Abstract
Tobacco smoke has both carcinogenic effects and anti-estrogenic properties and its inconsistent association with breast cancer risk in observational studies may be because of these competing effects across the lifecourse. We conducted a prospective study of prenatal smoke exposure, childhood household smoke exposure, and adult active smoke exposure and mammographic density, a strong intermediate marker of breast cancer risk, in an adult follow-up of existing US birth cohorts. Specifically, we followed up women who were born between 1959 and 1967 and whose mothers participated in either the Collaborative Perinatal Project (Boston and Providence sites) or the Childhood Health and Development Study in California. Of the 1134 women interviewed in adulthood (ranging in age from 39 to 49 years at interview), 79% had a screening mammogram. Cigarette smoking was reported by mothers at the time of their pregnancy; 40% of mothers smoked while pregnant. Women whose mothers smoked during pregnancy had a 3.1% (95% confidence interval (CI) = −6.0%, −0.2%) lower mammographic density than women whose mothers did not smoke during pregnancy. When we further accounted for adult body mass index and adult smoking status, the association remained (β = −2.7, 95% CI = −5.0, −0.3).When we examined patterns of smoking, prenatal smoke exposure without adult smoke exposure was associated with a 5.6% decrease in mammographic density (β = −5.6, 95% CI = −9.6, −1.6). Given the strength of mammographic density as an intermediate marker for breast cancer, the inverse associations between mammographic density and smoking patterns across the lifecourse may help explain the complex association between cigarette smoking and breast cancer risk.
Keywords: breast cancer, mammographic density, prenatal smoke exposure
Introduction
Cigarette smoke, a known carcinogen, increases the risk of cancer at many sites. The association between cigarette smoke and breast cancer is much more modest (as reviewed in1,2), though evidence suggests a stronger association in selected subgroups defined by genes related to poorer DNA repair (including BRCA1), carcinogen metabolism and oxidative damage.3–8 Cigarette smoke has both anti-estrogenic and carcinogenic properties, and its inconsistent association with breast cancer risk in observational studies may result from these competing effects.9,10 Timing and duration of exposure may be particularly important. For example, among 111,140 participants in the Nurses’ Health Study, smoking more than 30 pack-years between menarche and menopause increased breast cancer risk by 28%; conversely, smoking after menopause was inversely related to breast cancer incidence.11
Most studies of breast cancer etiology have focused on exposures and behaviors in adulthood. However, increasing epidemiologic evidence supports the impact of exposures much earlier in life, including in the prenatal period, on breast cancer susceptibility.12,13 Strong associations between maternal diethylstilbestrol exposure and the daughter’s subsequent risk of clear cell vaginal cancer provided the first epidemiologic evidence that an in utero exposure, particularly a hormonal one, could be a cause of cancer later in life.14–16 Prenatal exposures may increase breast cancer risk by increasing the number of mammary cells, increasing the rate of cell division, increasing the number of in utero mutations, imprinting the fetal ovary and altering epigenetic profiles.17,18 Evidence to support the prenatal environmental influences on breast cancer emerged from epidemiologic studies investigating proxies for the hormonal intrauterine environment such as older maternal age at birth,19–23 twinning24–29 and higher birth weight.26,30–32 These markers of the intrauterine environment are non-specific and many other prenatal exposures remain unexplored. Because most prenatal factors are not as easily or reliably recalled in adulthood,33 studies with prospectively collected data on specific exposures in early life are required to investigate these potential associations.
Prenatal smoke exposure has been associated with the risk of breast cancer and benign breast disease in some studies,31,34 although other studies have not observed an association.24,35,36 As breast cancer susceptibility may be affected by early life factors and smoking patterns can be influenced by socioeconomic and family environment across the lifecourse, we conducted a prospective study of both passive and active cigarette smoke exposure and breast cancer risk. We measured breast cancer risk using mammographic density, a strong intermediate marker of breast cancer risk, which has been associated with many hormonal risk factors for breast cancer.37–43 Adult cigarette smoking has been consistently, inversely associated with mammographic density, perhaps reflecting the anti-estrogenic effect of cigarette smoke.44–46 We conducted our study in an adult follow-up of existing US birth cohorts; these cohorts have prospectively collected data on prenatal smoke exposure, which were collected before smoking behavior (particularly during pregnancy) was widely stigmatized and subject to social desirability bias when reporting.
Methods
Study sample
The Early Determinants of Mammographic Density (EDMD) study is an adult follow-up of women born in two US birth cohorts – the Child Health and Development Study (CHDS), which was conducted in California from 1960 to 1967,47 and two sites of the Collaborative Perinatal Project conducted in Boston, Massachusetts, and Providence, Rhode Island, from 1959 to 1966.48 These birth cohorts were established to investigate prenatal and familial antecedents of childhood growth and development. Both cohorts recruited pregnant women who received prenatal care at a participating hospital. The CHDS recruited pregnant women receiving prenatal care at the Kaiser Family Health Plan in Oakland, California. The New England sites of the Collaborative Perinatal Project (referred to hereafter as the New England Family Study, NEFS) recruited pregnant women receiving prenatal care at one of the teaching hospitals of Harvard Medical School and Brown University. Both studies followed up the pregnant women prospectively through pregnancy and childbirth and followed up their children postnatally through early childhood. The EDMD study includes the Early Determinants of Adult Health (EDAH) sample (described in49), but greatly extended it with separate funding, in order to address specific questions about prenatal exposures and breast cancer risk across the continuum of birth size.
Adult follow-up eligibility
We restricted eligibility for the EDMD study to women who fulfiled the following criteria: (1) singleton birth; (2) survived to last childhood follow-up; (3) birth weight and length recorded at birth; (4) childhood height and weight measures for at least two time points; (5) third trimester serum available; (6) at least one sister in the original cohort meeting the same criteria. Please see Susser et al.49 in this issue for additional information on sampling from the overall EDAH. There were 2423 women across the NEFS and CHDS cohorts who met these criteria (1163 sibling sets). The sibling sample was designed to reduce bias due to family-level confounders such as socioeconomic status. We supplemented this sibling sample with an additional sample of women who met the first five criteria, but did not have a sibling who met these eligibility criteria. In total, 3256 women were eligible for inclusion in the EDMD study.
Recruitment for follow-up
We attempted contact with 1925 (59.1%) women who were randomly selected from the pool of 3256 eligible women. Of those we attempted to contact, we were able to successfully trace 68.8% of the women (n = 1314). Of the successfully traced women, 1134 (86.3%) participated in the EDMD study. This final sample consisted of 521 singletons and 296 sibling sets comprised 613 individuals (277 sets of 2 siblings, 17 sets of 3 siblings, 2 sets of 4 siblings). Tracing rates were higher for the CHDS cohort than for the NEFS cohort (80.2% v. 59.1%); however, participation rates were very similar across the CHDS and NEFS once the women were successfully traced (85% and 88%, respectively). The study was approved by the Institutional Review Boards at Columbia University Medical Center, Kaiser Permanente, Brigham and Women’s Hospital and Brown University.
Baseline childhood data
Childhood and prenatal data were based on direct measurements and maternal reports at exam visits. Data on prenatal smoke exposure (yes/no and intensity of smoking), maternal pre-pregnancy body size and maternal education were based on maternal reports during prenatal visits. Birth characteristics including birth weight, birth length and placental weight were measured using calibrated scales and standardized procedures. Placental weight (grams) was collected and recorded according to the Benirschke protocol.50 Gestational age was defined as the time elapsed from 1st day of last menstrual period (LMP) to the day of delivery. LMP was established at the initial prenatal registration interview by a trained interviewer. In the NEFS, childhood height and weight measures were collected by trained clinical staff at either 8 months or 12 months, and at either 4 years or 7 years. In the CHDS, height and weight measures were recorded from the information collected at pediatric visits from 1 to 5 years of age (±6 months).49
Adult interview data collection
Women who agreed to participate completed a 45-min computer-assisted telephone interview, in which data were collected on personal health history and medication use, first degree family history of cancer, sociodemographic factors, alcohol use, detailed reproductive history, anthropometric measures and lifecourse exposures to passive and active tobacco smoke. We used data from the adult interviews including exposure to childhood environmental tobacco smoke (ETS) from birth to age 18 (defined as report of smoking in the home by the mother, father or other household members), age at menarche (reported in increments of 0.5 years), own smoking behavior of the participants at the time of interview (included in this analyses as never, former and current), alcohol intake (included in this analyses as nondrinker, <3 drinks per week, 3–7 drinks per week and >7 drinks per week), highest completed education, current household income, current body mass index (BMI, kg/m2, calculated from self-reported height and weight) and age at last mammogram.
Mammographic density data
During the interview, we asked participants whether they had had a mammogram in the 2 years before the interview or whether they planned to have a mammogram in the following 12 months. We asked participants who responded affirmatively to the above questions to provide information about the facility where they had their most recent mammogram or where they planned to have a mammogram, and to sign medical release authorization forms allowing us to borrow their mammograms for density assessment. Researchers at the Columbia University requested films from radiological facilities of the participants, digitized the films using a Kodak Lumisys Film Digitizer (Kodak LS85) and promptly returned the films to the facilities. Of the 981 participants who had a previous or future planned mammogram (87% of all 1134 participants), 893 (91%) provided a signed medical release form. We were unable to obtain mammograms for 23 participants and excluded mammograms for 51 participants because of poor image quality. Of the 819 participants with available mammograms of sufficient quality, 119 participants only had digital mammograms and were not included in the analysis, leaving a sample size of 700 women. For our primary analysis, we further excluded 22 who were missing prenatal smoke data; thus, our analysis was restricted to 678 film mammograms. We assessed mammographic density using Cumulus, a computer-assisted thresholding program51 in which the reader outlines the total breast area and dense area, and the software measures the size by identifying the number of pixels within the outlined areas. We calculated absolute breast area and dense area by converting the measure from pixels to cm2. Percent mammographic density was calculated as dense area divided by breast area multiplied by 100. We read films in batches of approximately 50 films, and repeated readings for 10% of films from the same batch. We repeated an additional 10% of films in every batch to estimate batch-to-batch variability. Each batch included films from both sites and films from siblings were read in the same batch. All cranio-caudal (CC) films that were available for a participant were read in the same batch. If both left and right images were available, we only read left breast images. We used the film taken closest to the date of interview in our analyses, using the left CC if available and the right CC if the left was unavailable (the correlation between left and right side breast density measures is in the range of 0.92–0.9652). The overall within-batch correlation coefficient was 0.96 for percent density and the intraclass correlation coefficient for between-batch reliability was 0.95.
Statistical analysis
We compared early life, child and adult risk factors between those exposed to prenatal smoke and those unexposed to prenatal smoke separately by site (Table 1). We also investigated patterns of exposure to prenatal smoke, childhood ETS and adult smoking status at the time of interview (never, former and current; Table 2).
Table 1.
Distribution of early life, child and adult characteristics by prenatal smoke exposure, EDMD
| NEFS | CHDS | |||
|---|---|---|---|---|
| Prenatal smoke (n = 176), Mean ± s.d. or n (%) |
No prenatal smoke (n = 174), Mean ± s.d. or n (%) |
Prenatal smoke (n = 96), Mean ± s.d. or n (%) |
No prenatal smoke (n = 232), Mean ± s.d. or n (%) |
|
| Early life | ||||
| Maternal age at registration | 24.3 ± 5.4 | 26.9 ± 6.2 | 25.9 ± 5.3 | 26.8 ± 5.8 |
| Birth weight (kg) | 3.3 ± 0.6 | 3.5 ± 0.5 | 3.3 ± 0.5 | 3.6 ± 0.5 |
| Birth length (cm) | 50.4 ± 2.6 | 50.6 ± 3.9 | 51.2 ± 2.4 | 52.4 ± 2.4 |
| Maternal weight gain (kg) | 9.1 ± 4.1 | 8.9 ± 3.8 | 8.8 ± 3.7 | 9.8 ± 4.2 |
| Maternal education at registration | ||||
| <HS | 77 (44.0) | 46 (27.1) | 18 (18.8) | 33 (14.2) |
| HS graduate | 75 (42.9) | 89 (52.4) | 41 (42.7) | 74 (31.9) |
| HS+ grad, some college, ≥college grad | 23 (13.1) | 35 (20.6) | 37 (38.5) | 125 (53.9) |
| Maternal race | ||||
| Non-Hispanic White | 161 (91.5) | 159 (91.4) | 74 (77.1) | 164 (70.7) |
| Non-Hispanic Black | 15 (8.5) | 14 (8.0) | 13 (13.5) | 37 (15.9) |
| Hispanic | 0 (0.0) | 0 (0.0) | 5 (5.2) | 20 (8.6) |
| Non-Hispanic API/other | 0 (0.0) | 1 (0.6) | 4 (4.2) | 11 (4.7) |
| Child | ||||
| Child environmental tobacco smoke exposure | ||||
| Yes | 167 (94.9) | 115 (66.1) | 90 (94.7) | 124 (53.4) |
| No | 9 (5.1) | 59 (33.9) | 5 (5.3) | 108 (46.6) |
| Age at menarche | 12.7 ± 1.5 | 12.9 ± 1.7 | 13.0 ± 1.8 | 12.4 ± 1.4 |
| Adult | ||||
| Adult smoking status | ||||
| Never | 62 (35.2) | 80 (46.0) | 65 (68.4) | 160 (69.0) |
| Former | 77 (43.8) | 66 (37.9) | 22 (23.2) | 47 (20.3) |
| Current | 37 (21.0) | 28 (16.1) | 8 (8.4) | 25 (10.8) |
| Alcohol intake at interview | ||||
| Nondrinker | 67 (38.3) | 58 (33.5) | 32 (33.7) | 84 (36.2) |
| <3 drinks/week | 49 (28.0) | 44 (25.4) | 33 (34.7) | 79 (34.1) |
| 3–7 drinks/week | 29 (16.6) | 45 (26.0) | 21 (22.1) | 47 (20.3) |
| >7 drinks/week | 30 (17.1) | 26 (15.0) | 9 (9.5) | 22 (9.5) |
| Income | ||||
| <$25,000 | 13 (7.6) | 14 (8.3) | 6 (6.5) | 16 (7.2) |
| $25,000–$50,000 | 25 (14.6) | 26 (15.5) | 20 (21.5) | 37 (16.6) |
| $50,000–<$75,000 | 33 (19.3) | 30 (17.9) | 14 (15.1) | 46 (20.6) |
| $75,000–<$100,000 | 44 (25.7) | 31 (18.5) | 15 (16.1) | 44 (19.7) |
| $100,000–<$150,000 | 41 (24.0) | 44 (26.2) | 15 (16.1) | 59 (26.5) |
| ≥$150,000 | 15 (8.8) | 23 (13.7) | 23 (24.7) | 21 (9.4) |
| Education | ||||
| HS or less | 41 (23.3) | 33 (19.0) | 11 (11.5) | 36 (15.5) |
| Some college/technical/trade school | 50 (28.4) | 36 (20.7) | 30 (31.3) | 63 (27.2) |
| Associate degree | 23 (13.1) | 25 (14.4) | 9 (9.4) | 16 (6.9) |
| Bachelor’s degree | 49 (27.8) | 54 (31.0) | 29 (30.2) | 79 (34.1) |
| Masters or doctoral degree | 13 (7.4) | 26 (14.9) | 17 (17.7) | 38 (16.4) |
| BMI (kg/m2) | 27.6 ± 6.0 | 26.2 ± 4.9 | 27.6 ± 7.3 | 28.1 ± 7.0 |
| Age at mammogram | 43.1 ± 2.4 | 43.2 ± 2.5 | 43.2 ± 2.2 | 43 ± 2.1 |
| Adult race | ||||
| Non-Hispanic White | 155 (88.6) | 155 (89.6) | 71 (74.0) | 149 (64.2) |
| Non-Hispanic Black | 14 (8.0) | 14 (8.1) | 13 (13.5) | 42 (18.1) |
| Hispanic | 3 (1.7) | 2 (1.2) | 7 (7.3) | 25 (10.8) |
| Non-Hispanic API/Other | 3 (1.7) | 2 (1.2) | 5 (5.2) | 16 (6.9) |
| Menopausal status | ||||
| Premenopausal | 123 (71.1) | 117 (69.6) | 62 (67.4) | 161 (72.2) |
| Menopausal transition/perimenopausal | 22 (12.7) | 33 (19.6) | 18 (19.6) | 33 (14.8) |
| Postmenopausal | 28 (16.2) | 18 (10.7) | 12 (13.0) | 29 (13.0) |
EDMD, Early Determinants of Mammographic Density; API, Asian and Pacific Islanders; NEFS, New England Family Study; CHDS, Child Health and Development Study; HS, high school.
Table 2.
Relation between exposure to prenatal smoke, Child ETS and adult smoke, EDMD
| Prenatal smoke, n (%) | No prenatal smoke, n (%) | P-value | |
|---|---|---|---|
| Child ETS | <0.0001a | ||
| No | 14 (5.2) | 167 (41.1) | |
| Yes | 257 (94.8) | 239 (58.9) | |
| Mother and father | 164 (60.5) | 56 (13.8) | |
| Mother only | 75 (27.7) | 39 (9.6) | |
| Father only | 18 (6.6) | 129 (31.8) | |
| Other only | 0 (0.0) | 15 (3.7) | |
| Adult smoking status | 0.01 | ||
| Never | 127 (46.9) | 240 (59.1) | |
| Former | 99 (36.5) | 113 (27.8) | |
| Current | 45 (16.6) | 53 (13.1) |
ETS, environmental tobacco smoke; EDMD, Early Determinants of Mammographic Density.
For comparison of prenatal smoke and overall childhood ETS.
We assessed the association between prenatal smoke exposure and percent mammographic density (hereafter referred to as mammographic density) using generalized estimating equation models to account for the correlated nature of the outcome among sibling sets. This method enabled us to include all participants, including singletons. Because of the differences in overall density from film and digital mammography, we conducted the primary analyses using women who had film mammograms. We performed secondary analyses including all women with any mammogram, film or digital. The overall inferences did not change when we included women with digital mammograms (data not shown); however, we do not present these data so that the overall measures of density are comparable across all individuals. We also conducted all the analyses separately by geographic site; however, the overall inferences did not change by site, and thus we present all models with both sites combined.
We estimated three primary models. The first model (labeled Model 1 in Tables 3–6) was a model of prenatal smoke exposure and mammographic density adjusting for age at mammogram. The second model (labeled Model 2 in Tables 3–6) adjusted for potential confounders that altered the association between prenatal smoke exposure and mammographic percent density by more than 10 percent. We considered both maternal education and maternal weight gain as potential confounders; however, maternal weight gain was not a confounder of the association between prenatal smoke and mammographic density. This second model can be viewed as the model providing the overall total effect of prenatal smoke exposure. The third model (labeled Model 3 in Tables 3, 5 and 6) included further accounting for adult smoking status and adult body size, which are important a priori predictors of mammographic density and may also be potential mediators in the prenatal smoke exposure pathway. In addition, in Table 3 we report a separate overall model for adult smoking not adjusting for prenatal smoke exposure and other early life variables. In Table 4, we report models adjusting for adult smoking status and adult body size separately so that the reader can evaluate the impact of these potential mediators on the estimate of association between prenatal smoke exposure and mammographic density. We considered the following variables as potential mediators: birth weight, birth length, age at menarche, adult body size (measured as BMI) and adult smoking status. We also evaluated the association between the amount of prenatal smoke exposure (maternal cigarettes per day smoked during pregnancy: nonsmoker, >0 to pack/day, pack/day to <1 pack/day and ≥1 pack/day) and mammographic percent density for the three models described above (Table 5).
Table 3.
Linear regression models of the association between prenatal smoke and adult smoking status and percent mammographic density, EDMD
| Percent mammographic density β (95% CI) |
|
|---|---|
| Prenatal smoke exposure | |
| No | Ref. |
| Yes | |
| Model 1a | −3.80 (−6.69, −0.91) |
| Model 2b | −3.08 (−6.00, −0.15) |
| Model 3c | −2.65 (−5.01, −0.30) |
| Adult smoke exposured | |
| Never | Ref. |
| Former | −2.11 (−4.69, 0.46) |
| Current | −2.56 (−6.06, 0.94) |
CI, confidence interval; BMI, body mass index; EDMD, Early Determinants of Mammographic Density.
Adjusted for age at mammogram.
Adjusted for age at mammogram and maternal education.
Adjusted for age at mammogram, maternal education, adult smoking status, BMI.
Adjusted for age at mammogram, maternal education, BMI.
Table 6.
Multivariable models of patterns of smoke exposure, EDMD
| EDMD (both sites) | |||
|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Panel A: Prenatal and child ETS exposure and mammographic percent density | |||
| Prenatal and childhood smoke exposure | |||
| No smoke | Ref. | Ref. | Ref. |
| Prenatal onlyd | – | – | – |
| Child ETS only | −2.54 (−6.38, 1.30) | −2.34 (−6.26, 1.58) | −0.97 (−4.26, 2.31) |
| Prenatal + Child ETS | −5.04 (−8.78, −1.31) | −4.21 (−8.08, −0.34) | −3.00 (−6.07, 0.08) |
| Model 1e | Model 2f | Model 3g | |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Panel B: Prenatal and adult tobacco smoke exposure and mammographic percent density | |||
| Prenatal and adult tobacco smoke exposure | |||
| No prenatal, no adult | Ref. | Ref. | Ref. |
| Prenatal, no adult | −5.71 (−9.66, −1.76) | −5.58 (−9.59, −1.58) | −5.76 (−8.75, −2.76) |
| No prenatal, former adult | −4.30 (−8.40, −0.20) | −4.80 (−8.86, −0.75) | −4.65 (−8.05, −1.25) |
| No prenatal, current adult | −8.90 (−13.98, −3.82) | −8.62 (−13.77, −3.47) | −4.72 (−9.39, −0.05) |
| Prenatal, former adult | −6.11 (−10.53, −1.68) | −5.32 (−9.80, −0.83) | −3.63 (−7.18, −0.07) |
| Prenatal, current adult | −6.98 (−12.78, −1.17) | −6.23 (−11.97, −0.49) | −4.66 (−9.73, 0.41) |
| Model 1h | Model 2i | Model 3j | |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Panel C: Child ETS and adult tobacco smoke exposure and mammographic percent density | |||
| Child ETS and adult tobacco smoke exposure | |||
| No child ETS, no adult | Ref. | Ref. | Ref. |
| Child ETS, no adult | −3.93 (−8.12, 0.25) | −3.51 (−7.77, 0.75) | −3.34 (−6.66, −0.01) |
| No child ETS, former adult | −5.04 (−11.16, 1.08) | −4.90 (−10.96, 1.16) | −4.79 (−9.49, −0.08) |
| No child ETS, current adultk | – | – | – |
| Child ETS, former Adult | −6.07 (−10.51, −1.63) | −5.63 (−10.15, −1.12) | −4.27 (−7.89, −0.64) |
| Child ETS, current adult | −8.08 (−13.10, −3.05) | −7.25 (−12.38, −2.13) | −3.98 (−8.31, 0.36) |
EDMD, Early Determinants of Mammographic Density; ETS, environmental tobacco smoke.
Adjusted for age at mammogram; P for interaction = 0.32.
Adjusted for age at mammogram and maternal education; P for interaction = 0.26.
Adjusted for age at mammogram, maternal education, adult smoking status and BMI; P for interaction = 0.14.
Small sample size, not reported.
EDMD, Early Determinants of Mammographic Density.
Adjusted for age at mammogram; P for interaction = 0.13.
Adjusted for age at mammogram and maternal education; P for interaction = 0.08.
Adjusted for age at mammogram, maternal education and BMI; P for interaction = 0.02.
EDMD, Early Determinants of Mammographic Density; ETS, environmental tobacco smoke.
Adjusted for age at mammogram; P for interaction = 0.13.
Adjusted for age at mammogram and maternal education; P for interaction = 0.15.
Adjusted for age at mammogram, maternal education and BMI; P for interaction = 0.01.
Small sample size, not reported.
Table 5.
Linear regression models of the association between number of maternal cigarettes per day and mammographic percent density in the daughter, EDMD
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Number of maternal cigarettes per day | |||
| None | Ref. | Ref. | Ref. |
| 0+ to pack/day | −5.14 (−9.13, −1.16) | −4.62 (−8.60, −0.63) | −2.05 (−5.11, 1.02) |
| pack to <1 pack/day | −2.21 (−6.48, 2.07) | −1.45 (−5.79, 2.88) | −2.01 (−5.66, 1.64) |
| ≥1 pack/day | −3.66 (−7.98, 0.66) | −2.72 (−7.11, 1.68) | −3.74 (−7.11, −0.37) |
| P for trend | 0.05 | 0.16 | 0.02 |
CI, confidence interval; EDMD, Early Determinants of Mammographic Density.
Adjusted for age at mammogram.
Adjusted for age at mammogram and maternal education.
Adjusted for age at mammogram, maternal education, adult smoking status and BMI.
Table 4.
Linear regression models of the association between prenatal smoke and mammographic percent density exploring the role of potential intermediate variables, EDMD
| Model 1a | Model 2b | |
|---|---|---|
| β (95% CI) | β (95% CI) | |
| Prenatal smoke exposure | ||
| No | Ref. | Ref. |
| Yes | −3.80 (−6.69, −0.91) | −3.08 (−6.00, −0.15) |
| +Birth weight | −4.13 (−7.14, −1.13) | −3.41 (−6.42, −0.40) |
| + Birth length | −4.34 (−7.24, −1.44) | −3.63 (−6.56, −0.70) |
| +Age at menarche | −3.87 (−6.76, −0.98) | −3.18 (−6.10, −0.25) |
| +Current BMI | −3.12 (−5.43, −0.81) | −2.82 (−5.16, −0.48) |
| +Adult smoking status | −3.27 (−6.19, −0.34) | −2.72 (−5.68, 0.24) |
BMI, body mass index; CI, confidence interval; EDMD, Early Determinants of Mammographic Density.
Adjusted for age at mammogram.
Adjusted for age at mammogram and maternal education.
We assessed the impact on mammographic density of patterns of smoking for prenatal smoke exposure and (a) childhood ETS, (b) adult smoking exposure, (c) childhood ETS and adult smoking exposure (labeled panels a, b, c, respectively, in Table 6). To compare patterns of exposure with prenatal smoke and childhood ETS and mammographic density, we created a four-level variable: no smoke exposure; prenatal smoke exposure only; prenatal smoke and child ETS exposures; and child ETS exposure only. To compare patterns of exposures with prenatal smoke and adult smoke exposure and mammographic percent density, we created a six-level variable: no smoke exposure; prenatal smoke exposure only; adult current smoke only; adult former smoke only; prenatal smoke and adult former smoke; and prenatal smoke and adult current smoke. To compare patterns of childhood ETS and adult smoke exposure and mammographic percent density, we created a six-level variable: no smoke exposure; child ETS exposure only; adult current smoke only; adult former smoke only; child ETS and adult former smoke; and child ETS and adult current smoke. We assessed formal tests of statistical interaction in each of these three models. Because of sample size constraints, we were unable to compare three-way patterns of smoke exposure across the lifecourse (prenatal, child ETS and adult smoking status) and mammographic density. In addition to the primary analyses considering mammographic density as the outcome variable, we examined whether our overall inferences were altered when we considered dense tissue area (in cm2) as the outcome. We performed analyses using the SAS version 9.2.
Results
The majority of women in our cohort 79% had a mammogram. Women who had a mammogram were more likely to be never smokers and less likely to be current smokers than women without a mammograms. Table 1 summarizes the descriptive statistics for the women who had mammograms of sufficient quality for inclusion. Descriptive statistics are shown separately by NEFS and CHDS cohorts in Table 1. The average age of participants at the time of interview was 44.1 years (s.d. 1.8, range 39.2–49.2), and the average age at mammogram was 43.1 years (s.d. 2.3, range 30.4–48.6). Overall 40.1% of the mothers smoked during pregnancy in the EDMD birth cohort; the percentage was higher in NEFS than in CHDS, 50.3% and 29.3%, respectively. Women in either birth cohort who were exposed to prenatal smoke were more likely to have a lower birth weight, have a mother with less than a high school education and be exposed to childhood environmental smoke than women who were unexposed to prenatal tobacco smoke (Table 1). Prenatal smoke exposure was not associated with maternal race, a woman’s own education, age at mammogram and a woman’s own self-reported race in adulthood (Table 1). There were some descriptive differences across the two cohorts. Specifically, in the NEFS, maternal age and adult BMI varied by prenatal smoke exposure; whereas in the CHDS, birthlength, maternal weight gain, age at menarche, and income varied by prenatal smoke exposure (Table 1).
Table 2 summarizes the association between prenatal smoke exposure and smoke exposure during two other life periods, that is, childhood ETS and active smoking status in adulthood. Prenatal smoke exposure was strongly correlated with exposure to childhood ETS, with 94.8% of women with prenatal smoke exposure also exposed to childhood ETS through one or both the parents’ smoking. Only 14 women (5.2%) who were exposed to prenatal smoke were not exposed to childhood ETS, whereas 58.9% of women (n = 239) who were not exposed to prenatal smoke were exposed to childhood ETS. Childhood ETS exposure was more likely to be from both parents for women who were exposed to prenatal smoke and the father only if not exposed to prenatal smoke. Of women with prenatal smoke exposure, 53.1% were ever smokers in adulthood. In contrast, only 40.9% of women who were not exposed to prenatal smoke exposure were ever smokers in adulthood (Table 2).
Table 3 reports the association between prenatal smoke and mammographic density in the age-adjusted model (Model 1), age and maternal education adjusted model (Model 2) and the model further accounting for adult smoking and BMI (Model 3). Overall, in the age-adjusted model, those exposed to prenatal smoke had on average 3.8% lower mammographic density compared with those who were not exposed (β = −3.8, 95% CI = −6.7, −0.9). The association between prenatal smoke and mammographic density was weaker after further adjustment by maternal education (Model 2: β = −3.1, 95% CI = −6.0, −0.2). Model 3 reports the association further adjusted for adult BMI and adult smoking pattern. Model 3 findings support an independent effect of prenatal smoke exposure even after adjusting for adult smoke exposure (Model 3: β = −2.7, 95% CI = −5.0, −0.3). Table 3 also reports the overall effect of adult smoke exposure not adjusting for prenatal smoke exposure. There was an inverse, but not statistically significant, association between current smoke exposure and mammographic density (β = −2.6, 95% CI = −6.1, 0.9).
To further explore potential intermediaries between prenatal smoke exposure and mammographic density, Table 4 reports the age-adjusted (Model 1) and maternal education and age-adjusted (Model 2) for each potential intermediate including birth weight, birth length, age at menarche, current BMI and adult smoking status. As Table 4 illustrates, adult BMI and adult smoking status (but not birth weight, birth length or age at menarche) reduced the overall association between prenatal smoke exposure and adult smoking status.
Table 5 reports the association between intensity of prenatal smoke exposure and mammographic density across the three models. These models support a general decrease in mammographic density from any amount of prenatal smoke exposure, but do not support a dose–response association. Adult BMI was higher in those whose mothers smoked pack a day (average BMI = 28.5) than for those whose mothers smoked more (average BMI = 27.3 and 27.0 for to <1 pack/day and ≥1 pack a day, respectively); however, the overall association between adult BMI and intensity of prenatal smoke exposure was not statistically significant.
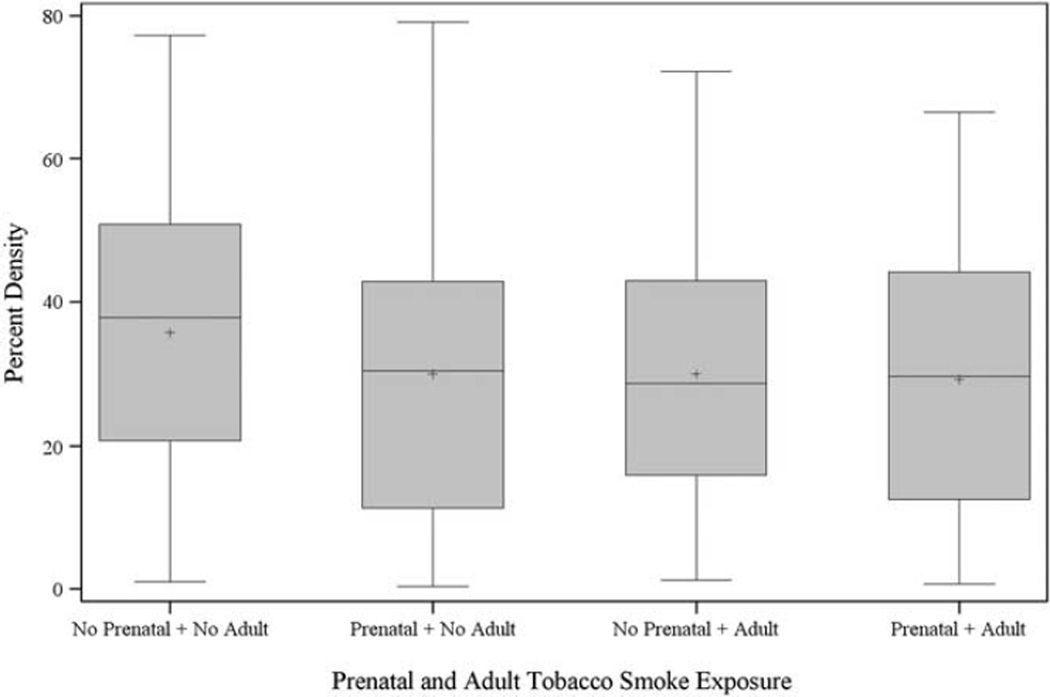
Table 6 reports the results from models focusing on combined measures of prenatal smoke exposure and childhood ETS exposure (panel a), prenatal smoke exposure and adult smoking status (panel b) and childhood ETS and adult smoking status (panel c). Overall, those exposed to either childhood ETS or both prenatal and childhood ETS combined had lower mammographic density. As reported in Table 2, only 14 women were exposed to prenatal smoke and not childhood ETS, thus we did not report estimates for this subset. Those exposed to prenatal smoke and childhood ETS had lower mammographic density (Model 2: β = −4.2, 95% CI = −8.1, −0.3). The effect of childhood ETS without prenatal smoke on mammographic density was weaker and not statistically significant (Model 2: β = −2.3, 95% CI = −6.3, 1.6). The overall test for statistical interaction was not significant for any of the three models in Table 6, panel a (P = 0.32, 0.26, 0.14 for Models 1, 2 and 3, respectively). Panel b of Table 6 reports the combination of prenatal smoke exposure and adult smoking status. As Table 6 (Model 2) reveals, when we examined these combined patterns of smoking, women who were exposed to prenatal smoke and not to adult smoke, had a 5.6% lower mammographic density (β = −5.6, 95% CI = −9.6, −1.6). Women who were not exposed to prenatal smoke but who were active smokers in adulthood also had lower mammographic density (β = −4.8, 95% CI = −8.9, −0.8 for former smokers; and β = −8.6, 95% CI = −13.8, −3.5 for current smokers). Women who had both prenatal and current adult smoke exposure had a 6.2% lower mammographic density (β = −6.2, 95% CI −12.0, −0.5). Women who had both prenatal and former adult smoke exposure had a 5.3% lower mammographic density (β = −5.3, 95% CI −9.8, −0.8). The overall test for statistical additive interaction was not significant for Models 1 and 2 (P = 0.13, 0.08), but was statistically significant for Model 3, shown (P = 0.02) in Table 6, panel b. Figure 1 summarizes the overall distribution of percent density by different categories of smoke exposure and supports that prenatal smoke exposure irrespective of active cigarette smoking in adulthood is associated with lower density of the magnitude of that for women only exposed to adult cigarette smoke. Panel c of Table 6 reports the combination of childhood ETS and adult smoking status. We did not report the estimate of association for women who were current adult smokers not exposed to childhood ETS, because there were too few women in this category. Women who had both childhood ETS and current adult smoke exposure had on average a 7.3% lower mammographic density (β = −7.3, 95% CI −12.4, −2.1, Model 2). Women who had both childhood ETS and former adult smoke exposure had a 5.6% lower mammographic density (β = −5.6, 95% CI −10.2, −1.1). The overall test for statistical additive interaction was not significant for Models 1 and 2 (P = 0.13, 0.15) and was significant for Model 3 (P = 0.01) in Table 6, panel c.
Fig. 1.
Distribution of percent mammographic density by category of smoke exposure.
In addition to examining percent density as an outcome, we examined the same models with dense area as the outcome. The overall inferences for prenatal smoke and mammographic density were similar and in some cases even stronger; prenatal smoking was statistically significantly, inversely associated with dense area in all models used in Tables 3–6. Table 7 reports the models for dense area from the primary analysis reported in Table 3 for percent density. The association between prenatal smoke and dense area was statistically significant after adjusting for maternal education (β = −4.4, 95% CI = −7.9, −0.9), and also for further adjusting for BMI and adult smoking status (β = −4.5, 95% CI = −8.1, −1.0). Adult smoking status was not associated with dense area (β = −1.4, 95% CI = −6.4, 3.6 for current, β = 0.6, 95% CI = −3.2, 4.3 for former).
Table 7.
Linear regression models of the association between prenatal smoke and adult smoking status and dense area (cm2), EDMD
| Dense area (cm2) | |
|---|---|
| β (95% CI) | |
| Prenatal smoke exposure | |
| No | Ref. |
| Yes | |
| Model 1a | −4.35 (−7.76, −0.94) |
| Model 2b | −4.38 (−7.92, −0.85) |
| Model 3c | −4.54 (−8.06, −1.01) |
| Adult smoke exposured | |
| Never | Ref. |
| Former | 0.58 (−3.15, 4.31) |
| Current | −1.35 (−6.35, 3.64) |
CI, confidence interval; EDMD, Early Determinants of Mammographic Density.
Adjusted for age at mammogram.
Adjusted for age at mammogram and maternal education.
Adjusted for age at mammogram, maternal education, adult smoking status and BMI.
Adjusted for age at mammogram, maternal education and BMI.
Discussion
Both prenatal smoke exposure and adult smoke exposure were inversely associated with mammographic density. Importantly, the association between prenatal smoke exposure and mammographic density was observed even in women who did not smoke in adulthood. Although one study observed a positive association between prenatal smoke exposure and breast cancer risk,31 other studies have not observed an association between prenatal smoke exposure and later breast cancer risk.24,35,36 Strohsnitter et al.,53 observed an inverse association between prenatal smoke exposure and breast cancer risk in a cohort study, with maternal report of smoking during pregnancy. The authors argued that previous case–control studies may have been biased because they relied on recall of prenatal smoke exposure by the daughter at diagnosis of cancer rather than a direct report from the mother. Both the study by Strohsnitter and our present study, collected pregnancy smoke exposure directly from the mothers, and thus have the distinct advantage of minimizing measurement error. Our study, however, collected the data from the mothers at the time of pregnancy rather than relying on maternal recall. Prenatal smoke exposure is also associated with later age at menarche,54 lower maternal estrogen levels,24,55 reduced maternal serum levels of growth factors56 and higher levels of maternal testosterone,57 all factors that may potentially reduce the risk of breast cancer.
We observed a statistically significant inverse association between current active cigarette smoking and mammographic density. Other cross-sectional studies of mammographic density have also examined the association between adult smoking status and density.44–46 These studies, which have been conducted in three different countries, reported an inverse association between adult cigarette smoking and mammographic density. Although the published literature supports a consistent inverse association between adult cigarette smoking and mammographic density, it is possible that studies with null findings have remained unpublished. A study by Jeffreys et al.,46 also suggested that smoking during early adulthood (as measured by college health interviews) was associated with lower mammographic density in a population of women with median age of 58.9 years (interquartile range (IQR) = 55.3–60.6). Collectively, these studies add support to the anti-estrogenic effects of cigarette smoke and the resulting decrease in mammographic density. Mammographic density has been shown to be sensitive to changes in the hormonal environment; for example, mammographic density increases with hormone replacement therapy and decreases with tamoxifen.58
We did not observe an association between adult cigarette smoke and dense area. Because current smokers had significantly higher BMI, the inverse association we observed for adult smoke and percent density may have resulted from residual confounding of the association from measurement error in body size rather than by a decreased area of dense tissue in adult smokers. The consistent, inverse association we observed for prenatal smoke exposure and density measured as percent density and dense area supports our overall inference that prenatal smoke has a persistent effect on mammographic density in adulthood.
Maternal education was a partial confounder of the association between prenatal smoke exposure and mammographic density. Parental education has been used as a broad proxy for early-life socioeconomic conditions.59 An inverse and strong socioeconomic gradient in smoking behavior has been documented for the past several decades. Specifically, although the prevalence of smoking has declined over time, this trend has occurred at a slower rate in women and in lower socioeconomic groups.60–62 One of the most convincing explanations for the emergence and persistence of a socioeconomic gradient in smoking involves the accumulation of evidence and public knowledge of health consequences of smoking beginning in the 1950s, which allowed those with more socioeconomic resources to more effectively avoid smoking than those with fewer resources.62,63 In our study, we observed a similar link between maternal education and maternal smoking, with higher proportions of smokers among mothers with a high school education or less. Although, maternal education and prenatal smoke exposure are both associated with lower birthweight,64 we observed an inverse association between prenatal smoke exposure and mammographic density even after accounting for these factors.64
A key strength of our study was the availability of prospective, valid measures of prenatal smoke, collected before the attachment of any stigma to the reporting of pregnancy smoking. Over a third of the mothers in our cohorts smoked during pregnancy, which allowed us to examine this exposure with sufficient power. In addition, we were able to assess breast cancer risk through the use of mammographic density, a strong intermediate marker of breast cancer risk. The high reliability of mammographic density assessment also increased our overall power to detect very modest associations. Additional strengths of our study include the availability of environmental tobacco exposure in childhood and active smoke exposure in adulthood. When we evaluated childhood ETS in the absence of prenatal smoke exposure, childhood ETS was not associated with mammographic density. However, when we investigated childhood ETS without adult smoke exposure, we observed that those exposed to childhood ETS had statistically, significantly lower mammographic density (Table 6, panel c). Because of numerical constraints, we were unable to explore the patterns of mammographic density in those exposed only to childhood ETS without prenatal and adult exposure to further tease the individual effects apart. Very few women in our study population were current smokers and were not exposed to childhood ETS.
Building on existing birth cohorts to address the role of early life factors on breast cancer risk has a number of distinct advantages including the availability of prospective measures that cannot be easily recalled in adulthood.65–67 However, there are a number of challenges that affect observational studies of this type. First, many exposures are more likely to remain constant over the lifecourse than to change, making it difficult to understand whether exposures are more important at specific susceptibility periods than in other periods. For example, almost all of the women exposed to prenatal smoke also reported being exposed to smoke in childhood; only 17 women were exposed to smoke prenatally, but not in childhood. Although these women were much more likely to have a lower mammographic density, the limits of any statistical regression model, and the potential for over-fitting the model, need to be recognized with such a small sample size. We were also able to consider a number of potential intermediaries of the prenatal smoke and mammographic density association and our overall inferences remained after accounting for these intermediaries, although there may be unaccounted for confounding of these intermediaries with our outcome.
Although the vast majority of women in our cohort (79%) had a mammogram, there were some differences between those who had a mammogram compared with those who did not, which may impact on the generalizability of our results. For example, participants who participated in the mammogram portion of the study were more likely to be never smokers and less likely to be current smokers than non-participants. However, there were no statistically significant differences between participants in the mammogram portion of the study compared with non-participants in the mammogram portion of the study by birth length, age at menarche, prenatal smoke exposure, age, maternal age at registration, paternal age at registration, birth weight, maternal pre-pregnancy BMI, family income at registration, maternal and paternal education, child ETS, alcohol intake at interview, adult income, BMI, menopausal status, hormone replacement therapy use, adult race, maternal race or height and weight at age 1 year. We were also able to achieve a high overall participation rate (86.3%) and a high overall tracing rate (68.3%). These participation rates are as high or higher than most birth cohorts of similar ages.65,68,69
In conclusion, our study suggests that both prenatal smoke exposure and active adult cigarette smoking are associated with lower mammographic density. Given the strength of the association between mammographic density and breast cancer, this study suggests that cigarette smoke exposure may lower overall breast cancer risk by reducing mammographic density. However, coupled against the carcinogenic effects of cigarette smoke, which increases many cancers and may increase breast cancer risk through a pathway other than mammographic density, the overall effect of cigarette smoke on breast cancer risk will likely vary across populations with different exposure timing of cigarette smoking and background mammographic density levels.
Acknowledgments
The authors greatly acknowledge the funding by the National Cancer Institute’s R01CA104842-03 and K07CA90685, the National Institute of Child Health and Development’s P01AG023028-01, as well as the insightful input from the following collaborators Drs Barbara Cohn, Ezra Susser, Lambert Lumey and Piera Cirillo. We thank Jill MacCrae for invaluable help with data collection.
References
- 1.Terry PD, Miller AB, Rohan TE. Cigarette smoking and breast cancer risk: a long latency period? Int J Cancer. 2002;100:723–728. doi: 10.1002/ijc.10536. [DOI] [PubMed] [Google Scholar]
- 2.Johnson KC, Miller AB, Collishaw NE, et al. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009) Tob Control. 2011;20:e2. doi: 10.1136/tc.2010.035931. 1–6. [DOI] [PubMed] [Google Scholar]
- 3.Terry PD, Goodman M. Is the association between cigarette smoking and breast cancer modified by genotype? A review of epidemiologic studies and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:602–611. doi: 10.1158/1055-9965.EPI-05-0853. [DOI] [PubMed] [Google Scholar]
- 4.Shen J, Gammon MD, Terry MB, et al. Polymorphisms in XRCC1 modify the association between polycyclic aromatic hydrocarbon–DNA adducts, cigarette smoking, dietary antioxidants, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:336–342. doi: 10.1158/1055-9965.EPI-04-0414. [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Terry MB, Gammon MD, et al. MGMT genotype modulates the associations between cigarette smoking, dietary antioxidants and breast cancer risk. Carcinogenesis. 2005;26:2131–2137. doi: 10.1093/carcin/bgi179. [DOI] [PubMed] [Google Scholar]
- 6.Terry MB, Gammon MD, Zhang FF, et al. Polymorphism in the DNA repair gene XPD, polycyclic aromatic hydrocarbon–DNA adducts, cigarette smoking, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:2053–2058. [PubMed] [Google Scholar]
- 7.Breast Cancer Family Registry, Kathleen Cuningham Consortium for Research into Familial Breast Cancer (Australasia) and Ontario Cancer Genetics Network (Canada). Smoking and risk of breast cancer in carriers of mutations in BRCA1 or BRCA2 aged less than 50 years. Breast Cancer Res Treat. 2008;109:67–75. doi: 10.1007/s10549-007-9621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissonauth V, Shatenstein B, Fafard E, et al. Weight history, smoking, physical activity and breast cancer risk among French–Canadian women non-carriers of more frequent BRCA1/2 mutations. J Cancer Epidemiol. 2009;2009:1–11. doi: 10.1155/2009/748367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol. 1990;162:502–514. doi: 10.1016/0002-9378(90)90420-c. [DOI] [PubMed] [Google Scholar]
- 10.Band PR, Le ND, Fang R, Deschamps M. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet. 2002;360:1044–1049. doi: 10.1016/S0140-6736(02)11140-8. [DOI] [PubMed] [Google Scholar]
- 11.Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med. 2011;171:125–133. doi: 10.1001/archinternmed.2010.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8:1088–1100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- 13.Forman MR, Cantwell MM, Ronckers C, Zhang Y. Through the looking glass at early-life exposures and breast cancer risk. Cancer Invest. 2005;23:609–624. doi: 10.1080/07357900500283093. [DOI] [PubMed] [Google Scholar]
- 14.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina: association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 15.Giusti RM, Iwamoto K, Hatch EE. Diethylstibestrol revisted: a review of the long-term health effects. Ann Intern Med. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hatch EE, Palmer JR, Titus-Ernstoff L, Noller KL, et al. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA. 1998;280:630–634. doi: 10.1001/jama.280.7.630. [DOI] [PubMed] [Google Scholar]
- 17.Anbazhagan R, Nathan B, Gusterson BA. Prenatal influences and breast cancer [letter; comment] Lancet. 1992;340:1477–1478. doi: 10.1016/0140-6736(92)92676-7. [DOI] [PubMed] [Google Scholar]
- 18.Anbazhagan R, Gusterson BA. Prenatal factors may influence predisposition to breast cancer. Eur J Cancer. 1994;30A:1–3. doi: 10.1016/s0959-8049(05)80006-1. [DOI] [PubMed] [Google Scholar]
- 19.Standfast SJ. Birth characteristics of women dying from breast cancer. J Natl Cancer Inst. 1967;39:33–42. [PubMed] [Google Scholar]
- 20.Rothman KJ, MacMahon B, Lin TM, et al. Maternal age and birth rank of women with breast cancer. J Natl Cancer Inst. 1980;65:719–722. doi: 10.1093/jnci/65.4.719. [DOI] [PubMed] [Google Scholar]
- 21.Thompson WD, Janerich DT. Maternal age at birth and risk of breast cancer in daughters. Epidemiology. 1990;1:101–106. doi: 10.1097/00001648-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Janerich DT, Hayden CL, Thompson WD, Selenskas SL, Mettlin C. Epidemiologic evidence of the perinatal influence in the etiology of adult cancers. Clin Epidemiol. 1989;42:151–158. doi: 10.1016/0895-4356(89)90088-7. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh CC, Tzonou A, Trichopoulos D. Birth order and breast cancer risk. Cancer Causes Control. 1991;2:95–98. doi: 10.1007/BF00053127. [DOI] [PubMed] [Google Scholar]
- 24.Weiss HA, Potischman NA, Brinton LA, et al. Prenatal and perinatal risk factors for breast cancer in young women. Epidemiology. 1997;8:181–187. doi: 10.1097/00001648-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Swerdlow AJ, De Stavola BL, Swanwick MA, Maconochie NES. Risks of breast and testicular cancer in young adult twins in England and Wales: evidence on prenatal and genetic aetilogy. Lancet. 1997;350:1723–1728. doi: 10.1016/s0140-6736(97)05526-8. [DOI] [PubMed] [Google Scholar]
- 26.Ekbom A, Trichopoulos D, Adami HO, Hsieh CC, Lan SJ. Evidence of prenatal influences on breast cancer risk. Lancet. 1992;340:1015–1018. doi: 10.1016/0140-6736(92)93019-j. [DOI] [PubMed] [Google Scholar]
- 27.Ekbom A. Growing evidence that several human cancers may originate in utero. Semin Cancer Biol. 1997;8:237–244. doi: 10.1006/scbi.1998.0073. [DOI] [PubMed] [Google Scholar]
- 28.Braun MM, Ahlbom A, Floderus B, Brinton LA, Hoover RN. Effect of twinship on incidence of cancer of the testis, breast, and other sites (Sweden) Cancer Causes Control. 1995;6:519–524. doi: 10.1007/BF00054160. [DOI] [PubMed] [Google Scholar]
- 29.Verkasalo PK, Kaprio J, Pukkala E, Koskenvuo M. Breast cancer risk in monozygotic and dizygotic female twins: a 20-year population-based cohort study in Finland from 1976 to 1995. Cancer Epidemiol Biomarkers Prev. 1999;8:271–274. [PubMed] [Google Scholar]
- 30.Michels KB, Trichopoulos D, Robins JM, et al. Birthweight as a risk factor for breast cancer. Lancet. 1996;348:1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson M, Williams MA, Malone KE, et al. Perinatal factors and risk of breast cancer. Epidemiology. 1996;7:34–37. doi: 10.1097/00001648-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Mogren I, Damber L, Tavelin B, Hogberg U. Characteristics of pregnancy and birth and malignancy in the offspring (Sweden) Cancer Causes Control. 1999;10:85–94. doi: 10.1023/a:1008813701634. [DOI] [PubMed] [Google Scholar]
- 33.Tehranifar P, Liao Y, Flom JD, Terry MB. Validity of self-reported birth weight by adult women: sociodemographic influences and implications for life-course studies. Am J Epidemiol. 2009;170:910–917. doi: 10.1093/aje/kwp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T, Gatsonis CA, Baylin A, Buka SL. Prenatal exposure to cigarette smoke and benign breast disease. Epidemiology. 2010;21:736–743. doi: 10.1097/EDE.0b013e3181e9c118. [DOI] [PubMed] [Google Scholar]
- 35.Sanderson M, Williams MA, Daling JR, et al. Maternal factors and breast cancer risk among young women. Paediatr Perinat Epidemiol. 1998;12:397–407. doi: 10.1046/j.1365-3016.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 36.Sandler DP, Everson RB, Wilcox AJ, Browder JP. Cancer risk in adulthood from early life exposure to parents’ smoking. Am J Public Health. 1985;75:487–492. doi: 10.2105/ajph.75.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 38.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–1144. [PubMed] [Google Scholar]
- 39.Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 40.Ciatto S, Zappa M. A prospective study of the value of mammographic patterns as indicators of breast cancer risk in a screening experience. Eur J Radiol. 1993;17:122–125. doi: 10.1016/0720-048x(93)90048-r. [DOI] [PubMed] [Google Scholar]
- 41.Byrne C, Schairer C, Wolfe J, et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87:1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 42.Kato I, Beinart C, Bleich A, et al. A nested case–control study of mammographic patterns, breast volume, and breast cancer (New York City, NY, United States) Cancer Causes Control. 1995;6:431–438. doi: 10.1007/BF00052183. [DOI] [PubMed] [Google Scholar]
- 43.Oza AM, Boyd NF. Mammographic parencymal patterns: a marker of breast cancer risk. Epidemiol Rev. 1993;15:196–208. doi: 10.1093/oxfordjournals.epirev.a036105. [DOI] [PubMed] [Google Scholar]
- 44.Butler LM, Gold EB, Conroy SM, et al. Active, but not passive cigarette smoking was inversely associated with mammographic density. Cancer Causes Control. 2010;21:301–311. doi: 10.1007/s10552-009-9462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bremnes Y, Ursin G, Bjurstam N, Gram IT. Different measures of smoking exposure and mammographic density in postmenopausal Norwegian women: a cross-sectional study. Breast Cancer Res. 2007;9(5):R73. doi: 10.1186/bcr1782. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeffreys M, Warren R, Gunnell D, McCarron P, Smith GD. Life course breast cancer risk factors and adult breast density (United Kingdom) Cancer Causes Control. 2004;15:947–955. doi: 10.1007/s10522-004-2473-3. [DOI] [PubMed] [Google Scholar]
- 47.Van den Berg BJ. The California child health and development studies. In: Mednick SA, Harway M, Finello KM, editors. Hanbook of Longitudinal Studies. New York: Praeger; 1984. pp. 166–179. [Google Scholar]
- 48.Broman S. The Collaborative Perinatal Project: an overview. In: Mednick SA, Harway M, Finello KM, editors. Handbook of Longitudinal Research. Vol. I. New York: Praeger Publishers; 1984. pp. 185–227. [Google Scholar]
- 49.Susser E, Buka SL, Schaefer CA, et al. The Early Determinants of Adult Health (EDAH) Study. DOHAD; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benirschke K. Examination of the placenta. Obstet Gynecol. 1961;18:309–333. [Google Scholar]
- 51.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–1638. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 52.Byng JW, Boyd NF, Little L, et al. Symmetry of projection in the quantitative analysis of mammographic images. Eur J Cancer Prev. 1996;5:319–327. doi: 10.1097/00008469-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Strohsnitter WC, Noller KL, Titus-Ernstoff L, et al. Breast cancer incidence in women prenatally exposed to maternal cigarette smoke. Epidemiology. 2005;16:342–345. doi: 10.1097/01.ede.0000158741.07645.9b. [DOI] [PubMed] [Google Scholar]
- 54.Ferris JS, Flom JD, Tehranifar P, Mayne ST, Terry MB. Prenatal and childhood environmental tobacco smoke exposure and age at menarche. Paediatr Perinat Epidemiol. 2010;24:515–523. doi: 10.1111/j.1365-3016.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacMahon B, Trichopoulos D, Cole P, Brown J. Cigarette smoking and urinary estrogens. N Engl J Med. 1982;307:1062–1065. doi: 10.1056/NEJM198210213071707. [DOI] [PubMed] [Google Scholar]
- 56.Evans P, Wheeler T, Anthony F, Osmond C. Maternal serum vascular endothelial growth factor during early pregnancy. Clin Sci. 1997;92:567–571. doi: 10.1042/cs0920567. [DOI] [PubMed] [Google Scholar]
- 57.Kandel DB, Udry JR. Prenatal effects of maternal smoking on daughters’ smoking: nicotine or testosterone exposure? Am J Public Health. 1999;89:1377–1383. doi: 10.2105/ajph.89.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin LJ, Minkin S, Boyd NF. Hormone therapy, mammographic density, and breast cancer risk. Maturitas. 2009;64:20–26. doi: 10.1016/j.maturitas.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 59.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1) J Epidemiol Community Health. 2006;60:7–12. doi: 10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- 61.Giovino GA, Schooley MW, Zhu BP, et al. Surveillance for selected tobacco-use behaviors – United States, 1900–1994. MMWR CDC Surveill Summ. 1994;43:1–43. [PubMed] [Google Scholar]
- 62.Link BG, Phelan J. The social shaping of health and smoking. Drug Alcohol Depend. 2009;104:S6–S10. doi: 10.1016/j.drugalcdep.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Link BG. Epidemiological sociology and the social shaping of population health. J Health Soc Behav. 2008;49:367–384. doi: 10.1177/002214650804900401. [DOI] [PubMed] [Google Scholar]
- 64.Mortensen LH, Diderichsen F, Smith GD, Andersen AM. The social gradient in birthweight at term: quantification of the mediating role of maternal smoking and body mass index. Hum Reprod. 2009;24:2629–2635. doi: 10.1093/humrep/dep211. [DOI] [PubMed] [Google Scholar]
- 65.Terry MB, Flom J, Tehranifar P, Susser E. The role of birth cohorts in studies of adult health: the New York women’s birth cohort. Paediatr Perinat Epidemiol. 2009;23:431–445. doi: 10.1111/j.1365-3016.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Susser E, Terry MB, Matte T. The birth cohorts grow up: new opportunities for epidemiology. Paediatr Perinat Epidemiol. 2000;14:98–100. doi: 10.1046/j.1365-3016.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- 67.Susser E, Terry MB. A conception-to-death cohort. Lancet. 2003;361:797–798. doi: 10.1016/S0140-6736(03)12721-3. [DOI] [PubMed] [Google Scholar]
- 68.Hemachandra AH, Howards PP, Furth SL, Klebanoff MA. Birth weight, postnatal growth, and risk for high blood pressure at 7 years of age: results from the Collaborative Perinatal Project. Pediatrics. 2007;119:e1264–e1270. doi: 10.1542/peds.2005-2486. [DOI] [PubMed] [Google Scholar]
- 69.Klebanoff MA, Zemel BS, Buka S, Zierler S. Long-term follow-up of participants in the Collaborative Perinatal Project: tracking the next generation. Paediatr Perinat Epidemiol. 1998;12:334–346. doi: 10.1046/j.1365-3016.1998.00125.x. [DOI] [PubMed] [Google Scholar]