Abstract
BACKGROUND
The long-term renal consequences of kidney donation by a living donor are attracting increased appropriate interest. The overall evidence suggests that living kidney donors have survival similar to that of nondonors and that their risk of end-stage renal disease (ESRD) is not increased. Previous studies have included relatively small numbers of donors and a brief follow-up period.
METHODS
We ascertained the vital status and lifetime risk of ESRD in 3698 kidney donors who donated kidneys during the period from 1963 through 2007; from 2003 through 2007, we also measured the glomerular filtration rate (GFR) and urinary albumin excretion and assessed the prevalence of hypertension, general health status, and quality of life in 255 donors.
RESULTS
The survival of kidney donors was similar to that of controls who were matched for age, sex, and race or ethnic group. ESRD developed in 11 donors, a rate of 180 cases per million persons per year, as compared with a rate of 268 per million per year in the general population. At a mean (±SD) of 12.2±9.2 years after donation, 85.5% of the subgroup of 255 donors had a GFR of 60 ml per minute per 1.73 m2 of body-surface area or higher, 32.1% had hypertension, and 12.7% had albuminuria. Older age and higher body-mass index, but not a longer time since donation, were associated with both a GFR that was lower than 60 ml per minute per 1.73 m2 and hypertension. A longer time since donation, however, was independently associated with albuminuria. Most donors had quality-of-life scores that were better than population norms, and the prevalence of coexisting conditions was similar to that among controls from the National Health and Nutrition Examination Survey (NHANES) who were matched for age, sex, race or ethnic group, and body-mass index.
CONCLUSIONS
Survival and the risk of ESRD in carefully screened kidney donors appear to be similar to those in the general population. Most donors who were studied had a preserved GFR, normal albumin excretion, and an excellent quality of life.
Kidney transplantation, particularly from a living donor, is the treatment of choice for most patients with end-stage renal disease (ESRD).1 The superior results achieved with kidney transplantation from living donors have resulted in an increase in this method of transplantation.2
The life expectancy of kidney donors appears to be similar to that of nondonors or perhaps even longer, as suggested by one study.3 However, at least two reports have described donors in the United States who were subsequently placed on the waiting list for kidney transplantation.4,5 Although the risk of ESRD among donors does not appear to be increased, and although cross-sectional studies have reported no major elevations in serum creatinine levels for up to 30 years after donation,6–10 such studies estimated the glomerular filtration rate (GFR) from the serum creatinine concentration, and the length of the follow-up period and the number of subjects studied were relatively limited. The present study ascertained vital status and the risk of ESRD in a large number of kidney donors and compared their health status with that of controls. To overcome the limitations of previous studies, kidney function was formally assessed by measurement of the GFR and urinary albumin excretion in 255 donors who had donated kidneys 3 to 45 years before the study began.
METHODS
STUDY POPULATION
From November 1963 through December 2007, a total of 3698 nephrectomies in living donors were performed at the University of Minnesota. Potential donors had to be free from diabetes and hypertension and have a GFR greater than 80 ml per minute per 1.73 m2 of body-surface area — requirements that reflect the practice at most transplantation centers. Donors provided a complete history and underwent a physical examination, as well as renal and vascular imaging. They underwent a comprehensive laboratory assessment to rule out liver disease, active infections, and systemic illnesses. No potential donor with any albuminuria (defined as a urinary albumin-tocreatinine ratio of >0.02 on more than one occasion) was accepted.
We ascertained the vital status of donors as of December 31, 2007, with the use of the payment records of the Social Security Administration Death Master File. A person is included in this file if his or her lump-sum benefit was paid as a result of a request from a family member, an attorney, or a mortuary. The presence of ESRD was ascertained through reports by the recipients and donors themselves.
In December 2003, we initiated a comprehensive effort to contact all persons who had donated a kidney after November 1963. We consulted telephone and internet directories and asked recipients for their specific donor’s contact information. We asked the donors we located to provide us with updates on their health status and to report the results, if available, of urinalysis and serum creatinine testing. At the beginning of this effort in 2003, we generated lists of donors who were known to be alive (as of December 2003) and stratified them according to sex and the number of years since donation (in 3-year intervals). A random starting point within each stratified list was used to generate random numbers to select 5 to 10% of donors (a total of 185 to 370) from each stratum who would be asked to undergo measurements of the GFR. Eighty percent of the donors who were designated in this way were successfully contacted, and 255 donors underwent measurement of the GFR. If the selected donor refused to participate, the same method was used to contact a new donor from the same stratum. Therefore, all donors who underwent measurement of the GFR, by design, had donated a kidney in the year 2000 or earlier. All studies were approved by the institutional review board at the University of Minnesota, and all participants provided written informed consent.
The GFR was determined on the basis of the plasma clearance of nonradioactive iohexol, and the urinary albumin excretion rate was calculated according to the ratio of albumin to creatinine in an early-morning urine sample11 (see the Supplementary Appendix, available with the full text of this article at NEJM.org). Blood pressure was measured three times while the donor was seated, and the mean value was recorded. We considered hypertension to be present when a donor required antihypertensive medications or when a donor who was not taking antihypertensive drugs had average blood-pressure readings above 140/90 mm Hg. At the time the GFR was measured, donors were asked to complete the Medical Outcomes Study 36-Item, version 1, or 12-Item, version 2, Short-Form General Health Survey (SF-36 and SF-12, respectively)12,13 in order to assess their quality of life (see the Supplementary Appendix).
STATISTICAL ANALYSIS
Categorical variables are presented as percentages, and continuous variables as means and standard deviations. We compared survival probabilities for kidney donors with those for the general population, using life tables from the Human Mortality Database, which combines data regarding survival and death rates from the National Center for Health Statistics with data from other sources.14 Expected survival probabilities were calculated separately according to sex in 1-year calendar intervals. For donors who were 40 years of age at the time of donation in 1990, the expected death rate in the first year would be the rate for people in the general population who were 40 years old in 1990, and the expected rate in the second year would be the rate for people in the general population who were 41 years old in 1991; this process was updated annually. The rate of ESRD in the general population was obtained from the 2007 annual data report of the United States Renal Data System.1 Since the majority of donors were white, we used the overall adjusted incidence rate for whites as of 2005 — 268 cases per 1 million persons per year.
Analyses of survival and ESRD were post hoc, and all the visits for determination of the GFR, as well as analyses related to quality of life, were prespecified. Albumin-to-creatinine ratios were logarithmically transformed. We fitted logistic-regression models using a backward selection method for a GFR of less than 60 ml per minute per 1.73 m2, albuminuria (microalbuminuria and macroalbuminuria were combined), and hypertension, with covariates of age, age at the time of donation, sex, time since donation, systolic blood pressure, diastolic blood pressure, body-mass index, smoking status, and baseline GFR, estimated with the use of the Modification of Diet in Renal Disease (MDRD) study equation. For inclusion in the logistic-regression models, P values of less than 0.10 were considered to indicate statistical significance; for all other analyses, P values of less than 0.05 were considered to indicate statistical significance. We used a paired t-test to compare the GFR, systolic and diastolic blood pressure, and albumin-to-creatinine ratio in donors who underwent repeated measurements. We used SAS software, version 9.1 (SAS Institute), and the R statistical package for all analyses.
Donors in whom the GFR was measured were compared with matched controls; the controls were subjects from the National Health and Nutrition Examination Surveys (NHANES) of 2003–2004 and 2005–2006 for whom data on fasting morning laboratory tests were available.15 Probability-based methods are used in NHANES to generate a study sample that is representative of the noninstitutionalized population of the United States. For this analysis, donors were matched with NHANES controls in a 1:1 ratio on the basis of age (in 1-year increments), sex, race or ethnic group, and body-mass index (the weight in kilograms divided by the square of the height in meters) at the time of the measurement of GFR (>30 vs. ≤30). A similar analysis was carried out among donors who had donated a kidney more than 20 years earlier to compare those in whom the GFR was measured (55 donors) with those in whom the GFR was not measured but for whom laboratory results and health status updates were available (1035 donors).
The SF-12 and SF-36 scores were summarized as a physical-health summary score and a mental-health summary score, with the use of the algorithms and the U.S. population norms provided in the SF-12 and SF-36 manuals, respectively.12,13 The values for the physical-health summary and the mental-health summary were standardized as T scores (with a mean [±SD] of 50±10). Age- and sex-adjusted difference scores for the physical-health summary and the mental-health summary were calculated by subtracting the norm for the relevant sex-by-age group from the observed T score. T scores and adjusted differences from the SF-36 and SF-12 were then pooled for analyses. One-sample, two-tailed z tests were used to compare the mean standardized T scores for the physical-health and mental-health summaries with the normative reference mean of 50. Pearson correlations were used to estimate associations between the SF-36 and SF-12 adjusted-difference summary scores and time since donation. All reported P values are two-sided and are not adjusted for multiple testing.
RESULTS
SURVIVAL
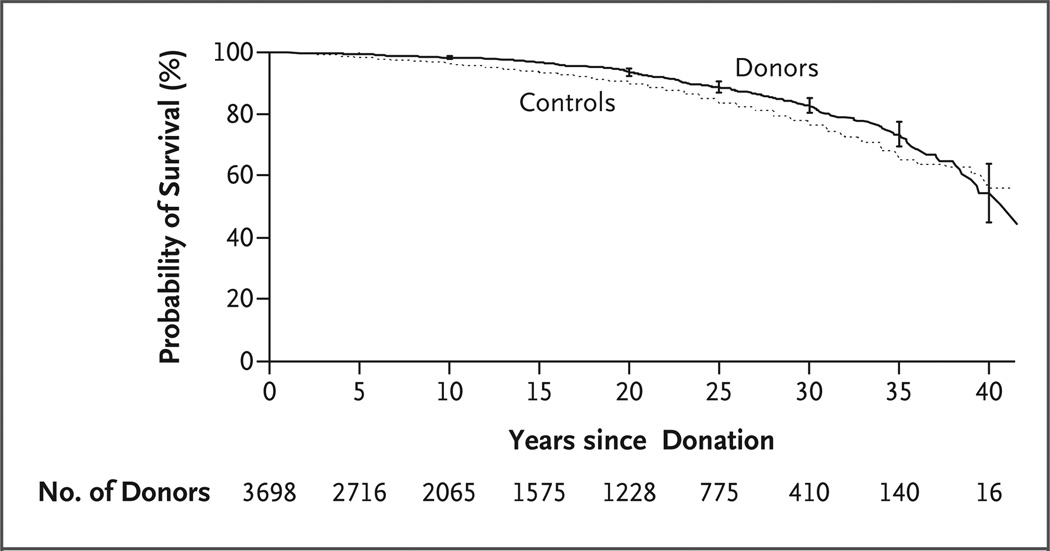
As of December 31, 2007, a total of 3404 of the 3698 donors were documented as being alive, 268 were documented as having died, and 26 were foreign nationals whose vital status was unknown. A total of 196 donors died before the initiation of this study, and 72 died during the study period (December 2003 through December 2007). The cause of death was unknown for 162 donors; among the remaining 106, cardiovascular disease accounted for 30% of all deaths. Excluding donors who were foreign nationals, the survival of donors appeared to be similar to that of the controls in the general population, but survival could not be formally compared, since life tables from the National Center for Health Statistics do not provide confidence intervals for the probability of survival in the general population (Fig. 1).
Figure 1. Survival of Kidney Donors and Controls from the General Population.
I bars at 5-year intervals indicate 95% confidence intervals for the probability of survival among kidney donors.
RISK OF ESRD
ESRD that necessitated dialysis or transplantation developed in 11 donors 22.5±10.4 years after donation; 7 of the donors were women and 8 were white. All of the donors had donated kidneys to relatives, 7 of whom were siblings. The causes of ESRD in the recipients of kidneys from these 11 donors were type 1 diabetes (4 recipients), hypertension (2), glomerulonephritis (2), obstructive uropathy (1), hemolytic–uremic syndrome (1), and interstitial nephritis (1). Three of the 11 donors had the same cause of ESRD as their sibling recipients: hemolytic–uremic syndrome (1 donor), hypertension (1) and glomerulonephritis (1). The causes of ESRD in the other 8 donors were hypertension (2 donors), renal-cell carcinoma (1), scleroderma (1), and unknown (4).
A measurement of the serum creatinine level for 232 donors who had died was available 1 to 10 years before their death. The mean level was 1.2±0.2 mg per deciliter (107±18 µmol per liter). No donors who had died had reported needing dialysis or a transplantation in our multiple contacts with them. Therefore, the estimated incidence of ESRD in donors would appear to be 180 per million persons per year, as compared with the overall adjusted incidence rate of 268 per million persons per year in the white population of the United States.
GFR AND URINARY PROTEIN EXCRETION
We were able to contact 2949 of the 3404 donors who were known to be alive as of December 31, 2007. At the beginning of this effort in 2003, a total of 3162 kidney donations from living donors had been performed; 2199 of the donors consented to give health status updates and report laboratory results. Of these 2199 donors, 255 (who represented 6.9% of the entire donor pool, 11.6% of those contacted, and 14.3% of the 1785 donors who were invited to undergo measurement of iohexol GFR) agreed to return for formal measurement of GFR.
The mean age of donors who underwent formal evaluation was 41.1±11.0 years at the time of donation and 53.2±10.0 years at the time that measurement of iohexol GFR was performed; 61.6% of the donors were women, and 98.8% were white. From the time of donation, 12.2±9.2 years had elapsed; 43.1% of the donations had occurred more than 10 years before. The 255 donors who returned for measurement of the iohexol GFR were older than the 3443 donors who did not (41.1±11.0 vs. 38.4±11.7 years, P<0.001), and they had donated more recently (13.7±9.2 vs. 16.3±11.0 years earlier, P<0.001); the two groups were otherwise similar.
The mean serum creatinine level at the time of donation was 0.9±0.2 mg per deciliter (88±74 µmol per liter); the GFR (as estimated from the MDRD study equation) was 84.0±13.8 ml per minute per 1.73 m2. At the time, the iohexol GFR was measured (12.2±9.2 years after donation), the mean serum creatinine level was 1.1±0.2 mg per deciliter (98±19 µmol per liter), and the estimated GFR was 63.7±11.9 ml per minute per 1.73 m2. The estimated GFR at the time of the measurement of the iohexol GFR was 76±12% of the estimated GFR at the time of donation. A younger age at the time of donation, a longer time since donation, and a higher estimated GFR at the time of donation were associated with a greater compensatory increase in the estimated GFR in the remaining kidney.
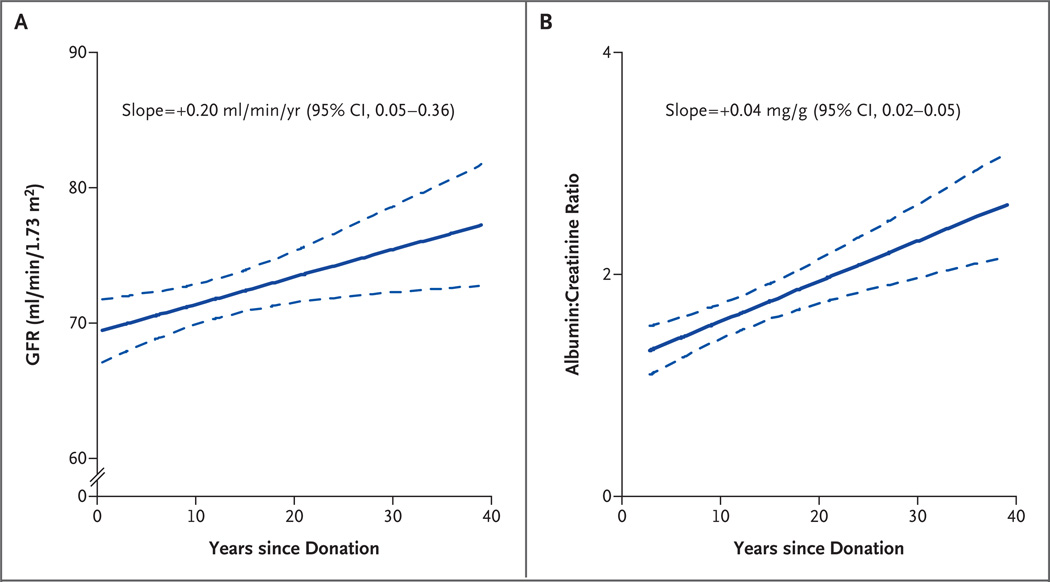
The majority of the donors in whom the GFR was measured (85.5%) had an iohexol GFR that was greater than 60 ml per minute per 1.73 m2; none had a rate that was less than 30 ml per minute per 1.73 m2. A longer time since donation was associated with a higher iohexol GFR but also with a higher albumin excretion rate (Fig. 2). The slope for the relationship between the iohexol GFR and the time since donation was 0.20 (95% confidence interval [CI], 0.05 to 0.36; P = 0.01), indicating that each year after donation was associated with an increase in the iohexol GFR of 0.20 ml per minute per 1.73 m2. Among the 255 donors, 87.3% had normoalbuminuria, 11.5% had microalbuminuria, and only 1.2% had macroalbuminuria. None of the 255 donors had both an iohexol GFR under 45 ml per minute per 1.73 m2 and albuminuria. Since albuminuria is the hallmark of hyperfiltration damage, we compared the estimated GFR at donation and at the last visit among donors with and those without albuminuria (microalbuminuria or macroalbuminuria). At the time of donation, those in whom albuminuria developed later had a higher baseline estimated GFR, as compared with those in whom albuminuria did not develop, although the difference was not significant (88.4±13.4 vs. 82.6±15.9 ml per minute per 1.73 m2, P = 0.08). Among donors in whom iohexol GFR was measured, the rate was 75.7±13.0 ml per minute per 1.73 m2 in those with albuminuria, as compared with 71.2±11.5 ml per minute per 1.73 m2 in those without albuminuria (P = 0.04).
Figure 2. Glomerular Filtration Rate (GFR) and Urinary Albumin Excretion According to Time since Donation.
Panel A shows the GFR, and Panel B shows log-transformed values for the ratio of urinary albumin to creatinine. In each panel, the solid line indicates the regression line, and the dotted line, the 95% confidence interval.
The measured iohexol GFR was inversely related to age; there was a decline of 0.49 ml per minute per 1.73 m2 per year (95% CI, 0.34 to 0.62) in the GFR. Among men, the decline was 0.34 ml per minute per 1.73 m2 per year (95% CI, 0.14 to 0.55), and in women, 0.60 (95% CI, 0.43 to 0.78).
All 255 donors were invited to return in 3 years for a second measurement of the iohexol GFR, and thus far, none have refused. To date, 38 donors who had donated a kidney 11.7±7.7 years previously have undergone two measurements of the iohexol GFR. As compared with those with a single measurement, these donors were older at the time of the first measurement (57.9±11.1 vs. 52.4±9.6 years, P<0.001) and older at the time of donation (43.8±8.4 vs. 41.1±11.0 years, P = 0.04). However, these 38 donors had creatinine levels and estimated GFRs at the time of donation that were similar to those in the rest of the 255 donors (serum creatinine level, 0.9±0.19 and 1.00±0.90 mg per deciliter [79.6±16.8 and 88.4±79.6 µmol per liter], respectively [P = 0.20]; estimated GFR, 82.6± 15.4 and 83.4±15.8 ml per minute per 1.73 m2, respectively [P = 0.80]). The iohexol GFR remained stable in the donors with two serial GFR measurements (69.4±12.7 and 67.7±8.5 ml per minute per 1.73 m2), with a decline of only 0.6±3.8 ml per minute per 1.73 m2 per year. Blood pressure, as well as the albumin-to-creatinine ratio, remained essentially unchanged in the donors with two serial GFR measurements (systolic pressure, 124.3±15.0 and 124.0±16.6 mm Hg; diastolic pressure, 71.5±6.7 and 72.5±7.0 mm Hg; albumin-tocreatinine ratio, 0.01±0.01 and 0.07±0.37). None of the differences were significant.
HYPERTENSION
The mean systolic blood pressure was 122.2±14.9 mm Hg, and the mean diastolic blood pressure was 73.3±9.0 mm Hg. Sixty-three donors (24.7% of those who underwent measurement of the GFR) required antihypertensive medication, and 19 (7.5%) had newly diagnosed hypertension, which was defined as blood pressure higher than 140/90 mm Hg. Among those receiving antihypertensive medications, 19.4% had poorly controlled hypertension. Since higher blood-pressure levels within the normal range are associated with increased cardiovascular risk,16 we further characterized blood pressure in the 173 donors who were not receiving antihypertensive treatment: 54.6% had a systolic pressure lower than 120 mm Hg, 35.4% a systolic pressure between 120 and 140 mm Hg, and 9.9% a systolic blood pressure higher than 140 mm Hg.
RISK OF REDUCED GFR, ALBUMINURIA, AND HYPERTENSION
The risk of having a GFR lower than 60 ml per minute per 1.73 m2 was associated with age (odds ratio, 1.15; 95% CI, 1.08 to 1.21; P<0.001), body-mass index (odds ratio, 1.12; 95% CI, 1.02 to 1.23; P = 0.02), and female sex (odds ratio, 3.11; 95% CI, 1.11 to 8.67; P = 0.03). The time since donation and, surprisingly, smoking status were not associated with this risk (Table 1). However, the time since donation was significantly associated with the development of albuminuria (odds ratio, 1.12, 95% CI, 1.05 to 1.20; P<0.001). Albuminuria was less likely to develop in women. The risk of hypertension increased with age (odds ratio, 1.09; 95% CI, 1.04 to 1.13; P<0.001) and with a higher body-mass index (odds ratio, 1.12; 95% CI, 1.04 to 1.21; P = 0.003).
Table 1.
Multivariable Risk of Reduced Iohexol Glomerular Filtration Rate (GFR), Albuminuria, and Hypertension in 255 Kidney Donors.*
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Iohexol GFR <60 ml/min/1.73m2 | ||
| Age, per year | 1.15 (1.08–1.21) | <0.001 |
| Time since donation, per year | 0.87 (0.79–0.95) | 0.003 |
| Body-mass index, per unit | 1.12 (1.02–1.23) | 0.02 |
| Current smoker | 0.42 (0.17–1.05) | 0.06 |
| Female sex | 3.11 (1.11–8.67) | 0.03 |
| Albuminuria | ||
| Time since donation, per year | 1.12 (1.05–1.20) | <0.001 |
| Female sex | 0.31 (0.12–0.79) | 0.01 |
| Hypertension requiring medication | ||
| Age, per year | 1.09 (1.04–1.13) | <0.001 |
| Body-mass index, per unit | 1.12 (1.04–1.21) | 0.003 |
Covariates include age, sex, time since donation, current body-mass index, creatinine level at the time of donation, smoking status, and systolic and diastolic blood pressures.
HEALTH STATUS AND QUALITY OF LIFE
The current health status of the 255 donors who underwent measurement of iohexol GFR was compared with that of 255 controls from NHANES who were matched for age, sex, race or ethnic group, and body-mass index.15 Donors had a lower estimated GFR, lower systolic blood pressure, and a lower urinary albumin excretion rate (Table 2). Hemoglobin, glucose, cholesterol, triglyceride and high-density lipoprotein cholesterol levels were lower in the donors than in the controls. Donors were less likely than controls to be smokers and were less likely to report having received a diagnosis of cancer. Self-reported diabetes and use of antihypertensive medications were similar in the two groups. These patterns in self-reported conditions and laboratory measurements persisted beyond the first 20 years after kidney donation. In 55 donors who had a measured iohexol GFR and had donated a kidney more than 20 years before, the serum creatinine level was 1.1±0.2 mg per deciliter (93±20 µmol per liter), and the iohexol GFR was 74.0±13.8 ml per minute per 1.73 m2. As compared with the NHANES controls, these 55 donors had a lower GFR but similar urinary albumin excretion. There was no significant difference in the prevalence of diabetes, use of antihypertensive medications, or cancer between the donors and the controls (Table 3). To strengthen this comparison, we used data that we obtained from the pool of donors who had donated a kidney more than 20 years before. There are 1445 such donors, and 1035 responded to our health survey, provided laboratory results, or did both. The findings in this larger group were similar to those in the two other groups (Table 3).
Table 2.
Current Health Status of Kidney Donors with Measured Glomerular Filtration Rate (GFR).*
| Variable | Kidney Donors (N = 255) |
Controls† (N = 255) |
P Value |
|---|---|---|---|
| Age (yr) | 52.9±9.9 | 52.9±9.9 | |
| Female sex (%) | 62.1 | 61.8 | |
| White race (%) | 99.2 | 99.2 | |
| Body-mass index >30 (%)‡ | 29.3 | 29.3 | |
| Blood pressure | |||
| Systolic (mm Hg) | 121.8±14.6 | 125.9±19.1 | 0.003 |
| Diastolic (mm Hg) | 73.0±8.9 | 71.0±16.5 | 0.07 |
| Systolic ≥140 mm Hg or diastolic ≥90 mm Hg (%) | 14.4 | 18.7 | 0.19 |
| GFR (ml/min/1.73 m2)§ | 63.7±11.3 | 81.6±18.5 | <0.001 |
| Urinary albumin-to-creatinine ratio | |||
| Natural-log–transformed value | 1.65±1.2 | 2.10±1.0 | <0.001 |
| >0.03 (%) | 9.1 | 8.9 | 1.00 |
| Hemoglobin (g/dl) | 13.7±1.2 | 14.5±1.2 | <0.001 |
| Glucose (mg/dl) | 90.9±11.9 | 102.8±33.1 | <0.001 |
| Cholesterol (mg/dl) | 186.2±33.1 | 205.2±41.1 | <0.001 |
| Triglycerides (mg/dl) | 124.5±95.6 | 174.3±182.5 | <0.001 |
| High-density lipoprotein cholesterol (mg/dl) | 50.4±16.5 | 54.5±16.4 | 0.001 |
| Clinical conditions (%)¶ | |||
| Diabetes | 3.1 | 5.9 | 0.10 |
| Cancer | 8.2 | 14.5 | 0.01 |
| Coronary heart disease | 4.3 | 3.9 | 0.81 |
| Cerebrovascular accident or transient ischemic attack | 0.4 | 1.9 | 0.10 |
| Use of antihypertensive drugs (%)¶ | 24.7 | 28.8 | 0.83 |
| Current smoker (%)¶ | 14.5 | 21.5 | 0.03 |
Plus–minus values are means ±SD. The paired t-test (for continuous variables) and McNemar’s test (for categorical variables) were used for between-group comparisons. To convert values for hemoglobin to millimoles per liter, multiply by 0.6206. To convert the values for glucose to millimoles per liter, multiply by 0.05551; to convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
Kidney donors were matched in a 1:1 ratio according to age, sex, race or ethnic group, and body-mass index with participants from the National Health and Nutrition Examination Surveys (NHANES) of 2003–2004 and 2005–2006 for whom data on fasting morning laboratory tests were available.15
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The GFR was estimated with the use of the Modification of Diet in Renal Disease (MDRD) study equation.
This variable was self-reported.
Table 3.
Health Status of Kidney Donors More Than 20 Years after Donation.*
| Kidney Donors with GFR Measurement (N = 55) |
Controls (N = 55) |
Kidney Donors without GFR Measurement (N = 1035)† |
P Value‡ | |
|---|---|---|---|---|
| Age (yr) | 57.7±9.8 | 57.7±9.8 | 61.9±11.6 | — |
| Female sex (%) | 64.1 | 64.1 | 57.6 | — |
| White race (%) | 98 | 98 | 98 | — |
| Body-mass index >30 (%)§ | 32.0 | 32.0 | 31.8 | — |
| Blood pressure | ||||
| Systolic (mm Hg) | 121.3±16.1 | 128.7±21.3 | 126.9±15.8 | 0.02 |
| Diastolic (mm Hg) | 72.5±10.5 | 68.5±17.9 | 75.8±9.7 | 0.16 |
| Systolic ≥140 mm Hg or diastolic ≥90 mm Hg (%) | 24.5 | 22.6 | 23.2 | 0.80 |
| GFR (ml/min/1.73 m2)¶ | 62.7±12.6 | 76.1±16.5 | 65.2±9.5 | <0.001 |
| Urinary albumin-to-creatinine ratio | ||||
| Natural-log–transformed value | 2.22±1.7 | 2.28±1.0 | NA | 0.81 |
| >0.03 (%) | 17.3 | 11.3 | NA | 0.36 |
| Hemoglobin (g/dl) | 13.8±1.3 | 14.5±1.3 | 14.0±1.9 | <0.001 |
| Glucose (mg/dl) | 90.9±9.8 | 102.3±16.2 | 100.6±25.9 | <0.001 |
| Cholesterol (mg/dl) | 186.4±38.1 | 205.4±35.1 | 200.5±41.4 | 0.01 |
| Triglycerides (mg/dl) | 112.7±60.3 | 153.8±80.3 | 138.1±93.2 | <0.01 |
| High-density lipoprotein cholesterol (mg/dl) | 50.3±17.4 | 54.4±16.4 | 54.7±17.6 | 0.12 |
| Clinical conditions (%)‖ | ||||
| Diabetes | 5.7 | 11.3 | 7.1 | 0.17 |
| Cancer | 11.3 | 15.1 | 13.4 | 0.56 |
| Coronary heart disease | 3.8 | 9.4 | 4.5 | 0.17 |
| Cerebrovascular accident or transient ischemic attack | 1.9 | 3.8 | 1.9 | 0.56 |
| Use of antihypertensive drugs (%)‖ | 39.6 | 37.7 | 40.4 | 0.85 |
| Current smoker (%)‖ | 15.1 | 11.3 | 15.7 | 0.52 |
Plus–minus values are means ±SD. Kidney donors in whom the glomerular filtration rate (GFR) was measured were matched in a 1:1 ratio according to age, sex, race or ethnic group, and body-mass index with participants from the National Health and Nutrition Examination Surveys (NHANES). The paired t-test (for continuous variables) and McNemar’s test (for categorical variables) were used for between-group comparisons. NA denotes not available. To convert the values for hemoglobin to millimoles per liter, multiply by 0.6206. To convert the values for glucose to millimoles per liter, multiply by 0.05551; to convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
Data were available for 391 to 1035 kidney donors. Total numbers of donors whose data were included for each variable are listed in the Supplementary Appendix.
The P value is for the comparison of donors in whom the GFR was measured with controls from NHANES only.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The GFR was estimated with the use of the Modification of Diet in Renal Disease (MDRD) study equation.
This variable was self-reported.
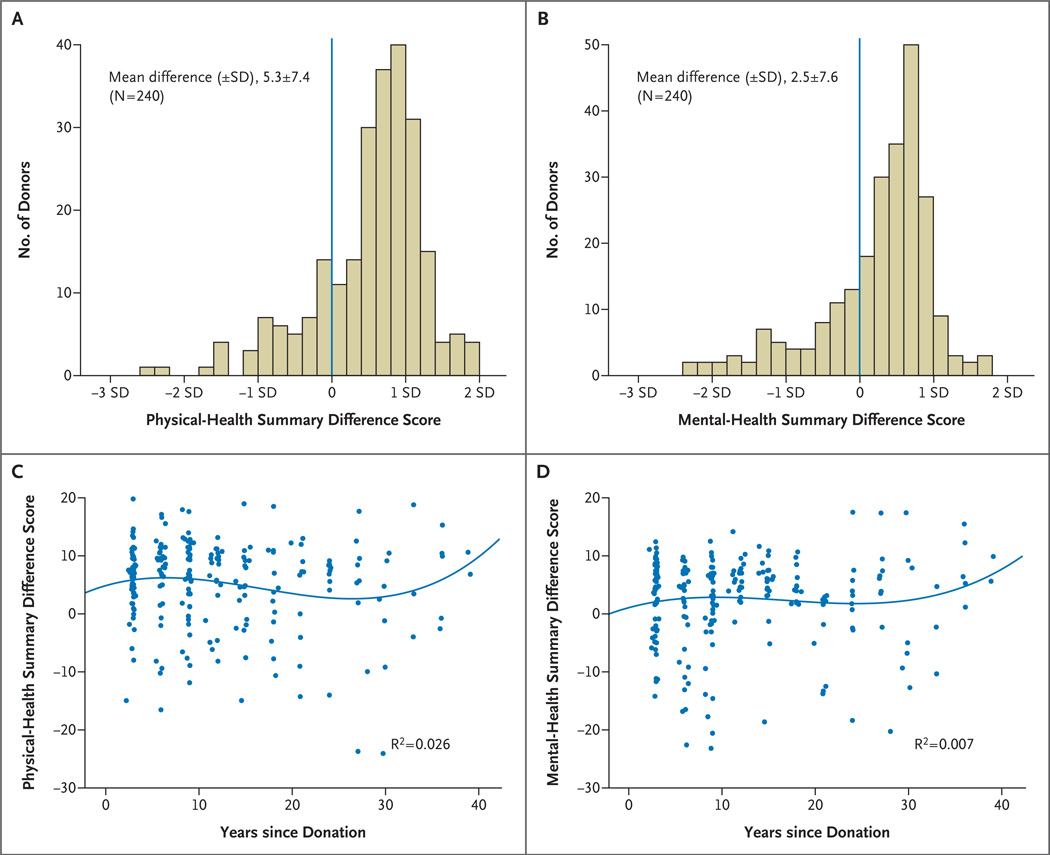
The physical-health summary score (53.6±7.4) and the mental-health summary score (52.6±7.7) for the 255 donors were significantly above the U.S. population norms (P<0.001 for both comparisons) (Fig. 3A and 3B). To determine whether donors, who were considered very healthy at the time of donation in order to donate, were “losing ground” over time, age- and sex-adjusted difference scores were plotted according to the time since donation (Fig. 3C and 3D). The bivariate correlations were small and not significant (physical-health summary score, r = −0.11 [P = 0.10]; mental-health summary score, r = 0.03 [P = 0.69]).
Figure 3. Quality-of-Life Scores for Kidney Donors.
The physical-health and mental-health summary scores, adjusted for age and sex, as compared with population norms, are shown in Panels A and B, respectively. In the case of the physical-health summary score, 40.4% of the scores were below the mean, and 59.6% were above the mean. In the case of the mental-health summary score, 38.0% of the scores were below the mean, and 62.0% were above the mean. The blue lines in Panels A and B represent the population norms. The relationship of the physical-health and mental-health summary scores, adjusted for age and sex, to time since donation is shown in Panels C and D, respectively. The lines in Panels C and D are the regression lines.
DISCUSSION
Our results indicate that the life span of kidney donors is similar to that of persons who have not donated a kidney. The risk of ESRD does not appear to be increased among donors, and their current health seems to be similar to that of the general population. In addition, their quality of life appears to be excellent. These outcomes may be a direct consequence of the routine screening of donors for important health conditions related to kidney disease at the time of donation. At follow-up, most of the donors in our study had an iohexol GFR higher than 60 ml per minute per 1.73 m2; only 12.7% had albuminuria, and none of the donors with albuminuria had an iohexol GFR lower than 45 ml per minute per 1.73 m2. Moreover, the rate of change in the GFR did not appear to accelerate over time. The prevalences of hypertension and albuminuria in kidney donors were similar to those in controls who were matched for age, sex, race or ethnic group, and body-mass index, even two decades after donation.
Uninephrectomy is followed by a compensatory increase in the GFR in the remaining kidney to about 70% of prenephrectomy values.17 We found that this compensatory increase was higher in donors who were younger at the time of donation. The direct relationship between time since donation and the GFR may reflect not only a young age at donation but also the assiduous screening for underlying kidney disease that prospective donors undergo, a screening that excludes persons with hypertension or albuminuria. Compensatory hemodynamic changes in some animal models after a reduction of 50% or more in renal mass have been reported to be ultimately deleterious.18–20 There has been a concern that kidney donors (who undergo a 50% reduction in renal mass with donation) might have hyperfiltration damage in addition to the normal loss of kidney function with age.21,22
Information regarding the long-term renal consequences of reduced renal mass in humans has come mainly from studies of children born with a reduced number of functioning nephrons, reports of focal sclerosis in patients with unilateral renal agenesis, and studies of World War II veterans who lost a kidney as a result of trauma.23–25 More relevant to the current study are numerous studies that have examined renal function in kidney donors.6–10 Although isolated cases of renal failure have been reported, to our knowledge, no large study has shown evidence of progressive deterioration of renal function.6–10,26–32 The present analysis suggests that there is no excess risk of ESRD in donors and confirms the view that factors linked to a reduced GFR in donors are the same as those that have been observed in the general population — namely, age and overweight. One might argue that the risk of ESRD among kidney donors should be much lower than the risk in the general population, since donors are screened very carefully. The risk appears to be lower, but hypertension and diabetes (the two most common causes of kidney disease) develop at a similar frequency among donors as in the general population, which probably explains some of the engendered risk.
Our study has certain limitations. By its nature, the study included only a small, though representative, proportion of all kidney donors to date. Only donors with available contact information who are still alive participated in this effort (a rate of one of seven selected donors). Therefore, our analysis is subject to both response and survival bias. Most of our living donors are white, a factor that may also limit the generalizability of our results, though only 14% of living kidney donors in the United States are nonwhite.5 The use of the MDRD study equation to estimate the GFR for the comparison of current and baseline rates is limited by the fact that this formula was developed in people with a GFR of less than 60 ml per minute per 1.73 m2, and the usefulness of the equation above this level is limited. Serum creatinine was calibrated at the time of measurement of the iohexol GFR but not at the time of donation, a factor that may have resulted in imprecise estimates of the change in the estimated GFR after donation.
The most important limitation of our study is the lack of an ideal control group. Our data offer prospective donors information regarding their life span and their risk of ESRD as compared with those of the general population, but it would be even more informative to compare their outcomes with those of sibling controls who were screened for donation and accepted as donors but did not donate a kidney, since kidney donors are carefully screened and are healthier, on average, than general-population controls. An ongoing study sponsored by the National Institutes of Health that includes three large transplantation centers (the University of Minnesota, the Mayo Clinic, and the University of Alabama) will provide information on more than 8000 kidney donors in the next few years (ClinicalTrials.gov number, NCT00608283).
In conclusion, our study indicates that kidney donors have a normal life span, a health status that is similar to that of the general population, and an excellent quality of life; they do not have an excessive risk of ESRD. The majority of donors in our study had a preserved GFR, and their rates of albuminuria and hypertension were similar to those of matched controls.
Acknowledgments
Supported by grants from the National Institutes of Health (PO1DK13083) and the General Clinical Research Centers (M01- RR00400) and, in part, by the Monica Libin Fund.
Dr. Ibrahim reports receiving consulting fees from the Chronic Disease Research Group, Minneapolis, and serving on an advisory board for Roche; Dr. Foley, receiving consulting fees from and serving on a paid advisory board for Affymax, Amgen, Luitpold Pharmaceuticals, Genzyme, 21st Services, and Abbott, and receiving lecture fees from Amgen and Janssen-Ortho; Dr. Gross, receiving consulting fees from and serving on a paid advisory board for Medtronic; and Dr. Matas, receiving grant support from Astellas Pharma, Wyeth, Bristol-Myers Squibb, and Genzyme.
We thank the donors for their participation in these studies, Olga Gurvich for her assistance with the statistical analysis of the quality-of-life data, Erin Leister for statistical help, and Mary Knatterud and Jensina Ericksen for their thoughtful review of the manuscript.
Footnotes
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.U.S. Renal Data System. USRDS 2007 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 2.The Organ Procurement and Transplantation Network. [Accessed January 5, 2009];United Network for Organ Sharing (UNOS) (at http://www.optn.org.) [Google Scholar]
- 3.Fehrman-Ekholm I, Elinder G, Stenbeck M, Tydén G, Groth C. Kidney donors live longer. Transplantation. 1997;64:976–978. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Ellison MD, McBride MA, Taranto SE, Delmonico FL, Kauffman HM. Living kidney donors in need of kidney transplants: a report from the Organ Procurement and Transplantation Network. Transplantation. 2002;74:1349–1351. doi: 10.1097/00007890-200211150-00025. [DOI] [PubMed] [Google Scholar]
- 5.Gibney EM, King AL, Maluf DG, Garg AX, Parikh CR. Living kidney donors requiring transplantation: focus on African Americans. Transplantation. 2007;84:647–649. doi: 10.1097/01.tp.0000277288.78771.c2. [DOI] [PubMed] [Google Scholar]
- 6.Fehrman-Ekholm I, Norden G, Lennerling A, et al. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006;82:1646–1648. doi: 10.1097/01.tp.0000250728.73268.e3. [DOI] [PubMed] [Google Scholar]
- 7.Bay WH, Hebert LA. The living donor in kidney transplantation. Ann Intern Med. 1987;106:719–727. doi: 10.7326/0003-4819-106-5-719. [DOI] [PubMed] [Google Scholar]
- 8.Najarian JS, Chavers BM, McHugh LE, Matas AJ. 20 Years or more of follow-up of living kidney donors. Lancet. 1992;340:807–810. doi: 10.1016/0140-6736(92)92683-7. [DOI] [PubMed] [Google Scholar]
- 9.Ramcharan T, Matas AJ. Long term (20–37 years) follow-up of living kidney donors. Am J Transplant. 2002;2:959–964. doi: 10.1034/j.1600-6143.2002.21013.x. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim HN, Rogers T, Tello A, Matas A. The performance of three serum creatinine-based formulas in estimating GFR in former kidney donors. Am J Transplant. 2006;6:1479–1485. doi: 10.1111/j.1600-6143.2006.01335.x. [DOI] [PubMed] [Google Scholar]
- 11.Gaspari F, Perico N, Ruggenenti P, et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6:257–263. doi: 10.1681/ASN.V62257. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Jr, Kosinski M, Turner-Bowker DM, Gandek B. How to score version 2 of the SF-12 Health Survey (with a supplement documenting version 1) Lincoln, RI: Quality Metric; 2002. [Google Scholar]
- 13.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: a user’s manual. Boston: Health Assessment Lab; 1994. [Google Scholar]
- 14.Human Mortality Database (HMD) [Accessed January 5, 2009]; home page. (at http://www.mortality.org.) [Google Scholar]
- 15.National Center for Health Statistics. [Accessed January 5, 2009]; home page. at http://www.cdc.gov/nchs/.)
- 16.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 17.Krohn AG, Ogden DA, Holmes JH. Renal function in 29 healthy adults before and after nephrectomy. JAMA. 1966;196:322–324. [PubMed] [Google Scholar]
- 18.Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 19.Robertson JL, Goldschmidt M, Kronfeld DS, Tomaszewski JE, Hill GS, Bovee KC. Long-term renal responses to high dietary protein in dogs with 75% nephrectomy. Kidney Int. 1986;29:511–529. doi: 10.1038/ki.1986.29. [DOI] [PubMed] [Google Scholar]
- 20.Bourgoignie JJ, Gavellas G, Hwang KH, Disbrow MR, Sabius SG, Antonovych TT. Renal function of baboons (Papio hamadryas) with a remnant kidney, and impact of different protein diets. Kidney. 1989;27(Suppl):S86–S90. [PubMed] [Google Scholar]
- 21.Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950;29:496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan C, Pasternack B, Shah H, Gallo G. Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol. 1975;80:227–234. [PMC free article] [PubMed] [Google Scholar]
- 23.Thorner PS, Arbus GS, Celermajer DS, Baumal R. Focal segmental glomerulosclerosis and progressive renal failure associated with a unilateral kidney. Pediatrics. 1984;73:806–810. [PubMed] [Google Scholar]
- 24.Baudoin P, Provoost A, Molenaar J. Renal function up to 50 years after unilateral nephrectomy in childhood. Am J Kidney Dis. 1993;21:603–611. doi: 10.1016/s0272-6386(12)80032-1. [DOI] [PubMed] [Google Scholar]
- 25.Narkun-Burgess DM, Nolan CR, Norman JE, Page WF, Miller PL, Meyer TW. Forty-five year follow-up after uninephrectomy. Kidney Int. 1993;43:1110–1115. doi: 10.1038/ki.1993.156. [DOI] [PubMed] [Google Scholar]
- 26.Sobh M, Nabeeh A, el-Din AS, et al. Long-term follow-up of the remaining kidney in living related kidney donors. Int Urol Nephrol. 1989;21:547–553. doi: 10.1007/BF02549594. [DOI] [PubMed] [Google Scholar]
- 27.Bohannon LL, Barry JM, Norman DJ, Bennett WM. Renal function 27 years after unilateral nephrectomy for related donor kidney transplantation. J Urol. 1988;140:810–811. doi: 10.1016/s0022-5347(17)41822-2. [DOI] [PubMed] [Google Scholar]
- 28.Watnick TJ, Jenkins RR, Rackoff P, Baumgarten A, Bia MJ. Microalbuminuria and hypertension in long-term renal donors. Transplantation. 1988;45:59–65. [PubMed] [Google Scholar]
- 29.Torres VE, Offord KP, Anderson CF, et al. Blood pressure determinants in living-related renal allograft donors and their recipients. Kidney Int. 1987;31:1383–1390. doi: 10.1038/ki.1987.153. [DOI] [PubMed] [Google Scholar]
- 30.Dunn JF, Nylander WA, Jr, Richie RE, Johnson HK, MacDonell RC, Jr, Sawyers JL. Living related kidney donors: a 14-year experience. Ann Surg. 1986;203:637–643. doi: 10.1097/00000658-198606000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg AX, Muirhead N, Knoll G, et al. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801–1810. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]
- 32.Boudville N, Prasad GV, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]