Abstract
During a pneumococcal disease outbreak in a pediatric psychiatric unit in a hospital in Rhode Island, USA, 6 (30%) of 20 patients and staff were colonized with Streptococcus pneumoniae serotype 15A, which is not included in pneumococcal vaccines. The outbreak subsided after implementation of antimicrobial drug prophylaxis and enhanced infection control measures.
Keywords: Streptococcus pneumoniae, pneumococcal infections, pneumonia, pneumococcal, serotype 15A, bacteria, antibiotic, antibacterial, disease outbreak, antimicrobial drugs
Streptococcus pneumoniae, or pneumococcus, causes an estimated 4 million illnesses in the United States annually (1). Pneumococcal disease outbreaks often occur in closed settings such as childcare facilities and hospitals. Control measures include vaccination, antimicrobial drug prophylaxis, and infection control (2). On January 26, 2011, the Rhode Island Department of Health was notified of 2 cases of invasive pneumococcal disease (IPD) and 2 cases of pneumonia that were associated with a unit (Unit 1) in a pediatric psychiatric hospital that had unusual infection control challenges. We investigated the outbreak to confirm the etiologic agent and prevent disease transmission.
The Study
Children who have developmental disabilities and aggressive or self-injurious behavior are treated in Unit 1. Inpatient capacity is 17, and <5 outpatients are sometimes present during the day for group and individual therapy. The unit employs >100 staff members. Patients require constant supervision and intensive help with activities of daily living.
At the beginning of this investigation, we established outbreak case definitions. We defined confirmed pneumococcal disease as IPD (isolation of S. pneumoniae from a normally sterile site such as blood) or noninvasive (laboratory confirmation of S. pneumoniae from a nonsterile site in the setting of a compatible clinical illness, such as isolation from ear drainage samples from a patient with otitis media). We defined confirmed pneumonia as pneumonia diagnosed by a clinician, using chest radiographs confirmed as showing pneumonia by a radiologist. We defined suspected pneumonia as pneumonia diagnosed by a clinician with no radiologic studies obtained. Cases occurred in Unit 1 staff, patients, and visitors during the study period. We maintained active surveillance through May 1, but no cases occurred after February 23.
To identify risk factors for pneumococcal disease (3,4), we abstracted medical records of all Unit 1 patients (n = 30) during November 1, 2010–January 30, 2011. Unit 1 staff members completed questionnaires about respiratory illnesses experienced during December–February. The hospital informed families of then-current Unit 1 patients of the outbreak. Family members or visitors who reported illness were interviewed. All pertinent medical records were reviewed.
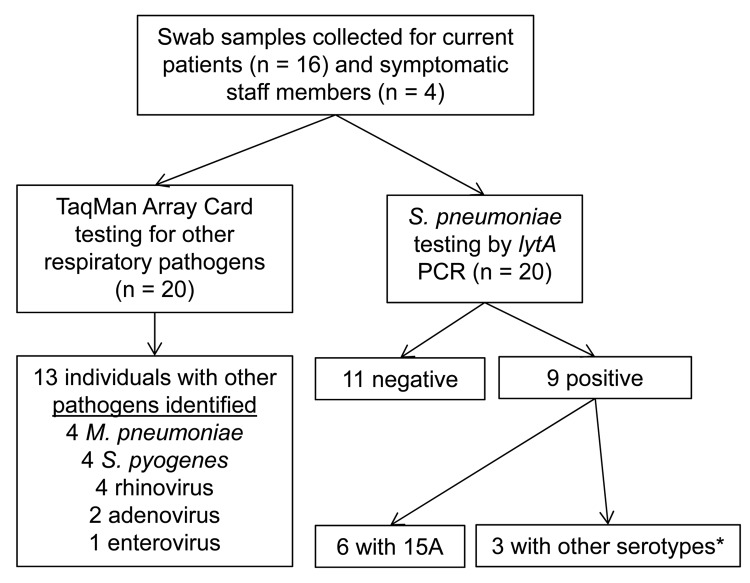
We collected available clinical specimens and conducted a survey to identify respiratory pathogens carried by patients and staff (Figure 1). We collected nasopharyngeal and oropharyngeal calcium alginate swab specimens from then-current Unit 1 patients (n = 16) and staff with ongoing respiratory symptoms (n = 4) during January 29–February 2. For pneumococcal carriage, swab specimens were processed as described (5,6). Three pneumococcal isolates were recovered (2 from blood, 1 from ear drainage samples). Swab specimens and isolates underwent specific real-time PCR that targeted the lytA gene (6), PCR-based serotyping (7,8), and multilocus sequence type determination (9). Antibacterial drug susceptibility testing was performed by using broth microdilution (10). Additional swabs stored in viral transport media were tested by solid-phase real-time PCR on TaqMan Array Cards (Life Technologies, Carlsbad, CA, USA), for 20 additional respiratory pathogen targets (11).
Figure 1.
Respiratory pathogen carriage survey related to Streptococcus pneumoniae serotype 15A outbreak in a pediatric psychiatric hospital, Rhode Island, USA, December 25, 2010–January 31, 2011, performed on Unit 1 patients (n = 16) and symptomatic staff (n = 4) during January 29–February 2. No visitors were screened for respiratory pathogen carriage. Nasopharyngeal (NP) and oropharyngeal (OP) swab specimens were taken from each participant. TaqMan Array Card (TAC) used to test for influenza A (H1 and H3) and B, respiratory syncytial virus, human parainfluenza viruses 1–3, human metapneumovirus, rhinovirus, enterovirus, parechovirus, adenovirus, Legionella species, Haemophilus influenzae, Streptococcus pyogenes, Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Bordetella pertussis. Asterisk indicates other pneumococcal serotypes identified in 3 persons, including 11A/D, 20, 34, and serotypes that were nontypeable by real-time PCR: 1, 2, 3, 4, 5, 6A/B/C, 6C, 7F/7A, 9V/9A, 11A/11D, 12F/(12A/44/46), 14, 15A/15F, 16F, 18/(18A/18B/18C/18F), 19A, 19F, 22F/22A, 23A, 23F, 33F/33A/37.
Twenty patients resided on Unit 1 during December 25, 2010–January 31, 2011 (Table 1). Among Unit 1 patients, staff, and visitors, the following cases were identified: 3 confirmed pneumococcal disease, 6 confirmed pneumonia, and 2 suspected pneumonia (Figure 2). Three case-patients were hospitalized (2 with IPD, 1 with confirmed pneumonia). Among the 20 patients, the cases of 5 (attack rate 25%) met an outbreak case definition (Table 2). In addition, 1 staff member had IPD, 1 had confirmed pneumonia, and 1 had suspected pneumonia (Table 2, attack rate <3%). These staff members provided direct care to all Unit 1 patients. Three visitors had confirmed pneumonia. Adults (3 staff and 3 visitors) who became ill during the outbreak and for whom ages were available (5 of 6) ranged in age from 27 to 56 years.
Table 1. Characteristics of 20 psychiatric unit patients during Streptococcus pneumoniae serotype 15A outbreak, Rhode Island, USA, December 25, 2010–January 31, 2011.
| Characteristic | No. (%) patients |
|---|---|
| Male sex | 14 (70.0) |
| Median age, y (range) | 13 (4–24) |
| Race | |
| White | 16 (80.0) |
| Black | 4 (20.0) |
| Other/unknown | 0 |
| Underlying medical conditions | |
| Asthma* | 6 (30.0) |
| Chronic heart disease | 2 (10.0) |
| Other risk factors for pneumococcal infection† | 0 |
| Median length of stay, days on Unit 1 as of January 30, 2011 (range) | 71 (1–706) |
| Receiving systemic antimicrobial drugs during January for illness unrelated to outbreak | 1 (5.0) |
| No. patients on Unit 1 as of January 30, 2011 | |
| Current in-patient | 15 (75.0) |
| Day program patient‡ | 1 (20.0) |
| Discharged (since December 25, 2010) | 4 (5.0) |
*None were treated with systemic steroids (i.e., none had an indication for vaccine) (3,4). †Includes chronic lung disease (other than asthma), diabetes mellitus, cerebrospinal fluid leaks, cochlear implant, systemic steroid use, sickle cell disease, congenital or acquired asplenia, HIV infection, chronic renal failure, nephritic syndrome or kidney disease with dialysis, bone-marrow or organ transplant, diseases associated with treatment with immunosuppressive drugs or radiation therapy, including malignant neoplasms, leukemia, lymphoma, and Hodgkin disease (3,4). ‡Day program patients were treated in Unit 1 during the day and returned home to stay overnight with their families.
Figure 2.
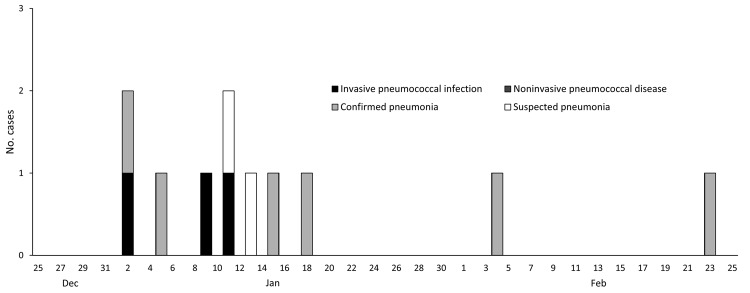
Epidemic curve of Streptococcus pneumoniae outbreak in a pediatric psychiatric hospital, Rhode Island, USA, December 25, 2010–January 31, 2011, for invasive pneumococcal disease, noninvasive pneumococcal disease, confirmed pneumonia, and suspected pneumonia. *Staff members; †family members.
Table 2. Cases, clinical isolates, and results of pneumococcal carriage and respiratory pathogen survey associated with Streptococcus pneumoniae serotype 15A outbreak, Rhode Island, USA, December 25, 2010–January 31, 2011*.
| Case status | Case no. | Onset date | Culture date, specimen and result | Chest imaging results | Treatment | Outcome | Carriage | Sero | Other pathogens by TAC† |
|---|---|---|---|---|---|---|---|---|---|
| IPD | S1 | Jan 9 | Jan 14, blood, S. pneumoniae 15A | Right upper lobe pneumonia | Moxifloxacin | Rec | Neg | Neg | |
| IPD | P1 | Jan 11 | Jan 25, blood, S. pneumoniae 15A | Negative | Ceftriaxone | Rec | Pos | 15A | Rhinovirus, adenovirus |
| Noninvasive pneumococcal disease | P2 | Jan 2 | Jan 28, otorrhea, S. pneumoniae 15A | Cefuroxime, topical ofloxacin | Rec | Pos | 15A | Mycoplasma pneumoniae | |
| Confirmed pneumonia | P3 | Jan 2 | Left lower lobe pneumonia | Amoxicillin/ clavulanate, azithromycin | Rec | Pos | NT‡ | Rhinovirus | |
| Confirmed pneumonia | S2 | Jan 5 | Left upper lobe pneumonia | Amoxicillin/ clavuanate, ciprofloxacin | Rec | NS | NS | ||
| Confirmed pneumonia | V1 | Jan 15 | Right basilar pneumonia | Moxifloxacin | Rec | NS | NS | ||
| Confirmed pneumonia | P4 | Jan 18 | Right lower lobe pneumonia | Ceftriaxone, ampicillin, amoxicillin | Rec | Neg | M. pneumoniae | ||
| Confirmed pneumonia | V2 | Feb 4 | Left lower lobe pneumonia | Doxycycline, levofloxacin | Rec | NS | NS | ||
| Confirmed pneumonia§ | V3 | Feb 23 | Left lower lobe pneumonia | Unk | Unk | NS | NS | ||
| Suspected pneumonia | S3 | Jan 11 | Azithromycin | Rec | NS | NS | |||
| Suspected pneumonia | P5 | Jan 13 | Clindamycin | Rec | Neg | Neg | |||
| Non-case | P6 | Neg | S. pyogenes | ||||||
| Non-case | P7 | Neg | Adenovirus | ||||||
| Non-case | P8 | Neg | Neg | ||||||
| Non-case | P9 | Pos | 34 | S. pyogenes | |||||
| Non-case | P10 | Neg | Neg | ||||||
| Non-case | P11 | Pos | 15A | Enterovirus | |||||
| Non-case | P12 | Pos | 15A | Neg | |||||
| Non-case | P13 | Pos | 11 A/D, 20 | S. pyogenes | |||||
| Non-case | P14 | Neg | Neg | ||||||
| Non-case | P15 | Pos | 15A | Rhinovirus, M. pneumoniae | |||||
| Non-case | P16 | Pos | 15A | M. pneumoniae | |||||
| Non-case | S4 | Neg | Rhinovirus | ||||||
| Non-case | S5 | Neg | Neg | ||||||
| Non-case | S6 | Neg | S. pyogenes |
*Sero, serotype; TAC, TaqMan Array Card; IPD, invasive pneumococcal disease; S, staff; rec, recovered; neg, negative; P, patient; pos, positive; NS, no carriage screen; V, visitor; NT, nontypeable; Unk, unknown. †TAC used to test for influenza viruses A (H1 and H3) and B, respiratory syncytial virus, human parainfluenza viruses 1–3, human metapneumovirus, rhinovirus, enterovirus, parechovirus, adenovirus, Legionella species, Haemophilus influenzae, Streptococcus pyogenes, Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Bordetella pertussis. ‡NT by real-time PCR (for serotypes: 1, 2, 3, 4, 5, 6A/B/C, 6C, 7F/7A, 9V/9A, 11A/11D, 12F/(12A/44/46), 14, 15A/15F, 16F, 18/(18A/18B/18C/18F), 19A, 19F, 22F/22A, 23A, 23F, 33F/33A/37). §Refused further involvement with investigation.
All 3 clinical isolates were identified as S. pneumoniae serotype 15A, sequence type 63, (Table 2) with matching antimicrobial drug susceptibility patterns. Nine (45%) of 20 persons tested carried pneumococcus; of those, 6 (30%) carried serotype 15A. Rhinovirus, Streptococcus pyogenes, and Mycoplasma pneumoniae were additional pathogens most frequently identified by using TaqMan Array Cards (Figure 1, Table 2).
We assessed Unit 1 infection control practices; in particular, for staff compliance with hand and respiratory hygiene. The hand hygiene audit revealed that staff members had performed hand hygiene in 4 (24%) of 17 instances before patient contact and 11 (79%) of 14 times after patient contact, for an overall compliance of 15 (48%) of 31 opportunities. In addition,, supplies (e.g., gloves) were kept in locked cabinets because of safety concerns. Staff reported that patients, because of their developmental delays, were often unable to appropriately manage their respiratory secretions.
The presence of 6 carriers of serotype 15A on Unit 1 during the carriage survey indicated the potential for continued transmission and disease. The hospital mandated hand and respiratory hygiene training for all Unit 1 staff, administered during February 4–8. We recommended high-dose amoxicillin prophylaxis (90 mg/kg/day divided into 2 doses, maximum of 1,000 mg, for 5 days) for all Unit 1 patients, which began on February 7. After control measures were fully implemented, 1 case of pneumonia was confirmed in a patient’s parent on February 23, but the etiology was not identified. No additional cases occurred among Unit 1 patients and staff members during the subsequent 3 months.
Conclusions
Laboratory and epidemiologic evidence indicates that an outbreak of S. pneumoniae serotype 15A infection occurred on Unit 1. Increased transmission opportunities likely resulted from infection control challenges in the unit. Because serotype 15A is not included in any current pneumococcal vaccine, we used antimicrobial drug prophylaxis as an immediate intervention to reduce transmission. However, infection control was the critical long-term control measure. We modified acute-care hospital infection control practices for the unique environment of Unit 1, such as scheduling hand hygiene sessions every 2–3 hours for all patients, rather than expecting patients to understand when to perform hand hygiene. Furthermore, other respiratory pathogens were present on Unit 1, which could have contributed to disease and facilitated pneumococcal transmission by increasing nasopharyngeal colonization (12). The same infection control lapses that led to the S. pneumoniae serotype 15A outbreak likely led to transmission of these respiratory pathogens.
Since the introduction of the 7-valent pneumococcal conjugate vaccine, nasopharyngeal carriage of serotype 15A has increased (13). Among Massachusetts children 3 months–7 years of age, serotype 15A carriage prevalence was 4% in 2007 (14), compared with 30% in this outbreak. Since the introduction of the 7-valent pneumococcal conjugate vaccine, serotype15A sequence type 63 has also caused an increasing proportion of penicillin-nonsusceptible IPD in the United States (15). The sequence type and antimicrobial drug susceptibilities of the serotype 15A strain from this outbreak are characteristic of a single, internationally disseminated serotype 15A strain (15). No other increases in IPD or serotype 15A were detected by the Rhode Island Department of Health during this period, indicating that the outbreak was restricted to Unit 1.
This investigation had several limitations. First, diagnostic tests were performed at the discretion of treating clinicians, and specific diagnostic testing was not performed for most pneumonia case-patients. In addition, we used TaqMan Array Cards <3 weeks after symptom onset, which decreased our ability to detect all bacterial and viral co-factors that possibly contributed to this outbreak. Because of the small size of the patient cohort, statistical tests would not have power to assess risk factors for pneumococcal disease or carriage. Finally, we did not assess the knowledge of hand hygiene among staff after the training to evaluate its effectiveness.
In conclusion, this outbreak was associated with S. pneumoniae serotype 15A, a serotype not included in available pneumococcal vaccines. The outbreak subsided after patients received antimicrobial drug prophylaxis. The hospital instituted a hand hygiene monitoring program in response to our recommendations and enhanced infection control practices were implemented, especially careful adherence to hand and respiratory hygiene. Hand and respiratory hygiene training and monitoring are critical for infection control in units that serve patients with special cognitive needs.
Acknowledgments
We gratefully acknowledge the patients, staff members, and family members of Unit 1 and the staff at the hospital who assisted with the investigation.
Biography
Dr Fleming-Dutra is an Epidemic Intelligence Service officer with the Respiratory Diseases Branch, Division of Bacterial Diseases, National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta. Her research interests include outbreaks of bacterial respiratory diseases and pneumococcal epidemiology.
Footnotes
Suggested citation for this article: Fleming-Dutra K, Mbaeyi C, Link-Gelles R, Alexander N, Guh A, Forbes E, et al. Streptococcus pneumoniae serotype 15A outbreak in psychiatric unit, Rhode Island, USA, 2011. Emerg Infect Dis [Internet]. 2012 Nov [date cited]. http://dx.doi.org/10.3201/eid1811.120454
References
- 1.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–412. 10.1016/j.vaccine.2011.02.088 [DOI] [PubMed] [Google Scholar]
- 2.Basarab M, Ihekweazu C, George R, Pebody R. Effective management in clusters of pneumococcal disease: a systematic review. Lancet Infect Dis. 2011;11:119–30. 10.1016/S1473-3099(10)70281-4 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102–6. [PubMed] [Google Scholar]
- 4.Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1–18. [PubMed] [Google Scholar]
- 5.O’Brien KL, Bronsdon MA, Dagan R, Yagupsky P, Janco J, Elliott J, et al. Evaluation of a medium (STGG) for transport and optimal recovery of Streptococcus pneumoniae from nasopharyngeal secretions collected during field studies. J Clin Microbiol. 2001;39:1021–4. 10.1128/JCM.39.3.1021-1024.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Gloria Carvalho M, Pimenta FC, Jackson D, Roundtree A, Ahmad Y, Millar EV, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J Clin Microbiol. 2010;48:1611–8. 10.1128/JCM.02243-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol. 2006;44:124–31. 10.1128/JCM.44.1.124-131.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. PCR deduction of pneumococcal serotypes [cited 2011 Jan 29]. http://www.cdc.gov/ncidod/biotech/strep/PCR.htm
- 9.Multi locus sequence typing. Streptococcus pneumoniae [cited 2011 Jan 29]. http://spneumoniae.mlst.net/
- 10.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-first information supplement. Wayne (PA): The Institute; 2011. [Google Scholar]
- 11.Kodani M, Yang G, Conklin L, Travis T, Whitney C, Anderson L, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49:2175–82. 10.1128/JCM.02270-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23(Suppl):S87–97. 10.1097/01.inf.0000108197.81270.35 [DOI] [PubMed] [Google Scholar]
- 13.Sá-Leão R, Nunes S, Brito-Avo A, Frazao N, Simoes AS, Crisostomo MI, et al. Changes in pneumococcal serotypes and antibiotypes carried by vaccinated and unvaccinated day-care centre attendees in Portugal, a country with widespread use of the seven-valent pneumococcal conjugate vaccine. Clin Microbiol Infect. 2009;15:1002–7. 10.1111/j.1469-0691.2009.02775.x [DOI] [PubMed] [Google Scholar]
- 14.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–11. 10.1542/peds.2008-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gertz RE Jr, Li Z, Pimenta FC, Jackson D, Juni BA, Lynfield R, et al. Increased penicillin nonsusceptibility of nonvaccine-serotype invasive pneumococci other than serotypes 19A and 6A in post-7-valent conjugate vaccine era. J Infect Dis. 2010;201:770–5. 10.1086/650496 [DOI] [PubMed] [Google Scholar]