Abstract
Background
In the Raloxifene Use for The Heart trial, 10 101 postmenopausal women with coronary heart disease (CHD) or multiple CHD risk factors were randomly assigned to 60 mg/d raloxifene or to placebo and followed for a median of 5.6 years. Raloxifene, a selective estrogen receptor modulator, was found to reduce the risk of invasive breast cancer and vertebral fractures but not the risk of cardiovascular events. Here, we provide further details about breast cancer incidence by tumor characteristics, duration of treatment, and subgroup.
Methods
Reported breast cancer was adjudicated by an independent committee based on medical records and pathology reports. The primary analyses used Cox proportional hazards models with time to first breast cancer as the outcome. Subgroup effects were analyzed using similar models with terms for treatment by subgroup. All statistical tests were two-sided.
Results
As previously reported, raloxifene reduced the incidence of invasive breast cancer by 44% (hazard ratio [HR] = 0.56; 95% confidence interval [CI] = 0.38 to 0.83; absolute risk reduction = 1.2 invasive breast cancers per 1000 women treated for 1 year). The lower incidence of invasive breast cancer reflected a 55% lower incidence of invasive estrogen receptor (ER)–positive tumors (HR = 0.45; 95% CI = 0.28 to 0.72). However, raloxifene treatment did not reduce the incidence of noninvasive breast cancer or of invasive ER-negative breast cancer. The reduced incidence of invasive breast cancer was similar across subgroups, including those defined by age, body mass index, family history of breast cancer, prior use of postmenopausal hormones, and 5-year estimated risk of invasive breast cancer.
Conclusion
Raloxifene reduces risk of invasive ER-positive breast cancer regardless of a woman's baseline breast cancer risk but does not reduce risk of noninvasive or ER-negative breast cancers. These results confirm those of the Multiple Outcomes of Raloxifene Evaluation, a previous randomized trial among women with osteoporosis.
Breast cancer is the most common cancer among women (1). Many risk factors for the development of breast cancer have been identified, including older age, early menarche, late menopause, nulliparity, higher breast density, atypical hyperplasia, family history of breast cancer, and mutation of the BRCA1 or BRCA2 genes (1). Many of these risk factors are not modifiable. However, lifestyle, behavioral, or dietary approaches to prevention of breast cancer, including a low-fat diet (2, 3), exercise (4, 5), and reduction in alcohol consumption (6), may hold some promise.
Chemoprevention with antiestrogens also appears to be effective in reducing risk of breast cancer. In a large placebo-controlled trial conducted by National Surgical Adjuvant Breast and Bowel Project in North America (NSABP P-1), treatment with tamoxifen reduced the incidence of invasive breast cancer by 49% (7). However, use of tamoxifen has been associated with increased risk of uterine cancer (7), stroke (8), and venous thromboembolic events in postmenopausal women (7). New, effective and safer approaches to prevention of breast cancer would, therefore, be useful.
Two major clinical trials have provided evidence that the selective estrogen receptor modulator (SERM) raloxifene is a promising drug for the prevention of breast cancer. In the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, 7705 postmenopausal women with osteoporosis who were treated for 4 years with raloxifene had a 72% reduction in the incidence of invasive breast cancer compared with women treated with a placebo (9), and protection against breast cancer persisted during 8 years of follow-up (10). More recently, the Raloxifene Use for The Heart (RUTH) trial enrolled 10 101 women with coronary heart disease (CHD) or at high risk of CHD to determine the effect of raloxifene on incidence of coronary events and invasive breast cancer (11). In 2006, the main results of the RUTH trial were published, including information on the effects of raloxifene on invasive and noninvasive breast cancer and on estrogen receptor (ER)–positive and -negative tumors (11). This report provides additional data from the RUTH trial, including the effects of raloxifene on risk for breast cancer by histological type, tumor stage, lymph node status, and tumor grade and size, as well as by duration of treatment and subgroup.
Methods
RUTH was a randomized, blinded, placebo-controlled trial conducted at 177 sites in 26 countries on five continents (11, 12). The major aims of the trial were to evaluate the effects of raloxifene on incidence of coronary events and invasive breast cancer. The institutional review board at each study site approved the protocol, and all participants gave written informed consent. The RUTH trial was registered at ClinicalTrials.gov (Registry Number: NCT00190593).
Participants
Between June 1998 and August 2000, 10 101 postmenopausal women were enrolled in the trial and randomly assigned to study drug. Participants were required to be 55 years of age or older, to be at least 1 year from their final menstrual period, and to have either documented CHD or increased risk for CHD based on the presence of established risk factors, such as older age, diabetes, hypertension, cigarette smoking, and hyperlipidemia. (12). Women were excluded if they were suspected of having breast cancer or if they had a history of breast cancer. Other reasons for exclusion included recent myocardial infarction, coronary artery bypass grafting or percutaneous coronary angioplasty, severe heart failure, history of venous thromboembolism, recent unexplained uterine bleeding, life expectancy less than 5 years, chronic liver or renal disease, recent use of oral or transdermal estrogens, or current use of other sex hormones or SERMs (11).
Study Medication
Eligible participants were randomly assigned to 60 mg raloxifene per day, orally (EVISTA; Eli Lilly and Company, Indianapolis, IN) or to an identical-appearing placebo and were asked to continue to take the study medication until the end of the trial. The study drug was permanently discontinued when a participant was unblinded or was diagnosed with breast cancer or a venous thromboembolism. The study medication was temporarily discontinued if the participant became immobilized for a prolonged time or if she took hormonal agents.
Random Assignment and Blinding
Random assignment was stratified by study site, and treatment was randomly assigned using an interactive voice response telephone system. Investigators, site staff, participants, laboratory staff, outcome adjudicators, and the sponsor were blinded to treatment assignment. Investigators were unblinded in only 26 cases in which safety was a concern.
Study Procedures
At baseline, all participants completed a breast cancer risk assessment (13). Breast cancer risk factors, including age, ethnicity, age at menarche, age at menopause, parity, family history of breast cancer, history of breast biopsy, history of ovariectomy, and use of hormone therapy were self-reported. Height and weight in which measured.
A clinical breast examination was performed at baseline and every 2 years thereafter. Mammograms had to have been obtained within 1 year before random assignment and every 2 years thereafter. Mammograms were performed and interpreted locally but rereviewed by a central radiologist when breast cancer was diagnosed.
Participants attended study visits or were contacted by telephone semiannually. At each contact, adherence to study medication and occurrence of adverse events and outcomes were ascertained. Participants were considered to have completed the trial if they had a final visit after at least 5 years of follow-up.
Breast Cancer
When breast cancer was diagnosed, the investigative site provided mammograms, medical records, and pathology reports to blinded independent adjudicators that included a medical and a surgical oncologist. Adjudicators reviewed the documents for each case and adjudicated the diagnosis of primary breast cancer, invasiveness of the cancer, stage, lymph node status, grade, type, and size. ER status was based on local immunocytochemical assay using local cut points. Consensus of the adjudicators was required for all breast cancer classifications.
Statistical Analyses
Analyses followed the intention-to-treat principle and included all women randomly assigned. If fewer than five events occurred in the placebo and raloxifene groups combined, no statistical tests were performed. Data collected before February 2, 2006, were included in the analyses.
Primary analyses measured time to first breast cancer. Data from women who did not experience a breast cancer event were censored on the date when study information was last collected or, for women who died of non – breast cancer causes during the study, on the date of the participant's death. A log-rank test was used to compare the cumulative incidence of breast cancers between treatment groups. Unadjusted Cox proportional hazards models were used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs). The proportionality of hazards was checked by testing the interaction of treatment by log of the time of follow-up and was found to hold.
Cox proportional hazards regression models were fit with treatment, subgroup, and treatment-by-subgroup interaction to determine if there were differential treatment effects by subgroup at a significance level of .10, as established a priori in our statistical analysis plan. Patient characteristics considered included age (<60, >60 and <70, ≥70 years), BMI (≤25, >25 and ≤30, >30 kg/m 2), current smoking and alcohol consumption status, age at menarche (<11, ≥11 years), parity (0, 1 – 2, ≥3), and age at first live birth (<20, ≥20 years). History of any first-degree relative with breast cancer, hysterectomy, ovariectomy, prior use of hormone replacement therapy (none, estrogen, estrogen plus progestin), and predicted breast cancer risk according to the Gail model were also considered. Tumor characteristics analyzed included invasiveness, ER status, histological type, stage, number of positive lymph nodes (0, 1–3, ≥4), tumor grade or differentiation, and tumor size (≤1.0, 1.1–2.0, 2.1–3.0, and ≥3.1 cm).
To explore the effect of treatment over time, post hoc year-by-year analyses were performed in which each participant was considered to be at risk of breast cancer if she had not been diagnosed with breast cancer by the beginning of the year. Hazard ratios and 95% confidence intervals were estimated using a Cox proportional hazards regression model that includes an interaction term for time and treatment effect.
To explore whether raloxifene treatment had a similar effect on incidence of both invasive and noninvasive breast cancers, interaction of treatment by breast cancer invasiveness was tested, assuming competing risks and independence of the first occurrence of either noninvasive or invasive breast cancer. (If a participant was diagnosed with both a noninvasive and an invasive breast cancer, only the event that occurred first was included in the analysis.) Specifically, we used a two-sided Z test to assess the statistical significance of the difference between the log of the hazard ratios for raloxifene vs placebo from separate Cox models for noninvasive and invasive breast cancer, divided by the standard error of the difference, computed under the independence assumption. Similar interaction tests were performed for treatment by subcategories of each tumor characteristic.
All reported P values are two-sided and were tested at a .05 significance level unless otherwise stated. No adjustments were made for multiple comparisons. Statistical analyses were performed using SAS software version 8.2 (SAS Institute, Cary, NC).
Results
Of the 10 101 women randomly assigned in the RUTH trial, 5057 were assigned to placebo and 5044 to raloxifene. The treatment groups had similar baseline characteristics (Table 1). Based on the Gail model (13), the mean estimated 5-year risk of developing invasive breast cancer in both groups was 1.7%.
Table 1.
Baseline Characteristics of Raloxifene Use for The Heart Participants*
| Characteristic | Placebo, (n = 5057) | Raloxifene, (n = 5044) | P value |
|---|---|---|---|
| Age, in y, mean (SD)† | 67.5 (6.7) | 67.5 (6.6) | .86 |
| White race, No. (%) | 4247 (84.0) | 4234 (84.0) | .96 |
| Body-mass index, kg/m2, mean (SD)† | 28.7 (5.1) | 28.8 (5.2) | .27 |
| Current smoker, No. (%) | 649 (12.8) | 607 (12.0) | .22 |
| Alcohol consumption, No. (%)‡ | 2177 (43.1) | 2150 (42.7) | .68 |
| Hysterectomy, No. (%) | 1175 (23.3) | 1144 (22.7) | .48 |
| Ovariectomy, No. (%) | 774 (15.5) | 800 (16.1) | .49 |
| Age at menarche, in y, mean (SD)† | 13.5 (1.8) | 13.5 (1.8) | .25 |
| Age at menopause, in y, mean (SD)† | 48.0 (6.1) | 48.1 (6.0) | .21 |
| Nulliparity, No. (%) | 521 (10.3) | 529 (10.5) | .76 |
| Age at first live birth, in y, mean (SD)† | 23.3 (4.5) | 23.4 (4.4) | .31 |
| Family history of breast cancer§, No. (%) | 445 (9.7) | 452 (9.8) | .85 |
| Prior breast biopsy, No. (%) | 468 (9.3) | 416 (8.3) | .07 |
| Prior use of estrogen, No. (%) | 702 (14.0) | 697 (14.0) | .93 |
| Prior use of estrogen plus progestin, No. (%) | 323 (6.5) | 282 (5.7) | .10 |
| Mean 5 year risk of invasive breast cancer, % (SD)†, ∥ | 1.73 (0.77) | 1.73 (0.76) | .85 |
Additional information concerning geographic distribution and cardiovascular risks of trial participants can be found in reference 11.
SD = standard deviation.
Defined as any consumption of alcohol.
Family history of breast cancer includes women with any first-degree relative with breast cancer.
Five-year risk of invasive breast cancer estimated based on recognized risk factors for breast cancer using the Gail model (13).
The trial was completed by 3979 women in the placebo group (79%) and by 4060 women in the raloxifene group (80%) (11). Median duration of follow-up was 5.6 years in both groups. Overall, 71% of women in the placebo group and 70% in the raloxifene group took at least 70% of the assigned study medication based on pill counts. At baseline, all but four participants had had a mammogram within 12 months before randomization. During follow-up, 92% of women had a mammogram at 2 years, 88% at 4 years, and 80% at 6 years. Clinical breast examination was performed for 91% of participants at 2 years, 88% at 4 years, and 86% at 6 years of follow-up. Adherence with mammography and completion of clinical breast examination did not differ by treatment group (data not shown).
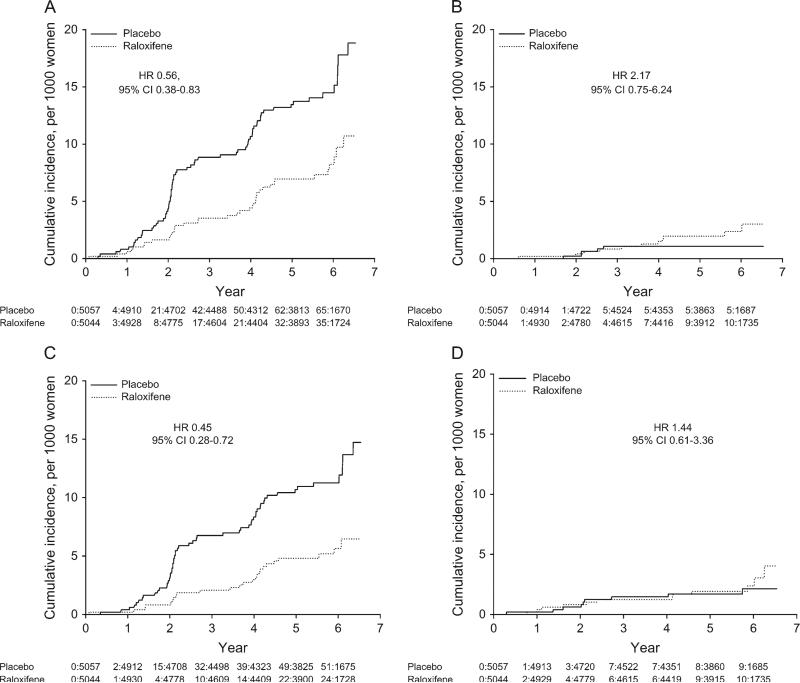
During follow-up, 76 women in the placebo group were diagnosed with breast cancer (annualized rate, 0.29%) compared with 52 in the raloxifene group (annualized rate, 0.20%) (11). Raloxifene treatment reduced the overall risk of breast cancer by one-third (HR = 0.67, 95% CI = 0.47 to 0.96; Table 2). Two women in the study died of breast cancer, both assigned to raloxifene. Raloxifene reduced the incidence of invasive breast cancer by 44% (HR = 0.56, 95% CI = 0.38 to 0.83; Table 2 and Figure 1, A), but there was no evidence that it had an effect on the incidence of noninvasive breast cancer (HR = 2.17, 95% CI = 0.75 to 6.24; Pinteraction = .02; Table 2 and Figure 1, B) (11).
Table 2.
Incidence of breast cancer by tumor characteristics and treatment group*
| No. of events (annualized rate, %) |
||||
|---|---|---|---|---|
| Tumor characteristic | Placebo (n = 5057) | Raloxifene (n = 5044) | HR (95% CI) | P interaction |
| All breast cancers | 76 (0.29) | 52 (0.20) | 0.67 (0.47 to 0.96) | .02 |
| Invasive | 70 (0.27) | 40 (0.15) | 0.56 (0.38 to 0.83) | |
| Noninvasive† | 5 (0.02) | 11 (0.04) | 2.17 (0.75 to 6.24) | |
| Invasiveness unknown | 1 (0.004) | 1 (0.004) | NA | |
| Invasive breast cancer | ||||
| ER status | .02 | |||
| Positive | 55 (0.21) | 25 (0.09) | 0.45 (0.28 to 0.72) | |
| Negative | 9 (0.03) | 13 (0.05) | 1.44 (0.61 to 3.36) | |
| Unknown | 6 (0.02) | 2 (<0.01) | 0.33 (0.07 to 1.63) | |
| Histological type | .92 | |||
| Infiltrating ductal carcinoma | 51 (0.19) | 27 (0.10) | 0.52 (0.33 to 0.83) | |
| Lobular carcinoma | 10 (0.04) | 5 (0.02) | 0.49 (0.17 to 1.44) | |
| Other‡ | 9 (0.34) | 8 (0.30) | 0.88 (0.34 to 2.29) | |
| Stage | .45 | |||
| I | 37 (0.14) | 19 (0.07) | 0.51 (0.29 to 0.88) | |
| II§ | 23 (0.09) | 13 (0.05) | 0.56 (0.28 to 1.10) | |
| III§ | 0 (0.00) | 3 (0.01) | NA | |
| IV§ | 1 (<0.01) | 1 (<0.01) | 0.98 (0.06 to 15.71) | |
| Unknown | 9 (0.03) | 4 (0.02) | 0.44 (0.13 to 1.42) | |
| Lymph nodes∥ | .41 | |||
| 0 positive | 42 (0.16) | 28 (0.11) | 0.66 (0.41 to 1.06) | |
| 1–3 positive§ | 12 (0.05) | 7 (0.03) | 0.58 (0.23 to 1.46) | |
| ≥4 positive§ | 8 (0.03) | 2 (<0.01) | 0.25 (0.05 to 1.18) | |
| Unknown | 0 (0.00) | 1 (<0.01) | NA | |
| Tumor grade | .39 | |||
| Well differentiated | 15 (0.06) | 10 (0.04) | 0.66 (0.30 to 1.46) | |
| Moderately differentiated | 34 (0.13) | 16 (0.06) | 0.46 (0.26 to 0.84) | |
| Poorly differentiated | 9 (0.03) | 9 (0.03) | 1.00 (0.39 to 2.51) | |
| Unknown | 12 (0.05) | 5 (0.02) | 0.41 (0.14 to 1.16) | |
| Tumor size, cm | .01 | |||
| ≤1.0 | 17 (0.07) | 15 (0.06) | 0.87 (0.44 to 1.74) | |
| 1.1–2.0 | 37 (0.14) | 10 (0.04) | 0.27 (0.13 to 0.54) | |
| 2.1–3.0 | 10 (0.04) | 11 (0.04) | 1.08 (0.46 to 2.55) | |
| ≥3.1 | 3 (0.01) | 3 (0.01) | 0.99 (0.20 to 4.90) | |
| Unknown | 3 (0.01) | 1 (<0.01) | 0.33 (0.03 to 3.16) | |
HR = hazard ratio; CI = confidence interval; ER = estrogen receptor.
All noninvasive cancers were ductal carcinoma in situ.
Other histological types included adenocarcinoma, medullary carcinoma, mucinous adenocarcinoma, papillary carcinoma, and tubular adenocarcinoma.
Due to few events, these categories were combined for tests of interaction of treatment by tumor characteristic; the unknown subcategory was excluded in these analyses.
Lymph nodes were evaluated for only 100 invasive breast cancer cases. Information was not available for the remaining 10 cases
Figure 1.
Cumulative breast cancer incidence rates. A) invasive breast cancer, B) noninvasive breast cancer, C) invasive estrogen receptor (ER)–positive breast cancer, and D) invasive ER-negative breast cancer. The cumulative number of events and number of participants at risk are shown below each plot.
Most invasive breast cancers were infiltrating ductal carcinoma, ER positive, stage I or II, lymph node negative, well or moderately differentiated, and smaller than 2 cm (Table 2). Treatment with raloxifene reduced the incidence of invasive ER-positive breast cancer by 55% (HR = 0.45, 95% CI = 0.28 to 0.72; Table 2 and Figure 1, C) (11) but had no effect on the incidence of invasive ER-negative cancer (HR = 1.44, 95% CI = 0.61 to 3.36, Pinteraction = .02; Table 2 and Figure 1, D). Raloxifene reduced absolute risk by 0.9 cases of any breast cancer, 1.2 cases of any invasive breast cancer, and 1.2 cases of invasive ER-positive breast cancer per 1000 women treated for 1 year.
Raloxifene reduced the incidence of invasive breast cancer regardless of histological type, stage, lymph node status, or tumor grade (all Pinteraction >.30) (Table 2). Raloxifene appeared to reduce the risk of tumors that were 1 – 2 cm in size more than the risk of either larger or smaller tumors (Pinteraction for treatment by tumor size = .02).
A statistically significant reduction in incidence of invasive breast cancer among women taking raloxifene compared with the placebo group was observed by the second year of treatment (Table 3). The incidence of invasive breast cancer was lower in the raloxifene group than in the placebo group during each of the first 4 years of treatment but was similar in the two treatment groups in years 5–7 (Table 3). However, the interaction between duration of treatment and effect of treatment on incidence of invasive breast cancer was not statistically significant (P = .55).
Table 3.
Incidence of invasive breast cancer by treatment group and by year*
| No. of events (annualized rate, %) |
|||
|---|---|---|---|
| Placebo (n =5057) | Raloxifene (n = 5044) | HR (95% CI)† | |
| Year 1 | 4 (0.08) | 3 (0.06) | 0.75 (0.17 to 3.35) |
| Year 2 | 17 (0.35) | 5 (0.10) | 0.29 (0.11 to 0.79) |
| Year 3 | 21 (0.46) | 9 (0.19) | 0.42 (0.19 to 0.92) |
| Year 4 | 8 (0.18) | 4 (0.09) | 0.49 (0.15 to 1.63) |
| Year 5 | 12 (0.29) | 11 (0.26) | 0.90 (0.40 to 2.04) |
| Year 6 | 3 (0.11) | 3 (0.11) | 0.98 (0.20 to 4.84) |
| Year 7 | 5 (0.09) | 5 (0.90) | 1.00 (0.29 to 3.44) |
Hazard ratios (HR) and 95% confidence intervals (CI) are from unadjusted Cox proportional hazards models.
Pinteraction of treatment effect and year of follow-up (from Cox proportional hazards model that included an interaction term for year and treatment effect) = .55.
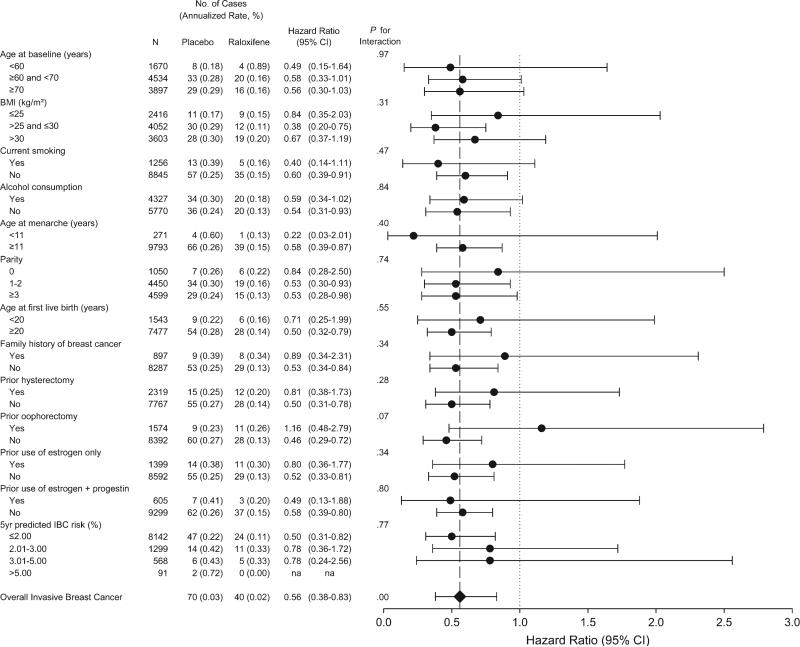
The effect of treatment on the incidence of invasive breast cancer did not differ across subgroups defined by age, body mass index, smoking, alcohol consumption, reproductive history, family history of breast cancer, prior hysterectomy, use of postmenopausal hormones, or 5-year predicted risk for invasive breast cancer (13) (Figure 2). Treatment with raloxifene appeared to reduce the incidence of invasive breast cancer in women with ovaries but not in those with bilateral ovariectomy (Pinteraction = .07).
Figure 2.
Invasive breast cancer (annualized percent) by baseline characteristics and treatment assignment. Hazard ratios (dots) and 95% confidence intervals (error bars) are from unadjusted Cox proportional hazards models, and Pinteraction values are from Cox proportional hazards models that include an interaction term for subgroup and treatment effect. Diamond indicates the overall hazard ratio. BMI = body mass index; IBC = invasive breast cancer.
Discussion
Among postmenopausal women at increased risk for major coronary events with an average estimated 5-year risk of developing breast cancer of 1.7%, treatment with 60 mg of raloxifene for 5.6 years resulted in a 44% reduction in incidence of invasive breast cancer and a 55% reduction in incidence of invasive ER-positive breast cancer (11). Treatment did not reduce the incidence of invasive ER-negative breast cancer or of noninvasive breast cancer. Raloxifene appeared to be most effective in reducing the incidence of breast cancer during the first 4 years of treatment. Moreover, the effect of treatment appeared to be similar across subgroups defined by age, reproductive history, and breast cancer risk status.
The reduction in incidence of invasive breast cancer and invasive ER-positive breast cancer in the RUTH trial confirms the findings of the MORE randomized trial, which assessed the effects of 60 and 120 mg of raloxifene on fractures in 7705 postmenopausal women with osteoporosis (9, 10, 14). After 4 years of follow-up in the MORE trial, the incidence of invasive breast cancer was 72% lower among women assigned to raloxifene compared with women assigned to placebo and the incidence of ER-positive breast cancer was 84% lower (9). By comparison, in the Breast Cancer Prevention Study, a randomized trial of 13 388 women with greater than 1.66% 5-year risk of breast cancer, treatment with 20 mg of tamoxifen daily reduced the incidence of invasive breast cancer by 50% after 4 years (7). Similar to the effect of raloxifene in the MORE and RUTH trials, the reduction in risk of breast cancer associated with tamoxifen was confined to ER-positive tumors (7). The effects of raloxifene and tamoxifen on breast cancer risk were compared directly in the Study of Tamoxifen and Raloxifene (STAR), a randomized trial in which 19 747 women with a 4% average 5-year risk of developing breast cancer were randomly assigned to treatment with 20 mg tamoxifen or 60 mg raloxifene daily for 4 years. In the STAR trial, there were no differences between the two active treatments in the incidence of either invasive breast cancer or ER-positive invasive breast cancer (15).
Raloxifene and tamoxifen are SERMs that bind to ERs and have estrogenic activity in some tissues and antiestrogenic activity in others. Both are thought to reduce the incidence of breast cancer by interfering with the effects of estrogen on the initiation, promotion, and/or proliferation of ER-positive cancers (16). A reduction in incidence of invasive breast cancer was observed in the RUTH trial within 2 years of beginning treatment. Given that several years are required from initiation to clinical detection of breast cancer (17), the RUTH findings are most consistent with an effect of raloxifene on estrogen-related tumor promotion or proliferation. A similar pattern of early reduction in incidence of breast cancer was observed in the MORE trial with raloxifene (14) and in the Breast Cancer Prevention Trial with tamoxifen (7).
Noninvasive breast cancers constituted only 7% of all breast cancers in the RUTH trial (0.2 cases per 1000 woman-years). Among women over 50 years old in the United States, the rate of noninvasive breast cancer is 0.85 per 1000, accounting for about 25% of all breast cancers (18). RUTH was an international trial, with the majority of participants in Europe. The low rate of noninvasive breast cancer in the RUTH trial may be due to a longer mammographic screening interval or to lower rates of detection of noninvasive breast cancer outside the United States. Noninvasive breast cancer is generally detected on screening mammography (19), which is commonly performed annually in the United States and biannually outside the United States. Following European standards, mammography was performed biannually in the RUTH trial, and detection of noninvasive cancer depended on local readings.
Treatment with raloxifene reduced the incidence of invasive breast cancer to a similar degree regardless of tumor stage, lymph node status, or tumor grade. Although there was a statistically significant interaction of treatment and tumor size, there was no consistent association of the effect of raloxifene on risk for invasive breast cancer by increasing tumor size (P for linear trend = .82).
Raloxifene did not reduce the risk of noninvasive breast cancer (11). In fact, more noninvasive breast cancers occurred in the raloxifene than in the placebo group (11 vs 5, HR = 2.17, 95% CI = 0.75 to 6.24), although the number of noninvasive breast cancers was small and this difference was not statistically different. There was also no reduction in the incidence of noninvasive breast cancer with raloxifene treatment in the MORE trial (HR = 0.90, 95% CI = 0.30 to 2.69) (9) or in the Continuing Outcomes Relevant to Evista (CORE) trial (HR = 1.78, 95% CI = 0.37 to 8.61) (10). In contrast, treatment with tamoxifen in the Breast Cancer Prevention Trial resulted in a 50% reduction in the incidence of both invasive and noninvasive breast cancer (7). In the STAR trial, in which raloxifene and tamoxifen were compared directly, there was no difference in the effect of tamoxifen and raloxifene on incidence of invasive breast cancer, but women assigned to raloxifene had a 40% higher incidence of noninvasive breast cancer than women in the tamoxifen group (HR = 1.40, 95% CI = 0.98 to 2.00) (15).
It is not clear why tamoxifen appears to reduce incidence of noninvasive breast cancer whereas raloxifene does not. One possible explanation is that tamoxifen may be more effective than raloxifene in preventing the development of noninvasive disease because it has a greater binding affinity for ERβ (20). Compared with proliferative lesions and noninvasive breast cancer, invasive disease is characterized by greater expression of ERα and less expression of ERβ (21), which appears to protect against tumor progression (21–24).
The effect of raloxifene on the incidence of invasive breast cancer was similar across subgroups. Because we examined a total of 20 subgroups, the borderline interaction between treatment effect and ovariectomy likely occurred by chance.
If SERMs such as tamoxifen and raloxifene are used for prevention of breast cancer, it is important to determine the optimal duration of treatment. Among women with lymph node–negative ER-positive breast cancer, a reduced risk of recurrence of cancer persists for up to 15 years after 5 years of treatment with tamoxifen (27). However, women treated with tamoxifen for longer than 5 years do not have improved outcomes (7). After 4 years of follow-up, 92% of women who received 5 years of tamoxifen treatment and were then randomly assigned to placebo were alive and free of breast cancer, compared with 86% of women randomly assigned to continue tamoxifen (P = .003) (28). These data resulted in the recommendation that adjuvant therapy with tamoxifen in women with breast cancer be discontinued after 5 years. In the Breast Cancer Prevention Trial, treatment with tamoxifen reduced risk of invasive breast cancer during each year of 6 years of follow-up (7).
In a post hoc analysis, the effect of raloxifene treatment on incidence of invasive breast cancer did not differ by year, although in years 5–7, the hazard ratios were close to 1. In the MORE trial, 4 years of treatment with raloxifene resulted in a 72% reduction of risk of invasive breast cancer (9), and in the CORE trial, after treatment was interrupted for an average of 11 months, an additional 4 years of blinded treatment with raloxifene resulted in a 59% reduction in risk of invasive breast cancer (HR = 0.41, 95% CI = 0.24 to 0.71) (10). These data from MORE and CORE provide the longest observation of the use of an SERM for breast cancer prevention. Taken together with the main finding in RUTH, that breast cancer risk was reduced over 5.6 years of treatment, it seems reasonable to conclude that treatment with raloxifene for up to 8 years is safe and effective in reducing breast cancer risk.
Participants in the RUTH trial were selected because they either had known coronary disease or were at high risk for coronary disease. Thus, it is possible that the findings of the RUTH trial may not be able to be generalized to women who are not at high risk for coronary disease. However, 41% of RUTH participants had a 5-year predicted risk of breast cancer of 1.66% or greater, which would have made them eligible for the Breast Cancer Prevention and STAR trials. In addition, the effectiveness of raloxifene in reducing risk of breast cancer did not vary by baseline risk for breast cancer (Figure 2).
Which women might consider taking raloxifene to reduce the risk of breast cancer? Although treatment with raloxifene in the RUTH trial reduced the incidence of breast cancer and vertebral fracture, it also increased the incidence of venous thromboembolic events and fatal stroke (11). Assuming that the relative risks from the RUTH trial apply to women in the general population, the best benefit to risk ratio would occur in women at high risk of breast cancer and osteoporosis and low risk of venous thrombosis and stroke. In the STAR trial, which directly compared the effects of 4 years of treatment with tamoxifen or raloxifene among women at high risk of breast cancer, rates of invasive breast cancer were the same, but women assigned to raloxifene had lower rates of venous thromboembolic events (HR = 0.70, 95% CI = 0.54 to 0.91), uterine cancer (HR = 0.62, 95% CI = 0.35 to 1.08), and cataracts (HR = 0.79, 95% CI = 0.68 to 0.92) compared with tamoxifen. The rates of stroke, fatal stroke, coronary events, and fractures did not differ in the two treatment groups.
CONTEXT AND CAVEATS.
Prior knowledge
Raloxifene was reported by the investigators of the RUTH (Raloxifene Use for the Heart) trial to reduce the risk of both breast cancer and vertebral fractures, but not of cardiovascular events.
Study design
In the RUTH trial, 10101 postmenopausal women at increased coronary risk were randomly assigned to 60 mg/d raloxifene or to a placebo, and data were analyzed after a median follow-up of 5.6 years. Here, the authors report breast cancer incidence by tumor characteristics, duration of treatment, and subgroup analysis.
Contribution
The 44% reduction in invasive breast cancers by raloxifene included a 55% reduction in invasive ER-positive breast cancers regardless of age, body mass, history of hormone replacement therapy, family or other breast cancer risk. Incidence of invasive ER-negative breast cancers and of noninvasive breast cancers were not affected.
Implications
Raloxifene appears to be effective in prevention of invasive ER-positive breast cancer but not in prevention of noninvasive cancers.
Limitations
Participants in the RUTH trial were selected to be at increased risk of coronary disease compared to the general population.
Acknowledgments
Funding
Eli Lilly and Company, Indianapolis, IN.
Footnotes
The RUTH trial was designed and monitored by an Executive Committee that included E. Barrertt-Connor, MD (principal investigator); N. K. Wenger, MD (coprincipal investigator); P. Collins, MD; D. Grady, MD, MPH; L. Mosca, MD, PhD; and M. Kornitzer, PhD. The sponsor participated in the study design, collected the data, monitored data quality, and conducted data analyses under the supervision of the manuscript authors. MJG. JS, and JM are employees of Eli Lilly and Company, the maker of raloxifene (Evista), and hold Eli Lilly stock. DG has received salary support for research from Eli Lilly via contracts with UCSF, but holds no stock options and received no speaking or consulting fees. JC has received research funding from Novartis, Merck and Pfizer, and has received consulting honoraria from Eli Lilly and Novartis. LM has received an honorarium from Eli Lilly for participation in the RUTH trial. PC has received consulting fees from Eli Lilly, Berlex, Merck, Pantarhei and Pfizer, lecture fees from Berlex, Merck, Pfizer, Novo Nordisk, and Organon, and grant support from Eli Lilly, Organon, and Merck. NKM has received grant support from Pfizer, Merck, and CV Therapeutics and consulting fees from Schering-Plough, AstraZeneca, Abbott, Merck, and Pfizer. KM has no conflict of interest.
Members of the RUTH Breast Cancer Adjudication Committee were Valerie Jackson, MD (radiologist), Kathy Miller, MD (medical oncologist), and C. Chace Lottich, MD (surgical oncologist).
The authors would like to thank Sherie Dowsett for expert help in preparing the manuscript, tables, and figures.
References
- 1.Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast cancer. Lancet. 2005;365:1727–1741. doi: 10.1016/S0140-6736(05)66546-4. [DOI] [PubMed] [Google Scholar]
- 2.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer. The Women's Health Initiative randomized controlled dietary modification trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the women's intervention nutrition study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 4.Lahmann PH, Friedenreich C, Schuit AJ, et al. Physical activity and breast cancer risk: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16:36–42. doi: 10.1158/1055-9965.EPI-06-0582. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein L, Patel AV, Gursin G, et al. Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst. 2005;97:1671–1679. doi: 10.1093/jnci/dji374. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SM, Lee I, Manson JE, Cook NR, Willett WC, Buring JE. Alcohol consumption and breast cancer risk in the women's health study. Am J Epidemiol. 2007;165:667–676. doi: 10.1093/aje/kwk054. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Costantino J, Wickerham D, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 8.Bushnell CD, Goldstein LB. Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta-analysis. Neurology. 2004;63:1230–1233. doi: 10.1212/01.wnl.0000140491.54664.50. [DOI] [PubMed] [Google Scholar]
- 9.Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple Outcomes of Raloxifene Evaluation. Breast Cancer Res Treat. 2001;65:125–134. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- 10.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing Outcomes Relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 11.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 12.Mosca L, Barrett-Connor E, Wenger NK, et al. Design and methods of the Raloxifene Use for The Heart (RUTH) study. Am J Cardiol. 2001;88:392–395. doi: 10.1016/s0002-9149(01)01685-x. [DOI] [PubMed] [Google Scholar]
- 13.Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–1548. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 15.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other dis ease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 16.Yager J, Davidson N. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 17.Spratt J, Meyer J, Sprat J. Rates of growth of human neoplasms: part II. J Surg Oncol. 1996;61:68–83. doi: 10.1002/1096-9098(199601)61:1<68::aid-jso2930610102>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18. [June 26, 2007];Surveillance Epidemiology, and End Results (SEER) incidence statistics. Section IV. :156, 158. http://seer.cancer.gov/cgi-bin/csr/1975_2004/search.pl#results.
- 19.Sumner WE, III, Koniaris LG, Snell SE, et al. Results of 23,810 cases of ductal carcinoma-in-situ. Ann Surg Oncol. 2007;14:1638–1643. doi: 10.1245/s10434-006-9316-1. [DOI] [PubMed] [Google Scholar]
- 20.Escande A, Pillon A, Servant N, et al. Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta. Biochem Pharmacol. 2006;71:1459–1469. doi: 10.1016/j.bcp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–2541. [PubMed] [Google Scholar]
- 22.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 23.Helguero LA, Faulds MH, Gustafsson JA, Haldosen LA. Estrogen receptors alpha (ERalpha) and beta (ERbeta) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- 24.Roy PG, Thompson AM. Cyclin D1 and breast cancer. Breast. 2006;15:718–727. doi: 10.1016/j.breast.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Key TJ, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 26.Cauley JA, Cummings SR, Black DM, Mascioli SR, Seeley DG. Prevalence and determinants of estrogen replacement therapy in elderly women. Am J Obstet Gynecol. 1990;163(5 pt 1):1438–1444. doi: 10.1016/0002-9378(90)90602-4. [DOI] [PubMed] [Google Scholar]
- 27.Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858–868. doi: 10.1016/S0140-6736(04)16981-X. [DOI] [PubMed] [Google Scholar]
- 28.Nolvadex [package insert] 2002 [Google Scholar]