Abstract
Background: To reduce the mortality and morbidity rates of cystic fibrosis (CF) patients, and to have an effective clinical management, it is important to monitor the progression of the disease. The aim of this study was to evaluate the progression of lung disease in CF patients by means of assessing the correlation of the CT scoring system with clinical status and pulmonary function test at the Pediatric Pulmonary Ward of Masih Daneshvari Hospital in 2008.
Methods: Pulmonary high resolution computed tomography (HRCT) was performed in 23 CF patients using the Brody's scoring system. Morphologic signs as well as the extent and severity of each sign were scored, and the total score was calculated. The correlation of HRCT scores (total score as well as the score for each parameter) with Shwachman Kuczycki scoring system and pulmonary function test were examined.
Results: The study included 9 female and 14 male patients with an age range of 5-23 years (mean: 13.42 years). Bronchiectasis (100%) and peribronchial wall thickening (100%) were the most frequent CT abnormalities. Mucus plugging, air trapping and parenchymal involvements were respectively seen in 95.7%, 91.3% and 47.8% of patients. The overall CT score for all patients was 57.6±24.2 (means±SD). The results of pulmonary function test showed a restrictive pattern; however, in 5.3% of the patients PFT was normal. The overall Shwachman-Kulczycki score was 53.48±13.8. There was a significantly (P=0.015) negative correlation between the total CT score and Shwachman-Kulczycki score; however, there was no significant correlation between total CT score and the results of PFT (P=0.481)
Conclusion: The Brody's scoring system for high resolution computed tomography seems to be a sensitive and efficient method to evaluate the progression of CF, and can be more reliable when we combine the CT scores with clinical parameters.
Key Words: Clinical status, pulmonary function test, cystic fibrosis
Introduction
Cystic fibrosis (CF) is the most common fatal genetic disorder in white population.1,2 Due to new and restrict modalities in the treatment of CF patients, their survival has increased. However, CF is still responsible for major complications, which increase the mortality and morbidity rates in such patients.3 The most common cause of mortality in CF patients is chronic pulmonary disease, which is the consequence of persistent infections and inflammations.1,2,4
To evaluate the pulmonary status in CF, a number of diagnostic procedures including chest radiography, high resolution computed tomography (HRCT), sputum culture and pulmonary function test are considered.1,2,4-7 Since CT Scan was found to be one of the best evaluation tools for cystic fibrosis progression, CT scoring system was proposed to make the evaluation more effective.8-11
A computed tomography scoring system is a tool to describe the abnormalities found by CT scan.12 The scoring system was introduced by Bhalla and colleagues 12. Since then, a number of other scoring systems have been proposed by Helbich,13,14 Santamaria,15 and Brody,16 and their colleagues.
Brody's scoring system is a lobar scoring system, which assigns a score to each lobe separately. This scoring system describes the following morphologic changes: bronchiectasis, peri-bronchial wall thickening, mucus plugging, air trapping and parenchymal involvement.16 Although different studies were conducted to show the usefulness of Brody CT scoring system in the assessment of the progression of the disease,16-19 possible correlation between the Brody scoring system and clinical status in patients with CF has not been examined. Therefore, the present study was designed to examine the correlation of the Brody scoring system with clinical parameters and pulmonary function test (PFT) in pediatric patients with CF. The study also aimed at evaluating the progression of lung disease in such patients.
Materials and Methods
This is a cross-sectional study, which was performed on 23 patients with CF admitted at the Pediatric Respiratory Ward of Masih Daneshvari Hospital in 2008. The study was approved by the Ethics Committee of Masih Daneshvari Hospital. Informed consent was obtained from all patients participating in the study.
Patients, who were diagnosed as CF based on two positive sweat tests as well as clinical manifestations compatible with CF, were included the study.20 Those with had negative Sweat Tests and no positive chromosomal analysis were excluded from the study. Four patients had chromosomal analysis test confirming the diagnosis.
High resolution computed tomography, PFT, and clinical findings (on the basis of Shwachman-Kulczycki scoring system) were used. High resolution computed tomography was performed in all patients, whereas PFT was carried out in only 20 of them. The patients under 6 years could not co-operate, and PFT was not performed in them. Computed tomography scan was obtained in all patients in supine position in both expiratory and inspiratory phases from lung apex to the below of costophrenic angles. Thin section (1 mm section thickness and 20 mm interval) CT scans were obtained with a spiral CT unit (Siemens SOMATOM Emotion, KVP 110).
The clinical status of all patients was evaluated by Shwachman-Kulczycki scoring system. This system determines the clinical severity of cystic fibrosis by scoring four parameters including general activity, physical examination, nutrition status and radiological findings. All examinations (HRCT, PFT and evaluation of clinical status) for evaluation each patient were conducted within two weeks.
An attending radiologist and two radiology residents reviewed the CT scans using the parameters listed in scoring system, determined the grades of morphologic signs of bronchiectasis, peri-bronchial wall thickening, mucus plugging and air trapping, and calculated the total score. They were not aware of the patients' clinical status and PFT results. In Brody scoring system,16 the severity and extent )central-peripheral) of bronchiectasis, peribronchial wall thickening, mucus plugging and air trapping were evaluated in right upper lobe, left upper lobe, right middle lobe, lingula, right lower lobe and Left lower lobe.
Pulmonay function test included forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1) and maximum expiratory flow at 50% and 25% of vital capacity. Results were described as the percentage of the predicted values based on reference values of PFT). Patients were divided into four groups including those with FEV1; <40%, FEV1; 40%-59%, FEV1; 60%-80%, and FEV1; >80% based on FEV1 results. Such groups were considered as severe, moderate, mild and normal, respectively. Shwachman-Kulczycki scoring system was used to evaluate the clinical status of all patients. This system assesses general activity, physical examination, nutrition and X ray findings. Scores for each parameter ranges from 5 to 25, and the total scores ranges from 20 (severely impaired) to 100 (normal).21
Spearman and correlation tests were used to examine the correlation between CT scores, pulmonary function tests and Shwachman-Kulczycki scores. The analysis of data was performed using Statistical Package for Social Sciences software (SPSS version.16). A P value of 0.05 or less was considered as statistically significant.
Results
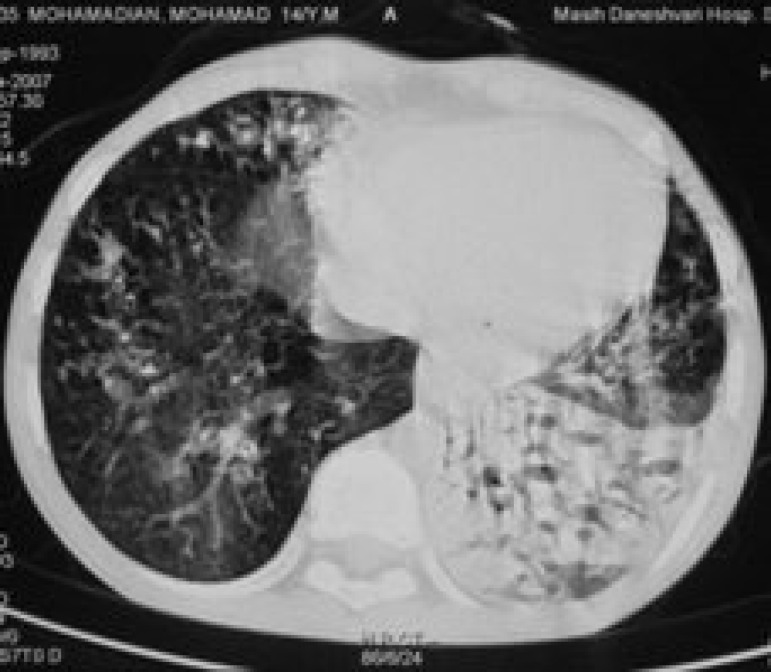
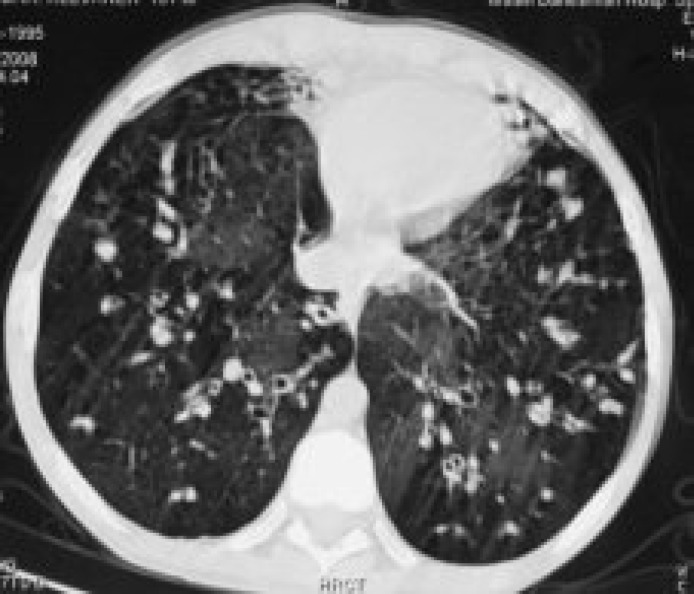
Twenty three (nine females and 14 males) patients with CF entered this prospective study. The range of the patients' age was 5-23 years (mean: 13.42 years). The overall CT score for all patients was 57.6±24.2. The most common findings in patients’ HRCT were bronchiectasia (100%), peribronchial thickening (100%), mucus plugging (95%) and air trapping (90%). A prototype of bronchiechtasia, peribronchial wall thickening and mucus plugging in patients' HRCT are shown in figures 1-3.
Figure 1.
Computed tomography from a 13-year-old girl. Bronchiectasia, peribronchial wall thickening, mucus plugging can be seen in both lungs.
Figure 3.
Computed tomography of a 14-year-old boy. Mucus plugging and bronchiectasia can be seen in the right lung.
Figure 2.
Computed tomography of a 9-year-old boy. Bronchiectasia is seen in right and left lungs.
A significant positive correlation was observed between the patients' age, and air trapping, bronchiectasis and total score. The results of PFT showed that the severity of restrictive pattern increased with the advancing age. In other words, the PFT results worsened significantly (P=0.006) with the increase of patients' ages.
The overall Shwachman-Kulczycki score was 53.48±13.8. There was no correlation between the Shwachman-Kulczycki scores and the patients’ age (P=0.136). Tables 1 and 2 summarize the PFT findings and Shwachman-Kulczycki scores. There was a significant (P=0.015) correlation between the total CT scores and Shwachman-Kulczycki scores; however, there was no significant (P=0.481) correlation between total CT score and the results of PFT (table 3).
Table 1.
The results of pulmonary function test in patients with cystic fibrosis.
| FEV1 | Frequency | Valid Percent | |
|---|---|---|---|
| <40% | 7 | 36.8% | |
| 40%-59% | 5 | 26.3% | |
| 60%-79% | 6 | 31.6% | |
| >80% | 1 | 5.3% | |
| Missing | 4 | ||
| Total | 23 | ||
FEV1 (forced expiratory volume in one second)
Table 2.
Schwachman-Kulczycki scores from patients with cystic fibrosis.
| Score | Frequency | Valid Percent |
|---|---|---|
| <40 | 5 | 21.7% |
| 41-55 | 9 | 39.1% |
| 56-70 | 7 | 30.4% |
| 71-85 | 2 | 8.7% |
| 86-100 | 0 | 0% |
Table 3.
Spearman Rank Correlation test results showing the correlation between high resolution computed tomography (HRCT) scores obtained by Brody's scoring system and pulmonary function test or Shwachman–Kulzcycki (S-K) score
| HRCT Findings |
FEV1
|
S-K Score
|
||
|---|---|---|---|---|
| R Correlation | P value | R Correlation | P value | |
| Bronchiectasis | -0.374 | 0.115 | -0.510* | 0.013 |
| Mucus plugging | 0.356 | 0.135 | -0.151 | 0.491 |
| Peribronchial wall thickening | 0.039 | 0.875 | -0.545 | 0.007 |
| Parenchyma | 0.215 | 0.377 | 0.064 | 0.773 |
| Air trapping | -0.179 | 0.463 | -0.271 | 0.212 |
| Total CT Score | -0.162 | 0.509 | -0.500* | 0.015 |
*P value <0.05 is considered significant, FEV1 (forced expiratory volume in one
Discussion
Cystic fibrosis is known as the most common fatal genetic disease among the white population.1,2 The evaluation of the disease progression by means of a routine monitoring will reduce the mortality and morbidity rates of the patients. This study evaluated the progression of lung disease in CF patients by means of assessing the relation between HRCT scoring system and non imaging parameters such as PFT and clinical scoring system. Recently, HRCT has been known as an extremely common diagnostic method to evaluate pulmonary involvement in CF patients. Hence, various scoring systems, which can evaluate the morphologic abnormalities found in HRCT, have been recommended.14,22-24
Although different studies have been performed to determine the importance of using CT scoring system to assess the progression of the disease,16-19 there are still some limitations such as the lack of a study evaluating the correlation of patients' clinical status with CT scoring system results. Twenty three children with CF were included in the present study. The mean age was 13.4 years indicating the age range of the participants was higher than those of similar studies.14 This might be due to delayed diagnosis. The lack of neonatal screening and high cost of evaluation for genetic mutation in Iran have led to the diagnosis of the disease on the basis of clinical manifestations and sweat test results in a higher range of age.
The evaluation of HRCT findings showed the following defects in decreasing order of frequency: bronchiectasis (100%), peribronchial wall thickening (100%), mucus plugging (95.7%), air trapping (91.3%) and parenchymal involvement (47.8%). A similar study conducted by Helbich et al.14 showed that bronchiectasis and peribronchial wall thickening were the most common findings on HRCT (80.3% and 76.1%, respectively). The other common findings were mucus plugging (63.9%) and mosaic perfusion (51.3%). The presence of all abnormalities in the majority of patients in the present study can be related to their high range of age. In other words, the higher the age of the patients, the higher the rate of lung involvements found on HRCT.
In this study, there was a significant (P=0.037) correlation between total CT scores and the patients' age. This indicates that CT scoring seem to be sensitive in the assessment of the disease progression. Moreover, there was a significant relationship between the most common abnormalities found on CT and the aggravation of the clinical manifestations of patients in this study. The progression of these abnormalities during the disease course could be explained by recurrent pulmonary infections and chronic inflammation.9,22,23 Long-term mucus plugging is accompanied by progressive bronchial destruction ending in bronchiectasis and bronchial wall thickening.9,22,23
In our study, a significant correlation was found between the advancement of age and the decrease of FEV1.This contradicts the results obtained from previous studies, which showed an increase in CT score in contrast to no change or improvement of respiratory test during the course of the disease.25,26
In this study there was no relationship between clinical score and the patients’ age, which can be due to imprecise reflection of lung status by Schwachman-Kulczycki score,24 and also the few number of patients recruited in this study.
The findings of the study show that there was a negative correlation between the overall HRCT scores and Schwachman-Kulczycki score. However, there was no significant relationship between CT score and PFT findings. This may be due to the four missing patients, whose absence may have affected the correlation. Less significant correlations between other CT findings and clinical manifestations of patients can indicate the importance of such a system, which evaluates a large number of morphologic findings. Therefore, the total score of abnormalities can be judged, but not each one alone.17
Conclusion
We recommend the widespread use of CT scoring system as a sensitive and effective method to monitor the status and progression of the disease among patients. Furthermore, it seems that this system is more sensitive than previous non-morphological assays. Additionally, it can play an important role in the determination of an appropriate therapeutic regimen, and the prognosis of the disease due to remarkable correlation of HRCT scoring and clinical scoring.
Acknowledgment
The authors would like to thank spirometry lab and statistical unit for their assistance in this study.
Conflict of Interest: None declared
References
- 1.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2005 Annual data report to the center directors. Bethesda, MD: Cystic Fibrosis Foundation; 2006. [Google Scholar]
- 2.Accurso FJ. Update in Cystic fibrosis 2007. Am J Respir Crit Care Med. 2008;177:1058–61. doi: 10.1164/rccm.200801-069UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flume PA, O'Sullivan BP, Robinson KA, et al. Cystic Fibrosis Pulmonary Guidelines,Chronic Medications for Maintenance of Lung Health. Am J Respir Crit Care Med. 2007;176:957–69. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 4.Gibson RL, Burns JL, Ramsey BW. Ransey. Pathophysiology and management of pulmonmary infection in Cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland RH, Neish AS, Zurakowaski D, et al. Cystic fibrosis: a system for assessing and predicting progression. AJR. 1998;170:1067–72. doi: 10.2214/ajr.170.4.9530060. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SM, Howatt WF, Grum CM. Spirometry and chest roentgenographic appearance in adult with cystic fibrosis. Chest. 1992;101:961–4. doi: 10.1378/chest.101.4.961. [DOI] [PubMed] [Google Scholar]
- 7.Taussig LM, Kattwinkel J, Friedewald WT, Di Sant'AgnesePA. A new prognostic score and clinical evaluation system for CF. J Pediatr. 1973;82:380–90. doi: 10.1016/s0022-3476(73)80110-6. [DOI] [PubMed] [Google Scholar]
- 8.Shah RM, Sexauer W, Ostrum BJ, et al. High resolution CT in the acute exacerbation of cystic fibrosis: Evaluation of acute findings, reversibility of those findings and clinical correlation. AJR Am J Roentgenol. 1997;169:375–80. doi: 10.2214/ajr.169.2.9242738. [DOI] [PubMed] [Google Scholar]
- 9.Maffessanti M, Candusso M, Brizzi F, Piovesana F. Cystic fibrosis in children: HRCT findings and distribution of disease. J Thorac Imaging. 1996;11:27–38. doi: 10.1097/00005382-199601110-00002. [DOI] [PubMed] [Google Scholar]
- 10.Nathanson I, Conboy K, Murphy S, et al. Ultrafast computerized tomography of the chest in cystic fibrosis: a new scoring system. Pediatr Pulmonol. 1991;11:81–6. doi: 10.1002/ppul.1950110112. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TE. Computed Tomography Scanning Techniques for the Evaluation of Cystic Fibrosis Lung Disease. Proc Am ThoracSoc. 2007;4:310–5. doi: 10.1513/pats.200612-184HT. [DOI] [PubMed] [Google Scholar]
- 12.Bhalla M, Turcios N, Aponte V, et al. Cystic fibrosis: scoring system with thin section CT. Radiology. 1991;179:783–8. doi: 10.1148/radiology.179.3.2027992. [DOI] [PubMed] [Google Scholar]
- 13.Helbich TH, Heinz-peer G, Fleischmann D, et al. Evolution of CT findings in patient with cystic fibrosis. AJR Am J Roentgenol. 173:81–88. doi: 10.2214/ajr.173.1.10397104. [DOI] [PubMed] [Google Scholar]
- 14.Helbich TH, Heinz-Peer G, Eichler I, et al. Cystic fibrosis CT: assessment of lung involvement in children and adult. Radiology. 1999;213:537–44. doi: 10.1148/radiology.213.2.r99nv04537. [DOI] [PubMed] [Google Scholar]
- 15.Santamaria F, Grillo G, Guidi G, et al. Cystic fibrosis: when should high resolution computed tomography of the chest be obtained? Pediatrics. 1998;101:908–13. doi: 10.1542/peds.101.5.908. [DOI] [PubMed] [Google Scholar]
- 16.Brody AS, Kosorok MR, Li Z, et al. Reproducibility of scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging. 2006;21:14–21. doi: 10.1097/01.rti.0000203937.82276.ce. [DOI] [PubMed] [Google Scholar]
- 17.de JongPA, Nakano Y, Lequin MH, et al. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J. 2004;23:93–7. doi: 10.1183/09031936.03.00006603. [DOI] [PubMed] [Google Scholar]
- 18.Brody AS, Suchare H, Campbeu JD, et al. Computed tomograghy correlates with pulmonary exacerbation s in children with Cystic Fibrosis. AMJ Respire Crit Care Med. 2005;172:1128–32. doi: 10.1164/rccm.200407-989OC. [DOI] [PubMed] [Google Scholar]
- 19.de JongPA, Lindblad A, Rubin L, et al. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax. 2006;61:80–5. doi: 10.1136/thx.2005.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klieman , Behrman , Jenson , Stanton . In: Nelson's Textbook of Pediatrics. 18 th ed. Kliegman, R.M, editor. Vol 2. 2007. pp. 1806–8. [Google Scholar]
- 21.Shwachman H, Kulczycki LL. Long term study of 105 patients with cystic fibrosis. Am J Dis Child. 1958;96:6–10. doi: 10.1001/archpedi.1958.02060060008002. [DOI] [PubMed] [Google Scholar]
- 22.Davis SD, Fordham LA, Brody AS, et al. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175:943–50. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 23.Klein JS, Quan J, Beanj A. High-resolution computed tomography in young patients with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function test. J Pediatr. 2004;145:32–8. doi: 10.1016/j.jpeds.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 24.Taccone A, Romano L, Marzoli A, Giroso D, Dell’Acqua A. High-resolution computed tomography in cystic fibrosis. Eur J Radiol. 1992;15:125–9. doi: 10.1016/0720-048x(92)90137-x. [DOI] [PubMed] [Google Scholar]
- 25.Alan SBrody. Scoring system for CT in cystic fibrosis: who cares. Radiology. 2004;231:296–8. doi: 10.1148/radiol.2312032097. [DOI] [PubMed] [Google Scholar]
- 26.Judge EP, Dodd JD, Masterson JB, Gallaghe CG. Pulmonary abnormalities on high resolution CT demonstrate more rapid decline than FEV1 in adult with cystic fibrosis. Chest. 2006;130:1424–32. doi: 10.1378/chest.130.5.1424. [DOI] [PubMed] [Google Scholar]