Abstract
Topical application of 400µg of the juvenile hormone analog, methoprene, to females of the penultimate instar of Leucophaea maderae failed to induce vitellogenin synthesis. However, last instar females showed an increasing response level in making vitellogenin as they aged during the first half of the instar. In the second half of the last instar the response to methoprene declined to nearly zero when the prothoracic glands have become highly active. Then, a few days before the metamorphic molt the responsiveness reached maximal levels, i.e., comparable to adult females. These data suggest that the fat body develops competency to produce vitellogenin during the last nymphal instar, but increasing titers of ecdysone then interfere with the action of methoprene and consequently production of vitellogenin is curtailed.
When prothoracic glands from the second half of the last instar were implanted into adult females, the normal activation of the corpora allata, or their accelerated activation induced by mating, did not occur. Likewise, an activation of the corpora allata due to the severance of the NCCI was not observed when prothoracic glands had been implanted prior to such operations. Thus, ecdysone released by the prothoracic glands appeared to directly inhibit the isolated corpora allata in vivo i.e. without the mediation by the brain.
Methoprene applied to allatectomized adult females induced vitellogenin synthesis in a dose dependent manner. This induction was, however, quantitatively reduced by implanted active prothoracic glands, particularly when low doses of methoprene had been applied. Methoprene higher than 5µg overcame the inhibitory potency of the implanted prothoracic glands. The effect of the prothoracic glands, i.e. ecdysone, appears to signal an interference with the action of methoprene at the target tissues, the fat body. The exposure of the fat body to a given juvenile hormone/ecdysone ratio dictates the apparent effectiveness of ecdysone. The precise mode of the interaction of juvenile hormone and ecdysone on the adult fat body is not known.
These data show that ecdysone inhibits vitellogenesis by two independent mechanisms: 1) inhibition of the corpora allata resulting in the inhibition of juvenile hormone production and 2) inhibition of vitellogenin synthesis by the fat body. Both of these mechanisms appear to be operative in immature and mature animals. However, the action of ecdysones on the fat body is only seen after it had acquired competency to make vitellogenin during the last nymphal instar.
Keywords: ecdysone, lipophorin, methoprene, vitellogenin, Leucophaea maderae, vitellogenesis
Introduction
The making of a yolky egg, i.e. vitellogenesis, requires the controlled massive synthesis of the yolk protein precursor, vitellogenin, as well as its controlled incorporation into the oocytes in a timely fashion. In most species of insects the predominant yolk protein, vitellin, is derived primarily from the extraovarially synthesized vitellogenin that is usually only identified in the adult female. The question then arises as to the details of the various control mechanisms involved in vitellogenin production and normal vitellogenesis in the mature female.
For the majority of the investigated insect species it has been documented that juvenile hormone stimulates the transcription of the vitellogenin genes and the consequent control of vitellogenin production (cf. Engelmann, 1983; Wyatt and Davey, 1996). However, it has also been shown that ecdysteroids (ecdysones) can be involved directly or indirectly in vitellogenin production in a limited number of insect species, particularly certain Diptera (Dhadialla and Raikhel, 1994; cf. Hagedorn, 1994). Both juvenile hormone and ecdysone occur in all immature insects simultaneously, but peak levels often do not coincide. We also know that vitellogenesis is not observed in most immature insects, even in the presence of high titers of juvenile hormone and ecdysone. This indicates that additional factors must play crucial roles in regulating the timing of vitellogenin synthesis in the adult female. For example, competency of the fat body to produce vitellogenin appears to be attained only during the pre-adult period as part of the metamorphic development of the animal. This has been shown conclusively, for example, for the cockroach Leucophaea maderae (Don-Wheeler, 1996). Also, high concentrations of free ecdysones do not occur in the hemolymph of the non-Dipteran adult insects in a timely pattern, and therefore, juvenile hormone appears to be the major controlling agent associated with vitellogenin synthesis in the mature female of most insect species.
In this present communication I will attempt to provide experimental information regarding juvenile hormone and ecdysone interactions in immature and adult females as it relates to the control of the corpora allata and the stimulation of vitellogenin synthesis in the target tissues and subsequent vitellogenesis. The model species is the cockroach Leucophaea maderae.
Materials and Methods
Animals and operations
Females of the cockroach Leucophaea maderae were used in all experiments and observations. Stock colonies and experimental animals were kept at 26°C and 70% relative humidity. All animals were fed rodent chow (Purina, www.purina.com) and water. Operations, such as allatectomy, severance of the cardiaca or allatal nerves, implantation of prothoracic glands or nymphal muscles (as controls), were performed under CO2 anesthesia. Routinely three pairs of prothoracic glands from the second half of the last instar nymphal period were implanted into the adult females. For females the average duration of the last nymphal instar is 38 days, with a range of 34 to 45 days.
Treatments
Topical applications of the juvenile hormone analog methoprene were performed under CO2 anesthesia. The appropriate amounts of methoprene were dissolved in acetone and 5µl or 10µl of the solution was applied to the abdominal terga. The amounts of applied methoprene varied and are indicated for each of the experiments in the respective result sections. Controls received acetone only.
Quantitative determinations of vitellogenin and lipophorin
Hemolymph was collected following the indicated intervals after operations or hormone treatments. For this the neck was ligated, a hole was cut posterior to the ligation and the animal was then centrifuged in a clinical centrifuge head down at low speed. Between 100µl and 200µl of hemolymph was collected from each animal. Hemolymph clots were removed by centrifugation in a clinical centrifuge at 3000g and the sera stored at −25°C until use. Following bleeding the experimental animals were dissected and the oocyte lengths were measured. For quantification of vitellogenin and lipophorin in these sera, rocket immunoelectrophoresis was employed as described earlier (Engelmann, 1978). Polyclonal specific anti-vitellogenin and anti-lipophorin was used respectively for these assays. The sensitivity limit in these assays was about 0.1mg protein/ml hemolymph. Total hemolymph proteins were determined by the method of Lowry et al., 1951, using bovine serum albumin (Sigma, www.sigmaaldrich.com) as a standard.
Rates of vitellogenin synthesis
Animals were injected with 50 µCi of Trans 35S-methionine/cysteine (specific activity 1,080 Ci/mmole; ICN, www.icnbiomed.com) four hours prior to bleeding. The hemolymph samples were diluted 10fold with Tris buffered saline (0.05M Tris pH 7.5, containing 0.4M NaCl) and the radiolabeled vitellogenin precipitated with anti-vitellogenin for two hours at 4°C. A few µg of unlabeled vitellogenin was added to facilitate precipitation when the quantity of vitellogenin in the sample was low. The precipitates were pelleted by centrifugation in a Beckman (www.beckman.com) Microfuge B (8,700 g) for four minutes and washed four times with immunoprecipitation buffer (0.1M Tris pH 7.5, 0.4M NaCl, 1% (w/v) Triton X-100, and 1% sodium deoxycholate). The pellets were solubilized in 25µl of 1N NaOH and added to one ml of EcoLite™ (ICN) scintillation cocktail. The radioactivity was determined by liquid scintillation spectrometry in a Beckman LS 7000.
Results
Vitellogenesis in nymphs of the cockroach Leucophaea
Vitellogenins and vitellogenesis have never been observed in any nymphal instars of the cockroach Leucophaea. Reasons for this observation may be several fold: inadequate titers of juvenile hormone, lack of competency of the fat body, interference by other hormones, or a combination of any of these factors. It was, therefore, of interest to know whether the fat body of immature animals is capable to synthesize vitellogenin when stimulated with very high doses of methoprene.
Methoprene (400 µg) was topically applied one time to precisely staged females of penultimate and last instar nymphs. The animals were injected with radiolabelled amino acids 5 days later, bled, the vitellogenin precipitated from the hemolymph by anti-vitellogenin, and quantified. The very high dose of the methoprene may have produced an analog titer within these animals of approximately 6.5 mM, assuming that most of the methoprene enters the hemolymph compartment. This concentration is considerably higher than the juvenile hormone titers measured in normal vitellogenic females (µM range) and exceeds by far what is necessary to induce vitellogenin synthesis in adult females. Even this high dose of methoprene did not induce vitellogenin synthesis in penultimate females. On the other hand, in last instar nymphs an increasing response level was observed as the animals aged within the instar (Fig. 1). During the second half of the last instar the level of the response declined to nearly zero, but then rose shortly before the metamorphic molt to the level seen in adult females. Untreated adult females normally produce vitellogenin earliest at day 7 after the metamorphic molt. The varying rates of vitellogenin synthesis were reflected in the concentration of vitellogenin in circulation (Fig. 2). In these experiments the animals were treated with 200µg of methoprene for six rather than five days. As is seen, the profile of the vitellogenin titer in the hemolymph paralleled that of the rate of vitellogenin synthesis. A limited vitellogenic oocyte growth occurred, but the incorporation of vitellogenin was quantitatively limited in spite of the high vitellogenin titers in the hemolymph; no mature eggs were produced. No fully grown eggs were made even after repeated methoprene applications and allowing two to three weeks of observation (data not shown). The rise in the inducibilty of vitellogenin synthesis during the first half of the instar in response to methoprene suggests a progressive development of fat body competency.
Figure 1.
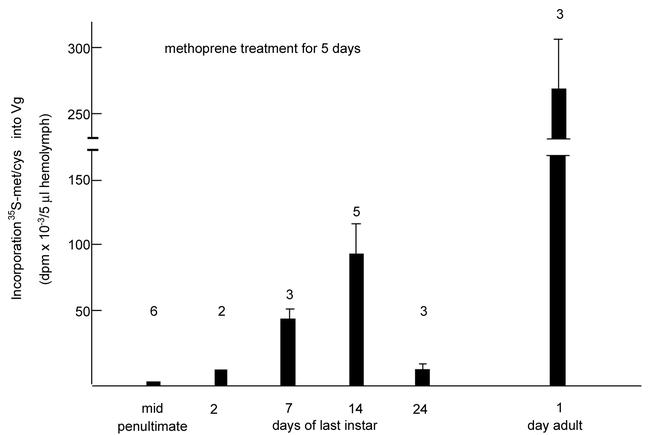
Vitellogenin synthesis in penultimate and last instar nymphs of Leucophaea maderae. A single dose of 400µg of methoprene was topically applied at the days indicated. Five days later the animals were radiopulsed for 4 hours, bled, and the vitellogenin precipitated from aliquots by anti-vitellogenin. Control animals had received 5µl of acetone by topical application. No vitellogenin synthesis was induced by acetone treatment. Columns represent averages ± SE of the mean. Numbers above the columns are the number of animals used. Unpublished data from Don-Wheeler, 1996.
Figure 2.
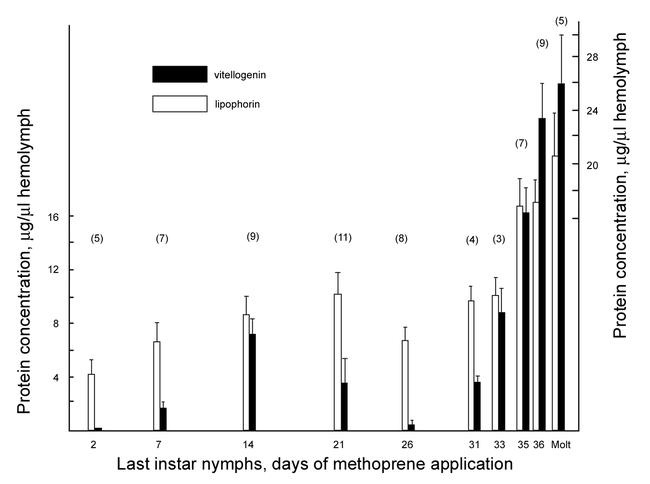
Vitellogenin and lipophorin titers in the hemolymph of last instar nymphs of Leucophaea maderae following the application of 200µg methoprene at the days indicated. The animals were bled 6 days after the methoprene application and the amounts of vitellogenin and lipophorin were determined in aliquots of the hemolymph by rocket immunoelectrophoresis. The metamorphic molt occured on average on day 38 of the last instar. Columns represent the averages ± SE of the mean of the number of animals indicated above the columns.
In parallel with the assays for vitellogenin content in the hemolymph, the titers of lipophorin were determined throughout the last nymphal instar period as well (Fig.2) in order to provide a complete picture as to the effect of the methoprene during this nymphal instar. Methoprene augmented the level of lipophorin in circulation during the few days before the metamorphic molt to twice that measured in normal (Engelmann and Mala, 2000) and treated females from the first half of the instar (Fig. 2). At emergence lipophorin titers of untreated last instar nymphs generally are below 1µg/µl hemolymph. This observation suggests an induced augmented synthesis of lipophorin during this time, just as it had been shown for the mature females (Engelmann and Mala, 2000).
We must ask the question as to what causes the decline of the response level during the second half of the last nymphal instar? Two possibilities come to mind: a decreased food intake prior to the metamorphic molt (Don-Wheeler, 1996), or the onset of a massive production of ecdysones by the prothoracic glands that interferes with the response to methoprene. In the late last instar nymphs vitellogenin production is indeed curtailed by a reduced feeding and by the high titers of ecdysones (see below).
Ecdysteroids inhibit the corpora allata in adult females
In an earlier publication (Engelmann, 1959) I showed that implantation of active prothoracic glands into adult females of Leucophaea or the application of 20-hydroxyecdysone reduced the activity of the corpora allata and consequently also vitellogenesis. The details regarding the mode of action of ecdysone were not known and we now ask whether ecdysone acts directly on the corpora allata or via the brain by decreasing the release of allatotropins or, in reverse, increasing the production and release of certain factors, such as allatostatin, that inhibit the corpora allata.
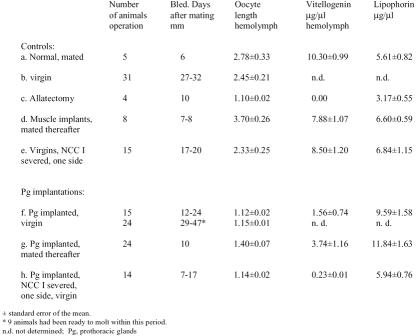
For an analysis of this question, a series of surgical interventions were performed in adult females of Leucophaea in conjunction with the implantation of active prothoracic glands from the second half of last nymphal instar. In a pilot study it was shown that prothoracic glands from the first half of this instar were only marginally effective, and therefore, only glands from the latter part of the instar were used for this further analysis. For most animals, oocyte lengths, and vitellogenin and lipophorin titers were determined upon conclusion of the experiments (Table 1).
Table 1.
Hemolymph vitellogenin and lipophorin titers and length of the basal oocytes of adult female Leucophaea maderae, following the implantation of active prothoracic glands.
Several types of controls are listed. On average females mate between days 8 and 14 after emergence. This stimulates complete oocyte growth (5.5 mm) within 21±0.3 days (Engelmann, 1960). In virgins, the rates of oocyte growth are highly variable beginning at the earliest on day 7 or 8. On average oocyte length is less than half of the mature size 4 to 5 weeks after emergence (Table 1,b). This is the result of a reduced rate of vitellogenin production and in some animals a delayed onset of vitellogenesis (Engelmann, 1960). Implantation of prothoracic glands into virgins further reduced oocyte growth to nearly zero, i.e. oocyte length was that of the newly emerged females; a very low vitellogenin titer was determined (Table 1,f). Severance of the cardiaca nerves in virgins removes the inhibition of the corpora allata by the brain and mimics the stimulatory effect of normal mating (Engelmann, 1957, 1960). As is seen (Table 1,e), unilateral severance of the NCCI, that caused the activation of the same side corpora allata (Engelmann 1957), induced an elevated vitellogenin titer in the hemolymph followed by a faster vitellogenic oocyte growth than in unoperated virgins (Table 1,b). About three weeks after this operation, oocyte lengths and vitellogenin titers were comparable to that of females, one week after mating. However, when prothoracic glands had been implanted into the abdomen prior to such operations the corpora allata appeared to remain relatively inactive and juvenile hormone titers must have been low since no or little vitellogenin was detected (Table 1,h). Mating after the implantation of prothoracic glands did not overcome the ecdysone-induced inhibition of the corpora allata either (Table 1,g). As a further control operation, muscle tissues from nymphs were implanted. These females responded to mating like normal animals, i.e., no inhibitory effect on vitellogenin production was observed (Table 1,d).
Table 1 contains a further finding of significance. All operated animals with active corpora allata exhibited an augmented hemolymph lipophorin titer compared to allatectomized females, reaching the elevated levels of normal mated females. Interestingly, in all of the series where active prothoracic glands had been implanted, lipophorin production was augmented as well and was expressed as elevated lipophorin concentration in the hemolymph. This occurred even though the corpora allata were either inactive or only moderately active (Table 1,f–h). This observation was not investigated further in the present context.
A substantial number of adult females had produced a complete new cuticle in preparation for another molt in response to the implanted prothoracic glands when the animals were allowed to live for five or more weeks after the implantation of the prothoracic glands (Table 1,f). The average molt interval for last instar nymphs is 5 to 6 weeks. This observation is taken as evidence that the implanted prothoracic glands were functional in the adult milieu and had released substantial amounts of ecdysone. These animals were, however, physically not able to shed their old cuticle, because adults lack the pronotal dorsal suture which is essential for splitting the old cuticle, and furthermore, wings lack the epithelium to produce a new cuticle. Also, the old cuticular lining of the tracheae of such animals cannot be removed and the animals eventually die due to suffocation.
The sum of these results shows that the prothoracic glands, i.e. the released ecdysone, directly inhibited the corpora allata and that the brain is not involved as an intermediary controlling organ in this case, unless one invokes the possibility that ecdysone caused a massive release of inhibitory substances, such as allatostatin, which then act via the circulation rather than the normal route through the nervous connection from the brain.
Ecdysteroids inhibit vitellogenin synthesis by the adult fat bodies
Application of juvenile hormone III to allatectomized adult females of Leucophaea induces vitellogenin synthesis (Engelmann, 1969). However, when 20-hydroxyecdysone was given simultaneously or within two or three days following a treatment with juvenile hormone such vitellogenin synthesis was curtailed in an ecdysone dose dependent manner (Engelmann, 1971). For a further analysis of the interaction of the two hormones, particularly the effect of ecdysone on vitellogenin synthesis, the time sequence of hormone treatments was reversed.
Allatectomized females received three pairs of active prothoracic glands and about two weeks thereafter methoprene was topically applied in the indicated doses. Three days after methoprene treatment the animals were bled and the vitellogenin in circulation quantified. Application of more than 5µg of methoprene resulted in a comparable rate of vitellogenin synthesis as in the control animals which had received only methoprene. Given enough time (two to three weeks) these latter animals produced fully grown eggs (data not shown). High doses of methoprene presumably resulted in a methoprene titer that exceeded by far the normal juvenile hormone titer and consequently the quantity of ecdysone secreted by the implanted prothoracic glands was out of balance. However, when lower doses of methoprene were given the quantity of ecdysone released by the implanted prothoracic glands caused a significant reduction in vitellogenin synthesis (Fig. 3) and nearly completely inhibited vitellogenin synthesis when the methoprene dose was as low as 0.5µg. Assuming an average hemolymph volume of 200µl for these animals, a methoprene titer of 9 × 10−6 M was estimated following the application of 0.5µg of the analog. This concentration is close to the juvenile hormone titer determined during the height of normal vitellogenesis in Leucophaea (Engelmann, 1986) or in Diploptera (Tobe et al., 1984). Interestingly, when methoprene was allowed to act longer than three days, the inhibitory potency of ecdysone was diminished even in these cases (data not shown). This latter observation may reflect the fact that methoprene is not readily metabolized and a high titer persisted and eventually overcame the effect of ecdysone (see Discussion).
Figure 3.
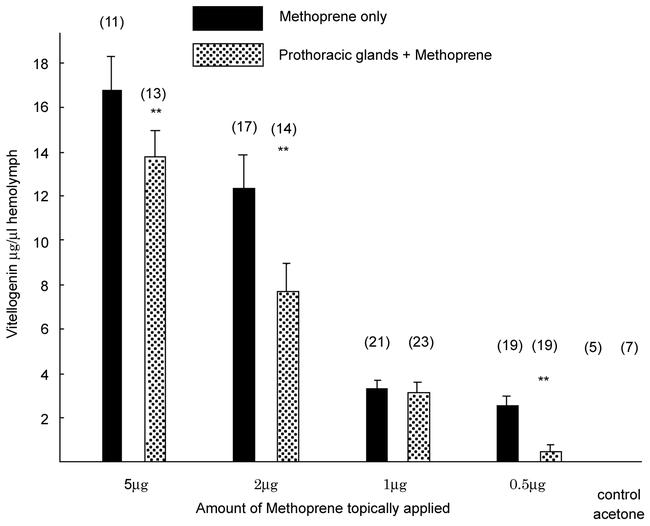
Methoprene stimulated vitellogenin levels in allatectomized adult virgin females of Leucophaea maderae. Each female received three active prothoracic glands about two weeks prior to the application of methoprene in the doses indicated. Controls received the solvent only. Three days after methoprene treatment the animals were bled and the vitellogenin content of aliquots of the hemolymph was determined by rocket immunoelectrophoresis. Columns represent the averages ± SE of the mean of the number of animals indicated above the columns. ** denotes a P-value of < 1‰ (student t-test).
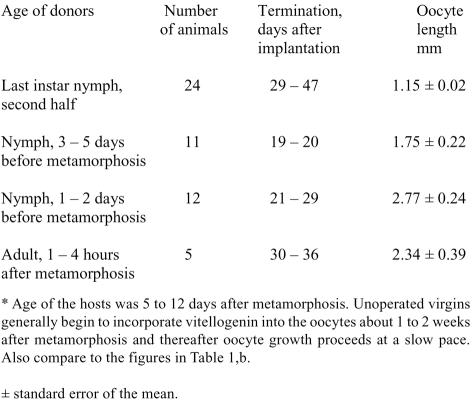
In a further experimental series, adult virgin females received prothoracic glands from nymphs that were about to undergo the metamorphic molt. A progressive loss of inhibitory potency of such prothoracic glands was seen beginning about 5 to 6 days prior to the final molt (Table 2). Four to five weeks after metamorphosis of the host, growth of the terminal oocytes in the host's ovaries was on average comparable to that of normal virgin females for that time interval (see Table 1,b). Obviously, prothoracic glands no longer release ecdysone at this time and glandular atrophy has been initiated prior to the actual metamorphic molt. At present we do not know the trigger for the functional atrophy of the prothoracic glands shortly before metamorphosis.
Table 2.
Prothoracic glands inhibit growth of the basal oocytes in Leucophaea maderae. Implantation of prothoracic glands into adult virgin females*
The data provided in this section show that the ecdysone secreted by the implanted prothoracic glands acts directly on the fat body and curtails the juvenile hormone induced synthesis of vitellogenin. It is also evident that after the fat body had attained the capability to produce vitellogenin during the last nymphal instar, and was subsequently inhibited by ecdysone, it had not lost the full responsiveness to juvenile hormone and synthesized vitellogenin: mature eggs were produced when stimulated by large amounts of methoprene.
Discussion
In the cockroach Leucophaea, ecdysteroids secreted by nymphal prothoracic glands, when introduced into the adult milieu, appear to inhibit vitellogenesis by two independent modes: direct inhibition of the corpora allata resulting in the lack of juvenile hormone production, and inhibition of vitellogenin synthesis by the fat body. The latter effect is not detectable in the early nymphal instars. However, after the females have attained the ability to synthesize vitellogenin, ecdysone appears to inhibit vitellogenin synthesis as in the adult female.
The product of the prothoracic glands which causes the inhibition of vitellogenesis in all likelihood is ecdysone, which has been shown to be secreted by this tissue (Borst and Engelmann, 1974). This is supported by the observation that the prothoracic glands induce a molt of the adult host and the effectiveness of these glands drops off toward the end of the last nymphal instar. This is the time when ecdysone production is reduced and the prothoracic glands are programmed to atrophy. Previous studies (Engelmann, 1971) had shown that treatment of adult Leucophaea with 20-hydroxyecdysone decreased vitellogenin synthesis in a dose dependent manner. Furthermore, ecdysone released by the implanted prothoracic glands effectively reduced vitellogenin production, particularly when low doses of methoprene had been applied (Fig. 3).
Ecdysones are known to inhibit vitellogenesis in a number of species from different insect orders. Among these are Gryllus domesticus (Thomas, 1964), Musca domestica (Robbins et al., 1968) Locusta migratoria (Cassier, 1970), Stomoxys calcitrans (Wright and Kaplanis, 1970), Danaus plexippus (Herman and Baker, 1976), Rhodnius prolixus (Garcia et al., 1979), and Diploptera punctata (Friedel et al, 1980). With the exception of Diploptera, most of these reports consist of primarily correlative observations of reduced activation or complete inhibition of egg production after ecdysone had been introduced into the adult females either by implantation of prothoracic glands, or injection/ingestion of ecdysones.
The introduction of ecdysteroids into the adult milieu may represent an anomaly for many adult insect species. Certainly, adults do no longer molt and the prothoracic glands (the main source for ecdysone) atrophy around the metamorphic molt, with the exception of Thysanuran species. However, for a number of species it has been shown that ovaries produce ecdysteroids and may release these molecules into the hemolymph where titers can reach high levels for short periods (cf. Bellés, 1998). Whether these latter observations represent artifacts or are part of a normal reproductive cycle has been debated in the literature (Bellés, 1998) and firm conclusions are difficult to reach. On the other hand, implantations of active prothoracic glands into the adult animal presumably generate a sustained high level of ecdysones in the host, and therefore, cannot be compared to the assumed normal phasic release of ecdysone by the ovaries. We deal with an anomaly that allows us to analyze the interaction of the two major insect hormones and extend these conclusions to events in the immature insects.
For Leucophaea it was thought that ecdysone inhibited the production of juvenile hormone by the corpora allata and thus curtailed yolk formation in the oocytes (Engelmann, 1959). The observation of a reduced size of the corpora allata, indicative for glandular inactivity, supported this conclusion. Further support for this idea was given for Gryllus (Thomas, 1964) and Diploptera (Friedel et al., 1980). For Diploptera it was indeed shown that ecdysone caused a reduced synthesis and release of juvenile hormone. For the latter species it was then conjectured that in response to the high ecdysone titers in circulation a humoral factor such as allatostatin was released by the brain or that ecdysone inhibited the corpora allata (Friedel et al., 1980) by acting like an allatostatin. However, no actual determinations of allatostatin titers were made in conjunction with these observations at that time. I show here that inhibition of the corpora allata may not be mediated by allatostatins released by the brain. The precise mechanism by which ecdysone inhibits the synthesis of juvenile hormone by the corpora allata is currently not understood. In Leucophaea it has been observed that following the implantation of prothoracic glands into the adult females nearly every cell of the corpora allata undergoes a mitotic division (Engelmann 1959). The massive mitotic activity in the corpora allata may render the cells temporarily incapable of producing large quantities of juvenile hormone.
Direct inhibition of vitellogenin synthesis by ecdysones was first suggested when ecdysone was applied to head ligated animals or isolated abdomina of Leucophaea (Engelmann, 1971). In these assays inhibition of vitellogenin synthesis was less than complete, presumably because of the short half-life of ecdysone in the milieu of an intact animal. I here reinvestigated and expanded these experiments by implanting highly active prothoracic glands, which were shown to sustain their activity and release ecdysone in the adult milieu because they were capable of inducing an additional molt. Furthermore, the use of the juvenile hormone analog, methoprene, which is known to have a long half-life, was used to allow a better insight into the mode of interaction of these hormones. Allatectomized females rather than head ligated animals were used for these long term observations.
The results suggest that the degree of stimulation of vitellogenin synthesis was determined by the ratio of juvenile hormone/ecdysone to which the fat bodies were exposed. The exact concentration of ecdysone in these experimental animals is, however, not known, but one may assume that it was rather high, since in a substantial number of these adult females the epidermis produced a new cuticle within 5 to 7 weeks. Concentrations of methoprene in the low micromolar range were only moderately effective in inducing vitellogenin synthesis in the presence of ecdysone, but levels in the millimolar range induced vitellogenin synthesis at a high rate. From these observations one may assume that juvenile hormone and ecdysone compete for the access to the machinery that responds to juvenile hormone in producing vitellogenin. High methoprene titers appear to out compete the available ecdysone molecules, but as was shown earlier, ecdysone did not compete for the same high affinity juvenile hormone binding sites which were extractable from cytosol of the vitellogenic fat body (Engelmann, 1981). Currently nothing is known about high affinity ecdysone binding sites in the fat body of adult females of Leucophaea.
Ecdysone titers have not been determined in normal adult females of Leucophaea. However, ecdysone titer determinations for the adults of several species have been made and evidence is given in a few cases that ecdysone originates in vitellogenic ovaries of these adult animals. In addition to findings in females of Aedes and some additional Dipteran species (cf. Hagedorn, 1994), both ecdysone and juvenile hormone titers have been monitored in some Orthopteran and Dictyopteran species (cf, Bellés, 1998). The observations in non-Dipteran insects lead to the speculation that ecdysone has a role in determination of a normal vitellogenic cycle (cf. Bellés, 1998). Generally, ecdysone levels become prominent towards the end of vitellogenesis after the peaks in juvenile hormone and vitellogenin synthesis. This suggests that ecdysone is involved in termination of vitellogenin synthesis, e.g. in Blattella germanica. The earlier experimental findings for Leucophaea and the results given here appear to support such a conclusion.
Juvenile hormone supported vitellogenin synthesis, i.e. vitellogenesis, and ecdysone-stimulated molting seem to be mutually exclusive events in non-Dipteran species. This is indeed documented for the Thysanuran Thermobia domestica in which egg production alternates with molting (Rousset and Bitsch, 1993). In this species, vitellogenin production occurs during the early part of the intermolt period when high juvenile hormone levels are observed, but at the time of high ecdysone titers before the molt juvenile hormone titers are low and virtually no vitellogenin synthesis occurs. From these observations one may deduce that ecdysone inhibits juvenile hormone synthesis and vitellogenin production during the second half of the intermolt period.
In adult insects the interaction of the two major hormones, juvenile hormone and ecdysone, appears to be at the target tissues, such as the corpora allata and/or the fat body. Ecdysone inhibits the production of juvenile hormone as well as the action of juvenile hormone in production of vitellogenin by the fat body. However, the reverse may occur in certain immature Lepidoptera, where juvenile hormone may stimulate the production of ecdysone. For example, in larvae or pupae of Mamestra brassicae the application of methoprene stimulated the prothoracic glands to produce more ecdysone and caused the animals to molt earlier than normal (Hiruma et al., 1978). However, this effect may not have been the result of a direct stimulation of the prothoracic glands but rather was the effect of the production of a hemolymph factor (not the prothoracicotropic hormone) which in turn stimulated the prothoracic glands. For Manduca sexta such a factor has been identified (Watson et al., 1988).
Acknowledgments
Some of the earlier works incorporated in this report was supported by grants from the National Science Foundation. The support is gratefully acknowledged. Support by ‘20th century graphics’ for much of the recent research is acknowledged. Mr. Sam Oda provided technical support. Methoprene was a gift by Professor David Schooley, University of Nevada, Reno, Nevada. I thank Dr. David Borst, Illinois State University, Normal, Illinois for reading a draft of this paper and making valuable suggestions.
Glossary
| Abbreviation: | |
|---|---|
| NCCI | The interior corpus cardiacum nerve: nervus corporis cardiacis interna |
This article was featured in the Ecdysone Workshop
References
- Bellés X. 1998 Endocrine effectors in insect vitellogenesis. In: Coast GM, Webster SG, editors. Recent Advances in Arthropod Endocrinology. pp. 71–90.Cambridge University Press, Cambridge. [Google Scholar]
- Borst DW, Engelmann F. In vitro secretion of a-ecdysone by prothoracic glands of a hemimetabolous insect, Leucophaea maderae (Blattaria) Journal of Experimental Zoology. 1974;189:413–419. doi: 10.1002/jez.1401890315. [DOI] [PubMed] [Google Scholar]
- Cassier P. 1970 Influence des conditions d'élevage sur la fécondité des femelles de Locusta migratoria migratorioides (R. et F.) et sur les caractéristiques de leur descendance: donnée endocrines. Colloques Internationaux du CNRS No 189. 87–111. [Google Scholar]
- Dhadialla TS, Raikhel AS. 1994 Endocrinology of mosquito vitellogenesis. In: Davey KG, Peter RE, Tobe SS, editors. Perspectives in Comparative Endocrinology. pp. 275–281.National Research Council of Canada, Ottawa. [Google Scholar]
- Don-Wheeler G. 1996 The development of the vitellogenic competence of the fat body and ovary to respond to juvenile hormone in Leucophaea maderae. Thesis. University of California, Los Angeles, California. [Google Scholar]
- Engelmann F. Die Steuerung der Ovarfunktion bei der ovoviviparen Schabe Leucophaea maderae (Fabr.) Journal of Insect Physiology. 1957;1:257–278. [Google Scholar]
- Engelmann F. Über die Wirkung implantierter Prothoraxdrüsen im adulten Weibchen von Leucophaea maderae (Blattaria) Zeitschrift für Vergleichende Physiologie. 1959;41:456–470. [Google Scholar]
- Engelmann F. Mechanisms of controlling reproduction in two viviparous cockroaches (Blattaria) Annals of the New York Academy of Sciences. 1960;89:516–536. [Google Scholar]
- Engelmann F. Female specific protein synthesis: biosynthesis controlled by corpus allatum in Leucophaea maderae. Science. 1969;165:407–409. doi: 10.1126/science.165.3891.407. [DOI] [PubMed] [Google Scholar]
- Engelmann F. Endocrine contol of insect reproduction, a possible basis for insect control. Acta Phytopathologica Academiae Scientiarum Hungaricae. 1971;6:211–217. [Google Scholar]
- Engelmann F. Synthesis of vitellogenin after long-term ovariectomy in a cockroach. Insect Biochemistry. 1978;8:149–154. [Google Scholar]
- Engelmann F. Heterogeneity of juvenile hormone binding compounds in fat bodies of a cockroach. Molecular and Cellular Endocrinology. 1981;24:103–112. doi: 10.1016/0303-7207(81)90082-4. [DOI] [PubMed] [Google Scholar]
- Engelmann F. 1983 Vitellogenesis controlled by juvenile hormone. In: Downer RGH, Laufer H, editors. Endocrinology of Insects. pp. 259–270.Alan R. Liss, Inc. New York. [Google Scholar]
- Engelmann F. Endocrine regulated insect vitellogenesis: A synthesis. Advances in Invertebrate Reproduction. 1986;4:31–37. [Google Scholar]
- Engelmann F, Mala J. The interactions between juvenile hormone (JH), lipophorin, vitellogenin, and JH esterases in two cockroach species. Insect Biochemistry and Molecular Biology. 2000;30:793–803. doi: 10.1016/s0965-1748(00)00051-5. [DOI] [PubMed] [Google Scholar]
- Friedel T, Feyereisen R, Mundall EC, Tobe SS. The allatostatic effect of 20-hydroxyecdysone on the adult viviparous cockroach, Diploptera punctata. Journal of Insect Physiology. 1980;26:665–670. [Google Scholar]
- Garcia MLM, Mello RP, Garcia ES. Ecdysone, juvenile hormone and oogenesis in Rhodnius prolixus. Journal of Insect Physiology. 1979;25:695–700. doi: 10.1016/0022-1910(79)90121-5. [DOI] [PubMed] [Google Scholar]
- Hagedorn HH. 1994 The endocrinology of the adult female mosquito. In. Advances in Disease Vector Research. 10:109–148. [Google Scholar]
- Herman WS, Baker JF. Ecdysterone antagonism, mimicry, and synergism of juvenile hormone action on the monarch butterfly reproductive tract. Jorunal of Insect Physiology. 1976;22:643–648. [Google Scholar]
- Hiruma K, Shimada H, Yagi S. Activation of the prothoracic gland by juvenile hormone and prothoracicotropic hormone in Mamestra brassicae. Jorunal of Insect Physiology. 1978;24:215–220. [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RH. Protein measurement with a folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Robbins WE, Kaplanis JN, Thompson MJ, Shortino TJ, Cohen CF, Joyner SC. Ecdysones and analogs: effects on development and reproduction of insects. Science. 1968;161:1158–1159. [PubMed] [Google Scholar]
- Rousset A, Bitsch C. Comparison between endogenous and exogenous yolk proteins along an ovarian cycle in the firebrat Thermobia domestica (Insecta. Thysanura) Comparative Biochemistry and Physiology. 1993;104B:33–44. [Google Scholar]
- Thomas A. Etude expérimentale relative au contrôle endocrine de l'ovogénèse chez Gryllus domesticus L. Bulletin de la Société Zoologique de France. 1964;89:835–854. [Google Scholar]
- Tobe SS, Stay BA, Baker FC, and Schooley DA. 1984 Regulation of juvenile hormone titer in the adult females cockroach Diploptera punctata. In: Hoffmann J, Porchet M, editors. Biosynthesis, Metabolism and Mode of Action of Invertebrate Hormones. pp. 397–406.Springer-Verlag Berlin Heidelberg. [DOI] [PubMed] [Google Scholar]
- Watson RD, Haire ME, Bollenbacher WE. Juvenile hormone regulates the titer of a hemolymph factor that enhances ecdysone synthesis by insect (Manduca sexta) prothoracic glands in vitro. Archives of Insect Biochemistry and Physiology. 1988;9:157–165. [Google Scholar]
- Wright JE, Kaplanis JN. Ecdysone and ecdysone-analogues: effects on fecundity of the stable fly, Stomoxys calcitrans. Annals of the Entomological Society of America. 1970;63:622–623. [Google Scholar]
- Wyatt GR, Davey KG. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormones in adult insects. Advances in Insect Physiology. 1996;26:1–155. [Google Scholar]