Abstract
Context
Older adults comprise the majority of new-onset heart failure (HF) diagnoses, but traditional risk-factor prediction models have limited accuracy in this population to identify those at highest risk for hospitalization or death.
Objectives
To determine if cardiac troponin T (cTnT) measured by a highly sensitive assay would be detectable in the majority of community-dwelling older adults, and if serial measures were associated with risk of HF hospitalization and cardiovascular death.
Design, Setting, and Participants
A longitudinal nationwide cohort study (Cardiovascular Health Study) of 4221 community-dwelling adults aged 65 years or older without prior HF who had cTnT measured using a highly sensitive assay at baseline (1989–1990) and repeated after 2 to 3 years (n = 2918).
Main Outcome Measures
New-onset HF and cardiovascular death were examined through June 2008 with respect to cTnT concentrations, accounting for clinical risk predictors.
Results
Cardiac troponin T was detectable (≥3.00 pg/mL) in 2794 participants (66.2%). During a median follow-up of 11.8 years, 1279 participants experienced new-onset HF and 1103 cardiovascular deaths occurred, with a greater risk of both end points associated with higher cTnT concentrations. Among those participants with the highest cTnT concentrations (>12.94 pg/mL), there was an incidence rate per 100 person-years of 6.4 (95% confidence interval [CI], 5.8–7.2; adjusted hazard ratio [aHR], 2.48; 95% CI, 2.04–3.00) for HF and an incidence rate of 4.8 (95% CI, 4.3–5.4; aHR, 2.91; 95% CI, 2.37–3.58) for cardiovascular death compared with participants with undetectable cTnT levels (incidence rate, 1.6; 95% CI, 1.4–1.8 and 1.1; 95% CI, 0.9–1.2 for HF and cardiovascular death, respectively). Among individuals with initially detectable cTnT, a subsequent increase of more than 50% (n = 393, 22%) was associated with a greater risk for HF (aHR, 1.61; 95% CI, 1.32–1.97) and cardiovascular death (aHR, 1.65; 95% CI, 1.35–2.03) and a decrease of more than 50% (n = 247, 14%) was associated with a lower risk for HF (aHR, 0.73; 95% CI, 0.54–0.97) and cardiovascular death (aHR, 0.71; 95% CI, 0.52–0.97) compared with participants with 50% or less change. Addition of baseline cTnT measurements to clinical risk factors was associated with only modest improvement in discrimination, with change in C statistic of 0.015 for HF and 0.013 for cardiovascular death.
Conclusion
In this cohort of older adults without known HF, baseline cTnT levels and changes in cTnT levels measured with a highly sensitive assay were significantly associated with incident HF and cardiovascular death.
Risk stratification for heart failure (HF) in older adults involves unique clinical challenges. Elderly individuals comprise the largest subgroup of patients hospitalized for HF, accounting for 80% of the more than 1.1 million US admissions per year.1,2 Once diagnosed with HF, older patients respond less well to guideline-based therapy than their younger counterparts, are more likely to require readmission, and are at higher risk for death.2–4 Furthermore, prediction models based on traditional cardiovascular risk factors are less adept at identifying cardiovascular risk in older adults compared with younger populations.5
Blood-based biomarkers, including C-reactive protein (CRP), natriuretic peptides, and troponins, have been advocated as adjuncts to clinical risk factors to identify community-dwelling older patients at high risk for adverse cardiovascular outcomes, but studies examining the additive prognostic value of these markers have reported inconsistent results.6–11 Prior studies have used standard troponin assays that are only able to detect circulating troponin levels in a small proportion of individuals.9–11 Recently, a highly sensitive cardiac troponin T (cTnT) assay has been developed, designed to improve low-end accuracy to meet and exceed myocardial infarction diagnosis guidelines.12,13 This assay has detected circulating cTnT in almost all patients with chronic HF or ischemic heart disease and provides independent prognostic information with respect to HF admission and cardiovascular death in these patients.14,15
We hypothesized that in community-dwelling ambulatory older adults without a prior HF diagnosis, a measurable cTnT concentration would be common using the highly sensitive cTnT assay and that higher concentrations would be associated with a greater risk of new-onset HF and cardiovascular death independent of traditional risk factors. Furthermore, we anticipated that serial measures of cTnT concentrations over time would change, potentially reflecting a dynamic change in risk.
METHODS
Study Population
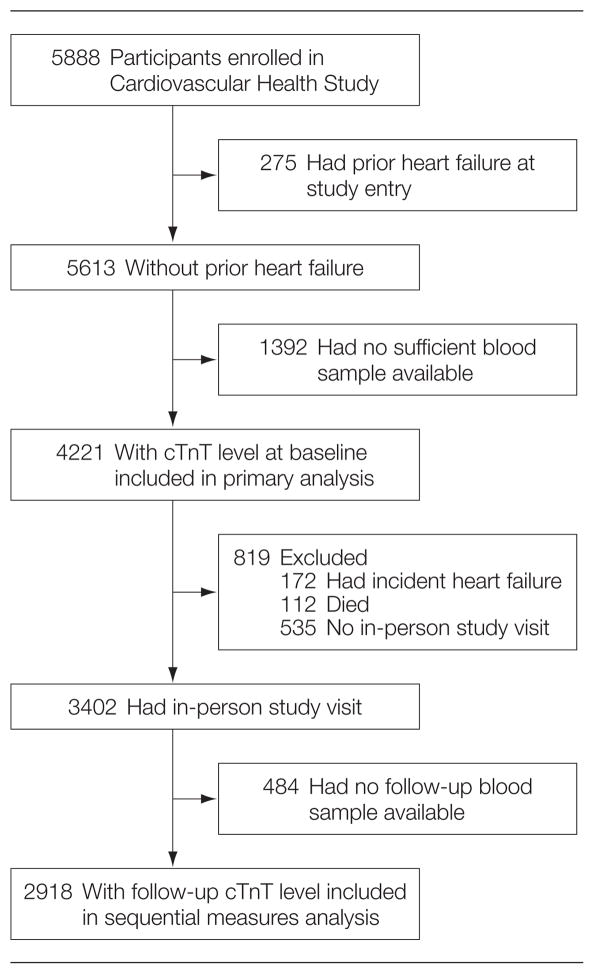
The Cardiovascular Health Study (CHS) is a multicenter prospective observational study of cardiovascular disease in older adults. For our analysis, participants with prior HF at study entry were excluded. Details of the study methods including data collection and definitions of comorbid conditions have been previously published.16,17 Figure 1 shows the numbers of participants with adequate serum and without prevalent HF for inclusion in the analysis at both baseline (1989–1990 for the main cohort and 1992–1993 for the supplemental black cohort) and follow-up periods (3 and 2 years later for the main and supplemental cohorts, respectively). These periods are the first follow-up visit for each cohort.
Figure 1.
Flow of Participants in the Cardiovascular Health Study
cTnT indicates cardiac troponin T.
The CHS was approved by the institutional review boards of the University of Washington, Seattle, and the participating centers. All participants gave written informed consent to participate at the time of study enrollment. Our study was approved by the institutional review board of the University of Maryland, Baltimore.
Biomarker Analysis
All measurements were performed on serum samples stored at −70°C to −80°C and thawed just before testing (maximum of 3 freeze-thaw cycles) in April 2010. Cardiac troponin T concentrations were measured with highly sensitive cTnT reagents on an Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, Indiana), with an analytical measurement range of 3 to 10 000 pg/mL. The value at the 99th percentile cutoff from a healthy reference population (n = 616) was 13.5 pg/mL.13 The 10% coefficient of variation concentration is reportedly close to or less than the 99th percentile of the reference population.13 Details of N-terminal protype B natriuretic peptide (NT-proBNP, expressed in pg/mL; to convert to nanograms per liter, multiply by 1.0) and CRP measurements in CHS have been described previously.6,8 Technologists recording the cTnT results were blind to participant outcomes.
Primary Outcome Measures
Outcome measures were incident HF and cardiovascular mortality. Incident HF events were ascertained by participant interview at semi-annual study visits and through examination of Medicare claims data. Potential HF events and determination of cause of death were assessed by an expert adjudication panel.18 The CHS Events Committee adjudicated HF by reviewing all pertinent data, including history, physical examination, chest radiography report, and medication use.19 An HF event was confirmed if, in addition to a physician diagnosis, there was (1) documentation in the medical record of a constellation of symptoms and physical signs; (2) supporting clinical findings; or (3) a record of medical therapy for HF. Incident HF in the current analysis was defined as adjudicated first HF event.
Cardiovascular mortality was defined as mortality related to atherosclerotic heart disease (fatal myocardial infarction and definite and possible fatal coronary heart disease [CHD]), mortality following cerebrovascular disease (fatal stroke), or mortality from other atherosclerotic and cardiovascular diseases including HF.18 Events ascertained through June 2008 were available for analysis.
Other Covariates
Race was self-identified and for the purposes of our analysis classified as black or other (primarily white and all other self-identified race). Coronary heart disease was defined as a history of angina, coronary revascularization, or myocardial infarction. Electrocardiograms were obtained at baseline and left ventricular hypertrophy was defined as previously reported.20 Echocardiograms were obtained during the year of the initial cTnT measure for the main cohort and 2 years following the initial cTnT measure for the black cohort. Left ventricular mass, left atrial diameter, and semi-quantitative left ventricular ejection fraction were defined using previously specified criteria.21
Statistical Methods
Participants were divided into categories of cTnT concentration, with individuals with undetectable levels in the first category (<3.00 pg/mL) and those with detectable levels divided into 4 equal-sized groups (3.00–5.44 pg/mL, 5.45–8.16 pg/mL, 8.17–12.94 pg/mL, and >12.94 pg/mL). Baseline participant characteristics were compared by category of cTnT concentration using 1-way analysis of variance tests for Gaussian continuous measures, Cuzik’s nonparametric trend test for non-Gaussian continuous variables,22 or the score test for trend in proportions, a test of linear trend of the log odds of a binary measure.23 Cumulative incidence of HF and cardiovascular death for each category of cTnT concentration were estimated using the Kaplan-Meier method and compared with the log-rank test for trend. Multivariate analyses were performed by using Cox proportional hazard regression models.
Three sets of covariates were selected for adjustment that included (1) demographics (age, sex, race [black vs other]); (2) traditional risk factors were selected from validated risk scores, which differ for HF and cardiovascular mortality.24,25 Heart failure models were adjusted for systolic blood pressure, glucose, albumin, CHD, smoking, creatinine, heart rate, and electrocardiographic-determined left ventricular hypertrophy; and cardiovascular death models were adjusted for systolic and diastolic blood pressure, use of antihypertensive medications, diabetes, CHD, smoking, and total and high-density lipoprotein cholesterol.24,25 (3) Other cardiovascular biomarkers (NT-proBNP and CRP). Because comorbid conditions such as hypertension and CHD are highly prevalent in elderly patients and could influence the association of cTnT with incident HF and cardiovascular death, a subgroup analysis was performed among those participants without either CHD or major HF risk factors.
Change in cTnT concentration was considered using 2 alternative categorizations. First, among all participants with cTnT measured at baseline and follow-up, we compared the risk of HF and cardiovascular death between those with and without detectable cTnT concentrations at follow-up, adjusting for baseline cTnT concentration and for the risk factors described above for each outcome.24,25 Second, among participants with detectable baseline cTnT (≥3.00 pg/mL), those with more than 50% relative increase in cTnT concentration or a more than 50% relative decrease were compared with those with longitudinal change of 50% or less with regard to new-onset HF and cardiovascular death, adjusting for baseline cTnT and for the risk factors described above. The choice of a 50% relative change threshold was specified a priori based on a prior study of short-term change in cardiac troponin I (measured with a highly sensitive assay) in healthy adults.26 In our analysis of more than 50% change, those participants with undetectable baseline cTnT were excluded.
The incidence rate of HF and cardiovascular death was estimated and compared across categories of cTnT change with the log-rank test, stratifying by baseline cTnT category. At-risk time was defined as time from the second cTnT measurement until the event of interest, death, or the most recent follow-up ( June 2008). Cox proportional hazard regression models stratified on cohort (main vs supplemental) were used for the analyses of change in cTnT level. For these multivariate models for new-onset HF and cardiovascular death, the risk factors described above, respectively for each end point, were entered as adjustment covariates using updated values from the study visit of the second cTnT measurement.24,25
We also conducted an exploratory analysis in which we considered cTnT as a continuous measure by imputing a concentration of 2.99 pg/mL for those participants (approximately 34%) with levels below the limit of detection for the assay, and assessed the association between natural log transformed cTnT values and incident HF and cardiovascular death.
Time-dependent C statistics were computed for survival regression models with and without baseline cTnT and interval change in cTnT27; the statistical significance of the improvement in the C statistics was determined using bootstrapping techniques. The net reclassification improvement (NRI) and the integrated discrimination improvement (IDI)28 were computed for the addition of cTnT to risk-factor adjusted models, and for the addition of change in cTnT to risk-factor adjusted models including only baseline cTnT. The NRI quantifies the extent to which a biomarker correctly reclassifies individuals to a higher or lower category of risk. For these calculations, individuals were categorized according to model-based 10-year risk of HF or cardiovascular death of less than 10%, 10% to 20%, or more than 20%. The IDI quantifies the improvement in predicted risk as a continuous measure.
Statistical analysis was performed by using Stata version 10 (StataCorp LP, College Station, Texas) and time-dependent C statistics were generated by using R version 2.1.1 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined a priori as P < .05.
RESULTS
Participant Characteristics
Baseline measures of cTnT were available in 3707 participants (74.6%) of the main cohort and 514 participants (80.1%) of the supplemental cohort without prevalent HF. Participants with sufficient sera volumes available for cTnT measurement were more likely to be women, black, and have diabetes and hypertension than those without sufficient sera available (eTable 1, available at http://www.jama.com). Concentrations of cTnT were equal to or more than the limit of detection (≥3.00 pg/mL) in 2794 participants (66.2%). Higher cTnT concentrations were associated with multiple traditional risk factors, known CHD, abnormal left ventricular ejection fraction, and increased left ventricular mass. In contrast, there was no association between cTnT level and race or body mass index, calculated as weight in kilograms divided by height in meters squared (Table 1). The linear correlation of cTnT with NT-proBNP was modest (Spearman rank ρ = 0.36, P < .001) and with CRP was minimal (ρ = 0.06, P < .001).
Table 1.
Characteristics of Study Sample by Baseline cTnT Concentration (N = 4221)a
| Characteristics | cTnT Concentration, pg/mL
|
P for Trend | ||||
|---|---|---|---|---|---|---|
| <3.00 (n = 1427) | 3.00–5.44 (n = 697) | 5.45–8.16 (n = 700) | 8.17–12.94 (n = 697) | >12.94 (n = 700) | ||
| Age, mean (SD), y | 70.4 (4.0) | 71.9 (4.7) | 72.9 (5.0) | 74.4 (5.7) | 76.3 (6.7) | <.001 |
|
| ||||||
| Men | 324 (22.7) | 234 (33.6) | 323 (46.1) | 362 (51.9) | 466 (66.6) | <.001 |
|
| ||||||
| Black | 250 (17.5) | 99 (14.2) | 97 (13.9) | 97 (13.9) | 139 (19.9) | .80 |
|
| ||||||
| Diabetes | 161 (11.3) | 98 (14.1) | 129 (18.4) | 146 (21.0) | 210 (30.0) | <.001 |
|
| ||||||
| Coronary heart disease | 167 (11.7) | 105 (15.1) | 134 (19.1) | 141 (20.2) | 193 (27.7) | <.001 |
|
| ||||||
| Hypertension | 700 (53.3) | 337 (54.8) | 363 (59.5) | 398 (67.8) | 364 (67.6) | <.001 |
|
| ||||||
| BP, mean (SD), mm Hg | ||||||
| Systolic | 132.6 (19.6) | 134.3 (19.8) | 138.0 (22.1) | 141.5 (21.8) | 142.8 (23.7) | <.001 |
|
| ||||||
| Diastolic | 70.4 (10.7) | 70.2 (10.4) | 70.7 (11.6) | 72.1 (11.5) | 72.0 (12.4) | <.001 |
|
| ||||||
| Estimated GFR <60 mL/min/1.73 m2 | 137 (13.1) | 101 (14.5) | 147 (21.0) | 179 (25.7) | 284 (40.6) | <.001 |
|
| ||||||
| Major ECG abnormality | 235 (17.0) | 156 (23.1) | 207 (30.3) | 237 (35.1) | 336 (50.5) | <.001 |
|
| ||||||
| Current or former smoker | 546 (38.3) | 286 (41.2) | 305 (43.6) | 299 (43.0) | 311 (44.4) | .003 |
|
| ||||||
| BMI, mean (SD) | 26.7 (4.7) | 26.5 (4.8) | 27.0 (5.0) | 27.0 (4.6) | 26.8 (4.6) | .97 |
|
| ||||||
| NT-proBNP, median (IQR), pg/mL | 79.5 (43.7–142.6) | 94.1 (50.6–178.3) | 116.5 (58.5–223.1) | 138.5 (75.9–301.6) | 228.7 (108.3–588.2) | <.001 |
|
| ||||||
| CRP, median (IQR) | 2.5 (1.3–4.3) | 2.3 (1.1–4.3) | 2.3 (1.3–4.2) | 2.5 (1.3–4.2) | 3.1 (1.6–6.7) | <.001 |
|
| ||||||
| LV mass by ECG, median (IQR), g | ||||||
| Men | 169.7 (156.9–184.2) | 171.4 (155.4–187.0) | 171.7 (155.8–188.2) | 171.0 (154.3–188.5) | 177.6 (159.0–202.0) | <.001 |
|
| ||||||
| Women | 133.5 (121.5–146.8) | 134.0 (121.2–148.2) | 136.5 (122.9–155.4) | 139.8 (124.4–157.2) | 136.8 (123.4–159.3) | <.001 |
|
| ||||||
| Left atrial diameter by echocardiogram, mean (SD), cm | 3.73 (0.59) | 3.84 (0.64) | 3.93 (0.65) | 3.96 (0.68) | 4.11 (0.72) | <.001 |
|
| ||||||
| Abnormal LVEF | 55 (4.1) | 35 (5.2) | 39 (5.8) | 69 (10.6) | 100 (15.8) | <.001 |
|
| ||||||
| LV mass index by echocardiogram, mean (SD), g/m2b | ||||||
| Women | 77.3 (19.5) | 79.4 (21.4) | 83.3 (23.5) | 86.5 (26.8) | 89.1 (23.7) | <.001 |
|
| ||||||
| Men | 85.9 (22.6) | 87.8 (23.3) | 90.9 (26.0) | 89.8 (24.1) | 96.9 (29.0) | <.001 |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; BP, blood pressure; CRP, C-reactive protein; cTnT, cardiac troponin T; ECG, electrocardiogram; GFR, glomerular filtration rate; IQR, interquartile range; LV, left ventricle; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-type B natriuretic peptide.
Data are presented as No. (%) unless otherwise indicated. A cTnT concentration of less than 3.00 pg/mL is undetectable.
A total of 2739 participants were assessed.
Outcomes by Initial cTnT Level
During a median follow-up of 11.8 years (interquartile range, 6.5–16.6 years) from the initial cTnT measurement, 1279 participants experienced new-onset HF and 1103 cardiovascular deaths occurred (among 2872 total deaths). The cumulative incidence of HF and cardiovascular death by cTnT category are shown in Figure 2. Differentiation of risk occurred by 1 to 2 years and continued through follow-up for both end points. The incidence rate for new-onset HF (per 100 person-years) ranged from 1.6 in those participants without detectable cTnT levels to 6.4 for those in the highest category (Table 2). After adjustment for clinical predictors of HF, the hazard ratio (HR) for HF for those participants with cTnT levels in the highest category was 2.48 (95% confidence interval [CI], 2.04–3.00) compared with participants with undetectable levels, with modest attenuation in risk after further adjustment for NT-proBNP and CRP. Similar findings were observed with respect to cardiovascular death (HR, 2.91; 95% CI, 2.37–3.58) (Table 2). Results were similar for the 1002 participants without CHD or major HF risk factors (eTable 2).
Figure 2.
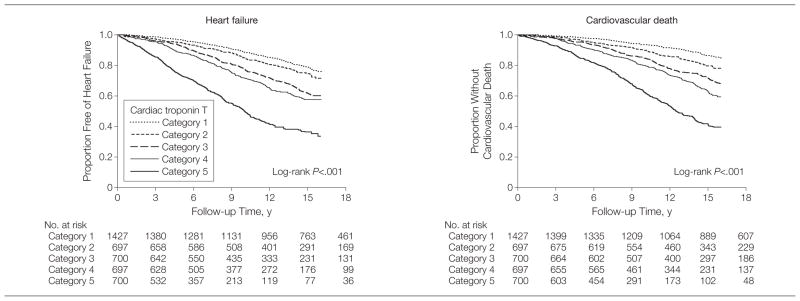
Kaplan-Meier Curves Reflecting Cumulative Proportion of Older Adults Free of Heart Failure and Without Cardiovascular Death by Baseline Cardiac Troponin T Concentration
Categories of cardiac troponin T concentrations were divided into category 1 (33.00 pg/mL), category 2 (3.00–5.44 pg/mL), category 3 (5.45–8.16 pg/mL), category 4 (8.17–12.94 pg/mL), and category 5 (312.94 pg/mL).
Table 2.
Association of Baseline cTnT Concentration With Incident Heart Failure and Cardiovascular Death
| cTnT Concentration, pg/mL
|
|||||
|---|---|---|---|---|---|
| <3.00 (n = 1427) | 3.00–5.44 (n = 697) | 5.45–8.16 (n = 700) | 8.17–12.94 (n = 697) | >12.94 (n = 700) | |
| Heart failure | (n = 311) | (n = 180) | (n = 235) | (n = 237) | (n = 316) |
|
| |||||
| Incidence rate (95% CI), per 100 person-years | 1.6 (1.4–1.8) | 2.1 (1.8–2.4) | 3.0 (2.6–3.4) | 3.4 (3.0–3.8) | 6.4 (5.8–7.2) |
|
| |||||
| Hazard ratio (95% CI) | |||||
| Unadjusted | 1 [Reference] | 1.33 (1.11–1.60) | 1.96 (1.65–2.31) | 2.27 (1.91–2.69) | 4.83 (4.12–5.66) |
|
| |||||
| Adjusted for demographic factorsa | 1 [Reference] | 1.21 (1.01–1.46) | 1.71 (1.44–2.03) | 1.79 (1.50–2.14) | 3.52 (2.95–4.21) |
|
| |||||
| Adjusted for demographic and traditional risk factorsb | 1 [Reference] | 1.13 (0.93–1.36) | 1.41 (1.18–1.69) | 1.47 (1.22–1.77) | 2.48 (2.04–3.00) |
|
| |||||
| Adjusted for demographic factors, traditional risk factors, and NT-proBNP and CRP | 1 [Reference] | 1.09 (0.90–1.32) | 1.27 (1.06–1.52) | 1.24 (1.03–1.50) | 1.84 (1.51–2.24) |
|
| |||||
| Cardiovascular death | (n = 222) | (n = 153) | (n = 204) | (n = 239) | (n = 285) |
|
| |||||
| Incidence rate (95% CI), per 100 person-years | 1.1 (0.9–1.2) | 1.6 (1.4–1.9) | 2.3 (2.0–2.7) | 3.0 (2.6–3.4) | 4.8 (4.3–5.4) |
|
| |||||
| Hazard ratio (95% CI) | |||||
| Unadjusted | 1 [Reference] | 1.59 (1.30–1.96) | 2.34 (1.93–2.82) | 3.14 (2.61–3.77) | 5.93 (4.96–7.08) |
|
| |||||
| Adjusted for demographic factorsa | 1 [Reference] | 1.41 (1.14–1.73) | 1.92 (1.58–2.33) | 2.24 (1.84–2.71) | 3.80 (3.12–4.64) |
|
| |||||
| Adjusted for demographic and traditional risk factorsc | 1 [Reference] | 1.35 (1.10–1.67) | 1.66 (1.36–2.02) | 1.91 (1.57–2.33) | 2.91 (2.37–3.58) |
|
| |||||
| Adjusted for demographic factors, traditional risk factors, and NT-proBNP and CRP | 1 [Reference] | 1.30 (1.05–1.60) | 1.45 (1.19–1.78) | 1.58 (1.29–1.93) | 2.10 (1.70–2.60) |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; cTnT, cardiac troponin T; NT-proBNP, N-terminal pro-type B natriuretic peptide.
Adjusted for age, sex, and race (black vs other).
Adjusted for demographic factors and systolic blood pressure, serum glucose, coronary heart disease, smoking status (never, current, former), creatinine, albumin, heart rate, and left ventricle hypertrophy by electrocardiogram.
Adjusted for demographic factors and systolic and diastolic blood pressure, antihypertensive medications, coronary heart disease, smoking status (never, current, former), diabetes, and total and high-density lipoprotein cholesterol.
In an exploratory analysis of cTnT as a continuous variable after natural log transformation, we found a continuous relationship with incident HF (per 1-ln unit increment; HR, 1.44; 95% CI, 1.33–1.55; P < .001) and with cardiovascular death (per 1-ln unit increment; HR, 1.54; 95% CI, 1.44–1.67; P < .001) in multivariate models including risk factors described in Table 2.
Outcomes by Change in cTnT Level During Interval Follow-up
Follow-up cTnT levels were available in 2918 of 3402 participants (85.8%) who returned for follow-up and did not develop HF between the baseline and follow-up period (Figure 1 and eTable 3). Within each baseline cTnT category, cTnT levels were similar among those participants who did or did not demonstrate subsequent changes in cTnT at the follow-up measurement (eTable 4). The rate of new-onset HF and cardiovascular death based on the presence or absence of a detectable cTnT on follow-up testing, stratified by initial cTnT category, are shown in eFigure 1 and eFigure 2. The risks of HF and cardiovascular death were higher among those participants with detectable compared with undetectable levels at follow-up, irrespective of the baseline level.
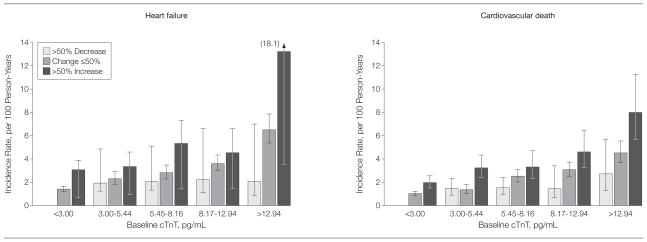
Differentiation of risk for both end points could be further refined by categorizing participants within each category by a more than 50% increase or decrease from baseline level (Figure 3). Within each category, risk of HF and cardiovascular death was highest for those with an increase in cTnT level of more than 50% and lowest for those with a more than 50% decrease in cTnT level on follow-up testing compared with participants with a change of 50% or less. For participants with measurable baseline cTnT levels, an increase of more than 50% was associated with an increased risk of HF and a greater risk of cardiovascular death, adjusting for baseline cTnT and risk factors. Results were modestly attenuated after adjusting for NT-proBNP and CRP (Table 3). In contrast, a decrease of more than 50% was associated with a risk-factor adjusted lower risk of HF and lower risk of cardiovascular death compared with those participants with 50% or less longitudinal change. Results were modestly attenuated and no longer significant after adjusting for NT-proBNP and CRP (Table 3).
Figure 3.
Incidence Rates of Heart Failure and Cardiovascular Death by Baseline cTnT Concentration and Subsequent Change in cTnT
cTnT indicates cardiac troponin T. Error bars represent 95% confidence intervals. Numbers of study participants in each cTnT category are shown in eTable 4. For those participants with initial undetectable concentrations of cTnT, a baseline value of 2.99 pg/mL (just below the lower limit of detection) was imputed for the calculation of relative change. For comparisons between the subsequent change in cTnT levels by log-rank test for trend for incidence rates of heart failure, P < .001 for less than 3.00 pg/mL, P= .02 for 3.00 to 5.44 pg/mL, P <.001 for 5.45 to 8.16 pg/mL, P= .02 for 8.17 to 12.94 pg/mL, and P <.001 for more than 12.94 pg/mL; and for incidence rates of cardiovascular death, P <.001 for less than 3.00 pg/mL, P <.001 for 3.00 to 5.44 pg/mL, P= .001 for 5.45 to 8.16 pg/mL, P= .001 for 8.17 to 12.94 pg/mL, and P= .004 for more than 12.94 pg/mL.
Table 3.
Association of Change in cTnT Concentration With Subsequent Heart Failure and Cardiovascular Death
| All Participants With Baseline cTnT (n = 2918)
|
Participants With Detectable Baseline cTnT Only (n = 1797)
|
||||
|---|---|---|---|---|---|
| Undetectable at Follow-up (n = 1036) | Detectable at Follow-up (n = 1882) | >50% Increase (n = 393) | Change ≤50% (n = 1157) | >50% Decrease (n = 247) | |
| Heart failure | (n = 182) | (n = 625) | (n = 155) | (n = 366) | (n = 56) |
|
| |||||
| Incidence rate (95% CI), per 100 person-years | 1.5 (1.3–1.7) | 3.7 (3.5–4.0) | 5.3 (4.5–6.2) | 3.5 (3.1–3.8) | 2.0 (1.5–2.6) |
|
| |||||
| Hazard ratio (95% CI) | |||||
| Unadjusteda | 1 [Reference] | 2.06 (1.70–2.50) | 1.73 (1.44–2.10) | 1 [Reference] | 0.57 (0.43–0.76) |
|
| |||||
| Adjusted for demographic factorsb | 1 [Reference] | 1.82 (1.50–2.21) | 1.67 (1.38–2.02) | 1 [Reference] | 0.65 (0.49–0.86) |
|
| |||||
| Adjusted for demographic and traditional risk factorsc | 1 [Reference] | 1.70 (1.39–2.07) | 1.61 (1.32–1.97) | 1 [Reference] | 0.73 (0.54–0.97) |
|
| |||||
| Adjusted for demographic factors, traditional risk factors, and NT-proBNP and CRP | 1 [Reference] | 1.55 (1.26–1.90) | 1.40 (1.14–1.71) | 1 [Reference] | 0.74 (0.55–1.00) |
|
| |||||
| Cardiovascular death | (n = 142) | (n = 534) | (n = 140) | (n = 321) | (n = 48) |
|
| |||||
| Incidence rate (95% CI), per 100 person-years | 1.1 (0.9–1.3) | 2.8 (2.6–3.1) | 4.1 (3.5–4.8) | 2.6 (2.4–3.0) | 1.6 (1.2–2.1) |
|
| |||||
| Hazard ratio (95% CI) | |||||
| Unadjusteda | 1 [Reference] | 1.94 (1.56–2.41) | 1.79 (1.47–2.19) | 1 [Reference] | 0.57 (0.42–0.77) |
|
| |||||
| Adjusted for demographic factorsb | 1 [Reference] | 1.63 (1.31–2.04) | 1.72 (1.40–2.11) | 1 [Reference] | 0.68 (0.50–0.93) |
|
| |||||
| Adjusted for demographic and traditional risk factorsd | 1 [Reference] | 1.57 (1.25–1.95) | 1.65 (1.35–2.03) | 1 [Reference] | 0.71 (0.52–0.97) |
|
| |||||
| Adjusted for demographic factors, traditional risk factors, and NT-proBNP and CRP | 1 [Reference] | 1.39 (1.12–1.74) | 1.38 (1.11–1.71) | 1 [Reference] | 0.75 (0.55–1.02) |
Abbreviations: CI, confidence interval; CRP, C-reactive protein; cTnT, cardiac troponin T; NT-proBNP, N-terminal protype B natriuretic peptide.
All models additionally adjusted for baseline cTnT and for Cardiovascular Health Study cohort (main vs supplemental).
Adjusted for age, sex, and race (black vs other).
Adjusted for demographic factors and systolic blood pressure, serum glucose, albumin and creatinine concentrations, coronary heart disease, smoking status (never, current, former), heart rate, and left ventricle hypertrophy on electrocardiogram.
Adjusted for demographic factors and systolic and diastolic blood pressure, use of antihypertensive medications, diabetes, coronary heart disease, total and high-density lipoprotein cholesterol, and smoking status (never, current, former).
Discrimination and Reclassification
For the prediction of both outcomes, the addition of baseline cTnT measurements to clinical risk factor models only modestly but statistically significantly improved classification (for HF: NRI=0.043 and IDI=0.026; and for cardiovascular death: NRI = 0.040 and IDI=0.021) and discrimination (for HF: difference in area under the curve [ΔAUC] = 0.015; and for cardiovascular death: ΔAUC = 0.013). The addition of change in cTnT level to clinical risk factors and baseline cTnT measures also significantly improved classification for both end points except for the NRI when predicting HF. However, the increment in discrimination was not significant for either end point (for HF: ΔAUC = 0.012, P= .09; and for cardiovascular death: ΔAUC = 0.007, P= .25) (eTable 5).
COMMENT
Low concentrations of cTnT, measured with a highly sensitive assay, were associated with a gradient of risk for new-onset HF and cardiovascular death in ambulatory community-dwelling individuals aged 65 years or older, independent of clinical variables associated with risk, as well as the cardiovascular risk biomarkers CRP and NT-proBNP. Furthermore, in this population, low cTnT concentrations are shown to frequently change over time. Independent of other risk factors as well as the baseline level of cTnT, these changes are associated with dynamic changes in risk of HF and cardiovascular death, concordant with the direction of change in biomarker level.
These findings expand upon prior studies using the highly sensitive cTnT assay outside the acute coronary syndrome setting in 3 ways. First, cTnT concentrations are detectable and of prognostic value in nearly two-thirds of a large geographically and ethnically diverse, stable, but at-risk population of ambulatory older individuals without a prior diagnosis of HF. The lower prevalence of detectable levels in the general population would be expected compared with studies of patients with stable coronary artery disease or symptomatic HF.14,15 Second, baseline levels of cTnT, below the range that would be expected to be detected with conventional assays, strongly associate with incident HF and cardiovascular death, independent of standard risk prediction variables. Addition of baseline cTnT to risk factor–adjusted models modestly improved discrimination, as measured by the C statistic and the IDI. Third, changes in cTnT during 2 to 3 years in older adults who remain free of HF, even when occurring at concentrations well below the 99th percentile of healthy younger blood donors,13 are prognostically significant.
These results should be considered in the context of prior findings with both conventional troponin T and I assays and other biomarkers for stratifying cardiovascular risk in community-dwelling older adults. In previous studies of older individuals, detectable levels of cardiac troponins were present in approximately 4% to 8%, and predictive of increased risk of HF, cardiovascular, and all-cause mortality.9–11 Similar to studies in patients with chronic but stable cardiovascular disease, the application of the highly sensitive cTnT assay increased the proportion of community-dwelling older adults with detectable cTnT levels approximately 10-fold.14,15 Compared with studies that used conventional troponin assays, the markedly increased range of measureable cTnT in our study enables estimation of a gradient of risk across the majority of older individuals, including those with an absence of clinical risk factors (other than age), and also permits examination of the significance of changing cTnT concentrations.
It is not possible from our study to determine the pathophysiology that results in detectable levels and frequent changes over time of cTnT in older adults. Prior evidence has shown that exercise-induced cardiac ischemia can lead to transient very low level increases in cardiac troponin levels as measured by a highly sensitive troponin I assay.29 Ischemia from known or unknown coronary artery disease must be considered in an older population, but magnetic resonance imaging in another stable older population does not support chronic ischemic heart disease as a predominant etiology linking low levels of troponins with subsequent development of HF.30 Furthermore, in a younger population with stable coronary artery disease small increases in cTnT levels measured by the highly sensitive cTnT assay were not predictive of myocardial infarction.15 Our findings also show that cTnT remains predictive of both HF and cardiovascular death in a subgroup of older adults with an absence of traditional risk factors, clinical history of heart disease, or an abnormal left ventricular ejection fraction (eTable 2).
Measurement of cTnT by the highly sensitive assay for risk stratification of older individuals has unique performance characteristics compared with other biomarkers that have been advocated by some for risk stratification in general populations, such as CRP and NT-proBNP. Although CRP has been associated with cardiovascular risk in younger populations, its prognostic value in older populations can be attenuated or absent.8,9,31 In contrast, natriuretic peptides, particularly NT-proBNP, perform better in higher-risk populations, including older adults compared with a younger general population.32 Our findings suggest that very low levels of cTnT provide prognostic information with respect to new-onset HF and cardiovascular mortality that is independent of NT-proBNP and CRP levels. The associations with HF were independent of other biomarkers such as renal function and electrocardiographic evidence of left ventricular hypertrophy.
The increment in the C statistic achieved by adding baseline cTnT to other clinical risk predictors is statistically significant, although more modest than in prior studies. In our analysis, we used as reference models outcome-specific clinical prediction models optimized for risk prediction in these individuals.24,25 The inclusion of variables such as albumin and left ventricular hypertrophy did optimize the clinical model but is beyond the traditional risk factors recommended for inclusion in statistical models when assessing the additional prognostic value of novel biomarkers.33 As a consequence, these reference models resulted in higher prognostic accuracy (C statistic, 0.75–0.78), even before adding cTnT than that reported in previous studies.9,15
Unique to our study is the finding that changes in very low levels of cTnT are common in this cohort of older adults and are independently associated with change in risk of both new-onset HF and cardiovascular death. Although it is possible that such changes reflect normal biological variation, the fact that these changes are associated with significant relative and absolute changes in risk regardless of baseline levels suggests that in fact they may represent a dynamic change in disease progression. Previously, we identified in the same population that change in NT-proBNP levels over time augmented prognostication above a single baseline measure.6 Taken together, these 2 findings suggest that serial measurements of both NT-proBNP and cTnT may improve risk assessment in elderly individuals.
Ultimately, the clinical importance of monitoring changes in cTnT levels for risk of progression to symptomatic HF is yet to be determined. This needs to be specifically considered in light of the conflicting findings with regard to improvement in discrimination, with significant improvement in one measure (the IDI) but not another (C statistic). However, the observation that changes in cTnT track with risk of HF and cardiovascular death reflects the dynamic nature of cardiovascular risk in older adults. Further studies are needed to assess whether monitoring low levels of cTnT may provide an opportunity to motivate specific changes in lifestyle or prompt medical interventions before progression to symptoms or cardiac structural abnormalities and to track the outcomes associated with these interventions.
Our study also has several limitations. First, samples were available in approximately three-fourths of the cohort at baseline, and differential absence of cTnT measures may have introduced bias into the estimates of associations with HF and cardiovascular death. Second, the duration of follow-up is a strength of our study; however, cardiovascular therapy has changed over time and it is possible that more ubiquitous use of medications such as statins could blunt the predictive value of the cTnT level. Third, unmeasured and residual confounding may have influenced our results; however, we demonstrate that cTnT concentration provides incremental prediction for HF and cardiovascular death beyond that provided by risk factors commonly used in clinical practice. Fourth, our choice of a more than 50% change in cTnT over time was based on biological variability in younger adults.26 Biological variability for cTnT using the highly sensitive cTnT assay has been reported to be higher in a small cohort of younger adults, for whom cTnT concentrations were mostly less than 3.00 pg/mL (the limit of detection of the current version of the highly sensitive cTnT assay).34 Therefore, these results may not be as relevant to our analysis. Biological variability in older adults with greater comorbidities remains to be determined.
CONCLUSIONS
Detectable cTnT levels as measured by a highly sensitive assay were present in the majority of community-dwelling older adults in this cohort, and higher concentrations—within a normal range established for a younger general population—reflect a greater burden of cardiovascular risk factors and imaging evidence of cardiac disease. Independent of these comorbidities, cTnT concentrations were associated with risk of new-onset HF and cardiovascular death. Furthermore, longitudinal changes in cTnT concentrations were common in this cohort and correspond with a dynamic change in risk over time.
Acknowledgments
Funding/Support: This study was supported by grants N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and U01 HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01 AG-15928, R01 AG-20098, and AG-027058 from the National Institute on Aging; R01 HL-075366 from the NHLBI; and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827. Additional funding was provided by Roche Diagnostics. Roche Diagnostics provided funding and laboratory reagents for the highly sensitive cardiac troponin T assay.
Role of the Sponsor: The NHLBI was involved in the study planning and limited data management. This study was also critically reviewed and approved by the Cardiovascular Health Study steering committee before submission. Roche Diagnostics had no role in the design and conduct of the study, in the collection, management, analysis, and data interpretation, or in the preparation, review, or approval of the manuscript.
Footnotes
A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Online-Only Material: eTables 1 through 5 and eFigures 1 and 2 are available at http://www.jama.com.
Author Contributions: Drs Zhan and Seliger had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: deFilippi, Gottdiener, Kop, Seliger.
Acquisition of data: Christenson, Gottdiener, Seliger.
Analysis and interpretation of data: deFilippi, de Lemos, Kop, Zhan, Seliger.
Drafting of the manuscript: deFilippi, Kop, Seliger.
Critical revision of the manuscript for important intellectual content: de Lemos, Christenson, Gottdiener, Kop, Zhan, Seliger.
Statistical analysis: Kop, Zhan, Seliger.
Obtained funding: deFilippi.
Administrative, technical, or material support: deFilippi, Christenson, Gottdiener.
Study supervision: deFilippi.
Financial Disclosures: Dr deFilippi reported receiving honorarium, consulting, and grant support from Roche Diagnostics and Siemens Healthcare Diagnostics, and consulting and grant support from Critical Diagnostics and BG Medicine. Dr de Lemos reported receiving research support from Biosite and Roche Diagnostics, and consulting fees, lecture honoraria, or both from Roche Diagnostics, Biosite/Inverness, and Siemens. Dr Christenson reported receiving research funding from Siemens Medical Diagnostics and Response Biomedical. Dr Seliger reported receiving grant and consulting support from Roche Diagnostics. Drs Gottdiener, Kop, and Zahn reported no financial disclosures.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2005;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121(15):1768–1777. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 3.Pfisterer M, Buser P, Rickli H, et al. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301 (4):383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 4.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 5.D’Agostino RB, Sr, Grundy S, Sullivan LM, et al. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 6.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people: the role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55(5):441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302(1):49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 9.Zethelius B, Berglund L, Sundström J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358(20):2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 10.Daniels LB, Laughlin GA, Clopton P, et al. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52(6):450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundström J, Ingelsson E, Berglund L, et al. Cardiac troponin-I and risk of heart failure: a community-based cohort study. Eur Heart J. 2009;30(7):773–781. doi: 10.1093/eurheartj/ehp047. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 13.Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(2):254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 14.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116(11):1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 15.Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361(26):2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 17.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 18.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 19.Schellenbaum GD, Rea TD, Heckbert SR, et al. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol. 2004;160(7):628–635. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 20.Rautaharju PM, Manolio TA, Siscovick D, et al. Utility of new electrocardiographic models for left ventricular mass in older adults. Hypertension. 1996;28(1):8–15. doi: 10.1161/01.hyp.28.1.8. [DOI] [PubMed] [Google Scholar]
- 21.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 22.Cuzick JA. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 23.Clayton D, Hills M. Statistical Models in Epidemiology. Oxford, England: Oxford University Press; 1993. [Google Scholar]
- 24.Kalogeropoulos APB, Psaty BM, Vasan RS, et al. Validation of the health ABC heart failure model for incident heart failure risk prediction: the Cardiovascular Health Study. Circ Heart Fail. 2010;3(4):495–502. doi: 10.1161/CIRCHEARTFAILURE.109.904300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pencina MJ, D’Agostino RB, Sr, Larson MG, et al. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119(24):3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu AHB, Lu QA, Todd J, et al. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52–58. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 27.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 29.Sabatine MS, Morrow DA, de Lemos JA, et al. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J. 2009;30(2):162–169. doi: 10.1093/eurheartj/ehn504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eggers KM, Lind L, Ahlström H, et al. Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population-based sample of elderly subjects. Eur Heart J. 2008;29(18):2252–2258. doi: 10.1093/eurheartj/ehn327. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 32.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120(22):2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 33.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119(17):2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56(7):1086–1090. doi: 10.1373/clinchem.2009.140616. [DOI] [PubMed] [Google Scholar]