Abstract
Background
Exposure to prenatal tobacco smoke (PTS) has been associated with a number of health outcomes in the offspring, including some childhood cancers. Lower levels of genomic DNA methylation have also been associated with several types of cancers. We investigated whether PTS was associated with global DNA methylation levels in the offspring.
Methods
Our sample was drawn from a birth cohort of women born between 1959 and 1963 in New York City (n = 90). We measured methylation of repetitive elements (Sat2, Alu, LINE-1) from peripheral blood granulocytes. We combined prospectively collected data on PTS with adult epidemiologic data and blood samples collected in 2001 to 2007 (mean age, 43 years). We used linear regression to assess the association between PTS and repetitive element methylation.
Results
Thirty-six percent of mothers smoked during pregnancy. We observed an inverse association between PTS and Sat2 methylation. This inverse association remained even after adjustment for potential mediators including child environmental tobacco smoke exposure, birth size, postnatal weight and height changes, and adult smoking status and alcohol intake (β = −0.22, 95% confidence interval = −0.40 to −0.03 for ever exposed to PTS vs. never exposed using models of log-transformed methylation levels). PTS exposure was not statistically significantly associated with LINE-1 or Alu methylation.
Conclusions
PTS exposure, measured at the time of pregnancy and not retrospectively reported, was associated with a decrease in Sat2 methylation but not LINE-1 or Alu methylation.
Impact
If replicated in larger studies, this study supports a persistent effect of PTS on DNA methylation levels, as measured by Sat2, in adulthood.
Introduction
Intrauterine exposures impact health throughout life and have been associated with several cancers including breast cancer (1). Prenatal tobacco smoke (PTS) exposure has been associated with increased risk of disease in the offspring including respiratory illness, type 2 diabetes, cardiovascular disease, and some childhood cancers (reviewed in refs. 2, 3).
DNA methylation is one type of epigenetic change which has been observed in aging, respiratory and auto-immune conditions, and cancers including breast cancer (4). Genomic DNA demethylation is associated with lower levels of methylation and increased mutation rates in animal models (5) and may contribute to carcinogenesis through promotion of chromosomal instability, activation of oncogenes and/or transposable elements, and loss of imprinting (6). Genomic DNA demethylation has been observed in chronic lymphocytic leukemia, multiple myeloma, and breast, ovarian, gastrointestinal, colorectal, prostate, head and neck, and bladder cancers (reviewed in ref. 4).
One measure of genomic DNA methylation is measurement of methylation of repetitive elements [including long interspersed nuclear elements (LINE), satellite repeats (e.g., satellite 2), and short interspersed nuclear elements (SINE)]. These repetitive elements account for approximately 45% of the genome (7, 8), and up to 80% of CpG dinucleotides exist in repetitive elements and CpG islands. Genomic DNA demethylation of these sequences has been observed in cancer cells (9), and methylation of repetitive elements including LINE-1, satellite 2 (Sat2), and Alu has been used as a proxy for measuring genomic DNA methylation changes. Sat2 demethylation has been observed in several cancers including extrahepatic cholangiocarcinoma, hepatocellular carcinoma, and ex-adenoma carcinoma of the large intestine (10–12). In one study, the authors reported that tissue from cirrhotic and hepatic livers had lower methylation levels than normal liver tissue and that tissue from hepatocellular carcinoma had the lowest methylation levels (12). Cho and colleagues observed an association between genomic DNA demethylation of the Sat2 repetitive element and breast cancer (13).
There is mounting evidence that PTS has a persistent impact on DNA methylation throughout life. A study recently observed a persistent impact of PTS on DNA methylation of the brain-derived neurotrophic factor-6 exon, with altered methylation patterns observed in adolescents who were exposed to PTS (14). Another study reported that exposure to PTS was associated with lower levels of Alu methylation in DNA from kindergarten and first-grade children (15). We previously reported a positive association between PTS and genomic DNA methylation in granulocytes using the methyl acceptor assay (16). Here, we investigate other measures of genomic DNA methylation using data from the same multiethnic birth cohort.
Methods
Study population
We used epidemiologic data and blood samples from members of the New York Women's Birth Cohort, a follow-up of female participants in the New York site of the National Collaborative Perinatal Project (NCPP; refs. 17, 18). All participants were born at Columbia Presbyterian Medical Center between 1959 and 1963. Detailed information was collected prospectively during the prenatal period, childbirth, and through age 7. The New York Women's Birth Cohort adult follow-up was initiated in 2001. Of 841 eligible women, we traced 44.5% (n = 374) and 70.1% (n = 262) completed the epidemiologic questionnaire. The study was approved by the Institutional Review Board at Columbia Medical Center.
Early life data
At enrollment in the NCPP study, mothers provided information about age, parity, smoking, race, height, prepregnancy weight, and maternal and paternal education, occupation, and income that were used to calculate a socioeconomic status (SES) score. Birth weight was obtained within 1 hour of delivery and birth length was measured crown to heel within 24 hours of delivery according to a standardized protocol. Child body size was measured during study visits at birth, 4 months, and 1 and 7 years; calculation of change in percentile rank is described in our previous work (19).
Adult data
Women self-reported height, weight, health conditions, first-degree history of breast cancer, age at menarche, parity, hormone use, alcohol intake, and a detailed personal history of smoking and exposure to environmental tobacco smoke (ETS) exposure at home during childhood (child ETS).
Blood collection
Ninety-two (35%) participants completed the blood portion of the study (16). There were no statistically significant differences in demographic (age, race, and SES), prenatal [maternal age, prepregnancy body mass index (BMI), pregnancy weight gain, pregnancy smoking, and infant and child anthropometry], and adult (parity, alcohol intake, and smoking and oral contraceptive use) measures between women giving and not giving blood specimens. Samples from 90 participants had sufficient DNA for analysis.
Methylation assays
Repetitive element methylation was assessed in granulocytes from peripheral blood cells. All assays were conducted blinded to exposure and outcome data.
DNA extraction and bisulfite treatment
Genomic DNA was extracted from granulocytes by a salting out procedure. Aliquots of DNA (500 ng) were bisulfite treated with the EZDNA Methylation Kit (Zymo Research) to convert unmethylated cytosines to uracils whereas leaving methylated cytosines unmodified. The DNA was resuspended in 20 μL of distilled water and stored at −20°C until use.
MethyLight assay
We used the sequences of probes and forward and reverse primers of LINE1-M1, Sat2-M1, and Alu-M2 described by Weisenberger and colleagues (20). Standard curves for the AluC4 repeat control reaction were generated from 1:25 serial dilutions of bisulfite-converted CpGenome universal methylated and unmethylated DNAs. Assays were run on an ABI Prism 7900 sequence detection system (PerkinElmer). The interassay coefficients of variation (CV) based on duplicate threshold cycle measures were 0.8, 0.6, and 0.9 for LINE-1, Sat-2M1, and Alu-M2, respectively.
Universal methylated DNA served as a methylated reference, and an Alu-based control reaction (AluC4) was used to measure the levels of input DNA to normalize the signal for each methylation reaction. MethyLight data specific for the repetitive elements were expressed as a percentage of methylated reference (PMR) values (21).
Each MethyLight reaction was conducted in duplicate and the PMR values represent the mean. Intra- and interassay coefficients of variation in the threshold cycles (Ct) of a pooled quality control sample were 1.2% and 1.9%, respectively, showing good reproducibility. The percentage of methylation value is based on 4 Ct values using the formula: PMR = 100% × 2 exp –[ΔCt (target gene in sample – control gene in sample) – ΔCt (100% methylated target in reference sample – control gene in reference sample)]. This results in CV in percentage of methylation with laboratory values of 25.2% for samples run on the same day and 28.5% for samples run on different days.
Statistical analyses
We excluded repetitive element methylation measures that were greater than 3 SDs from the mean (n = 3, 2, and 1 for Sat2, Alu, and LINE-1, respectively).
We used ANOVA to assess the bivariable associations between PTS and child ETS (any vs. none) and adult smoke exposure (never, former, and current), and other early life and adult variables and Sat2, Alu, and LINE-1 methylation. We report repetitive element methylation in PMR. Because repetitive element methylation was not normally distributed, we transformed the outcome variables (PMR values of Sat2, Alu, and LINE-1) by taking the natural logarithm. The P values reported are for log-transformed methylation.
We investigated the association between PTS and methylation and added child ETS and then adult smoke exposure to the model. To test confounding of the association between PTS and methylation, we added potential confounders (maternal race, family SES at registration, maternal and paternal age at registration, maternal prepregnancy BMI, and maternal weight gain during pregnancy) to the models and examined whether the point estimate for PTS changed by more than 10%. We also examined potential mediation of the association between PTS and methylation by adding selected covariates that occurred temporally after PTS (birth weight and birth length, weight and height percentile rank changes, birth order, age at menarche, BMI at interview, parity, age at first full-term pregnancy, oral contraceptive use, menopausal status, and first-degree family history of breast cancer) individually and observing whether they changed the point estimate on PTS and each repetitive element by more than 10%. We replicated the final Sat2 models using Alu and LINE-1 as the outcome. All analyses were conducted using SAS 9.2.
Results
Participants were on average 43.6 years of age (SD = 1.9) at blood draw. Thirty-two (36.0%) participants were exposed to PTS. In bivariable analyses, PTS was associated with a lower level of Sat2 methylation; however, the association was not statistically significant (see Table 1). Birth order was significantly associated with Sat2 methylation; women who were at least fourth-born had higher levels of Sat2 methylation (see Table 1). Current smokers had nonsignificantly lower levels of Alu and LINE-1 methylation than former and never smokers (see Table 1). In addition, women who drank any alcohol had lower levels of Sat2 (Table 1). Race/ethnicity was not associated with genomic methylation levels (Table 1). Methylation levels for the 3 repetitive elements were statistically significantly correlated with each other [Pearson correlation (r) = 0.31 between Sat2 and LINE-1, r = 0.33 between Sat2 and Alu, and r = 0.49 between LINE-1 and Sat2].
Table 1.
Univariable associations between prenatal, early life and adult exposures and repetitive element methylation, New York Women's Birth Cohort
| Sat2 |
Alu |
LINE-1 |
|||||
|---|---|---|---|---|---|---|---|
| N | Mean Sat2 (PMR) ± SD | P a | Mean Alu (PMR) ± SD | P a | Mean LINE-1 (PMR) ± SD | P a | |
| Prenatal | |||||||
| Prenatal smoke | |||||||
| Any | 32 | 76.9 ± 20.6 | 104.2 ± 40.8 | 159.6 ± 54.7 | |||
| None | 56 | 88.4 ± 33.4 | 0.17 | 109.7 ± 36.5 | 0.40 | 167.8 ± 55.9 | 0.62 |
| Child ETS | |||||||
| Any | 70 | 85.8 ± 30.7 | 106.2 ± 38.8 | 168.3 ± 55.7 | |||
| None | 19 | 77.6 ± 25.8 | 0.38 | 111.3 ± 35.5 | 0.52 | 152.9 ± 51.9 | 0.22 |
| Adult smoke | |||||||
| Current | 19 | 81.7 ± 16.3 | 97.1 ± 27.2 | 144.9 ± 30.6 | |||
| Former | 29 | 84.2 ± 35 | 112.9 ± 46.1 | 170.6 ± 56.5 | |||
| Never | 41 | 85 ± 31.2 | 0.94 | 107.9 ± 35.5 | 0.57 | 170.3 ± 61.3 | 0.43 |
| Early life | |||||||
| Maternal race | |||||||
| White | 32 | 82.4 ± 36.1 | 110.2 ± 40.5 | 165.4 ± 58.6 | |||
| Black | 32 | 86.6 ± 25.1 | 112 ± 36.6 | 163.4 ± 60.4 | |||
| Puerto Rican | 25 | 82.8 ± 27.3 | 0.53 | 97.2 ± 35.8 | 0.26 | 166.7 ± 44 | 0.82 |
| Maternal age at registration, y | |||||||
| 18–21 | 17 | 85.2 ± 23 | 97.7 ± 31.8 | 171.2 ± 58.6 | |||
| 22–25 | 27 | 75.1 ± 28.5 | 108.5 ± 38.9 | 163.9 ± 49.8 | |||
| 26–30 | 24 | 86.4 ± 32.1 | 115.2 ± 44.6 | 167.2 ± 48.6 | |||
| >30 | 22 | 92.3 ± 32.1 | 0.14 | 105.4 ± 34.4 | 0.63 | 158.9 ± 67.4 | 0.72 |
| Birth order | |||||||
| 1 | 34 | 80.4 | 101.7 ± 33.4 | 163.6 ± 43.5 | |||
| 2 | 24 | 76.3 | 109.6 ± 43.9 | 159.6 ± 67.1 | |||
| 3 | 16 | 83.6 | 108.4 ± 37.9 | 151.8 ± 45.4 | |||
| 4+ | 13 | 107.8 | 0.05 | 119.1 ± 40.3 | 0.61 | 180.2 ± 48.5 | 0.43 |
| Adult | |||||||
| BMI, kg/m2 | |||||||
| <25 | 39 | 81.9 ± 31 | 106.1 ± 37 | 176.5 ± 63.5 | |||
| ≥25 | 47 | 87.3 ± 29.3 | 0.28 | 107.1 ± 37.4 | 0.88 | 156.3 ± 46.1 | 0.24 |
| Alcohol | |||||||
| Nondrinker | 43 | 90.6 ± 31.6 | 116.2 ± 41.5 | 171.3 ± 54.7 | |||
| 1–6 drinks/wk | 38 | 81.5 ± 25.8 | 98.3 ± 31.5 | 163.1 ± 56 | |||
| ≥7 drinks/wk | 8 | 66 ± 30.2 | 0.02 | 101.3 ± 42.3 | 0.14 | 156.7 ± 36.6 | 0.79 |
P values based on log-transformed methylation levels.
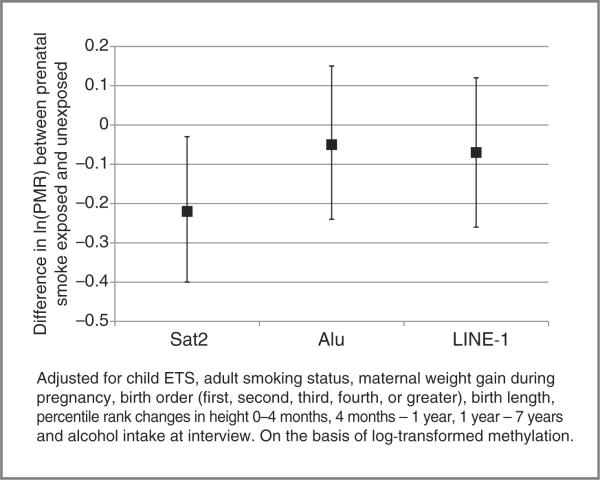
We observed inverse associations between PTS and both LINE-1 and Alu methylation; however, after adjusting for child ETS and adult smoke, the associations were not statistically significant (Fig. 1). Thus, we focused our modeling on Sat2. Table 2 reports the multivariable linear regression models of PTS and the natural log of Sat2 methylation. In an unadjusted model, PTS was inversely associated with Sat2 methylation, and after adjusting for child ETS, the association became stronger but was still not statistically significant. In models simultaneously adjusted for child ETS and adult smoke exposure, PTS was statistically significantly associated with lower levels of Sat2 methylation [β = −0.19, 95% confidence interval (CI) = −0.376 to −0.004]. Child ETS had a positive, non-statistically significant association with Sat2 methylation (β = 0.17, 95% CI = −to 0.37). After further adjusting for maternal weight gain, birth order, and infant and childhood growth variables, and alcohol intake, the inverse association with PTS remained (β −0.22, 95% CI = −0.40 to −0.03). We did not observe an association between adult smoke exposure and Sat2 methylation levels in adulthood. When we conducted supplemental analyses examining intensity of PTS (≤½ pack a day, >½ pack a day), we observed lower Sat2 methylation levels for both categories compared with no PTS, but there was no statistically significant difference between the 2 categories of PTS (data not shown).
Figure 1.
Difference (β) and 95% CI in repetitive element methylation between those exposed to PTS and those not exposed to PTS, New York Women's Birth Cohort.
Table 2.
Results from linear regression predicting ln(Sat2) methylation, New York Women's Birth Cohort
| PTS (n = 86) | PTS + child ETS (n = 86) | PTS + child ETS + adult smoke (n = 86) | Further adjusted modela (n = 84) | |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Prenatal smoke exposed | −0.11 (−0.27 to 0.05) | −0.17 (−0.34 to 0.01) | −0.19 (−0.38 to −0.004) | −0.22 (−0.40 to −0.03) |
| vs. unexposed | ||||
| Child ETS exposed | 0.16 (−0.04 to 0.36) | 0.17 (−0.03 to 0.37) | 0.14 (−0.06 to 0.34) | |
| vs. unexposed | ||||
| Adult smoking status | ||||
| Former: never smoke | −0.02 (−0.19 to 0.16) | −0.01 (−0.20 to 0.18) | ||
| Current: never smoke | 0.07 (−0.14 to 0.28) | 0.15 (−0.05 to 0.36) |
Adjusted for maternal weight gain during pregnancy, birth order (first, second, third, fourth, or greater), birth length, percentile rank changes in height 0–4 months, 4 months–1 year, 1–7 years, and alcohol intake at interview.
Discussion
Our results support an association between PTS and lower levels of genomic DNA methylation, measured in repetitive elements, in adulthood. Although PTS was associated with lower levels of repetitive element methylation in adjusted models, the association was only statistically significant in Sat2. Thirty-six percent of our sample were exposed to PTS, and whereas the prevalence of maternal smoking during pregnancy is significantly lower today, it remains high with more than 16% of women smoking during pregnancy (22).
There is mounting evidence to support persistent effects of PTS on DNA methylation throughout life (14–16). Only one other study has examined the association between PTS and repetitive element DNA methylation (15). The study supported an association between PTS and lower levels of Alu methylation in DNA from buccal cells in kindergarten and first-grade children. The authors also reported lower levels of LINE-1 methylation in children who were glutathione S-transferase (GST) M1 null (15).
We previously assessed the association between early life exposures and genomic DNA methylation in adulthood in the same study population using the methyl acceptor assay to measure genomic DNA methylation (16) and we reported that PTS was associated with higher levels of genomic DNA methylation. We have previously reported large differences in methylation levels by cell type and assay type within individuals, which may explain the conflicting results reported here (21). There is still some uncertainty about the best assay to measure methylation, and mounting evidence suggests that genomic methylation assays may not all measure the same construct (21). However, there is evidence that Sat2 methylation is a meaningful marker of risk, as we observed an association between lower levels of Sat2 methylation in white blood cells and breast cancer in another study population (13) and studies have reported that Sat2 demethylation is an early event in breast cancer (23).
Our overall power to detect statistically significant associations was limited by a relatively small sample of participants who provided blood. In addition, all of the participants who were exposed to PTS were also exposed to child ETS and we were thus unable to disentangle the effects of prenatal smoke and child ETS exposure. Because child ETS was positively associated with Sat2 methylation, this suggests that individuals exposed to only PTS may have even lower levels of Sat2 methylation, although much larger studies are needed to examine discordant patterns in smoking. Our measures of child ETS and adult smoking status were limited to self-report.
Our study has several strengths, in particular that prenatal and child measures, including our measure of PTS, were collected prospectively. PTS was reported prior to the 1964 Surgeon General's report on health consequences of smoking and is thus unlikely to be affected by underreporting bias due to the stigma that has been subsequently attached to smoking during pregnancy. The validity of maternal report of PTS exposure was confirmed in the New England site of the NCPP study with serum cotinine assays for ever/never exposure, although the study supports less accuracy for the number of cigarettes smoked which may partially explain the lack of dose–response seen in our study (24). Participants who provided blood did not differ significantly from those who did not in key exposures (16). Finally, we conducted all assays blinded to exposure data, including prenatal smoke exposure, and the assays had a very low coefficient of variation.
These results add to a growing body of literature supporting lifelong effects of prenatal exposures including birth length (16), lead (25), and prenatal famine (26, 27) on DNA methylation (28). If replicated in larger samples, these results suggest that PTS may have persistent effects on selective measures of DNA methylation and thus may provide a potential mechanism linking these early life exposures to adult diseases later in life.
Acknowledgments
Grant Support This work was supported by an award from the Breast Cancer Research Foundation and grants from the National Cancer Institute K07CA90685, Department of Defense (DoD) DAMD170210357, and National Institute of Environmental Health Science (NIEHS) ES009089.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Forman MR, Cantwell MM, Ronckers C, Zhang Y. Through the looking glass at early-life exposures and breast cancer risk. Cancer Invest. 2005;23:609–24. doi: 10.1080/07357900500283093. [DOI] [PubMed] [Google Scholar]
- 2.Doherty SP, Grabowski J, Hoffman C, Ng SP, Zelikoff JT. Early life insult from cigarette smoke may be predictive of chronic diseases later in life. Biomarkers. 2009;14(Suppl 1):97–101. doi: 10.1080/13547500902965898. [DOI] [PubMed] [Google Scholar]
- 3.Ng SP, Zelikoff JT. Smoking during pregnancy: subsequent effects on offspring immune competence and disease vulnerability in later life. Reprod Toxicol. 2007;23:428–37. doi: 10.1016/j.reprotox.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells: association with risk factors in epidemiologic studies. Epigenetics. 2011;6:828–37. doi: 10.4161/epi.6.7.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen RZ, Pettersson U, Beard C, Jackson-Grusby L, Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–93. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- 6.Fruhwald MC, Plass C. Global and gene-specific methylation patterns in cancer: aspects of tumor biology and clinical potential. Mol Genet Metab. 2002;75:1–16. doi: 10.1006/mgme.2001.3265. [DOI] [PubMed] [Google Scholar]
- 7.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 8.Jordan IK, Rogozin IB, Glazko GV, Koonin EV. Origin of a substantial fraction of human regulatory sequences from transposable elements. Trends Genet. 2003;19:68–72. doi: 10.1016/s0168-9525(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 9.Yoder JA, Walsh CP, Bestor TH. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997;13:335–40. doi: 10.1016/s0168-9525(97)01181-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim B-H, Cho N-Y, Shin S, Kwon H-J, Jang J, Kang G. CpG island hypermethylation and repetitive DNA hypomethylation in premalignant lesion of extrahepatic cholangiocarcinoma. Virchows Archiv. 2009;455:343–51. doi: 10.1007/s00428-009-0829-4. [DOI] [PubMed] [Google Scholar]
- 11.Kwon H-J, Kim J, Bae J, Cho N-Y, Kim T-Y, Kang G. DNA methylation changes in ex-adenoma carcinoma of the large intestine. Virchows Archiv. 2010;457:433–41. doi: 10.1007/s00428-010-0958-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee H, Kim B-H, Cho N-Y, Yoo E, Choi M, Shin S-H, et al. Prognostic implications of and relationship between CpG island hypermethylation and repetitive DNA hypomethylation in hepatocellular carcinoma. Clin Cancer Res. 2009;15:812–20. doi: 10.1158/1078-0432.CCR-08-0266. [DOI] [PubMed] [Google Scholar]
- 13.Cho Y, Yazici H, Wu H, Terry MB, Gonzalez K, Qu M, Dalay N, Santella RM. Aberrant promoter hypermethylation and genomci hypomethylation in tumor, adjacent normal tissues and blood from breast cancer patients. Anticancer Research. 2010;30:2489–96. [PMC free article] [PubMed] [Google Scholar]
- 14.Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, et al. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1350–4. doi: 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- 15.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–7. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terry MB, Ferris JS, Pilsner R, Flom JD, Tehranifar P, Santella RM, et al. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:2306–10. doi: 10.1158/1055-9965.EPI-08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terry MB, Flom J, Tehranifar P, Susser E. The role of birth cohorts in studies of adult health: the New York women's birth cohort. Paediatr Perinat Epidemiol. 2009;23:431–45. doi: 10.1111/j.1365-3016.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broman S. The Collaborative Perinatal Project: an overview. In: Mednick SA, Harway M, Finello KM, editors. Handbook of longitudinal research. 1st vol. Praeger Publishers; New York: 1984. pp. 185–227. [Google Scholar]
- 19.Terry MB, Wei Y, Esserman D. Maternal, birth, and early-life influences on adult body size in women. Am J Epidemiol. 2007;166:5–13. doi: 10.1093/aje/kwm094. [DOI] [PubMed] [Google Scholar]
- 20.Weisenberger DJ, Campan M, Long TI, Kim M, Woods C, Fiala E, et al. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33:6823–36. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu HC, Delgado-Cruzata L, Flom JD, Kappil M, Ferris JS, Liao Y, et al. Global methylation profiles in DNA from different blood cell types. Epigenetics. 2011;6:76–85. doi: 10.4161/epi.6.1.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Substance Abuse and Mental Health Services Administration, Office of Applied Studies . Results from the 2007 National Survey on drug use and health: national findings. NSDUH; Rockville, MD: 2008. (NSDUH Series H-34). DHHS Publication No. SMA 08-4343. [Google Scholar]
- 23.Jackson K, Yu MC, Arakawa K, Fiala E, Youn B, Fiegl H, et al. DNA hypomethylation is prevalent even in low-grade breast cancers. Cancer Biol Ther. 2004;3:1225–31. doi: 10.4161/cbt.3.12.1222. [DOI] [PubMed] [Google Scholar]
- 24.Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. Am J Epidemiol. 1998;148:259–62. doi: 10.1093/oxfordjournals.aje.a009633. [DOI] [PubMed] [Google Scholar]
- 25.Pilsner JR, Hu H, Ettinger A, Sanchez BN, Wright RO, Cantonwine D, et al. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ Health Perspect. 2009;117:1466–71. doi: 10.1289/ehp.0800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–53. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–9. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]