Abstract
Lipophorin is the major hemolymph protein responsible for lipid transport between tissues of insects. Lipophorins from several insect species in order Diptera (the fruit fly Drosophila melanogaster from the suborder Brachycera, the mosquito Aedes aegypti; the phantom midges Chaoborus maximus and minimus; the black fly Simulium vittatum; the crane fly Nephrotoma abbreviata, all from the suborder Nematocera) were isolated and characterized. All lipophorins consisted of two protein subunits of approximately 240 and 75 kDa each. The density of each lipophorin was in the high-density lipoprotein range (1.112 to 1.128 g/ml). The predominant neutral lipid carried by lipophorin from insects belonging to the infraorder Culicomorpha was triacylglycerol. Lipophorin from the crane fly Nephrotoma abbreviata, which belongs to the infraorder Tipulomorpha, carried approximately equivalent amounts of diacylglycerol and triacylglycerol. Lipophorin from D. melanogaster was found to carry diacylglycerol as the predominant neutral lipid.
Keywords: hemolymph, fly, mosquito, insect, lipid, lipoprotein, diacylglycerol
Introduction
Lipophorin is the major lipid transporting lipoprotein in the hemolymph of insects. In general, lipophorin occurs as a high-density lipophorin (HDLp, D≅1.063 to 1.21 g/ml) containing two apolipoproteins, apolipophorin-1 (apoLp-I, ∼250 kDa) and apolipophorin-II (apoLp-II, ∼70–80 kDa). In some insect species, several molecules of a third subunit, apolipophorin-III (apoLp-III, ∼18 kDa) can bind reversibly to lipophorin, which increases its lipid carrying capacity. All lipophorins characterized thus far contain a significant amount of phospholipid that is thought to occur as a monolayer on the surface of the particle. Lipophorin carries neutral lipid in a hydrophobic “core”, which, in most insects, consists predominantly of sn-1, 2-diacylglycerol (DAG). Lipophorins also have the capacity to carry other neutral lipids such as sterols, fatty acids, and hydrocarbons (Arrese et al, 2001; Canavoso et al, 2001).
Insect lipophorins can act as a reusable shuttle. Lipophorin is able to take up lipid and deliver it to target tissues without internalization and degradation of the particle. For example, a single lipophorin particle, synthesized in the fat body, can take up dietary lipid at the midgut in the form of DAG, diffuse through the hemolymph to the fat body, and deliver the DAG for storage in the fat body without being internalized and degraded. This same particle can then return to the midgut surface and repeat the process (Canavoso et al, 2001).
Mosquito lipophorin has a unique characteristic: it carries triacylglycerol (TAG) rather than DAG as the principle neutral lipid (Ford and Van Heusden, 1994). Lipophorin particles from several mosquito species have been characterized and all were found to exist as HDLp. In addition, all consisted of similar lipid compositions, with the predominant neutral lipid being TAG. The apolipoprotein content of each species was similar to that of other insects: two subunits of ∼ 240 kDa and ∼ 70 kDa (Ford and Van Heusden, 1994; Capurro, et al, 1994). Even though the uptake and delivery of DAG to and from insect tissues has been characterized to a significant degree, little is known about the mechanism of TAG uptake, transport, and delivery by mosquito lipophorin. A previous study (Pennington et al, 1996) compared the capacity of HDLp from Aedes aegypti and Manduca sexta to take up lipid from both A. aegypti and M. sexta fat body. This study indicated that mosquito lipophorin might not function efficiently as a reusable shuttle.
Studies of dipteran lipophorin have been conducted in the housefly, Musca domestica (Bianchi et al, 1987); the blowfly, Lucilla cuprina (Trowell et al, 1994); the Tsetse fly Glossina morsitans (Ochanda et al, 1991); and the fruitfly, Drosophila melanogaster (Fernando-Warnakulasuriya and Wells, 1988; Pho et al, 1996). In each case, these studies demonstrated that lipophorin from consisted of the same apoprotein composition of typical insect lipophorin. D. melanogaster lipophorin was found to have a relatively high density of 1.16g/ml and contained very little neutral lipid (Fernando-Warnakulasuriya and Wells, 1988). D. melanogaster lipophorin consisted of 7.4% DAG and 5.4 % TAG based on the total mass percent of the particle.
Because the presence of TAG in mosquito lipophorin seemed to be a unique, we characterized the lipid compositions of several other dipteran species to determine the prevalence of TAG-rich lipophorins in this order.
Materials and Methods
All reagents were obtained from Sigma Chemicals (St. Louis, MO) unless otherwise noted.
Insects
Aedes aegypti (NIH-Rockefeller) were maintained at 28°C at 70–80% humidity with a light: dark cycle of 16:8. Larvae were maintained on a diet consisting of rat chow (Sunburst Pet Foods, Phoenix, AZ), lactalbumin hydrolysate (USB, Cleveland, OH, www.usbweb.com) and yeast hydrolysate (USB). Fourth instar larvae were collected for analysis.
Chaoborus maximus/minimus: A pond water sample that consisted predominantly of a mixture of Chaoborus maximus and minimus was obtained from Live Aquatics (Staples, MN). Fourth instar C. maximus and minimus were separated from the other organisms in the sample and used for analysis.
Simulans vittatum: Insects were maintained according to the procedure of Bernardo et al (1986). Fourth instar larvae were collected and used for analysis.
Nephrotoma abbreviata were obtained from Dr. James LaFountain at The State University of New York at Buffalo. Larvae were maintained according to the procedure of A. Forer (1982) to the fourth instar in 100×20 petri dishes that contained moistened nonbleached toilet paper and a sufficient amount of nettle leaves (Frontier Natural Products, Norway IA, www.frontiernaturalbrands.com) to provide a continuous food source. Rearing dishes were replaced every 48 hours and larvae were sorted to prevent overcrowding during development.
Drosophila melanogaster: Insects were obtained from the Dr. Therese Markow and maintained according to the procedure of Fernando-Warnakulasuriya and Wells (1988). Third instar larvae were used for analysis.
Lipophorin isolation
Generally, lipophorin was isolated from homogenates of larvae using a modification of method of Fernando-Warnakulasuriya and Wells (1988). All samples were processed in triplicate. Five grams of insects were homogenized in 25 ml buffer using a Tissue Terror homogenizer (Fisher Scientific, Los Angeles, CA, http://www.fishersci.com) for two minutes on ice, with 15 second pauses every 30 seconds. The homogenization buffer consisted of 20 mM NaHPO4, pH 7.2, 150 mM NaCl, 20 mM Glutathione, 4 mM diisopropylfluorophosphate (DFP), 4 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM Benzamidine, 20 mM EDTA, 2 µg/ml Chymostatin, 2 µg/ml Pepstatin, 2 µg/ml Leupeptin, and 2 µg/ml Bestatin.
In the case of N. abbreviata larvae, lipophorin was isolated from hemolymph. Hemolymph was obtained by puncturing the larvae and collecting hemolymph in 5 ml of homogenization buffer. The collected hemolymph was centrifuged at 500×g for 10 minutes to remove hemocytes. The supernatant was collected and further processed in the same manner as the total insect homogenates.
KBr gradient ultra-centrifugation
The homogenate was centrifuged at 5000×g for 20 minutes at 4°C. The infranatant below the fat cake was collected and KBr was added to a final concentration of 0.4 mg/ml in 20 ml. This solution was overlayed with 20 ml of 150 mM NaCl and subjected to ultracentrifugation at 50,000 rpm using a VTi 50 vertical rotor in a Beckman L8-55 centrifuge for 16 hours at 4°C (Ford and Van Heusden, 1994). Fractions were collected from the top to the bottom of each gradient in 1 ml aliquots. Hemolymph was fractionated in the same way
Fractions from each gradient were dialyzed against 20 mM Na2HPO4, pH 7.2, containing 150 mM NaCl. The fractions containing lipophorin were identified by SDS-PAGE. Those fractions containing lipophorin, as evidence by the presence of apoLp-I and -II, were then subjected to a second ultracentrifugation for further purification to remove contaminating storage proteins. The densities of the purified lipophorin samples were determined by refractometry.
Lipid analysis
Purified lipophorin samples were dialyzed as described above. Lipids were extracted using the procedure of Bligh and Dyer (1959). Neutral lipid classes were separated by thin layer chromatography on Si 250 silica plates (JT Baker, Phillipsburg NJ, www.jtbaker.com) using the procedure of Freeman and West (1966) followed by iodine visualization. The iodine on the plate was allowed to sublimate completely and then lipids were visualized by charring as described by Ford and Van Heusden (1994). Briefly, TLC plates were sprayed with 3% solution of copper acetate in 8% phosphoric acid. The plates were then incubated at 180°C until the charred lipid spots were fully resolved. Lipid classes were quantified on the silica plates by scanning and analysis using Kodak 1D analysis software (Kodak Scientific Imaging Systems, New Haven CT).
Results
SDS-PAGE analysis of the fractions from the KBr density gradients demonstrated the presence of lipoproteins in each species studied. A representative SDS-PAGE of the C. maximus/minimus KBr gradient fractionation is shown in Figure 1. Fractions containing lipophorin-like proteins were pooled and subjected to a second density gradient ultracentrifugation. Purified lipoproteins from all species consisted of two protein subunits corresponding to apoLp-I and apoLp-II, with Mr of approximately 240 kDa and 75 kDa (Figure 2). An apoLp-III like protein could not be found in any of the species tested.
Figure 1.
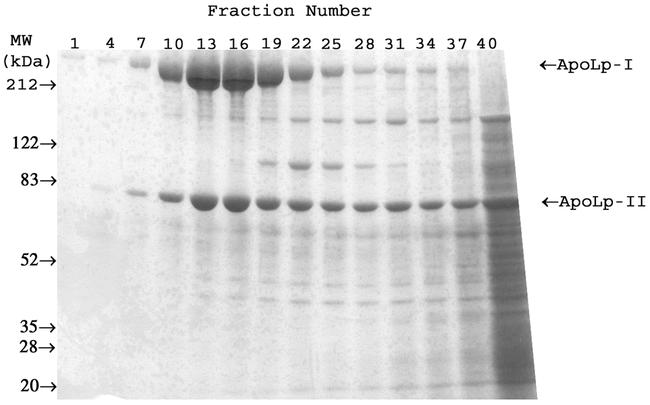
SDS-PAGE analysis of fractionated KBr Density Gradient Ultracentrifugation of Chaoborus maximus/minimus larval homogenate on 4–15% Coomassie-blue stained gel.
Figure 2.
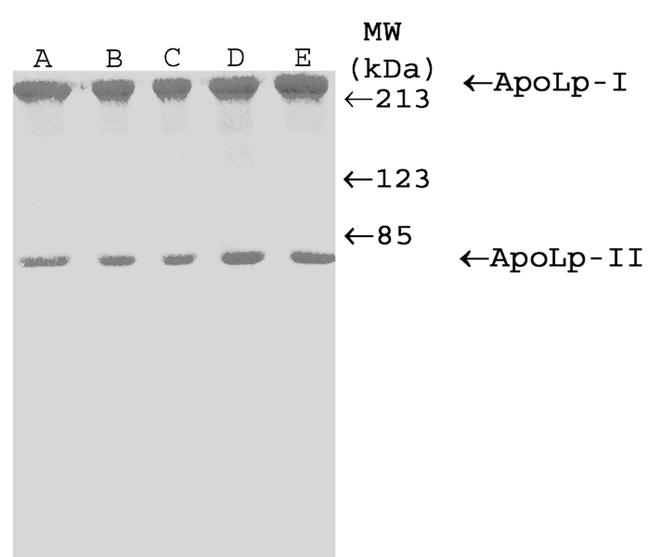
SDS-PAGE analysis of purified lipophorins on Coomassie-blue stained 8% gel. (A) Ae. aegypti, (B) C. maximus/minimus, (C) S. vittatum, (D) N. abbreviata, (E) D. melanogaster. Numbers indicate molecular weight in kDa.
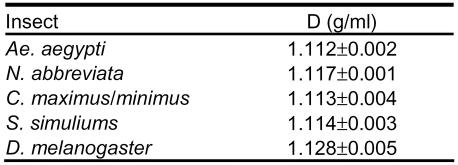
All of the lipophorins had a density in the high-density lipoprotein range (Table 1).
Table 1.
Densities of purified Dipteran HDLp. Densities are reported as mean of three determinations from three separate isolations (±SD).
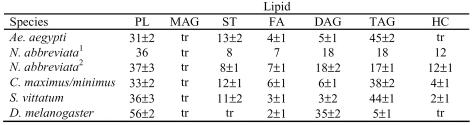
Lipid compositions are reported in Table 2. Triacylglyercerol was the major lipid found in HDLp from C. maximus/minimus, Ae. aegypti and S. vittatum. HDLp from another nematoceran, the crane fly N. abbreviata, consisted of roughly equivalent amounts of DAG and TAG. Hemolymph from N. abbreviata was also used as a source material for lipophorin isolation and analysis. The lipid composition of HDLp isolated from hemolymph did not differ from that of HDLp isolated from total homogenate. The neutral lipid composition of D. melanogaster HDLp consisted primarily of diacylglycerol.
Table 2.
Lipid Composition of Dipteran HDLp. Values are presented as weight % of lipid content. Data are the average ± SD of three determinations except for N. abbreviata hemolymph, which is a single determination.
One interesting observation was that another putative lipoprotein was found in the SDS-PAGE analysis of the C. maximus/minimus density gradient as seen in figure 1. This protein is approximately 100 kDa and has a density of 1.168 g/ml. This could be a lipoprotein due to its presence in some of the higher density fractions that lipophorin also appears, and this putative lipoprotein's apparent absence in the subphase fractions of the gradient. Lipid analysis will need to be performed on a purified sample to determine if this is indeed a lipoprotein.
Discussion
The previously report of the presence of TAG as the principal neutral lipid in mosquito lipophorin (Ford and Van Heusden, 1994) presented several interesting questions about the mechanism of lipid transport in mosquitoes. Uptake, transport, and delivery of DAG by lipophorin have been characterized extensively in several insect species (see recent reviews, Arrese et al, 2001; Canavoso et al, 2001). The transport of TAG in mosquitoes, however, has not yet been characterized. Intuitively, it would seem that uptake and delivery of TAG would be much more difficult energetically than that of DAG. Using in vitro techniques, a previous report concluded that the mosquito lipophorin is much less efficient at lipid uptake from A. aegypti and M. sexta fat body than the M. sexta lipophorin (Pennington et al, 1996).
Before embarking on a study of the mechanism of TAG transport in mosquitoes, we wanted to know whether the occurrence of a TAG-rich HDLp in mosquitoes was unique. We chose four species from suborder Nemotacera: the midge C. maximus/minimus, the black fly S. vittatum, and the mosquito Ae. aegypti from the infraorder Culicomorpha, as well as the crane fly N. abbreviata from infraorder Tipulomorpha. We also analyzed HDLp from the higher dipteran D. melanogaster. Even though the properties of HDLp from Ae. aegypti and D. melanogaster were reported previously, we analyzed HDLp from these insects as a comparison with the HDLp of those insects that are first described in this report.
Of the insects studied in this report, the presence of TAG-rich lipophorins was restricted to those species belonging to suborder Nematocera, infraorder Culicomorpha - Ae. aegypti, C. maximus/minimus, and S. vittatum. HDLp from the other Nematoceran species, N. abbreviata, consisted of roughly equivalent amounts of DAG and TAG, as well as a higher hydrocarbon content than found in HDLp from the other species.
The analysis of D. melanogaster HDLp yielded results that did not correspond to an earlier report. The density of D. melanogaster HDLp (1.128 g/ml) was considerably lower than the density of 1.16 g/ml reported by Fernando-Warnakulasuriya and Wells (1988). In addition, the lipid composition of D. melanogaster HDLp was different from that of the previous report by Fernando-Warnakulasuriya and Wells (1988). DAG was determined to be the predominant neutral lipid and was in much greater amounts than was described in the previous report. The source of the differences in density and lipid composition is not clear at this time, but different colonies were used and it is possible that the feeding behavior of the insects at the time of isolation may play a role. The density of lipophorin from dipterans has been shown to be affected by feeding in Ae. aegypti adults (Van Heusden et al, 1997). The current data do bring D. melanogaster lipophorin more in line with other dipteran lipophorins in terms of density, and its lipid composition does distinguish it from the lipophorins of insects belonging to the infraorder Culicomorpha
A consideration that must be made for the presence of high TAG levels in the lipophorin of insects from the Culicomorpha infraorder is the possibility of experimental artifacts caused by using total insect homogenates as the source for HDLp purification. In the previous report on lipophorin from D. melanogaster, an experiment was performed in which HDLp purified from M. sexta hemolymph was added to a fat body homogenate and then re-purified (Fernando-Warnakulasuriya and Wells, 1988). There was no significant change in the lipid composition of HDLp and the authors concluded that TAG from fat body stores could not be incorporated into HDLp during homogenization. It is still possible that some factors released from other tissues may be causing the transformation of DAG to TAG in lipophorin. To rule out this possibility, we collected hemolymph from N. abbreviata larvae and isolated HDLp from this source. Since the lipid composition of HDLp purified in this manner did not differ significantly from that of HDLp isolated from total homogenate, we concluded that there are not artifacts associated with using total homogenates.
Based on these studies we conclude that TAG-rich lipophorins are found only in the Culicomorpha infraorder, but not in higher Diptera. This means that TAG-rich lipophorins are not unique to mosquitoes, but must have arisen in the ancestor of the Culicomorpha. The presence of TAG-rich lipophorins is also not unique to blood feeding insects because the non-blood feeding Chaoborus sp also contains them. Future studies will concentrate on the mechanism(s) of biosynthesis and metabolism of these unique TAG-rich lipophorins.
Acknowledgments
The authors thank Frank Ramburg, Barry Thoele, Therese Markow and James LaFountain for kindly providing insects for this study. We would also like to thank Mary Hernandez for the excellent support she has provided to make this work possible. This work was supported by NIH grant GM50008.
Glossary
| Abbreviation: | |
|---|---|
| HDLp | High Density Lipophorin |
| DAG | Diacylglycerol |
| TAG | Triacylglycerol |
| MAG | Monoacylglycerol |
| ApoI | Apolipophorin I |
| ApoII | Apolipophorin II |
| ApoIII | Apolipophorin III |
References
- Arrese EL, Canavoso LE, Jouni ZE, Pennington JE, Tsuchida K, Wells MA. Lipid Storage and Mobilization in Insects: Current Status and Future Directions. Insect Biochemistry and Molecular Biology. 2001;31:7–17. doi: 10.1016/s0965-1748(00)00102-8. [DOI] [PubMed] [Google Scholar]
- Bernardo JM, Cupp EW, Kiszewski AE. Rearing black flies (Diptera: Simuliidae) in the laboratory: colonization and life table statistics for Simulium vittatum. Annals of the Entomological Socociety of America. 1986;79:610–621. [Google Scholar]
- Bianchi AG, Capurro M de L, Marinotti O. Lipophorin in the larval and adult stages of Musca domestica. Archives of Insect Biochemistry and Physiology. 1987;6:39–48. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Capurro M de L, Bianchi AG, Marinotti O. Aedes aegypti lipophorin. Comparative Biochemistry and Physioliology. 1994;108B(1):35–39. doi: 10.1016/0305-0491(94)90161-9. [DOI] [PubMed] [Google Scholar]
- De Bianchi AG, Capurro M de L. Musca Domestica larval lipoprotein. Archives of Insect Biochemistry and Physiology. 1991;17(1):15–27. doi: 10.1002/arch.940170104. [DOI] [PubMed] [Google Scholar]
- Fernando-Warnakulasuriya GJP, Wells MA. Isolation and characterization of lipophorin from Drosophila melanogaster larvae. Archives of Insect Biochemistry and Physiology. 1988;8:243–248. [Google Scholar]
- Ford PS, Van Heusden MC. Triglceride-Rich lipophorin in Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 1994;31(3):435–441. doi: 10.1093/jmedent/31.3.435. [DOI] [PubMed] [Google Scholar]
- Forer A. Crane fly spermatocytes and spermatids: A system for studying cytoskeletal components. Methods in Cell Biology. 1982;25:227–252. doi: 10.1016/s0091-679x(08)61427-2. [DOI] [PubMed] [Google Scholar]
- Freeman CP, West D. Complete separation of lipid classes on a single thin-layer plate. Journal of Lipid Research. 1966;7:324–327. [PubMed] [Google Scholar]
- Ochanda JO, Osir EO, Nguu EK, Olembo NK. Lipophorin from the tsetse fly, Glossina morsitans morsitans. Comparative Biochemistry and Physiology. 1991;99B(4):811–814. doi: 10.1016/0305-0491(91)90146-5. [DOI] [PubMed] [Google Scholar]
- Pennington JE, Nussenzveig RH, Van Heusden MC. Lipid transfer from insect fat body to lipophorin: comparison between a mosquito triacylglycerol-rich lipophorin and a sphinx moth diacylglycerol-rich lipophorin. Journal of Lipid Research. 1996;37:1144–1152. [PubMed] [Google Scholar]
- Pho DB, Pennanec'h M, Jallon JM. Purification of adult Drosophila melanogaster lipophorin and its role in hydrocarbon transport. Archives of Insect Biochemistry and Physiology. 1996;31:289–303. doi: 10.1002/(SICI)1520-6327(1996)31:3<289::AID-ARCH4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Trowell SC, Hines ER, Herlt AJ, Rickards RW. Characterization of a Juvenile hormone binding lipophorin from the blowfly Lucilia cuprina. Comparative Biochemistry and Physiology. 1994;109B(2/3):339–357. doi: 10.1016/0305-0491(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Van Heusden MC, Erickson BA, and Pennington JE. 1997. Lipophorin levels in the yellow fever mosquito, Aedes aegypti, and the effect. [DOI] [PubMed] [Google Scholar]