Abstract
Background: Brillantaisia lamium is an erect branched herb, which grows to a height of 1.50 m in moist tropical areas, both in full sun and partial shade. In , the aerial part of this plant is used in the treatment of various microbial infections such as skin diseases and infections of urinary tract. The aim of this study was to evaluate the antimicrobial activities of CH2Cl2: MeOH (1:1) extract, fractions and compounds from the aerial part of B. lamium.
Methods: The plant was dried and extracted by maceration in CH2Cl2: MeOH (1:1 v/v). Structures of the compounds from the CH2Cl2: MeOH (1:1) soluble fraction were determined by spectroscopic methods and compared with published data. The broth micro dilution method was used to evaluate the antimicrobial activities against bacteria and fungal species.
Results: Four known compounds: aurantiamide acetate (1), lupeol (2), lespedin (3), sitosterol 3-O-β-D-glucopyranoside (4) and a mixture of sterols: campesterol (5), stigmasterol (6) and β-sitosterol (7) were isolated from CH2Cl2: MeOH (1:1) extract of B. lamium aerial parts. The crude extract, fractions and isolated compounds exhibited both antibacterial and antifungal activities that varied with microorganism (MIC=6.25 – 1000 µg/ml). Compound 3 was the most active (MIC=6.25 – 100 µg/ml) while Staphylococcus aureus, Enterococcus faecalis, Candida tropicalis and Cryptococcus neoformans were the most sensitive to all the tested compounds.
Conclusion: The overall results of this study indicate that the CH2Cl2: MeOH (1:1) extract and some of isolated compounds have interesting antimicrobial properties and can be used for the treatment of fungal and bacterial infections.
Key Words: Antifungal, antibacterial, phytochemicals
Introduction
The emergence of human pathogenic microorganisms that are resistant to major classes of antibiotics has increased in recent years, due to the indiscriminate use of antimicrobial drugs.1 This has caused many clinical problems in the treatment of infectious diseases, and the antibiotics commonly used are sometimes associated with adverse effects such as hypersensitivity, allergic reaction and immunosuppression in the host.2 Thus, the search for the discovery of new antimicrobial agents is an urgent need. Cameroonian traditional medicine is increasingly solicited through tradipractitioners and herbalists in the treatments of infectious diseases. On the other hand, about 80% of citizens in developing countries use traditional medicine based on plant products.3 Traditionally, dry herbs are used either boiled in water like tea or as an infusion to treat systemic bacterial and fungal infections, or are directly applied on the skin or nails in a plaster form to treat local infections.4
Some species of the Acanthaceae family present antimicrobial activities.5 Previous chemical studies with species of this family were related to the isolation of alkaloids, iridoids, lignans, flavonoids, terpenoids and phenylpropanoids glycosides.6 Brillantaisia lamium (Nees) Benth is an erect branched herb from Acanthaceae family, which has a height of about and is found in moist tropical areas growing both in full sun and partial shade.7 The aerial part of this plant is used in Cameroon in the treatment of various microbial infections such as skin diseases and infections of urinary tract. However, no phytochemical and biological studies have been reported on B. lamium. As a continuation of our chemical and biological studies of Cameroonian medicinal plants,8-13 we have investigated the CH2Cl2: MeOH (1:1) extract of the aerial part of B. lamium, and have reported herein the isolation of a mixture of three sterols and four pure compounds together with their antibacterial and antifungal activities.
Materials and Methods
Plant Material
The aerial parts of B. lamium Benth, were collected in January Korup, South-west region of (figure 1). The botanical identification of the plant was done at the National Herbarium, , (voucher specimen No. 34376/HNC). The plant material was air-dried at room temperature and ground into fine powder.
Figure 1.
Aerial parts of Brillantaisia lamium
Microorganisms
The test microorganisms included three bacteria and two fungi. They were mostly reference strains obtained from the American Type Culture Collection (ATCC, ): Staphylococcus aureus ATCC25922, Enterococcus faecalis ATCC10541, Salmonella typhi ATCC6539, Candida albicans ATCC9002 and Candida tropicalis ATCC750. Also included, were one clinical isolate of Proteus mirabilis and one strain of Cryptococcus neoformans IP95026 collected from Pasteur Institute of Yaoundé () and Paris () respectively. They were maintained at +4°C on Agar slants in the Laboratory of Microbiology and Antimicrobial Substances (LAMAS) of the Faculty of Sciences, , where the antimicrobial tests were performed. The strains were subcultured on fresh appropriate agar plate 24 hours prior to any antimicrobial test.
Extraction, Fractionation and Isolation of Compounds
Previously dried and powdered aerial parts of B. lamium () were macerated with CH2Cl2: MeOH (1:1 v/v) (3 × , 72 hours) at room temperature (25±1°C) to obtain a crude extract () after evaporation of solvent under reduced pressure at 40°C. One hundred and seventy nine grams () of this extract were successively extracted with n-hexane (), followed by CH2Cl2 (). Thin layer chromatography (TLC) analysis showed that the n-hexane and CH2Cl2 extracts were qualitatively the same. They were thus combined and a portion of was subjected to silica gel column chromatography (Ø x L ) and eluted with n-hexane-EtOAc (10:0, 9:1, 8:2, 7:3, 1:1 and 0:10 each ) and EtOAc-MeOH (10:0, 19:1, 9:1 and 0:10 each ). Fifty five fractions of 300 ml each were collected and combined on the basis of TLC profile to give five major fractions A - E (A: 1–12; B: 13–25; C: 26–38; D: 39–45; E: 46–55). Fraction A () contained mostly fatty material and was not further investigated. Fraction B () was purified on a silica gel column (Ø × L ) with n-hexane-EtOAc (10:0, 90:10 and 9:1 each ) to afford lupeol (2) (20 mg; Rf=0.60, n-hexane-EtOAc, 9:1) and a mixture of campesterol (5), stigmasterol (6) and β-sitosterol (7) (22 mg; Rf=0.53, n-hexane-EtOAc, 9:1) in an estimated proportion of 1:4:1.50 (GC-MS). Fraction C () was subjected to silica gel column chromatography (Ø × L ) eluted with n-hexane-EtOAc (10:0, 9:1, 4:1, 3:2, 2:3 and 0:10, each ) to yield aurantiamide acetate (1) (30 mg; Rf=0.46, n-hexane-EtOAc, 7:3). Fraction D crystallized in methanol to give sitosterol 3-O-β-D-glucopyranoside (4) (160 mg; Rf=0.70, CH2Cl2-MeOH, 9:1). Fraction E () was submitted to silica gel column chromatography and eluted with CH2Cl2:MeOH (19:1, 17:3, 4:1, 7:3, each ) followed by purification through Sephadex LH-20 gel column chromatography using CH2Cl2:MeOH (1:1) to yield lespedin (3) (21 mg, Rf=0.35, CH2Cl2-MeOH, 9:1).
Identification of isolated compounds
The structural identification of compounds 1-4 was established using spectroscopic analysis, especially, NMR spectra in conjunction with 2D experiments and direct comparison with published information,6,11,14,15 and authentic specimens obtained in our laboratory for some cases. Melting points of isolated compounds were uncorrected and determined on a Büchi SMP-20 melting point apparatus and with a Reichert microscope. Infra-red spectra were measured on a Shimadzu FTIR-8400S spectrophotometer and the UV spectra were recorded with a Shimadzu UV-3101 PC, spectrophotometer. Electron impact-mass spectrometry (ionization voltage 70 eV) and High resolution-electron impact-mass spectrometry spectra were measured with a Finnigan MAT double focusing spectrometer Model 8230.1 H-NMR (500 MHz) and,5 C-NMR (125 MHz) spectra were recorded in CDCl3 using a Bruker-Avance-500 MHz NMR spectrometer and Trimethylsilyl as internal standard. The mixture of sterols was only identified by gas chromatography-mass spectrometry. Gas chromatography-mass spectrometry (GC-MS) data were obtained with an Agilent 6890N Network GC system/5975 Inert X L Mass Selective Detector at 70 eV and 20°C. The GC column was a CP- Sil 8 CB LB, fused silica capillary column ( × , film thickness 0.25 µm). The initial temperature was 50°C for 1 min, and then heated at 10°C/min to 300°C. For the carrier gas, helium was used with a flow rate of 1.20 ml/min. Kovat’s retention index (KI) was determined using a calibration curve of n-alkanes.
Antimicrobial Assays
Determination of Diameters of Inhibition Zones
The diameters of inhibition zones were determined by disc diffusion method as described by Tamokou and co-workers,10 with some modifications. Stock solutions of test samples were prepared in 10% v/v aqueous dimethylsulfoxide (DMSO) solution (Fisher chemicals) at concentrations of 100 mg/ml (for crude extract and fractions) and 10 mg/ml (for pure compounds). The inocula of microorganisms were prepared from 24 h old broth cultures. The absorbance was read at 600 nm and adjusted with sterile physiological solution to match that of a 0.5 McFarland standard solution. From the prepared microbial solutions, other dilutions with sterile physiological solution were prepared to give a final concentration of 106 colony-forming units (CFU) per milliliter for bacteria and 2x105 spores per milliliter for yeasts. Bottles containing 19.80 ml of sterile molten Sabouraud Dextrose Agar (, , ) or Mueller Hinton Agar (MHA) () were maintained in a water bath at 45°C to prevent solidification of the medium, and were aseptically inoculated with 200 µl of bacteria or yeast suspension. Microbial inoculum and medium were well mixed and dispensed into sterile diameter Petri dishes and allowed to solidify at room temperature in a sterile cupboard. After solidification, discs of in diameter previously impregnated with 10 µl of test samples were placed aseptically on the solid plates. This gave a charge of 1 mg or 100 µg of test substances per disc. The Petri dishes were left at +4°C for 2 h to allow extracts and compounds to diffuse from the discs into the medium. The test media were then incubated at 35°C for 24 h (for bacteria) and 48 h (for yeasts). Antimicrobial activity was evaluated by measuring the clear zone of growth inhibition on agar surface around the discs. The assay was done in triplicates. Gentamicin (Sigma-Aldrich, , ) and nystatin (Merck, ) were used as positive controls for bacteria and yeasts respectively at 10 µg per disc. Dimethylsulfoxide solution (10% v/v) was used as a negative control.
Determination of minimum inhibitory concentrations (MICs) and minimum microbicidal concentrations (MMCs)
Minimum inhibitory concentration values were determined by broth micro dilution method as reported by Nyaa and co-workers.16 The test samples were first of all dissolved in DMSO. The solution obtained was then added to Mueller Hinton Broth (MHB) for bacteria or Sabouraud Dextrose Broth (SDB) for yeasts to give a final concentration of 2000 µg/ml. This was serially diluted two folds to obtain a concentration range of 0.48 to 2000 µg/ml. One hundred microliters of each concentration was added into each well (96- wells microplate) containing 95 µl of MHB or SDB and 5 µl of inoculum (106 CFU/ml for bacteria and 5x105 spores/ml for yeasts) to obtain final concentrations varying from 0.12 to 1000µg/ml. The final concentration of DMSO in the well was less than 1% (v/v). Preliminary analysis with 1% (v/v) DMSO did not inhibit the growth of the test organisms. The negative control wells consisted of 195 µl of MHB or SDB and 5 µl of the inoculum. The plates were covered with sterile lids, then agitated to mix the contents of wells using a plate shaker and incubated at 35°C for 24 hours for bacteria, 48 hours for Candida sp, or 72 hours for Cryptococcus neoformans. The assays were repeated thrice. The MICs of samples were detected following the addition of 50 µl of a 0.20 mg/ml p-iodonitrotetrazolium violet (INT) solution followed by incubation at 35°C for 30 min. The colorless tetrazolium salt acts as an electron acceptor and is reduced to a red-colored formazan product by biologically active microorganisms. Where microbial growth was inhibited, the solution in the well remained clear after incubation with INT. Minimum inhibitory concentrations were defined as the lowest sample concentrations that prevented this color change indicating a complete inhibition of microbial growth.
For the determination of MMCs, a portion of liquid (5 µl) from each well that showed no growth of microorganism was plated on MHA or SDA and incubated at 35°C for 24 hours for bacteria, 48 hours for Candida sp, or 72 hours for Cryptococcus neoformans. The lowest concentration that yielded no revival of growth after this subculturing was taken as the MMCs.16 Gentamicin and nystatin were used as positive controls for bacteria and yeasts respectively.
Statistical Analysis
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) for Window software version 12.0. The inhibition diameters of test substances were expressed as meanstandard deviation. Group comparisons were done using one way analysis of variance (ANOVA) followed by Waller-Duncan Post Hoc test. A value of P<0.05 was considered statistically significant.
Results
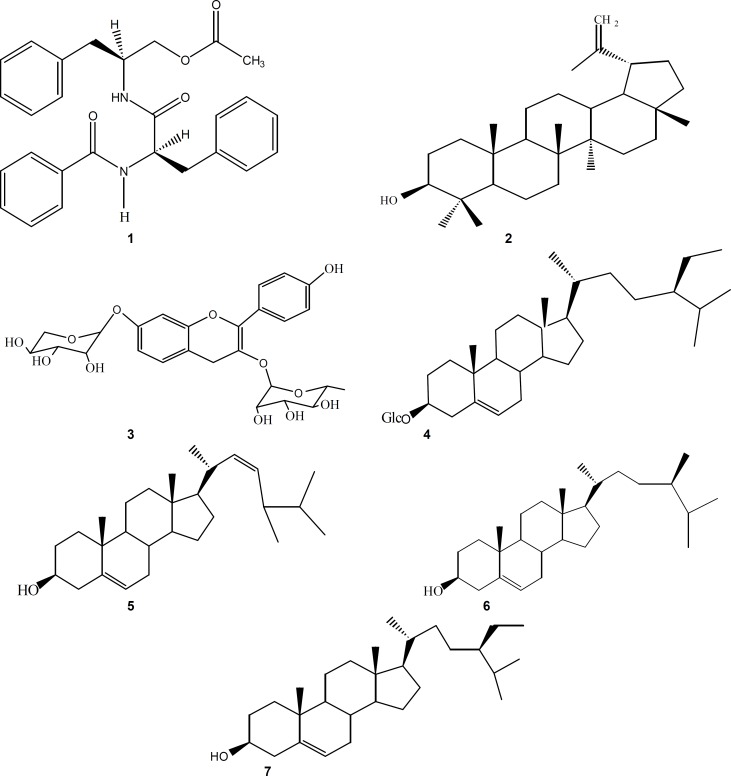
Four known compounds: aurantiamide acetate (1), lupeol (2), lespedin (3), sitosterol 3-O-β-D-glucopyranoside (4) and a mixture of sterols: campesterol (5), stigmasterol (6) and β-sitosterol (7) were isolated from CH2Cl2: MeOH (1:1) extract of B. lamium aerial parts (figure 2).
Figure 2.
Chemical structures of aurantiamide acetate (1), lupeol (2), lespedin (3), sitosterol 3-O-β-D-glucopyranoside (4), a mixture of sterols: campesterol (5), stigmasterol (6) and β-sitosterol (7) isolated from B. lamium.
The results of the antimicrobial activity showed that the CH2Cl2: MeOH (1:1) extract, fractions B-E and all the isolated compounds showed both antifungal and antibacterial activities that varied among the microbial strains (tables 1-3). Gram-positive bacteria were more sensitive to the test samples as compared with Gram-negative bacteria (table 1). Fraction A was found to be not active, and Salmonella typhi and Candida albicans were respectively the most resistant strains for bacteria and yeasts against all the tried samples. Fractionation enhanced the antimicrobial activity of the crude extract in fraction D (MIC=62.50-125 µg/ml). However, these activities decreased in other fractions. The results of MIC and MMC values were in agreement with the above observations (tables 2-3). The antimicrobial activity of compound 1 (MIC=50-200 µg/ml) was almost found to be comparable to that of gentamicin (MIC=12.50-100 µg/ml), but lower than that of nystatin (MIC=1.56-6.25 µg/ml) (table 2). Compound 3 (MIC=6.25-25 µg/ml) was the most active substance among the test samples (MIC=12.50-400 µg/ml). Moreover, its antibacterial activity was higher than that of gentamicin (MIC=12.50-100 µg/ml), which was used as a reference drug.
Table 1.
Diameters (mean±SD) of inhibition zones of the CH2Cl2: MeOH (1:1) extract and fractions and compounds isolated from Brillantaisia lamium
|
Test
substances |
Diameters of inhibition zones (in mm)
|
||||||
|---|---|---|---|---|---|---|---|
|
Bacteria
|
Fungi
|
||||||
| Enterococcus faecalis | Staphylococcus aureus |
Salmonella
typhi |
Proteus mirabilis |
Candida
albicans |
Candida tropicalis | Cryptococcus neoformans | |
| Crude extract | 20.00±0.50b | 18.00±1.0a | 21.00±0.50a | 20.00±0.00a | 15.00±0.00b | 17.50±1.00a | 15.50±1.00a |
| Fraction A | na | na | na | na | na | na | na |
| Fraction B | 14.50±1.0a | 16.00±0.00b | na | na | na | 15.00±0.00b | na |
| Fraction C | 17.50±0.50c | 15.00±0.50c | na | na | 13.50±1.00a | 14.50±0.50b | 12.50±1.00d |
| Fraction D | 21.00±0.50b | 22.50±0.50d | 23.50±0.50d | 24.00±0.00e | 17.50±0.50c | 20.00±1.0c | 18.00±0.00b |
| Fraction E | na | 12.50±0.50e | na | na | 11.00±0.50d | 14.50±0.50b | na |
| Mixture of sterols | 14.50±0.50a | 13.00±0.00e | na | 12.50±0.50b | na | 11.00±0.00d | na |
| 1 | 11.0 0± 0.00d | 16.00±0.00b | 13.00±0.50b | 15.00±0.00c | 15.50 ±0.50b | 13.00±0.00e | 17.0 0±0.50c |
| 2 | 10.00±0.00e | 12.50±1.00e | na | 12.00±0.00b | 11.50±0.50d | na | 12.50±1.00d |
| 3 | 20.50±0.50b | 26.50±0.50f | 26.00±0.00c | 23.50±1.00e | 15.50 ±0.50b | 15.00±0.00b | 18.00±0.50b |
| 4 | 11.50±0.00d | 13.50±0.50e | na | 14.50±0.50c | 13.00±0.00a | 15.00±0.50b | 15.00±1.00a |
| Gentamicin | 20.50±1.50b | 25.50±1.00f | 24.00±0.50d | 19.00±0.00d | nt | nt | nt |
| Nystatin | nt | nt | nt | nt | 25.50±0.50e | 22.00±1.00c | 26.00±0.50f |
Zone diameters were determined at 1 mg (for the crude extract and fractions), 100 µg (for the isolated compounds and mixture of sterol) or 10 µg (for gentamicin and nystatin) per spot; 1 mg, 100 µg and 10 µg were the minimum charges of a disc. For the same column, mean diameters of inhibition zones of the test samples bearing different superscript letters (a, b, c, d, e, f) are significantly different (p<0.05) compared to other mean values, and those carrying the same superscripts are not significantly different. The results are the mean values of triplicate tests measured in two directions after 24-48 h incubation at 35 °C.; na: not active; nt: not tested; aurantiamide acetate (1); lupeol (2); lespedin (3); sitosterol 3-O-β-D-glucopyranoside (4); a mixture of sterols: campesterol (5); stigmasterol (6); β-sitosterol (7)
Table 2.
Minimum inhibitory concentrations (MIC) of the CH2Cl2: MeOH (1:1) extract, fractions and compounds of Brillantaisia lamium
| Test substances |
Minimum inhibitory concentrations (in µg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
|
Bacteria
|
Fungi
|
||||||
| Enterococcus faecalis | Staphylococcus aureus |
Salmonella
typhi |
Proteus mirabilis |
Candida
albicans |
Candida tropicalis | Cryptococcus neoformans | |
| Crude extract | 125 | 250 | 125 | 62.50 | 500 | 250 | 250 |
| Fraction A | na | na | na | na | na | na | na |
| Fraction B | 1000 | 500 | na | na | na | 500 | na |
| Fraction C | 250 | 500 | na | na | 1000 | 500 | 1000 |
| Fraction D | 125 | 125 | 62.50 | 62.50 | 125 | 62.50 | 125 |
| Fraction E | na | 500 | na | na | 1000 | 500 | na |
| Mixture of sterols | 50 | 100 | na | 200 | na | 400 | na |
| 1 | 100 | 50 | 50 | 100 | 50 | 100 | 12.50 |
| 2 | 400 | 200 | na | 200 | 200 | na | 200 |
| 3 | 25 | 6.25 | 12.50 | 12.50 | 50 | 50 | 6.25 |
| 4 | 100 | 100 | na | 100 | 200 | 100 | 50 |
| Gentamicin | 12.50 | 50 | 50 | 100 | nt | nt | nt |
| Nystatin | nt | nt | nt | nt | 1.56 | 6.25 | 1.56 |
Tabulated values are the means of three trials which did not show any variation (SD=0); na: not active at concentrations up to 400 µg/ml (for pure compounds and mixture of sterols) or 1 mg/ml (for crude extract and fractions); nt: not tested; gentamycin and nystatin are used as reference antibiotics for bacteria and yeasts respectively; aurantiamide acetate (1); lupeol (2); lespedin (3); sitosterol 3-O-β-D-glucopyranoside (4); a mixture of sterols: campesterol (5); stigmasterol (6); β-sitosterol (7).
Table 3.
Minimum microbicidal concentrations (MMC) of the CH2Cl2: MeOH (1:1) extract, fractions and compounds of Brillantaisia lamium
| Test substances |
Minimum microbicidal concentrations (in µg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
|
Bacteria
|
Fungi
|
||||||
| Enterococcus faecalis | Staphylococcus aureus |
Salmonella
typhi |
Proteus mirabilis |
Candida
albicans |
Candida tropicalis | Cryptococcus neoformans | |
| Nystatin | nt | nt | nt | nt | 1.56 | 6.25 | 1.56 |
| Crude extract | 250 | 250 | 250 | 125 | 500 | 500 | 250 |
| Fraction A | na | na | na | na | na | na | na |
| Fraction B | 1000 | 500 | na | na | na | 1000 | na |
| Fraction C | 500 | 500 | na | na | 1000 | 1000 | 1000 |
| Fraction D | 125 | 250 | 125 | 62.50 | 250 | 125 | 125 |
| Fraction E | na | 500 | na | na | 1000 | 500 | na |
| Mixture of sterols | 100 | 100 | na | 200 | na | 400 | na |
| 1 | 100 | 50 | 50 | 100 | 50 | 100 | 25 |
| 2 | 400 | 200 | na | 200 | 200 | na | 200 |
| 3 | 25 | 12.50 | 12.50 | 25 | 100 | 50 | 12.50 |
| 4 | 200 | 100 | na | 100 | 200 | 200 | 100 |
| Gentamicin | 12.50 | 50 | 50 | 100 | nt | nt | nt |
Tabulated values are the means of three trials which did not show any variation (SD=0); na: not active at concentrations up to 400 µg/ml (for pure compounds and mixture of sterols) or 1 mg/ml (for crude extract and fractions); nt: not tested; gentamycin and nystatin are used as reference antibiotics for bacteria and yeasts respectively; aurantiamide acetate (1); lupeol (2); lespedin (3); sitosterol 3-O-β-D-glucopyranoside (4); a mixture of sterols: campesterol (5); stigmasterol (6); β-sitosterol (7).
Discussion
The findings of the present study showed that there were differences between the antimicrobial activities of crude extract and those of fractions. This suggests that the aerial part of B. lamium contains several antifungal and antibacterial principles with different polarities as shown by the phytochemical study. The fractionation of the crude extract enhanced its antimicrobial activity in fraction D, and reduced those of other fractions. This indicates that the active principles might be more concentrated in fraction D and more diluted in other fractions. All the isolated compounds showed antimicrobial activities on at least one microorganism. Such a finding supports the traditional use of this plant in the treatment of infectious diseases. The antimicrobial properties of some individual flavonoids, sterols and triterpenes of plant origin were documented.9,16-19 Compounds 1 and 2 displayed antibacterial as well as antifungal activities. Comparable results were obtained by Ragasa et al.19 and Singh and Singh.20
The known antimicrobial mechanisms associated to each group of chemicals to which the isolated compounds belong may explain the antimicrobial potency of the crude extract, fractions and compounds from B. lamium. Membrane disruption has been suggested as one of the likely mechanisms of action.21,22 This might also explain the antimicrobial activities of compounds 2 (triterpene), 4, 5, 6 and 7 (sterols).21,22 The activity of flavonoids such as compound 3 might be due to their ability to complex with bacteria cell wall,21 and therefore, inhibiting the microbial growth.
The antimicrobial activities varied with the bacterial and fungal species. These variations may be due to genetic differences between the microorganisms. The results of MMC values (table 3) indicated that a cidal effect of many of the tried samples could be expected. Moreover, a keen look at the results of MIC (table 2) and MMC (table 3), showed that the MIC values obtained were four times lesser than the MMCs on the corresponding (sensitive) microorganisms, confirming the microbicidal effects of the concerned samples.9,23 This is interesting in view of the perspective of developing new antibacterial drugs from natural products. To the best of our knowledge, this is the first report on the antimicrobial activities of the crude extract, fractions and compounds from B. lamium.
The overall results of this study can be considered as very promising in the perspective of new drugs discovery from plant sources, especially when the medical importance of tested microorganisms is considered. Staphylococcus aureus is a major cause of community and hospital-associated infection with an estimated mortality of around 7-10%.24 Moreover, about 2% of patients in Cameroon, are infected by Staphylococcus spp.25 Each year, some 500,000 patients in American hospitals contract a staphylococcal infection.24 Such findings stress the importance of finding an antibiotic against which the organism (Staphylococcus aureus) is sensitive. This pathogen was found to be sensitive to the crude extract, fractions and isolated compounds.
About 77% of immune-deficient patients' death is attributable to microscopic fungi, such as Candida species and Cryptococcus neoformans.26 Also, 75% of women, during their lives, would have had at least one episode of vulvo-vaginitis candidiasis, which is considered as the second most common form of vaginitis after bacterial vaginosis.27 Typhoid fever caused by Salmonella typhi continues to be a significant public health problem in developing countries in general and in Sub-Saharan Africa in particular. Generally, at least three of the samples tested (i.e. Fraction D, compounds 1 and 3) in this study prevented the growth of all microbial strains.
Conclusion
The results of this study indicate that the CH2Cl2: MeOH (1:1) extract from aerial parts of B. lamium might represents a potential source of plant drugs for the treatment of fungal and bacterial diseases. Also, all the isolated compounds found active in this study could be useful for the development of new antimicrobial drugs. However, pharmacological and toxicological studies, currently going on in our laboratory, will be necessary to confirm this hypothesis.
Acknowledgment
This research was supported by the International Sciences Program, Uppsala University, Sweden (ISP, Grant No CAM: 02, to Prof Tane), the International Foundation for Science, Stockholm, Sweden, and the Organization for the Prohibition of Chemical Weapons, The Hague, The Netherlands (IFS-OPCW, Grant No F/ 4238-1, to Dr Tene). We thank Pr. Karsten Krohn, Department of Chemistry, , and Pr. SF Kouam of "Ecole Normale Supérieure de Yaoundé", University of Yaounde 1, Cameroon for the NMR spectra analyses.
Conflict of Interest: None declared
References
- 1.Karaman I, Sahin F, Güllüce M, et al. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J Ethnopharmacol. 2003;85:231–5. doi: 10.1016/s0378-8741(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee PK, Saritha GS, Suresh B. Antimicrobial potential of two different Hypericum species available in India. Phytother Res. 2002;16:692–5. doi: 10.1002/ptr.1016. [DOI] [PubMed] [Google Scholar]
- 3.Sofowora A.E. Medicinal plants and traditional medicine in Africa. 2nd edition. Ibadan, Nigeria: Spectrum Books Ltd; 1993. p. 289. [Google Scholar]
- 4.Yang X, Summerhurst DK, Koval SF, et al. Isolation of an antimicrobial compound from Impatiens balsamina L using bioassay-guided fractionation. Phytother Res. 2001;15:676–80. doi: 10.1002/ptr.906. [DOI] [PubMed] [Google Scholar]
- 5.Meurer-Grimes B, McBeth DL, Hallihan B, et al. Antimicrobial activity in medicinal plants of the Scrophulariaceae and Acanthaceae. Pharm Biol. 1996;34:243–8. [Google Scholar]
- 6.Berrondo LF, Teixeira GF, Sidnei BO, et al. Dirhamnosyl flavonol and other constituents from Brillantaisia palisatii. Química Nova. 2003;26 [Google Scholar]
- 7.Csurhes S, Edwards R. Australia: Candidate species for preventative control. Canberra, Australia: Biodiversity Group, Environment Australia; 1998. Potential environmental weeds; p. 208. [Google Scholar]
- 8.Tamokou JD, Kuiate JR, Tene M, Tane P. Antimicrobial clerodane diterpenoids from Microglossa angolensis Oliv. et Hiern. Indian J Pharmacol. 2009;41:60–3. doi: 10.4103/0253-7613.51340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamokou JD, Tala FM, Wabo KH, et al. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J Ethnopharmacol. 2009;124:571–5. doi: 10.1016/j.jep.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 10.Tamokou JD, Kuiate JR, Njateng GSS, et al. Antimicrobial activity of dichloromethane-methanol (1:1 v/v) extract from the stem bark of Coula edulis Bail. (Olacaceae) Res J Microbiol. 2008;3:414–22. [Google Scholar]
- 11.Tene M, Ndontsa BL, Tane P, et al. Antimicrobial diterpenoids and triterpenoids from the stem bark of Croton macrostachys. Int J Biol Chem Sci. 2009;3:538–44. [Google Scholar]
- 12.Kuiate JR, Mouokeu S, Wabo KH, et al. Antidermatophytic triterpenoids from Syzygium jambos (L.) Alston (Myrtaceae) Phytother Res. 2007;21:149–52. doi: 10.1002/ptr.2039. [DOI] [PubMed] [Google Scholar]
- 13.Gatsing D, Tchakounte V, Ngamga D, et al. In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran J Med Sci. 2009;34:126–36. [Google Scholar]
- 14.Banerji A, Ray R. Aurantiamides: A new class of modified dipeptides from Piper aurantiacum. Phytochemistry. 1981;20:2217–20. [Google Scholar]
- 15.Tene M, Tane P, Tamokou JD, et al. Degraded diterpenoids from the stem bark of Neoboutonia mannii. Phytochemistry Lett. 2008;1:120–4. [Google Scholar]
- 16.Nyaa TBL, Tapondjou AL, Barboni L, et al. NMR assignment and Antimicrobial/ antioxidant activities of 1β-hydroxyeuscaphic acid from the seeds of Butyrospermum parkii. Nat Prod Sci. 2009;15:76–82. [Google Scholar]
- 17.Kuete V, Nguemeving JR, Penlap BengV, et al. Antimicrobial activity of the methanolic extracts and compounds from Vismia laurentii De Wild (Guttiferae) J Ethnopharmacol. 2007;109:372–9. doi: 10.1016/j.jep.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 18.Djoukeng JD, Abou-Mansour E, Tabacchi R, et al. Antibacterial triterpenes from Syzygium guineense (Myrtaceae) J Ethnopharmacol. 2005;101:283–6. doi: 10.1016/j.jep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Ragasa CY, Penalosa BA, Rideout JA. A bioactive dipeptide derivative from Malachra fasciata. Philippine J Sci. 1998;127:267–76. [Google Scholar]
- 20.Singh B, Singh S. Antimicrobial activity of terpenoids from Trichodesma amplexicaule Roth. Phytother Res. 2003;17:814–6. doi: 10.1002/ptr.1202. [DOI] [PubMed] [Google Scholar]
- 21.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arvind Sh, Reg FC, Enzo AP. Identification of antimicrobial component of an ethanolic extract of Australian medicinal plant, Eremophila duttonii. Phytother Res. 2004;18:615–8. doi: 10.1002/ptr.1507. [DOI] [PubMed] [Google Scholar]
- 23.Mims CA, Playfair JHL, Roitt IM, et al. Antimicrobials and chemotherapy. Med Microbiol Rev . 35:1–34. [Google Scholar]
- 24.Bowersox J. Experimental Staph Vaccine Broadly Protective in Animal Studies. NIH, 1999-05-27. [Retrieved on 2007-07-28].
- 25.CPC. Rapport d’activités du Centre Pasteur du Cameroun 2001-2002. 2002. p. 189. [Google Scholar]
- 26.Mohammad AH, Shigefumi M, Kataro M, et al. In vitro and in vivo activities of SCH56592 against Cryptococcus neoformans. J Antimicrob Chemother. 1999;44:827–9. doi: 10.1093/jac/44.6.827. [DOI] [PubMed] [Google Scholar]
- 27.Fidel P, Lynch M, Sobel J. Candida-specific TH1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–7. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]