33,34 However, it is difficult to determine whether the reduced blood flow was a preexisting pathological change or a consequence of glaucomatous optic atrophy—a “chicken or egg” question.15 More disturbingly, a large portion of axons are likely to have already become degenerated even at an “early” stage of glaucoma diagnosis.35,36 The result showing reduced ONH blood flow in BOA suggests that there is likely a similar situation in glaucoma. At least in part, reduced blood flow in the glaucomatous ONH could be a result of optic nerve degeneration after chronic axonal loss and reduced energy demands, though this does not preclude the possibility that it might act as preexisting condition to further progression of damage.
Interestingly, although ONH blood flow was closely correlated with RNFLT overall and for all but the nasal sector, the reduction of blood flow observed in the temporal quadrant in BOA eyes was not significantly worse than the reduction within the other quadrants, even though the RNFLT was an additional 30% thinner compared with relative loss in the other quadrants (Table). Instead, blood flow was evenly reduced across sectors of the ONH. One possible reason for this uniform blood flow reduction follows from results shown in Figure 5: All capillaries in the optic nerve reside within septa surrounding axonal fascicles. However, in regions of ONH with significant axonal loss in BOA eyes, the axon fascicles were significantly smaller, and the capillaries within the corresponding regions appeared to be more densely distributed compared to those in regions with less axonal loss in BOA or normal eyes. Thus, since the LSFG blood flow estimate represents the average flow density within the scattering volume of the tissue, it is possible that the slightly higher capillary density within the areas of greatest axonal degeneration led to a slightly higher flow estimate in the temporal quadrant than would be derived from a ideal measure of capillary blood flow (i.e., independent of density). This disparity between capillary density change and neuronal tissue damage in the optic nerve has been observed previously in experimental glaucoma in monkeys.37 However, this explanation should be considered with caution because the histological observations of capillaries were made approximately 3 mm behind the globe whereas the LSFG blood flow measurement was derived from approximately 1 mm of the anterior optic nerve.38 An additional possible reason for the uniform blood flow decrease is the rich capillary anastomosis in ONH,39 whereby blood flow across sectors could be partially equilibrated. The applicability of this theory would depend ultimately on what volume of the ONH can be considered functionally isolated with regard to blood flow autoregulation. If applicable, the theory might also explain why the nasal quadrant had the least damage as indicated by RNFLT yet a similar reduction of blood flow as compared with normal values.
It is also clear that the correlations between ONH blood flow and RNFLT would be weaker if assessed only in normal eyes (see Fig. 4). This is likely due to variance in normal RNFLT that is not strictly related to neural density (e.g., branching patterns of large vessel trunks, glial tissue volume, density of parapapillary capillary plexus).40–43 The moderate to severe neuronal loss observed in BOA extends the range of RNFLT and thus becomes the dominant source of variance in the data. This indicates that basal ONH blood flow is more closely related to decreasing axonal density in pathological states than it is in normal eyes.
Another interesting observation is the blood flow autoregulation changes in the ONH of BOA eyes. When the blood flow autoregulation system in normal eyes was challenged by a step IOP increase from 10 to 30 mm Hg (the corresponding OPP was decreased from approximately 60 to 40 mm Hg), no blood flow changes were observed. This is in agreement with previous observations in the ONH of monkey (Wang L, et al. IOVS 2012;53:ARVO E-abstract 6842) and human eyes,14,44,45 in which the lower limit of autoregulation, or the critical level of OPP below which blood flow starts to decline, is below 40 mm Hg. In BOA eyes, however, the same extent of IOP change at equivalent OPP levels caused a significant blood flow decrease. This suggests that the autoregulation capacity in the ONH of BOA eyes failed to maintain blood flow at OPPs that were otherwise normal.
This passive blood flow change following the perfusion pressure decrease in the BOA eyes is similar to the autoregulation changes reported in certain brain diseases,17,46–48 and in the ONH of experimentally induced diabetes9 and hypercholesterolemia10 in rabbits. Failed autoregulation may cause serious pathological consequences when perfusion pressure fluctuates even within a “normal” range: If perfusion pressure fluctuates to become lower, the tissue may suffer ischemia; if perfusion pressure fluctuates to become higher, it may cause “over-perfusion” or edema, even hemorrhage.7,8,49,50 Accordingly, a vicious cycle may develop under diseased conditions, causing affected tissue to be more vulnerable to perfusion pressure changes and thus accelerating ongoing neural degeneration.
This pathological mechanism in hemodynamics has been proposed as a cause of glaucomatous optic neuropathy in glaucoma.11–15 To date, however, solid evidence of autoregulation dysfunction in glaucomatous ONH is still absent. The current results show that autoregulation dysfunction may develop after neural degeneration as demonstrated in the ONH of BOA eyes. Although the specific cause of BOA remains unknown, a strong suspicion is that it is an inherited disease analogous to dominant optic atrophy or Leber's hereditary optic neuropathy in humans.1 Thus, the current observations suggest that a similar autoregulation change may occur in glaucoma. However, further study in a more controlled experimental optic atrophy condition, such as optic nerve transection, may be necessary to validate the findings.
The mechanisms underlying autoregulation dysfunction in the ONH of BOA are unclear. Several cell types are thought to be involved in normal blood flow autoregulation, including pericytes, smooth muscle cells, vascular endothelial cells, and increasingly also astrocytes.5,13,51–54 It is possible that the gliotic ONH changes observed here in BOA (e.g., Fig. 5) exacerbate cell signaling and communication and thus disrupt autoregulation. For example, in a recent study by Shibata et al.,55 failure of ONH autoregulation was demonstrated in experimental diabetes in rabbits—similar to our current observation in BOA monkeys—using the same LSFG technique. Interestingly, also in that study, intravitreal injection of a gap junction blocker to interrupt direct intracellular coupling in normal rabbits induced similar autoregulation changes. Since gap junctions are distributed largely within the endfeet of astrocytes in the ONH,56 which tightly ensheathe the blood vessels,57,58 this suggests that astrocytes were likely involved in autoregulation dysfunctions in the pathological ONH. Indeed, it has been demonstrated that astrocytes are associated with at least normal blood flow autoregulation53,59 in coupling to neuronal activities.60–63 Though no studies have specifically documented the functional communication between astrocytes and blood vessels in the ONH, close structural relationships have been demonstrated.57,58,64,65 In BOA eyes, evidence of massive “activation” of astrocytes with enhanced expression of glial fibrillary acidic protein (GFAP) was shown in this and our previous studies.1 Together, these findings suggest that the roles of astrocytes in neurovascular coupling and in the autoregulation system within the ONH in both normal and diseased conditions warrant further investigation.
The LSFG technique used in this study has certain limitations when applied in order to compare blood flow differences in tissues with different light absorbance/scattering properties.23 This is the case because two key components of the blur rate calibration underlying the LSFG measurement, a constant and the zero offset, are unknown in vivo and may vary between tissues with different absorbance/scattering properties38,66 such as in the pathological condition of BOA. It is possible that the absorption and/or scattering properties of ONH differ enough in BOA eyes to become a confounding factor in LSFG measurement (comparison of basal blood flow between BOA and controls). However, in a very recent study in our lab, blood flow changes measured with LSFG in the ONH of monkeys with experimental glaucoma, which exhibit pathological changes similar to those in BOA, were highly correlated with ONH blood flow measured simultaneously by the “gold standard” microsphere technique.16 This suggests that the basal ONH blood flow differences between BOA and healthy control eyes observed in this study using LSFG reflect true basal blood flow differences and are not due to potential artifact or confounding effects of altered tissue optical properties in the atrophied ONH.16
In summary, the present study characterized hemodynamic features in the ONH of eyes with BOA and demonstrated that the basal blood flow and autoregulation capacity in the ONH of BOA were significantly reduced with no regional preferences despite prominent temporal sector neural damage. It also showed that the reduction of basal blood flow was closely correlated with structural changes in the ONH. These findings suggest that similar hemodynamic changes may develop in other diseases with optic nerve atrophy, such as glaucoma.
Acknowledgments
The authors thank Claude F. Burgoyne for identifying two BOA animals used in the study and Yi Liang for technical assistance.
Footnotes
Supported by NIH Grant R01-019939 (LW), NIH Grant R01-EY019327 (BF), Good Samaritan Foundation, and unrestricted research funds from Pfizer, Inc.
Disclosure: C. Piper, None; B. Fortune, None; G. Cull, None; G.A. Cioffi, None; L. Wang, None
References
- 1. Fortune B, Wang L, Bui BV, Burgoyne CF, Cioffi GA. Idiopathic bilateral optic atrophy in the rhesus macaque. Invest Ophthalmol Vis Sci. 2005; 46: 3943–3956 [DOI] [PubMed] [Google Scholar]
- 2. Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Inc.; 2006. [Google Scholar]
- 3. Filosa JA. Vascular tone and neurovascular coupling: considerations toward an improved in vitro model. Front Neuroenergetics. 2010; 2: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhong Z, Huang G, Chui TY, Petrig BL, Burns SA. Local flicker stimulation evokes local retinal blood velocity changes. J Vis. 2012; 12: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiel JW. The Ocular Circulation. San Rafael, CA: Morgan & Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 6. Lindauer U, Dirnagl U, Fuchtemeier M, et al. Pathophysiological interference with neurovascular coupling – when imaging based on hemoglobin might go blind. Front Neuroenergetics. 2010; 2: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990; 2: 161–192 [PubMed] [Google Scholar]
- 8. Vavilala MS, Lee LA, Lam AM. Cerebral blood flow and vascular physiology. Anesthesiol Clin North America. 2002; 20: 247–264 [DOI] [PubMed] [Google Scholar]
- 9. Shibata M, Oku H, Sugiyama T, et al. Disruption of gap-junctions is possibly involved in impairment of autoregulation in optic nerve head blood flow of diabetic rabbits. Invest Ophthalmol Vis Sci. 2011; 52: 2153–2159 [DOI] [PubMed] [Google Scholar]
- 10. Shibata M, Sugiyama T, Hoshiga M, et al. Changes in optic nerve head blood flow, visual function, and retinal histology in hypercholesterolemic rabbits. Exp Eye Res. 2011; 93: 818–824 [DOI] [PubMed] [Google Scholar]
- 11. Grunwald JE, Riva CE, Stone RA, Keates EU, Petrig BL. Retinal autoregulation in open-angle glaucoma. Ophthalmology. 1984; 91: 1690–1694 [DOI] [PubMed] [Google Scholar]
- 12. Pillunat LE, Stodtmeister R, Wilmanns I, Christ T. Autoregulation of ocular blood flow during changes in intraocular pressure. Preliminary results. Graefes Arch Clin Exp Ophthalmol. 1985; 223: 219–223 [DOI] [PubMed] [Google Scholar]
- 13. Anderson DR. Introductory comments on blood flow autoregulation in the optic nerve head and vascular risk factors in glaucoma. Surv Ophthalmol. 1999; 43 (suppl 1): S5–9 [DOI] [PubMed] [Google Scholar]
- 14. Riva CE, Falsini B. Functional laser Doppler flowmetry of the optic nerve: physiological aspects and clinical applications. Prog Brain Res. 2008; 173: 149–163 [DOI] [PubMed] [Google Scholar]
- 15. Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002; 21: 359–393 [DOI] [PubMed] [Google Scholar]
- 16. Wang L, Grant A, Piper C, Burgoyne CF, Fortune B. Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and microsphere method. Invest Ophthalmol Vis Sci. 2012; 53: 8303–8309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995; 26: 1014–1019 [DOI] [PubMed] [Google Scholar]
- 18. Werner C, Lu H, Engelhard K, Unbehaun N, Kochs E. Sevoflurane impairs cerebral blood flow autoregulation in rats: reversal by nonselective nitric oxide synthase inhibition. Anesth Analg. 2005; 101: 509–516 [DOI] [PubMed] [Google Scholar]
- 19. Kremser PC, Gewertz BL. Effect of pentobarbital and hemorrhage on renal autoregulation. Am J Physiol. 1985; 249: F356–360 [DOI] [PubMed] [Google Scholar]
- 20. Paterno R, Heistad DD, Faraci FM. Potassium channels modulate cerebral autoregulation during acute hypertension. Am J Physiol Heart Circ Physiol. 2000; 278: H2003–2007 [DOI] [PubMed] [Google Scholar]
- 21. Preckel MP, Leftheriotis G, Ferber C, Degoute CS, Banssillon V, Saumet JL. Effect of nitric oxide blockade on the lower limit of the cortical cerebral autoregulation in pentobarbital-anaesthetized rats. Int J Microcirc Clin Exp. 1996; 16: 277–283 [DOI] [PubMed] [Google Scholar]
- 22. Fujii H, Nohira K, Yamamoto Y, Ikawa H, Hjura T. Evaluation of blood flow by laser speckle image sensing, part 1. Applied Optics. 1987; 26: 5321–5325 [DOI] [PubMed] [Google Scholar]
- 23. Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010; 88: 723–729 [DOI] [PubMed] [Google Scholar]
- 24. Liang Y, Downs JC, Fortune B, Cull GA, Cioffi GA, Wang L. Impact of systemic blood pressure on the relationship between intraocular pressure and blood flow in the optic nerve head of non-human primates. Invest Ophthalmol Vis Sci. 2009; 50: 2164–2160 [DOI] [PubMed] [Google Scholar]
- 25. Liang Y, Fortune B, Cull G, Cioffi GA, Wang L. Quantification of dynamic blood flow autoregulation in optic nerve head of rhesus monkeys. Exp Eye Res. 2010; 90: 203–209 [DOI] [PubMed] [Google Scholar]
- 26. Takayama J, Tomidokoro A, Ishii K, et al. Time course of the change in optic nerve head circulation after an acute increase in intraocular pressure. Invest Ophthalmol Vis Sci. 2003; 44: 3977–3985 [DOI] [PubMed] [Google Scholar]
- 27. Pasley BN, Freeman RD. Neurovascular coupling. Scholarpedia. 2008; 3: 5340 [Google Scholar]
- 28. Falsini B, Riva CE, Logean E. Flicker-evoked changes in human optic nerve blood flow: relationship with retinal neural activity. Invest Ophthalmol Vis Sci. 2002; 43: 2309–2316 [PubMed] [Google Scholar]
- 29. Formaz F, Riva CE, Geiser M. Diffuse luminance flicker increases retinal vessel diameter in humans. Curr Eye Res. 1997; 16: 1252–1257 [DOI] [PubMed] [Google Scholar]
- 30. Riva CE, Cranstoun SD, Petrig BL. Effect of decreased ocular perfusion pressure on blood flow and the flicker-induced flow response in the cat optic nerve head. Microvasc Res. 1996; 52: 258–269 [DOI] [PubMed] [Google Scholar]
- 31. Garhofer G, Resch H, Weigert G, Lung S, Simader C, Schmetterer L. Short-term increase of intraocular pressure does not alter the response of retinal and optic nerve head blood flow to flicker stimulation. Invest Ophthalmol Vis Sci. 2005; 46: 1721–1725 [DOI] [PubMed] [Google Scholar]
- 32. Geijer C, Bill A. Effects of raised intraocular pressure on retinal, prelaminar, laminar, and retrolaminar optic nerve blood flow in monkeys. Invest Ophthalmol Vis Sci. 1979; 18: 1030–1042 [PubMed] [Google Scholar]
- 33. Yokoyama Y, Aizawa N, Chiba N, et al. Significant correlations between optic nerve head microcirculation and visual field defects and nerve fiber layer loss in glaucoma patients with myopic glaucomatous disk. Clin Ophthalmol. 2011; 5: 1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiba N, Omodaka K, Yokoyama Y, et al. Association between optic nerve blood flow and objective examinations in glaucoma patients with generalized enlargement disc type. Clin Ophthalmol. 2011; 5: 1549–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harwerth RS, Crawford ML, Frishman LJ, Viswanathan S, Smith EL III, Carter-Dawson L. Visual field defects and neural losses from experimental glaucoma. Prog Retin Eye Res. 2002; 21: 91–125 [DOI] [PubMed] [Google Scholar]
- 36. Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000; 41: 741–748 [PubMed] [Google Scholar]
- 37. Furuyoshi N, Furuyoshi M, May CA, Hayreh SS, Alm A, Lutjen-Drecoll E. Vascular and glial changes in the retrolaminar optic nerve in glaucomatous monkey eyes. Ophthalmologica. 2000; 214: 24–32 [DOI] [PubMed] [Google Scholar]
- 38. Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H. Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp Eye Res. 1995; 60: 373–383 [DOI] [PubMed] [Google Scholar]
- 39. Zhao DY, Cioffi GA. Anterior optic nerve microvascular changes in human glaucomatous optic neuropathy. Eye. 2000; 14: 445–449 [DOI] [PubMed] [Google Scholar]
- 40. Scoles D, Gray DC, Hunter JJ, et al. In-vivo imaging of retinal nerve fiber layer vasculature: imaging histology comparison. BMC Ophthalmol. 2009; 9: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harwerth RS, Wheat JL. Modeling the effects of aging on retinal ganglion cell density and nerve fiber layer thickness. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 305–314 [DOI] [PubMed] [Google Scholar]
- 42. Frenkel S, Morgan JE, Blumenthal EZ. Histological measurement of retinal nerve fibre layer thickness. Eye (Lond). 2005; 19: 491–498 [DOI] [PubMed] [Google Scholar]
- 43. Hood DC, Fortune B, Arthur SN, et al. Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. J Glaucoma. 2008; 17: 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Riva CE, Hero M, Titze P, Petrig B. Autoregulation of human optic nerve head blood flow in response to acute changes in ocular perfusion pressure. Graefes Arch Clin Exp Ophthalmol. 1997; 235: 618–626 [DOI] [PubMed] [Google Scholar]
- 45. Pillunat LE, Anderson DR, Knighton RW, Joos KM, Feuer WJ. Autoregulation of human optic nerve head circulation in response to increased intraocular pressure. Exp Eye Res. 1997; 64: 737–744 [DOI] [PubMed] [Google Scholar]
- 46. Eames PJ, Blake MJ, Panerai RB, Potter JF. Cerebral autoregulation indices are unimpaired by hypertension in middle aged and older people. Am J Hypertens. 2003; 16: 746–753 [DOI] [PubMed] [Google Scholar]
- 47. Panerai RB. Assessment of cerebral pressure autoregulation in humans--a review of measurement methods. Physiol Meas. 1998; 19: 305–338 [DOI] [PubMed] [Google Scholar]
- 48. Strebel S, Lam AM, Matta BF, Newell DW. Impaired cerebral autoregulation after mild brain injury. Surg Neurol. 1997; 47: 128–131 [DOI] [PubMed] [Google Scholar]
- 49. Brian JE Jr, Faraci FM, Heistad DD. Recent insights into the regulation of cerebral circulation. Clin Exp Pharmacol Physiol. 1996; 23: 449–457 [DOI] [PubMed] [Google Scholar]
- 50. Vaquero J, Chung C, Blei AT. Cerebral blood flow in acute liver failure: a finding in search of a mechanism. Metab Brain Dis. 2004; 19: 177–194 [DOI] [PubMed] [Google Scholar]
- 51. Anderson DR. Glaucoma, capillaries and pericytes. 1. Blood flow regulation. Ophthalmologica. 1996; 210: 257–262 [DOI] [PubMed] [Google Scholar]
- 52. Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond). 1999; 96: 313–326 [PubMed] [Google Scholar]
- 53. Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2008; 294: H622–632 [DOI] [PubMed] [Google Scholar]
- 54. Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003; 6: 43–50 [DOI] [PubMed] [Google Scholar]
- 55. Shibata M, Oku H, Sugiyama T, et al. Disruption of gap junctions may be involved in impairment of autoregulation in optic nerve head blood flow of diabetic rabbits. Invest Ophthalmol Vis Sci. 2011; 52: 2153–2159 [DOI] [PubMed] [Google Scholar]
- 56. Kerr NM, Johnson CS, de Souza CF, et al. Immunolocalization of gap junction protein connexin43 (GJA1) in the human retina and optic nerve. Invest Ophthalmol Vis Sci. 2010; 51: 4028–4034 [DOI] [PubMed] [Google Scholar]
- 57. Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010; 58: 1094–1103 [DOI] [PubMed] [Google Scholar]
- 58. Triviño A, Ramírez JM, Salazar JJ, Ramírez AI, García-Sánchez J. Immunohistochemical study of human optic nerve head astroglia. Vision Res. 1996; 36: 2015–2028 [DOI] [PubMed] [Google Scholar]
- 59. Saez JC. Astrocytes as connexin-dependent signaling cells for local blood flow regulation. Am J Physiol Heart Circ Physiol. 2008; 294: H586–587 [DOI] [PubMed] [Google Scholar]
- 60. Allan S. The neurovascular unit and the key role of astrocytes in the regulation of cerebral blood flow. Cerebrovasc Dis. 2006; 21: 137–138 [DOI] [PubMed] [Google Scholar]
- 61. Rossi DJ. Another BOLD role for astrocytes: coupling blood flow to neural activity. Nat Neurosci. 2006; 9: 159–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wolff JR, Stuke K, Missler M, et al. Autocellular coupling by gap junctions in cultured astrocytes: a new view on cellular autoregulation during process formation. Glia. 1998; 24: 121–140 [DOI] [PubMed] [Google Scholar]
- 63. Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008; 294: H2855–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006; 7: 41–53 [DOI] [PubMed] [Google Scholar]
- 65. Dai C, Khaw PT, Yin ZQ, Li D, Raisman G, Li Y. Structural basis of glaucoma: the fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia. 2011; 60: 13–28 [DOI] [PubMed] [Google Scholar]
- 66. Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H. Noncontact, two-dimensional measurement of retinal microcirculation using laser speckle phenomenon. Invest Ophthalmol Vis Sci. 1994; 35: 3825–3834 [PubMed] [Google Scholar]