Abstract
Background and Purpose
Raloxifene, a selective estrogen receptor modulator, reduces risk of invasive breast cancer and osteoporosis, but the effect on risk for stroke and venous thromboembolism in different patient subgroups is not established. The purpose of this analysis was to evaluate the effect of raloxifene on the incidence of all strokes, stroke deaths, and venous thromboembolic events according to participant subgroups.
Methods
This was a secondary end point analysis of an international, randomized, placebo-controlled clinical trial of 10 101 postmenopausal women with or at increased risk of coronary heart disease followed a median of 5.6 years. Strokes, venous thromboembolic events, and deaths were adjudicated by expert centralized committees. Strokes were categorized as ischemic, hemorrhagic, or undetermined and venous thromboembolic events were subclassified.
Results
The incidences of all strokes did not differ between raloxifene (incidence rate per 100 woman-years=0.95) and placebo (incidence rate=0.86) treatment groups (P=0.30). In women assigned raloxifene versus placebo, there was a higher incidence of fatal strokes (incidence rates=0.22 and 0.15, respectively, P=0.0499) and venous thromboembolic events (incidence rates=0.39 and 0.27, respectively, P=0.02). No significant subgroup interactions were found except that there was a higher incidence of stroke associated with raloxifene use among current smokers.
Conclusions
In postmenopausal women at increased risk for coronary events, the incidences of venous thromboembolism and fatal stroke but not all strokes were higher in those assigned raloxifene versus placebo. Raloxifene's effect did not differ across subgroups, except that the risk of stroke differed by smoking status. Treatment decisions about raloxifene should be based on a balance of projected absolute risks and benefits.
Keywords: clinical trial, stroke, venous thromboembolism
Raloxifene, a nonsteroidal selective estrogen receptor modulator (SERM), was recently shown to significantly reduce the incidence of invasive breast cancer and clinical vertebral fractures and to have no significant effect on the incidence of coronary events in postmenopausal women at increased risk of coronary events.1 In this same trial, Raloxifene Use for The Heart (RUTH), no difference in the incidence of all strokes was found between placebo and treatment with raloxifene.1 However, the incidence of fatal stroke was higher in the raloxifene group (P=0.0499). Consistent with previous trial findings, raloxifene use was associated with an increased risk of venous thromboembolism (VTE).
All of the effects of raloxifene must be considered when making treatment decisions about raloxifene, and it has been suggested that the risk-to-benefit ratio of raloxifene may vary depending on the risk profile of women receiving treatment.2 It is not clear whether women who are at increased risk of stroke leading to death when treated with raloxifene can be identified. Although the risk of VTE associated with menopausal hormone therapy and SERMs has been documented, subgroups of women at heightened risk for VTE when treated with raloxifene have not been identified.3–5
The RUTH cohort represents a diverse international population of women, approximately half with established coronary heart disease and half at increased risk for coronary heart disease, which can provide insight into potential interactions between individual risk profiles and important clinical outcomes associated with long-term use of raloxifene. The purpose of these analyses was to determine the effect of raloxifene on the incidence of stroke, stroke deaths, and VTE in women with different risk profiles at baseline.
Materials and Methods
Design Overview
The design and methods of the RUTH trial have been previously reported in detail.6 Briefly, RUTH was an international randomized, controlled clinical trial conducted between 1998 and 2006 with the primary objective of comparing the effect of raloxifene with placebo on the incidence of coronary events and invasive breast cancer among women at increased risk of coronary events.
Prespecified secondary end points included stroke, VTE, and all-cause mortality. The RUTH protocol was approved by the ethical review board at each investigative site. All women gave written informed consent for study participation in accordance with the principles of the Declaration of Helsinki. RUTH was registered at ClinicalTrials.gov (Registry No NCT00190593).
RUTH enrolled 10 101 participants from 177 sites in 26 countries. Eligible women were aged ≥55 years, ≥1 year postmenopausal, and at increased risk for a major coronary event. Exclusion criteria were myocardial infarction or coronary artery bypass grafting, within 3 months of randomization; percutaneous coronary intervention within 6 months of randomization; a history of cancer or VTE; life expectancy <5 years; unexplained uterine bleeding within 6 months of randomization; Class III or IV heart failure; chronic liver or renal disease; use of oral or transdermal estrogens within 6 months; or current use of other sex hormones or SERMs.
Randomization and Interventions
Eligible women were randomized to receive 60 mg raloxifene per day (Evista; Eli Lilly and Company) orally (N=5044) or an identical-appearing placebo (N=5057). Overall, 70.4% in the raloxifene group and 70.9% of women in the placebo group took at least 70% of assigned medication and were classified as adherent to treatment (P=0.62).
Participants, investigators, laboratory and clinical center staff, and the sponsor were blinded to participants’ treatment assignment. The investigator was unblinded to treatment only if needed for participant safety. The study drug was permanently discontinued when a participant was unblinded (n=26) or diagnosed with breast cancer (n=128) or with VTE (n=174). The study drug was temporarily discontinued during periods of prolonged immobilization or if the participant took estrogen-containing preparations, other hormonal agents, or a SERM.
At each biannual clinical visit or telephone contact, adherence to study medication, adverse events, and outcomes were collected.
Outcomes and Follow-Up
Participants were followed a median of 5.6 years (range, 0.01 to 7.1 years) and the median study drug exposure was 5.1 years for both treatment groups. Among women assigned to placebo, 3979 completed the study (595 died and 483 discontinued study). In the raloxifene arm, 4060 women completed the trial (554 died and 430 discontinued the study). Primary and secondary outcomes were adjudicated by committees comprised of therapeutic experts, who were blinded to treatment assignment and not employees of the sponsor. The Stroke End Point Committee adjudicated the secondary end points of stroke. A stroke was defined as the rapid onset of a persistent neurological deficit attributed to an obstruction in cerebral blood flow and/or cerebral hemorrhage not due to trauma, tumor, infection, or other certain etiology. The deficit must have lasted more than 24 hours unless death occurred or there was a demonstrable lesion compatible with an acute stroke on imaging. Transient ischemic attacks were not a study end point but were reported as adverse events. Strokes were classified by cause (ie, ischemic, hemorrhagic, undetermined).
Noncoronary cardiovascular deaths, including deaths due to a cerebrovascular cause or VTE, were adjudicated by the Secondary End Point Committee Chair independent of the Stroke End Point Committee. Cause of death was assigned based on available clinical information, death certificate, and/or autopsy information. For cerebrovascular deaths, the committee adjudicating deaths did not classify a death due to stroke by cause (ischemic or hemorrhagic). To ascertain whether a death due to stroke was ischemic or hemorrhagic, the sponsor conducted a retrospective assessment of the last adjudicated stroke for each participant who died due to stroke.
The VTE End Point Committee adjudicated all reported venous thromboembolic events. These included deep vein thrombosis, pulmonary embolism, and intracranial thrombosis (ie, retinal vein thrombosis). Modified World Health Organization criteria7 were used for a definitive diagnosis of a deep vein thrombosis or pulmonary embolism. For a definitive diagnosis of deep vein thrombosis, supporting Doppler study or venogram findings were required. For a definitive diagnosis of pulmonary embolism, supporting pulmonary angiogram or ventilation perfusion scan findings were required. When diagnostic testing results were unavailable, a diagnosis of probable VTE could be based on the clinical diagnosis alone.
Statistical Analysis
The majority of analyses were prespecified. All analyses followed the intention-to-treat principle and were based on independently adjudicated events. Baseline characteristics were compared between treatment groups using one-way analysis of variance for continuous variables and χ2 tests for categorical variables.
A log-rank test was used to compare the incidences of VTE and stroke outcomes between treatment groups. Statistical significance was tested at the 2-sided alpha level of 0.05. For all analyses, unadjusted Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and their 95% CIs. Time to event was defined as the number of days between randomization and the first diagnosis of the event. Participants not experiencing the event were censored at the last date at which study information was collected or their date of death.
Prespecified and post hoc subgroup analyses were performed to explore for potential differential treatment effects on incidence of stroke, death due to stroke, and VTE in participants in different subgroups. Interaction of treatment and subgroup classification was tested at the 0.1 significance level. No adjustments for multiple testing were made.
At the beginning of each year, a participant was considered at risk for the event of interest if she was an active study participant and had not experienced the event by that time point. Hazard ratios and 95% CIs were estimated from a Cox proportional hazard regression model. All statistical analyses were performed using the SAS software version 8.2 (SAS Institute).
Results
The baseline characteristics of the RUTH trial participants are listed in Table 1 and were similar between treatment groups (data not shown). Their mean age was 67.5 years, approximately half had established coronary heart disease, and 46% had diabetes. Twenty percent of the women had previously used hormone therapy, and the average duration of use of this therapy was 4.1 (±SD 5.3) years.
Table 1.
Baseline Characteristics of Women (N=10 101) Randomized in RUTH
| Characteristic | Overall (N=10 101), No. (%) |
|---|---|
| Age | |
| ≤65 years | 3699 (36.6) |
| >65 and <70 years | 2471 (24.5) |
| ≥70 years | 3931 (38.9) |
| White | 8481 (84.0) |
| Region of the world | |
| Western Europe | 4679 (46.3) |
| Eastern Europe | 2310 (22.9) |
| Latin/South America | 1370 (13.6) |
| North America | 1029 (10.2) |
| Asia Pacific | 498 (4.9) |
| Africa | 215(2.1) |
| Body mass index | |
| ≤25 kg/m2 | 2416 (23.9) |
| >25 and ≤30 kg/m2 | 4052 (40.1) |
| >30 kg/m2 | 3603 (35.7) |
| *Current smoker | 1256 (12.4) |
| †Cardiovascular risk score category | |
| ≤5 | 3671 (36.3) |
| >5 and ≤9 | 3469 (34.3) |
| >9 | 2961 (29.3) |
| History of coronary heart disease | 5034 (49.8) |
| Prior estrogen therapy | 1399 (13.9) |
| Prior estrogen progestin therapy | 605 (6.0) |
| ‡Diabetes mellitus | 4607 (45.6) |
| Lower extremity arterial disease | 1083 (10.7) |
| Prior stroke | 860 (8.5) |
| History of atrial fibrillation | 482 (4.8) |
| §Hypertension | 7863 (77.8) |
| ∥Hyperlipidemia | 7381 (73.1) |
| Baseline medication use | |
| Statin | 4743 (47.0) |
| Beta blockers | 4794 (47.5) |
| Calcium channel blockers | 3579 (35.4) |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 4893 (48.4) |
| Diuretic | 3992 (39.5) |
| Warfarin | 417(4.1) |
| Aspirin | 5711 (56.5) |
| Nonaspirin antiplatelet | 298 (3.0) |
| Family history of deep vein clot | 650 (6.4) |
| Prior visible swelling in legs >24 hours | 452 (4.5) |
| Systolic blood pressure, mm Hg | 146 (20) |
| Diastolic blood pressure, mm Hg | 82 (10) |
| ¶Low-density lipoprotein cholesterol, mg/dL | 121.9 (37) |
| #triglyceride level, mg/dL | 158.7 (110) |
Smoking, self-reported smoking of an average of ≥10 cigarettes/day over the 6 months before Visit 1.
Cardiovascular risk score category, cardiovascular risk score calculated based on the presence of risk factors for a major coronary event.6
Diabetes, self-reported diabetes mellitus and use of oral hypoglycemic medication or insulin or fasting serum glucose >140 mg/dL at Visit 1.
Hypertension, self-reported hypertension and use of antihypertensives or systolic blood pressure >160 mm Hg or diastolic blood pressure >95 mm Hg on at least 2 measurements.
Hyperlipidemia, use of lipid-lowering medications or a fasting low-density lipoprotein cholesterol> 160 mg/dL or fasting high-density lipoprotein cholesterol <45 mg/dL with fasting triglycerides >250 mg/dL.
To convert values for low-density lipoprotein cholesterol to mmol/L, multiply by 0.0259.
To convert values for triglyceride level to mmol/L, multiply by 0.0112.
Stroke End Points
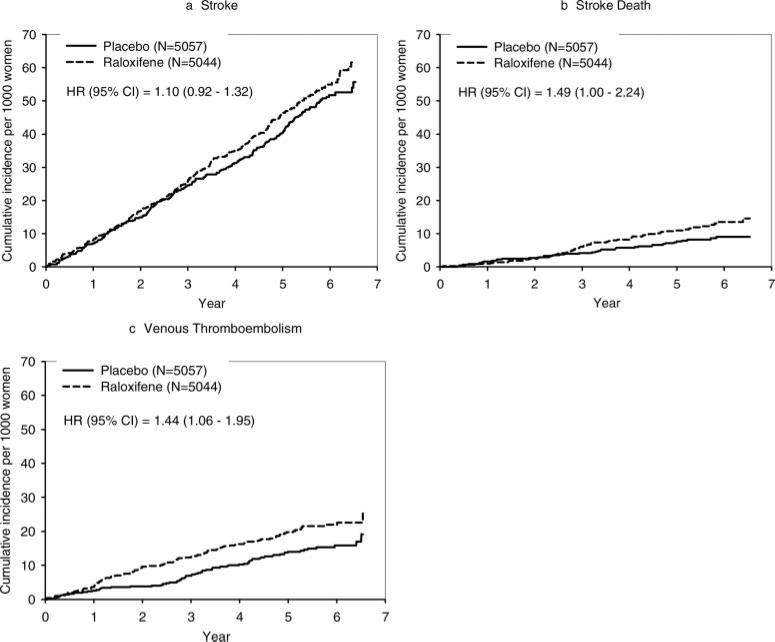
In the RUTH trial, 473 women experienced a stroke adjudicated as such by the Stroke End Point Committee. There was no statistically significant difference between treatment groups in the incidence of all strokes or hemorrhagic and ischemic strokes separately (Table 2; Figure 1a). Of the 473 women with an adjudicated stroke, 165 (raloxifene, 91; placebo, 74; P=0.21) died during the trial and 89 of these deaths were adjudicated as a death due to stroke by the Secondary End Point Committee Chair. An additional 9 deaths were adjudicated to have been caused by stroke without an independently adjudicated stroke being reported for a total of 98 deaths due to stroke. There were more cerebrovascular deaths in the raloxifene versus placebo group (59 versus 39; HR, 1.49; 95% CI, 1.00 to 2.24), attributable to an excess in ischemic and undetermined strokes (Table 2). The absolute risk increase for fatal stroke was 0.07 per 100 women treated for 1 year. The increased risk emerged after 3 years and remained constant thereafter (Figure 1B). There was no difference between treatment groups in all-cause mortality.
Table 2.
Incidence and HRs for Stroke and VTE Events
| No. of Events (annualized rate, %) |
||||
|---|---|---|---|---|
| End Point | Placebo (N=5057) | Raloxifene (N=5044) | HR (95% CI) | P Value |
| Stroke | 224 (0.86) | 249 (0.95) | 1.10(0.92–1.32) | 0.30 |
| Hemorrhagic | 30 (0.11) | 18 (0.07) | 0.59 (0.33–1.06) | 0.07 |
| Ischemic | 171 (0.65) | 198 (0.75) | 1.15 (0.93–1.41) | 0.19 |
| Undetermined | 30 (0.11) | 39 (0.15) | 1.28 (0.80–2.07) | 0.30 |
| VTE event | 71 (0.27) | 103 (0.39) | 1.44 (1.06–1.95) | 0.02 |
| Pulmonary embolism | 24 (0.09) | 36 (0.14) | 1.49 (0.89–2.49) | 0.13 |
| Deep vein thrombosis | 47 (0.18) | 65 (0.24) | 1.37 (0.94–1.99) | 0.10 |
| Intracranial (retinal vein) thrombosis | 6 (0.02) | 8 (0.02) | 1.32 (0.46–3.80) | 0.61 |
| Other | 1 (0.004) | 2 (0.004) | NA | NA |
| All-cause mortality | 595 (2.25) | 554 (2.07) | 0.92 (0.82–1.03) | 0.16 |
| All cardiovascular deaths | 355 (1.34) | 362 (1.35) | 1.01 (0.87–1.17) | 0.91 |
| Noncoronary | 81 (0.31) | 107 (0.40) | 1.31 (0.98–1.74) | 0.07 |
| Cerebrovascular (stroke) | 39 (0.15) | 59 (0.22) | 1.49 (1.00–2.24) | 0.05 |
| Hemorrhagic | 12 | 10 | 0.82 (0.36–1.90) | 0.65 |
| Ischemic | 16 | 29 | 1.79 (0.97–3.30) | 0.06 |
| Undetermined | 11 | 19 | 1.71 (0.81–3.59) | 0.15 |
| VTE | 5 (0.10) | 10 (0.20) | 1.98 (0.68–5.79) | 0.20 |
NA indicates not applicable.
Figure 1.
Cumulative incidence rates for strokes, fatal strokes, and venous thromboembolic events.
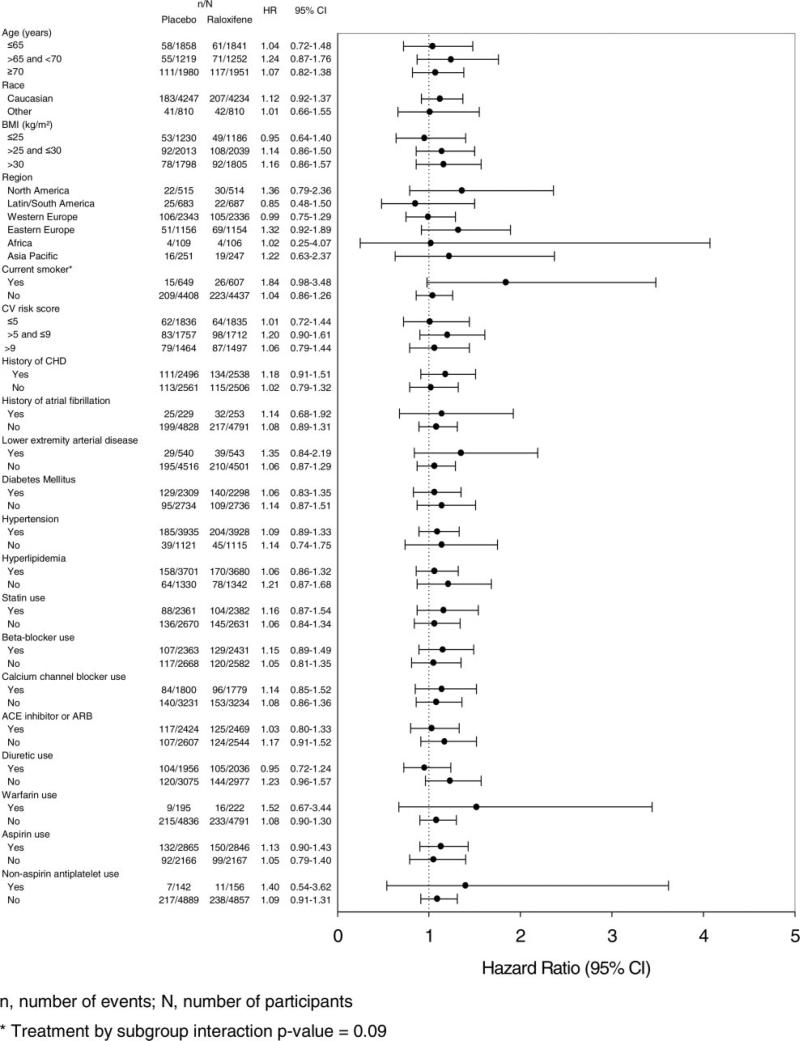
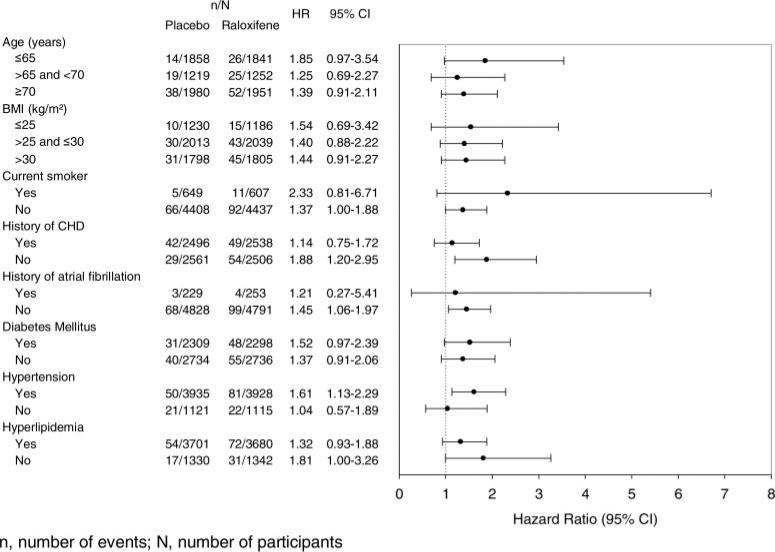
Results of the prespecified subgroup analyses for all strokes are shown in Figure 2. There were no significant treatment group interactions except in the case of smoking; current smokers had an 84% increased risk of all strokes associated with raloxifene versus placebo (HR, 1.84; 95% CI, 0.98 to 3.48), whereas nonsmokers had no increased risk with treatment (HR, 1.04; 95% CI, 0.86 to 1.26; interaction probability value 0.09). In post hoc analyses, there were no significant treatment group interactions for subgroups defined by systolic blood pressure (≤140 versus >140 and <160 versus ≥160 mm Hg; ≤160 versus >160 mm Hg), diastolic blood pressure (≤80 versus >80 and <90 versus ≥90 mm Hg), low-density lipoprotein cholesterol (≤100 versus >100 and <130 versus ≥130 mg/dL), total cholesterol (≤200 versus >200 and <240 versus ≥240 mg/dL), or triglycerides (<150 versus ≥150 mg/dL; interaction probability value >0.1 in all cases).
Figure 2.
Prespecified subgroup analyses for all strokes.
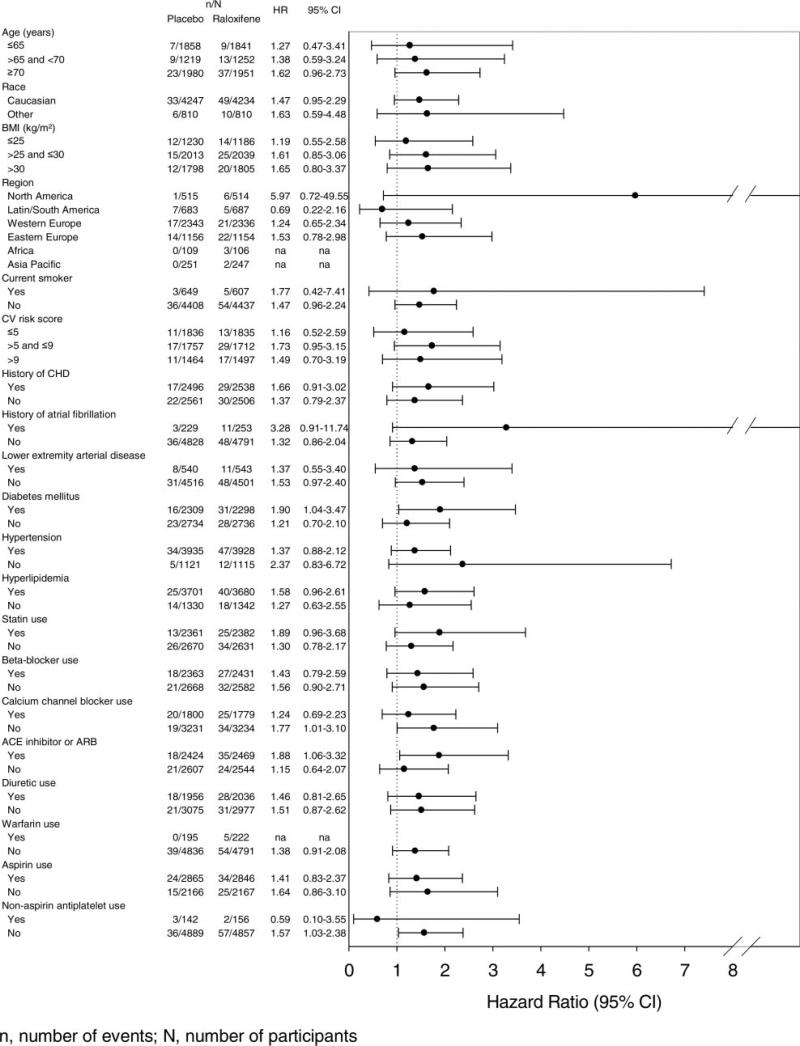
Results of the post hoc subgroup analyses for fatal strokes are shown in Figure 3. There were no significant treatment interactions for any subgroup shown or for those defined by systolic blood pressure, diastolic blood pressure, low-density lipoprotein cholesterol, total cholesterol, or triglycerides (interaction probability value >0.1 in all cases).
Figure 3.
Post hoc subgroup analyses for fatal strokes.
There was no significant difference between raloxifene and placebo groups in the proportion of women who reported transient ischemic attacks (1.7 versus 1.8; P=0.78) or atrial fibrillation (6.4 versus 6.6%; P=0.84).
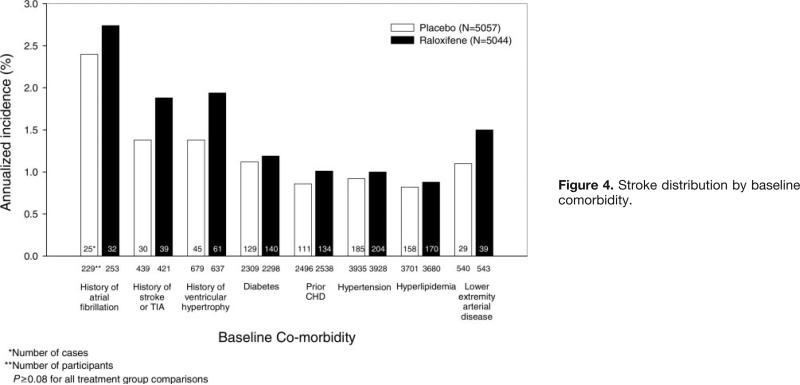
The incidence of strokes in women with established risk factors for stroke is illustrated in Figure 4 according to treatment group. Women with a history of atrial fibrillation, stroke, or transient ischemic attack or left ventricular hypertrophy on baseline electrocardiogram had increased stroke incidence with raloxifene versus placebo, but the differences between treatment groups were not statistically significant.
Figure 4.
Stroke distribution by baseline comorbidity.
Venous Thromboembolic Events
In the RUTH trial, 174 women had a VTE, and there were significantly more in the raloxifene than placebo group (103 versus 71; HR, 1.44; 95% CI, 1.06 to 1.95). The absolute increased risk for VTE associated with raloxifene was 0.12 per 100 woman-years (Table 2; Figure 1C). The incidence of pulmonary embolism, deep vein thrombosis, or retinal vein thrombosis individually did not differ significantly between treatment groups. All 15 fatal venous thromboembolic events were the result of pulmonary embolism. Post hoc analyses showed no significant treatment interactions by subgroup, including no association with smoking (Figure 5).
Figure 5.
Post hoc subgroup analyses for venous thromboembolic events.
Discussion
In postmenopausal women at increased risk of major coronary events, 60 mg raloxifene daily compared with placebo was associated with significant excess risks of stroke deaths (HR, 1.49; 95% CI, 1.00 to 2.24; absolute risk increase 0.07 per 100 women treated for 1 year) and venous thromboembolic events (HR, 1.44; 95% CI, 1.06 to 1.95; absolute risk increase 0.12 per 100 women). However, total mortality and total stroke incidence did not differ significantly between active treatment and placebo groups. The effect of raloxifene on stroke risk differed in smokers versus nonsmokers. Current smokers appeared to be at greater risk for stroke when treated with raloxifene versus placebo, but the difference between treatment groups did not reach statistical significance. We could not identify any specific characteristics that would put women at increased risk of fatal stroke or VTE due to raloxifene therapy.
Although not observed with raloxifene, clinical trials have documented an increased risk for all strokes with hormone therapy and tibolone, a synthetic steroid with estrogenic, progestogenic, and androgenic effects.8–13 In the Women's Health Initiative clinical trials, enrolling generally healthy women, there was an increased stroke incidence among women randomized to conjugated estrogen alone (HR, 1.39; 95% CI, 1.10 to 1.77; absolute risk increase 0.12 per 100 women treated for 1 year) or estrogen combined with progestin (HR, 1.41; 95% CI, 1.07 to 1.85; absolute risk increase 0.08 per 100 women) compared with placebo. A recent meta-analysis of 28 hormone therapy trials involving nearly 40 000 women, including those with a history of stroke, documented an increased risk of all strokes associated with therapy (OR, 1.29; 95% CI, 1.13 to 1.47); risk for death or dependency due to stroke was also significantly higher in women randomly assigned to active hormone treatments (OR, 1.56; 95% CI, 1.11 to 2.20).13 In the Women's Estrogen for Stroke Trial (WEST), enrolling 664 postmenopausal women with prior stroke or transient ischemic attack, women randomized to estrogen had a significantly increased risk of fatal stroke (relative risk, 2.9; 95% CI, 0.9 to 9.0).10 Tibolone has been shown to be associated with a 2.3-fold increased risk of stroke compared with placebo (P=0.02) in a randomized trial of over 4000 women with osteoporosis.11,12
In the Multiple Outcomes of Raloxifene Evaluation (MORE) trial of 7705 postmenopausal women with osteoporosis, there was no significant difference in the incidences of stroke (HR 0.69; 95% CI, 0.40 to 1.18) or fatal stroke (HR, 0.50; 95% CI, 0.13 to 1.96) among women randomized to 60 mg raloxifene per day compared with placebo.14 In a post hoc subgroup analysis of women in MORE who were at increased cardiovascular risk, stroke incidence was significantly less in women assigned 60 mg raloxifene per day versus placebo (HR, 0.38; 95% CI, 0.15 to 0.94) and there was no difference between treatment groups in the incidence of fatal stroke (HR, 0.59; 95% CI, 0.10 to 3.43). Baseline characteristics differed between women participating in MORE and those participating in RUTH. In MORE, less than 1% had a history of stroke, less than 3% of women had diabetes, 30% had hypertension15; in RUTH, 9% had a history of stroke, 50% had diabetes, and 80% had hypertension. In a recent large randomized trial comparing the effects of bazedoxifene (a SERM in clinical development), raloxifene, and placebo in postmenopausal women with osteoporosis, the incidences of ischemic and hemorrhagic strokes in the raloxifene group at 3 years were 0.6% and 0.1%, respectively, compared with 0.7% and 0.3% in the placebo group.16
In contrast to raloxifene, tamoxifen has been shown to increase stroke risk in clinical trials.17,18 In a recent randomized trial of tamoxifen compared with raloxifene among nearly 20 000 women at increased risk of breast cancer, there was no difference in incidence of invasive breast cancer or stroke between the 2 treatment groups; this trial was not powered to detect a difference in stroke incidence between raloxifene and tamoxifen.19
It is difficult to explain why the incidence of fatal stroke but not the incidence of all strokes might be higher in the raloxifene group. It is possible that the increased risk of fatal stroke associated with raloxifene treatment is spurious and due to the multiple number of analyses that was performed. However, an increase in risk of fatal stroke associated with raloxifene treatment is plausible based on the findings of trials of menopausal hormone therapy, tibolone, and tamoxifen. Of note, the fatal strokes seen with raloxifene were predominantly ischemic. Increased risk of some ischemic strokes as well as VTE, a recognized adverse event with raloxifene, may be related to alterations in coagulation factors, including promotion of coagulation and impaired anticoagulation.20–22 The effect of SERMs and hormone therapy on the pathophysiology of VTE and cerebral ischemia deserves further study.
In accordance with findings from other trials, raloxifene did increase the risk for VTE in RUTH. The risk of VTE is increased 2- to 3-fold with estrogen plus progestin or tamoxifen3,4,17; in the STAR trial, the risk of VTE was lower in women assigned raloxifene versus tamoxifen (relative risk, 0.70; 95% CI, 0.54 to 0.91).19 The totality of evidence suggests that menopausal hormone therapy and SERMs are associated with increased risk of VTE, including pulmonary embolism and deep vein thrombosis, and that the absolute risk increase varies depending on the type of agent and the underlying risk of VTE.
There are limitations to this analysis. We conducted multiple subgroup analyses and significant findings could occur due to chance alone. Moreover, although these subgroup analyses are hypothesis-generating, they cannot be used to identify women not at risk for an adverse event. We did not collect information on the severity or long-term outcomes of nonfatal strokes and therefore do not know if the strokes sustained by women assigned raloxifene were more severe or had greater associated comorbidity than those experienced by women on placebo. Also, the definition of cerebrovascular death was not prespecified and the type (ie, ischemic or hemorrhagic) of fatal stroke was determined by a post hoc review and was not classified by the adjudicating committee. However, adjudication of stroke and fatal stroke was systematic and blinded so the impact on findings should be minimal.
The excess risk of fatal stroke (0.07 per 100 women treated for 1 year) and VTE (0.12 per 100) should be evaluated in the context of clinical trial results documenting a reduced risk of invasive breast cancer (absolute risk reduction 0.12 per 100) and clinical vertebral fracture (absolute risk reduction 0.13 per 100) in the RUTH population.1
Our data show that the effect of raloxifene did not differ across subgroups for fatal strokes and VTEs, suggesting that no single risk factor can be used with confidence to identify women at highest risk for these adverse events. Decisions about the use of raloxifene to prevent or treat osteoporosis or to prevent invasive breast cancer in postmenopausal women should consider the absolute risk of stroke and venous thromboembolic events based on individual characteristics, including cigarette smoking and history of VTE or prolonged immobilization.
Acknowledgments
We are indebted to the 10 101 women and the investigators and staff for their contributions to the RUTH trial.
Role of the Funding Source
This study was sponsored by Eli Lilly and Company, Indianapolis, Ind. The executive committee had unrestricted request-based access to the study data, which were retained by the sponsor. The sponsor conducted the analysis under the direction of the lead author with input from coauthors. Data reported are those available as of February 2, 2006. L.M. drafted the initial paper and revised it based on input from all authors. The sponsor assisted with tables and graphs and made suggestions for text.
Footnotes
Disclosures
M.J.G., S.A.D., and M.A.-A. are full-time employees of and own stock or stock options for Eli Lilly and Company. L.M., M.K., and B.L.A. have had consultancies and honoraria through Eli Lilly and Company. L.M. has had consultancies and honoraria through Organon, Inc. D.G. has grants both received and pending from Eli Lilly and Company. E.B.-C. has had consultancies and honoraria, and received grants from Eli Lilly and Company. P.C. has had consultancies and honoraria and received grants from Eli Lilly and Company. L.M., D.G., E.B.C., P.C., N.W., and M.K. were paid external investigators for the RUTH trial.
References
- 1.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady G, Kornitzer M, McNabb MA, Wenger NK. Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–237. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 2.Stefanick ML. Risk–benefit profiles of raloxifene for women. N Engl J Med. 2006;355:190–192. doi: 10.1056/NEJMe068120. [DOI] [PubMed] [Google Scholar]
- 3.Grady D, Wenger NK, Herrington D, Khan S, Furberg C, Hunninghake D, Vittinghoff E, Hulley S. Postmenopausal hormone therapy increases risk for venous thromboembolic disease: the Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2000;132:689–696. doi: 10.7326/0003-4819-132-9-200005020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Grady D, Ettinger B, Moscarelli E, Plouffe L, Jr, Sarkar S, Ciaccia A, Cummings S, Multiple Outcomes of Raloxifene Evaluation Investigators Safety and adverse effects associated with raloxifene: Multiple outcomes of raloxifene evaluation. Obstet Gynecol. 2004;104:837–844. doi: 10.1097/01.AOG.0000137349.79204.b8. [DOI] [PubMed] [Google Scholar]
- 6.Mosca L, Barrett-Connor E, Wenger N, Collins P, Grady D, Kornitzer M, Moscarelli E, Paul S, Wright TJ, Helterbrand JD, Anderson PW. Design and methods of the Raloxifene Use For the Heart (RUTH) Study. Am J Cardiol. 2001;88:392–395. doi: 10.1016/s0002-9149(01)01685-x. [DOI] [PubMed] [Google Scholar]
- 7.A multinational case–control study of cardiovascular disease and steroid hormone contraceptives. Description and validation of methods. WHO Collaborative Study of Cardiovascular and Steroid Hormone Contraception. J Clin Epidemiol. 1995;48:1513–1547. doi: 10.1016/0895-4356(95)00516-1. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix SL, Wassertheil-Smoller, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J, WHI Investigators Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 9.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ, WHI Investigators Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative. JAMA. 2003;289:2717–2719. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 10.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, Mol-Arts M, Kloosterboer L, Mosca L, Christiansen C, Bilezikian, Kerzberg EM, Johnson S, Zanchetta J, Grobbee DE, Seifert W, Eastell W. The effects of tibolone in older postmenopausal women. Results of the Long-Term Intervention on Fractures with Tibolone (LIFT) Randomized Trial. N Engl J Med. 2008;359:697–708. doi: 10.1056/NEJMoa0800743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobbee DE, LIFT Steering Committee LIFT study to continue as planned. BMJ. 2005;333:843. doi: 10.1136/bmj.331.7520.843-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bath PMW, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta-analysis. BMJ. 2005;330:342. doi: 10.1136/bmj.38331.655347.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, Rautaharju P, Harper KD, MORE Investigators Raloxifene and cardiovascular events in osteoporotic postmenopausal women. Four-year results from the MORE randomized trial. JAMA. 2002;287:847–857. doi: 10.1001/jama.287.7.847. [DOI] [PubMed] [Google Scholar]
- 15.Ensrud K, Genazzani AR, Geiger MJ, McNabb M, Dowsett SA, Cox DA, Barrett-Connor E. Effect of raloxifene on cardiovascular adverse events in postmenopausal women with osteoporosis. Am J Cardiol. 2006;97:520–527. doi: 10.1016/j.amjcard.2005.09.083. [DOI] [PubMed] [Google Scholar]
- 16.Adachi JD, Chesnut CH, Brown JP, Christiansen C, Russo LA, Fernandes CE, Menegoci JC, Kung A, Chines AA, Bessac L, Chakrabarti D. ASBMR 29th annual meeting program; September 16–19, 2007; Honolulu. ASBMR Publications; Durham, NC: Safety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial [Abstract]. p. 133. Abstract W385. [Google Scholar]
- 17.Fisher B, Costantino JP, Wickerham DL, Redmon CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N, other National Surgical Adjuvant Breast and Bowel Project Investigators Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 18.Dignam JJ, Fisher B. Occurrence of stroke with tamoxifen in NSABP B-24. Lancet. 2000;355:848–849. doi: 10.1016/S0140-6736(05)72466-1. [DOI] [PubMed] [Google Scholar]
- 19.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, III, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N, for the National Surgical Adjuvant Breast and Bowel Project (NSABP) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP study of tamoxifen and raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 20.Azevedo GD, Franco RF, Baggio MS, Maranhão TM, Ferriani RA, Silva de Sá MF. Effects of raloxifene therapy on the anticoagulant system in postmenopausal women. Climacteric. 2003;6:140–145. [PubMed] [Google Scholar]
- 21.Sgarabotto M, Baldini M, Dei Cas A, Manotti C, Luciana Barilli A, Rinaldi M, Benassi L, Bacchi Modena A. Effects of raloxifene and continuous combined hormone therapy on haemostasis variables: a multicenter, randomized, double-blind study. Thromb Res. 2007;119:85–91. doi: 10.1016/j.thromres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Cosman F, Baz-Hecht M, Cushman M, Vardy MD, Cruz JD, Nieves JW, Zion M, Lindsay R. Short-term effects of estrogen, tamoxifen and raloxifene on hemostasis: a randomized-controlled study and review of the literature. Thromb Res. 2005;116:1–13. doi: 10.1016/j.thromres.2004.09.014. [DOI] [PubMed] [Google Scholar]