SUMMARY
Our understanding of vitamin D metabolism and biological effects has grown exponentially in recent years and it has become clear that vitamin D has extensive immunomodulatory effects. The active vitamin D generating enzyme, 1α-hydroxylase, is expressed by the airway epithelium, alveolar macrophages, dendritic cells and lymphocytes indicating that active vitamin D can be produced locally within the lungs. Vitamin D generated in tissues is responsible for many of the immunomodulatory actions of vitamin D. The effects of vitamin D within the lungs include increased secretion of the antimicrobial peptide cathelicidin, decreased chemokine production, inhibition of dendritic cell activation and alteration of T cell activation. These cellular effects are important for host responses against infection and the development of allergic lung diseases like asthma. Epidemiological studies do suggest that vitamin D deficiency predisposes to viral respiratory tract infections and mycobacterial infections and that vitamin D may play a role in the development and treatment of asthma. Randomized, placebo controlled trials are lacking but ongoing.
Keywords: Vitamin D, lung, immune, infection, obstructive
I. INTRODUCTION
Emerging information on vitamin D physiology has revealed that Vitamin D is not merely a micronutrient that plays a role in calcium homeostasis but a pluripotent hormone with extensive immunomodulatory functions. Studies have shown that the enzyme 1α-hydroxylase, which catalyzes the last and rate limiting step in the synthesis of active 1,25-dihydroxyvitamin D3 (1,25D), and the vitamin D receptor (VDR), which mediates the actions of vitamin D, are expressed widely in the body, including the lungs and cells of the immune system. These observations have led to a surge of epidemiological and basic research studies examining the effects of vitamin D on immune responses, lung infections and the development of lung diseases. Vitamin D insufficiency has been linked to increased risk of infections, in particular viral respiratory tract infections (Wayse, Yousafzai et al. 2004; Cannell, Vieth et al. 2006; Laaksi, Ruohola et al. 2007; Cannell, Zasloff et al. 2008; Roth, Jones et al. 2008; Ginde, Mansbach et al. 2009) and tuberculosis (Wilkinson, Llewelyn et al. 2000; Bornman, Campbell et al. 2004; Roth, Soto et al. 2004; Liu, Stenger et al. 2007; Martineau, Wilkinson et al. 2007; Martineau, Wilkinson et al. 2007). Vitamin D may also play a role in the development of obstructive lung diseases like asthma and COPD (Janssens, Lehouck et al. 2009; Brehm, Schuemann et al. 2010; Sutherland, Goleva et al. 2010). This review focuses on lung-specific vitamin D metabolism, immune effects of vitamin D and the potential role of vitamin D in the development and treatment of lung diseases.
II. LUNG IMMUNE FUNCTIONS
The respiratory tract has a surface area of approximately 70 m2 and is in direct and continuous contact with the surrounding environment. Despite continuous exposure to potential pathogens only rarely do the lungs become colonized or infected. A local defense system with components of both innate and adaptive immunity has evolved to discriminate between non-pathogenic antigens and potential pathogens and to clear pathogens.
The innate immune system involves a rapid, non-specific, recognition of and response to almost any pathogen. Only those antigens that penetrate the innate immune responses evoke the more specific adaptive immune responses. The main players in innate immunity in the lungs include the airway epithelium itself, alveolar macrophages and dendritic cells. They all express pattern recognition receptors (PRR’s) and ligand engagement results in activation of intracellular signaling pathways that mobilize antimicrobial defenses, inflammation and adaptive immune responses (Basu and Fenton 2004). The airway epithelium is the first line of defense and functions as a physical barrier to prevent the entry of inhaled pathogens. When the airway epithelium recognizes the presence of a pathogen it responds by releasing antimicrobials, chemokines and cytokines. Alveolar macrophages recognize, phagocytose and remove inhaled material. They are activated either in response to pathogens or through an autocrine/paracrine response to cytokines. Activation leads to enhanced phagocytosis and killing of pathogens as well as coordination of both innate and adaptive immune responses. The third major innate immune effector cells in the lung are dendritic cells. Dendritic cells use PRR’s to monitor the local environment for pathogens. When a pathogen is encountered it is ingested and its proteins are processed into peptides which are then presented at the surface of the dendritic cell. Activated dendritic cells produce chemokines and migrate to local lymph nodes where they present the antigenic peptides bound to major histocompatibility complex (MHC) molecules to naive T-cells (CD4+ T-helper cells and CD8+ T-cytotoxic cells) and induce their activation and differentiation. Dendritic cells thus serve as a link between innate and adaptive immune responses. Vitamin D can influence all three innate immune effectors in the lungs and thus may play an important role in how the lung recognizes and responds to pathogens.
Activation of the innate immune system drives activation of the long term adaptive immune system (Iwasaki and Medzhitov 2010). Adaptive immune responses involve the ability of T and B lymphocytes to produce cytokines and immunoglobulins respectively. All phases of the adaptive immune response are specific to unique antigen, from recognition of the antigen by antibody (humoral) or T-lymphocyte (cell mediated) through lymphocyte activation, to effector function (elimination of antigen) and the development of immunologic memory (Mak and Saunders 2005). Upon activation memory T cells down regulate lymphoid-tissue-homing receptors and up regulate tissue-specific-adhesion molecules and can now migrate to non-lymphoid tissues like the lungs (Holt, Strickland et al. 2008). Furthermore once activated TH (CD4+) cells differentiate into TH1, TH2 or TH17 effector cells. The effector cells are characterized by the production of distinct set of cytokines (Medzhitov 2007). Activation of B cells and their differentiation into antibody-secreting plasma cells can be triggered directly by antigen but usually requires helper T cells. Lastly regulatory T cells (TRegs) are important for the control of peripheral T-cell responses. In relation to the lungs they are believed to have key roles in protection against the inflammatory sequela of airway infections and in the protection against the induction and expression of atopic disease (Holt, Strickland et al. 2008). There is data to support both indirect (dendritic cells) and direct (T- and B-lymphocytes) effects of vitamin D on adaptive immune responses.
The respiratory tract in continuously exposed to antigens, some of which are pathogenic and some of which are not. A specialized lung immune system has evolved that can recognize and respond to potential pathogens but does not get activated by non-pathogenic antigens which would result in chronic inflammation and tissue damage. The following chapters will focus on how vitamin D may affect cells involved in lung immune responses at all levels i.e. airway epithelium, alveolar macrophages, dendritic cells and T- and B cells and thus can have significant overall immunomodulatory effects in the lungs.
III. 1,25-DIHYDROXYVITAMIN D IS GENERATED LOCALLY IN THE LUNGS
Humans get vitamin D through synthesis in the skin following UVB exposure and to a lesser extent from limited dietary sources. Vitamin D from the skin or diet is metabolized primarily in the liver to 25-hydroxyvitamin D3 (25D) (Ponchon, Kennan et al. 1969). 25Dis the “storage form” of vitamin D and is used to determine the vitamin D status of individuals. The last and rate limiting step in the synthesis of “active” 1,25-dihydroxyvitamin D3 (1,25D) is catalyzed by the mitochondrial enzyme 1α-hydroxylase and is conventionally known to take place in the kidneys. Renal 1α-hydroxylase activity is under stringent regulation by parathyroid hormone, calcium, calcitonin, phosphorus and 1,25D itself (ZEHNDER, BLAND et al. 1999). Vitamin D is inactivated by the ubiquitous enzyme, 24-hydroxylase, whose expression is inducible by 1,25D, thus creating a negative feedback loop (Holick 2007). The biological effects of vitamin D are achieved through the regulation of gene expression mediated by the vitamin D receptor (VDR) (Baker, McDonnell et al. 1988). Active vitamin D binds to VDR and upon ligand binding, the receptor dimerizes with the retinoic X receptor (RXR) (MacDonald, Dowd et al. 1993). The VDR/RXR complex binds to vitamin D responsive elements (VDREs) within the promoter regions of vitamin D regulated genes (Sutton and MacDonald 2003).
It is increasingly recognized that localized synthesis of 1,25D rather than systemic production is responsible for many of the immune effects of vitamin D. Extra-renal expression of 1α-hydroxylase has been found in various cells of the immune system including alveolar macrophages (Adams, Sharma et al. 1983; Reichel, Koeffler et al. 1987), dendritic cells (Fritsche, Mondal et al. 2003; Hewison, Freeman et al. 2003; Sigmundsdottir, Pan et al. 2007) and lymphocytes (Chen, Sims et al. 2007; Sigmundsdottir, Pan et al. 2007) as well as in airway epithelia (Hansdottir, Monick et al. 2008) (Table 1). Locally formed 1,25D acts in an autocrine or paracrine fashion to modulate cell proliferation, cell differentiation and immune function (Bell 1998; Hewison, Burke et al. 2007; White 2008).
Table 1.
Local Production and Effects of 1,25D in the Respiratory Tract
| Cell type | Conversion of 25D → 1,25D | 1.25D Effects | References |
|---|---|---|---|
| Airway Epithelium | Constitutive | Increases CD14 and cathelicidin. Dampens IFN-β and chemokine response during viral infection | Hansdottir et al. |
| Alveolar Macrophages | Upon activation | Increases the antimicrobial peptide cathelicidin | Liu et al. |
| Dendritic Cells | Increases with differentiation | Inhibits dendritic cell differentiation, maturation and function, decreases IL-12 and increases IL-10, alters T cell activation | Fritsche et al., Sigmundsdottir et al., Piemonti et al., Penna et al |
| T lymphocytes | At least when activated | Inhibits proliferation, modulates cytokine production - inhibits Th1 and Th17 cytokines but induces Tregs | Sigmundsdottir et al., Lemire et al., Daniel et al., Mora et al., Penna et al. |
| B lymphocytes | Unclear | Inhibits proliferation of activated B cells and generation of plasma cells | Chen et al. |
A. Airway epithelium
Defense systems have evolved to clear and inactivate inhaled pathogens so that despite continual exposure to potential antigens, the lung is generally maintained in a quiescent, non inflamed state (Kohlmeier and Woodland 2008). The airway epithelium is constantly exposed to potentially pathogenic microorganisms. Recognition of pathogens by airway epithelial cells results in activation of intracellular signaling pathways and the end result is transcription of genes for a variety of effector molecules, including antimicrobials, type I interferons and proinflammatory cytokines and chemokines (Bartlett, Fischer et al. 2008).
Recent work has found that airway epithelium exposed to the “storage” form of vitamin D is able to generate “active” vitamin D, potentially creating localized areas with higher 1,25D levels than are seen in serum (Hansdottir, Monick et al. 2008). Primary human airway epithelial cells express relatively high mRNA levels of 1α-hydroxylase and lower levels of the inactivating 24-hydroxylase at baseline. Unlike alveolar macrophages, which need to be stimulated to convert 25D to 1,25D, airway epithelial cells constitutively generate 1,25D. Not only do airway epithelial cells produce active vitamin D at baseline, they also respond to pathogens by increasing the machinery needed to convert 25 D to 1,25D. Viral infection induces expression of 1α-hydroxylase and increases conversion of 25D to 1,25D, which may be of benefit to the host response against the virus (Hansdottir, Monick et al. 2008).
Local generation of active vitamin D in the lung potentially regulates pulmonary immune responses. Active vitamin D, generated by airway epithelium, directly increases expression of VDR-regulated genes that are involved in recognition and killing of pathogens, including the TLR co-receptor CD14 and the antimicrobial peptide cathelicidin (Hansdottir, Monick et al. 2008). When airway epithelium is infected with a virus 1,25D modulates the expression of inflammatory chemokines and cytokines in response to the virus (Hansdottir, Monick et al. 2010). This will be discussed further in the section on vitamin D and respiratory infection.
B. Alveolar macrophages
Like airway epithelium, alveolar macrophages (AMs) can also generate active vitamin D. In contrast to the constitutive activity of 1α-hydroxylase in airway epithelium, alveolar macrophages need to be stimulated before converting inactive to active vitamin D. The first description of extrarenal 1α-hydroxylase was in patients with the granulomatous disease, sarcoidosis. It had been noted that some patients with sarcoidosis had hypercalcemia and high vitamin D levels (Papapoulos, Clemens et al. 1979; Barbour, Coburn et al. 1981). Subsequently Adams et al. (Adams, Sharma et al. 1983) showed that AMs from patients with sarcoidosis converted 25D to 1,25D whereas AMs from patients with idiopathic pulmonary fibrosis did not. It has since been shown that AMs from normal subjects do not constitutively express 1α-hydroxylase and convert 25D to 1,25D but can do so if activated with TLR 2/1 ligands, IFNγ or LPS (Reichel, Koeffler et al. 1987; Reichel, Koeffler et al. 1987; Liu, Stenger et al. 2006). This is different from renal 1α-hydroxylase which is mainly regulated by mediators of calcium and bone homeostasis. Moreover activated macrophages lack negative feedback by 25D and 1,25D (Dusso, Kamimura et al. 1997). A nonfunctional alternatively spliced form of 24-hydroxylase has been found in the cytoplasm of macrophages that may be responsible for impeding the access of 25D and 1,25D to the enzyme and preventing their catabolism (Ren, Nguyen et al. 2005). The lack of a negative feedback system contributes to the increased serum vitamin D levels in patients with granulomatous diseases.
Expression of 1α-hydroxylase by stimulated alveolar macrophages, production of 1,25D and lack of active 24-hydroxylase, can have beneficial effects on host defense but also has pathological implications. On one hand TLR 2/1 ligands (mycobacterial antigen) activate alveolar macrophages, induce 1α-hydroxylase and increase 1,25D which leads to an increase in the vitamin D regulated antimicrobial peptide cathelicidin. Cathelicidin facilitates killing of mycobacterium tuberculosis (Liu, Stenger et al. 2006). On the other hand overproduction of 1,25D in macrophages in sarcoidosis, tuberculosis and various other granulomatous conditions can result in hypercalcemia (Adams, Sharma et al. 1983). Epidemiological data suggests that low vitamin D is associated with susceptibility to tuberculosis and severity of disease(Gao, Tao et al. 2010). Available studies on vitamin D and tuberculosis will be reviewed in the following section on vitamin D and mycobacterial infections.
C. Dendritic cells
Dendritic cells play a key role in the initiation and regulation of adaptive immune responses to inhaled antigens. Dendritic cells form a contiguous network throughout the airway epithelium. In the steady state, dendritic cells are specialized for uptake and processing of environmental antigens but lack the capacity for efficient antigen presentation (Holt, Strickland et al. 2008). If dendritic cells sense an abnormal state they mature. Maturation is characterized by migration to regional lymph nodes, down-regulation of antigen uptake and an enhanced capacity to activate naive T cells. This process of dendritic cell maturation involves changes in the expression of chemokine receptors and is associated with up regulation of costimulatory molecules and markers of dendritic cell activation (Upham 2003).
Human blood monocytes can be differentiated to dendritic cells by in vitro culture with GM-CSF, IL-4 or IL-13. Monocyte derived dendritic cells constitutively express 1α-hydroxylase. Following terminal differentiation induced by TNFα, IFNγ, polyI:C or LPS there is marked increase in expression and function of 1α-hydroxylase (Fritsche, Mondal et al. 2003; Hewison, Freeman et al. 2003). Furthermore dendritic cells metabolize vitamin D precursors to active 1,25D (Fritsche, Mondal et al. 2003; Hewison, Freeman et al. 2003; Sigmundsdottir, Pan et al. 2007). In contrast VDR expression may be down-regulated during the maturation process (Hewison, Freeman et al. 2003). 1,25D generated by the dendritic cells themselves and exogenous 1,25D inhibit dendritic cell differentiation, maturation and function by decreasing the expression of MHC class II and costimulatory molecules, decreasing production of IL-12 and increasing secretion of IL-10 (D’Ambrosio, Cippitelli et al. 1998; Penna and Adorini 2000; Piemonti, Monti et al. 2000; Mora, Iwata et al. 2008). By modulating dendritic cell activation 1,25D alters T cell activation favoring the induction of regulatory T cells and leads to T cell hyporesponsiveness (Penna and Adorini 2000; Penna, Roncari et al. 2005). It has been postulated that inhibition of dendritic cell maturation and T cell hyporesponsiveness may explain some of the immunosuppressive activities of 1,25D including control of autoimmune diseases and transplantation tolerance (Gregori, Casorati et al. 2001; Griffin, Lutz et al. 2001; Adorini and Penna 2008).
D. Lymphocytes
Vitamin D not only affects lymphocytes indirectly via its effects on dendritic cells as described above but also has direct effects on T cells and likely B cells. Activated T lymphocytes and B lymphocytes have been found to express VDR and 1α-hydroxylase and to convert 25D to 1,25D (Bhalla, Amento et al. 1983; Provvedini, Tsoukas et al. 1986; Chen, Sims et al. 2007; Sigmundsdottir, Pan et al. 2007).
Antigen-mediated activation of naive T helper (TH) cells results in the generation of pluripotent TH0 lymphocytes that synthesize a broad spectrum of cytokines (IL-2, IL-4, IL-10 and IFNγ) (Adams and Hewison 2008). Proliferating TH0 lymphocytes are then able to differentiate into TH1 (IL-2, IFNγ, TNF), TH2 (IL-3, IL-4, IL-5 and IL-10) or TH17 (IL-17) lymphocytes with a more distinct cytokine profile. Vitamin D suppresses the production of TH1 and TH17 (Lemire, Archer et al. 1995; Daniel, Sartory et al. 2008; Mora, Iwata et al. 2008) cytokines but its effects on the production of TH2 cytokines is less clear. Early studies suggested that 1,25D enhanced the development of TH2 cells (Boonstra, Barrat et al. 2001) but subsequent studies indicate that 1,25D dose not favor the TH2 phenotype (Pichler, Gerstmayr et al. 2002; Staeva-Vieira and Freedman 2002). Recent studies have shown that the effects of vitamin D are complex and include the generation of IL-10 producing T-regulatory lymphocytes (previously known as suppressor T cells) (Penna, Roncari et al. 2005). IL-10 is a major antiinflammatory and immunosuppressive cytokine that inhibits both TH1 and TH2 immune responses (Moore, de Waal Malefyt et al. 2001). In general the immunomodulatory effects of vitamin D on T cell medicated immunity may be beneficial for conditions in which the immune system is directed at self i.e. autoimmune diseases and graft rejection in transplantation (Gregori, Casorati et al. 2001; Barrat, Cua et al. 2002; Bikle 2009). In direct relation to the lungs there is evidence that TReg function is impaired in allergic and asthmatic disease (Lloyd and Hawrylowicz 2009). Vitamin D has been shown to reverse steroid resistance, through induction of IL-10 secreting T cells, in patients with asthma (Xystrakis, Kusumakar et al. 2006). The role of vitamin D in asthma pathogenesis and treatment will be discussed in the section on vitamin D and obstructive lung diseases.
The actions of 1,25D on B cells are not well studied. A recent study found that 1,25D suppresses immunoglobulin production and B cell proliferation and differentiation (Chen, Sims et al. 2007). This study also found that patients with systemic lupus erythematosus have low vitamin levels and hypothesized that low vitamin D levels may contribute to the B cell hyperactivity that is seen in this disease.
IV. VITAMIN D AND LUNG INFECTIONS
A. Mycobacteria
It was first noted over one century ago that UV light seemed to help in the treatment of mycobacterial infections. The 1903 Nobel Prize in Medicine was awarded to Niels Finsen for demonstrating that UV light is beneficial to patients with lupus vulgaris (tuberculosis of the skin). In the late 19th century Hermann Brehmer built the first sanatorium for the treatment of tuberculosis (Liu, Krutzik et al. 2007). Patients were exposed to plentiful amounts of high altitude, fresh air, and good nutrition. It has since been speculated that patients with tuberculosis benefitted from sanatoriums because of UV light exposure and increased production of vitamin D precursors in the skin. The first in vitro studies looking at vitamin D and M. tuberculosis were published in the 1980s. These studies demonstrated that adding 1,25D to M. tuberculosis infected human monocytes and macrophages reduced the intracellular bacterial load (Rook, Steele et al. 1986; Crowle, Ross et al. 1987). This observation has been followed by a series of observational studies suggesting that individuals with low 25D levels are more susceptible to M. tuberculosis infection and often have a more severe course of disease (Wilkinson, Llewelyn et al. 2000; Ustianowski, Shaffer et al. 2005; Gibney, MacGregor et al. 2008; Nnoaham and Clarke 2008). Case-control studies have also found an association between VDR polymorphisms and susceptibility to tuberculosis, in particular in individuals with low 25D levels (Wilkinson, Llewelyn et al. 2000; Bornman, Campbell et al. 2004; Roth, Soto et al. 2004; Lewis, Baker et al. 2005).
Several different mechanisms have been proposed for how vitamin D may increase antimicrobial actions of monocytes and macrophages. A multiplicity of studies has been published recently indicating that a vitamin D induced antimicrobial peptide, cathelicidin, plays a key role. The first study was a translational study published in 2006 showing that adequate 25D levels are required for TLR2/1 activation (by a mycobacterial ligand) and subsequent 1α-hydroxylase and VDR dependent expression of cathelicidin. This study also revealed increased killing of mycobacteria by macrophages in the presence of 25D (Liu, Stenger et al. 2006). In a subsequent study of peripheral blood monocytes infected with recombinant mycobacteria, vitamin D strongly induced cathelicidin mRNA and reduced the growth of mycobacteria in a dose dependent fashion (Martineau, Wilkinson et al. 2007). Another study showed a direct correlation between serum 25D levels and monocyte expression of cathelicidin following treatment with TLR 2/1 and TLR 4 ligands. In the same study, in vivo supplementation of vitamin D enhanced ex vivo innate immune responses by rescuing TLR-mediated suppression of cathelicidin expression (Adams, Ren et al. 2009). Lastly a study using human monocytic cells found that siRNA knockdown of 1,25D induced cathelicidin resulting in complete loss of antimicrobial activity (Liu, Stenger et al. 2007) (Figure 1). Alternative mechanisms that have been proposed for the effects of vitamin D include 1,25D induction of superoxide burst and enhancement of phagolysosome fusion both of which are mediated through the phosphatidylinositol 3-kinase pathway (Sly, Lopez et al. 2001; Hmama, Sendide et al. 2004).
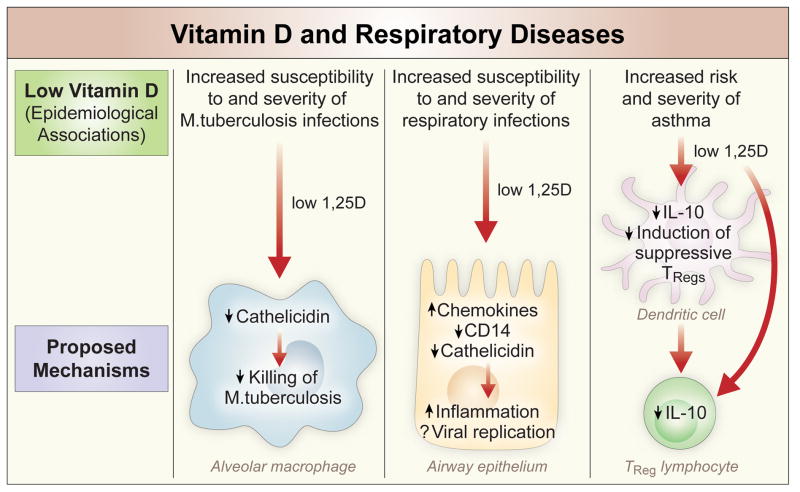
Figure 1. Epidemiological associations between vitamin D deficiency and lung diseases and proposed mechanisms.
Vitamin D deficiency appears to increase susceptibility to TB infections due to lack of induction of the cathelicidin antimicrobial peptide. Vitamin D deficient individuals also report more frequent respiratory tract infections perhaps due to less production of cathelicidin and/or increased production of chemokines leading to uncontrolled inflammatory response. Lastly vitamin D deficiency has been associated with higher prevalence of asthma and a more severe course of this disease. Two mechanisms have been proposed: i) Increased risk of respiratory viral infection. ii) Lack of vitamin D suppressive effects on adaptive immunity, in particular dendritic cells and T regulatory cells.
Human trials looking at vitamin D for prevention or treatment of tuberculosis have been performed. In a double blinded randomized controlled trial, 192 healthy adult TB contacts were randomized to receive a single oral dose of vitamin D (2.5 mg = 100,000 IU) or placebo. 6 weeks later a functional whole blood assay to assess growth of recombinant reporter mycobacteria in vitro (BCG-lux assay) was performed. IFN-γ responses to M. tuberculosis antigens were also determined. The investigators found that vitamin D significantly enhanced the ability of participants’ whole blood to restrict growth of the reporter mycobacteria but did not affect antigen-stimulated IFN-γ secretion (Martineau, Wilkinson et al. 2007). Two small randomized studies have looked at adding vitamin D to treatment regimens for tuberculosis and showed faster resolution of symptoms and earlier sputum conversion to culture negativity in patients given vitamin D (Morcos, Gabr et al. 1998; Nursyam, Amin et al. 2006). A larger randomized, double blind, placebo control trial included 365 patients with TB starting treatment and gave 100,000 IU of vitamin D at inclusion and again 5 and 8 months after the start of treatment. No differences were found in a clinical severity score (TB score), sputum conversion or 12-month mortality between patients treated with vitamin D or placebo (Wejse, Gomes et al. 2009). Of note is that 25D levels in the two groups were similar when measured at 2 and 8 months suggesting that perhaps the dose of vitamin D used was insufficient.
To date there is ample evidence that vitamin D inhibits growth of mycobacteria in vivo. Epidemiological studies suggest that low vitamin D levels increase the susceptibility to and severity of tuberculosis. Clinical trials looking at vitamin D for the treatment of tuberculosis have provided conflicting results and it remains unclear whether vitamin D supplementation is beneficial. Several clinical trials are ongoing that are investigating the impact of vitamin D supplementation on response to treatment of Mycobacterium Tuberculosis (www.clinicaltrials.gov).
B. Respiratory infections
Seasonal variation in the incidence of communicable diseases, in particular respiratory tract infections, is among the oldest observations in population biology, dating back to ancient Greece (Lipsitch and Viboud 2009). Several mechanisms have been hypothesized to explain this observation one of which is seasonal variation in vitamin D levels. It has been noted that the peak incidence of respiratory tract infections, coincides with the time of the year when there is insufficient UV-B light to produce vitamin D, and vitamin D levels in the population are at a low (Cannell, Vieth et al. 2006; Cannell, Zasloff et al. 2008). As our understanding of the role of vitamin D in innate immunity has increased, this hypothesis has gained increased popularity. Further circumstantial evidence supporting the role of vitamin D comes from epidemiological studies that have shown that children with rickets are at increased risk of respiratory infections (Rehman 1994; Muhe, Lulseged et al. 1997). More recently several epidemiological studies have consistently found an association between low vitamin D levels and increased susceptibility to respiratory infections (Wayse, Yousafzai et al. 2004; Aloia and Li-Ng 2007; Laaksi, Ruohola et al. 2007; Ginde, Mansbach et al. 2009). The largest of those studies was a secondary analysis of the Third National Health and Nutrition Examination Survey (NHANES-III) (Ginde, Mansbach et al. 2009). This study looked at the association between 25D levels of nearly 19,000 participants and self reported upper respiratory tract infections. After adjusting for demographic and clinical characteristics, lower 25D levels were independently associated with recent respiratory tract infections. Preliminary evidence also suggests an association between VDR polymorphisms and acute lower respiratory tract infection in children. A study of 56 children hospitalized with lower respiratory tract infection (predominantly viral bronchiolitis) found that the odds of infection were higher in children with the FokI ff VDR genotype (Roth, Jones et al. 2008) when compared with the FokI FF genotype (Roth, Jones et al. 2008).
At the basic science level, we have recently shown that airway epithelium converts 25D to 1,25D which raises the possibility of higher levels of 1,25D locally in the lungs than are seen in serum (Hansdottir, Monick et al. 2008). We have also shown that viral infection increases the amount of 1,25D generated by the airway epithelium. We believe that the increase in local 1,25D in airways will contribute to decreased tissue damage, while maintaining viral clearance. The studies supporting this conclusion are described below.
When examining the role of vitamin D in airway anti-viral responses, the transcription factor, nuclear factor-κB (NF-κB) is a potential regulatory point. NF-κB is a well established key player in multiple physiological processes including innate- and adaptive immune responses and inflammation (Holt, Strickland et al. 2008). IκBα inhibits the NF-κB pathway by binding to NF-κB subunits in the cytoplasm and inhibiting translocation to the nucleus (Li and Verma 2002). Relevant to vitamin D control of airway epithelial cell immune responses, we have shown that vitamin D induces IκBα in airway epithelium, leading to less induction of NF-kB driven genes during viral infection. The end result is decreased secretion of inflammatory chemokines but no change in viral clearance (Hansdottir, Monick et al. 2010). While vitamin D dampens expression of inflammatory chemokines we have also shown that it increases expression of CD14 and cathelicidin which serve a role in recognizing and eliminating pathogens, including viruses. Combined these results suggest that vitamin D may potentiate innate immunity while controlling the potentially harmful inflammatory response (Hansdottir, Monick et al. 2008; Hansdottir, Monick et al. 2010) (Figure 1).
Two randomized placebo controlled trials looking at vitamin D supplementation on respiratory tract infections were recently published. In the former study 162 adults were given 2000 IU units of vitamin D daily or placebo for 12 weeks. A questionnaire was administered bi-weekly to record the incidence and severity of upper respiratory tract infection symptoms. This study found no difference in the incidence or severity between the groups (Li-Ng, Aloia et al. 2009). The second randomized trial looked at the incidence of influenza A in school children treated with 1200 IU vitamin D daily or placebo. In this study, influence A occurred in 18/167 (10.8%) of children in the vitamin D group compared with 31/167 (18.6) in the placebo group (relative risk 0.58; 95% CI 0.34–0.99; P=0.04).
More rigorously designed randomized, placebo controlled, clinical trials are warranted to further explore and establish the role of vitamin D in preventing and/or treating respiratory tract infections. A trial of vitamin D supplementation for the prevention of influenza and other respiratory infections is ongoing (www.clinicaltrials.gov).
V. VITAMIN D AND OBSTRUCTIVE LUNG DISESES
A. Asthma
Asthma is a chronic inflammatory disorder that causes an increase in airways hyperresponsiveness leading to recurrent episodes of wheezing and shortness of breath. The prevailing consensus is that the immunological bases of allergic disease like asthma results from inappropriate TH2 responses to common, harmless, airborne antigens. These reactions are normally suppressed by TRegs which maintain airway tolerance (Lloyd and Hawrylowicz 2009). There is increasing evidence that one mechanism for the development of asthma is imbalance between regulatory and effector T cells and that the ability to enhance regulatory function may represent an effective treatment for asthma (Lloyd and Hawrylowicz 2009; Robinson 2009).
The prevalence of asthma has been steadily increasing over the last several decades and over the same period of time vitamin D insufficiency has been on the rise. The prevalence of both conditions have been linked to African American race, obesity and immigration to westernized countries (Litonjua and Weiss 2007). These observations have prompted the hypothesis that vitamin D deficiency is an important contributor to the asthma epidemic. Epidemiological studies have found that vitamin D insufficiency is common in asthmatics and is associated with increased asthma severity and hospitalizations (Brehm, Celedon et al. 2009; Brehm, Schuemann et al. 2010; Freishtat, Iqbal et al. 2010; Sutherland, Goleva et al. 2010). If such an association exists it may be mediated through increased risk of respiratory viral infection in vitamin D deficient individuals or by the effects of vitamin D on adaptive immunity, in particular T regulatory cells (Litonjua 2009).
Vitamin D modulates adaptive immunity both indirectly via inhibition of dendritic cell maturation and directly via its effects on TRegs. Regulatory T cells can either develop as a normal part of the immune system (naturally occurring TRegs) or in response to particular antigenic exposure (induced/adaptive TRegs) (Xystrakis, Urry et al. 2007). Naturally occurring TRegs are characterized by the expression of the forkhead winged transcription factor FoxP3 whereas induced TRegs may be FoxP3+ or FoxP3- (Dimeloe, Nanzer et al. 2010). Pretreatment of dendritic cells with vitamin D and subsequent co-culture with CD4+ cells leads to induction of CD4+FoxP3+ TRegs with suppressive activity (Penna, Roncari et al. 2005). 1,25D also acts directly on CD4+ T cells and promotes an IL-10 secreting TReg population (Barrat, Cua et al. 2002) (Figure 1). IL-10 inhibits many functions relevant to asthma and has been proposed to play a role in maintaining immune homoeostasis in the airways (Hawrylowicz and O’Garra 2005). An inverse correlation exists between the presence of IL-10 and the incidence and severity of asthma.
Glucocorticosteroids are the principal controller therapy for patients with persistent asthma but there is a significant variability in the response to this treatment and a proportion of patients do not achieve optimal asthma control despite high doses (Sutherland, Goleva et al. 2010). Glucocorticosteroids increase TRegs and IL-10 synthesis (Karagiannidis, Akdis et al. 2004) and the induction may be enhanced by 1,25D (Barrat, Cua et al. 2002; Xystrakis, Kusumakar et al. 2006; Xystrakis, Urry et al. 2007). CD4+ T cells from steroid resistant asthmatics fail to demonstrate increased IL-10 synthesis following stimulation in the presence of a glucocorticosteroid (Hawrylowicz, Richards et al. 2002). This defect in steroid induced IL-10 can be overcome by the addition of 1,25D to the T cell culture (Xystrakis, Kusumakar et al. 2006). Epidemiological evidence supports a role for vitamin D on the effects of glucocorticosteroids. Low vitamin D levels have been associated with increased use of corticosteroids and reduced in vitro glucocorticoid response (Brehm, Celedon et al. 2009; Searing, Zhang et al. 2010; Sutherland, Goleva et al. 2010).
To summarize, vitamin D deficiency is common in asthmatic patient and vitamin D supplementation may result in improvement in asthma severity and treatment response to corticosteroids, likely via induction of TRegs and secretion of IL-10. It should be noted that not all the data supports a positive role for vitamin D on the development of asthma. The hypothesis that vitamin D may cause asthma because of inhibition of TH1 responses also exists (Hypponen, Sovio et al. 2004; Gale, Robinson et al. 2008). Several clinical trials are on going that are looking at vitamin D and asthma, ranging from maternal supplementation during pregnancy and prevention of childhood asthma to the use of vitamin D as a treatment in individuals with asthma (www.clinicaltrials.gov).
B. Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation that is not fully reversible. The airflow limitation is progressive and associated with an abnormal inflammatory response of the lungs to noxious stimulus or gases, like cigarette smoke. In addition to slow progressive loss of lung function, patients with COPD can have acute exacerbations that lead to a faster decline in FEV1. Exacerbations are most often triggered by viral or bacterial infection. (Papi, Bellettato et al. 2006). Vitamin D deficiency is highly prevalent in COPD and correlates with the severity of COPD (Janssens, Bouillon et al. 2010). In line with new insights into the immunomodulatory effects of vitamin D, including anti-inflammatory and possibly anti-microbial effects, it has been postulated that vitamin D may affect the pathogenesis of COPD (Janssens, Lehouck et al. 2009). Epidemiological studies in healthy subjects and patients with COPD have suggested a dose dependant association between serum 25D levels and lung function (FVC and FEV1) (Black and Scragg 2005; Janssens, Bouillon et al. 2010). It is unclear at this time how vitamin D may affect lung function but variants in the vitamin D-binding gene have been linked to vitamin D deficiency and COPD risk (Janssens, Bouillon et al. 2010). These population based studies do not prove that there is an association between vitamin D deficiency and lung function but they do provide preliminary data and justification for randomized controlled trials of vitamin D supplementation in COPD. A randomized, multi-centre, double-blind, placebo-controlled trial of vitamin D supplementation in COPD is currently underway (www.clinicaltrials.gov).
VI. CONCLUSIONS AND FUTURE DIRECTIONS
Vitamin D deficiency is on the rise in western countries including the US (Ginde, Liu et al. 2009). Our understanding of vitamin D metabolism and function has grown exponentially over the last decade. It has become clear that vitamin D is not only important for bone and muscle health but has a wide spectrum of biological actions including significant immunomodulatory effects (Holick 2007). The enzyme 1α-hydroxylase is expressed by a variety of cells and the 1,25D that is produced locally in tissues may have direct effects on nearby cells and be responsible for the broad actions of vitamin D.
Epidemiological studies suggest an association between low vitamin D levels and mycobacterial infections, respiratory viral infections and asthma (Figure 1). The enzyme 1α-hydroxylase is expressed by airway epithelium, macrophages, dendritic cells and lymphocytes in the respiratory tract indicating that active vitamin D may be produced locally within the lungs (Table 1). Mechanistic studies have found the 1,25D influences cellular mechanisms that are important for recognition and killing of pathogens, inflammation and control of adaptive immune functions within the lungs (Figure 1).
Epidemiological and mechanistic studies indicate that vitamin D may play an important role in the development of respiratory diseases but many questions remain. Important clinical trials are on-going looking at the effects of vitamin D supplementation on mycobacterial infections, respiratory tract infections, asthma and COPD.
Acknowledgments
This work was supported by National Institutes of Health Grant KL2 RR024980
References
- Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Ren S, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Sharma OP, et al. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72(5):1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4(8):404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135(7):1095–1096. doi: 10.1017/S0950268807008308. author reply 1097–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AR, McDonnell DP, et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988;85(10):3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour GL, Coburn JW, et al. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981;305(8):440–443. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)-and Th2-inducing cytokines. J Exp Med. 2002;195(5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JA, Fischer AJ, et al. Innate immune functions of the airway epithelium. Contrib Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):L887–892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- Bell NH. Renal and nonrenal 25-hydroxyvitamin D-1alpha-hydroxylases and their clinical significance. J Bone Miner Res. 1998;13(3):350–353. doi: 10.1359/jbmr.1998.13.3.350. [DOI] [PubMed] [Google Scholar]
- Bhalla AK, Amento EP, et al. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- Bikle D. Nonclassic actions of vitamin d. J Clin Endocrinol Metab. 2009;94(1):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128(6):3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- Boonstra A, Barrat FJ, et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Bornman L, Campbell SJ, et al. Vitamin D receptor polymorphisms and susceptibility to tuberculosis in West Africa: a case-control and family study. J Infect Dis. 2004;190(9):1631–1641. doi: 10.1086/424462. [DOI] [PubMed] [Google Scholar]
- Brehm JM, Celedon JC, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm JM, Schuemann B, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell JJ, Vieth R, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell JJ, Zasloff M, et al. On the epidemiology of influenza. Virol J. 2008;5:29. doi: 10.1186/1743-422X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sims GP, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(3):1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- Crowle AJ, Ross EJ, et al. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987;55(12):2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio D, Cippitelli M, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101(1):252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C, Sartory NA, et al. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- Dimeloe S, Nanzer A, et al. Regulatory T cells, inflammation and the allergic response-The role of glucocorticoids and Vitamin D. J Steroid Biochem Mol Biol. 2010;120(2–3):86–95. doi: 10.1016/j.jsbmb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Dusso AS, Kamimura S, et al. gamma-Interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab. 1997;82(7):2222–2232. doi: 10.1210/jcem.82.7.4074. [DOI] [PubMed] [Google Scholar]
- Freishtat RJ, Iqbal SF, et al. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156(6):948–952. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche J, Mondal K, et al. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102(9):3314–3316. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- Gale CR, Robinson SM, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62(1):68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Tao Y, et al. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14(1):15–23. [PubMed] [Google Scholar]
- Gibney KB, MacGregor L, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46(3):443–446. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- Ginde AA, Liu MC, et al. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginde AA, Mansbach JM, et al. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori S, Casorati M, et al. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167(4):1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Lutz W, et al. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98(12):6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S, Monick MM, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181(10):7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S, Monick MM, et al. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184(2):965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylowicz C, Richards D, et al. A defect in corticosteroid-induced IL-10 production in T lymphocytes from corticosteroid-resistant asthmatic patients. J Allergy Clin Immunol. 2002;109(2):369–370. doi: 10.1067/mai.2002.121455. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5(4):271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- Hewison M, Burke F, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- Hewison M, Freeman L, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170(11):5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- Hmama Z, Sendide K, et al. Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1alpha,25-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J Cell Sci. 2004;117(Pt 10):2131–2140. doi: 10.1242/jcs.01072. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holt PG, Strickland DH, et al. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8(2):142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- Hypponen E, Sovio U, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens W, Bouillon R, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- Janssens W, Lehouck A, et al. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179(8):630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- Karagiannidis C, Akdis M, et al. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114(6):1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Kohlmeier JE, Woodland DL. Immunity to Respiratory Viruses. Annu Rev Immunol. 2008 doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- Laaksi I, Ruohola JP, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC, et al. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125(6 Suppl):1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Baker I, et al. Meta-analysis of vitamin D receptor polymorphisms and pulmonary tuberculosis risk. Int J Tuberc Lung Dis. 2005;9(10):1174–1177. [PubMed] [Google Scholar]
- Li-Ng M, Aloia JF, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137(10):1396–1404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- Li Q, I, Verma M. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Lipsitch M, Viboud C. Influenza seasonality: lifting the fog. Proc Natl Acad Sci U S A. 2009;106(10):3645–3646. doi: 10.1073/pnas.0900933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009;9(3):202–207. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120(5):1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Liu PT, Krutzik SR, et al. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13(3):117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, et al. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31(3):438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PN, Dowd DR, et al. Retinoid X receptors stimulate and 9-cis retinoic acid inhibits 1,25-dihydroxyvitamin D3-activated expression of the rat osteocalcin gene. Mol Cell Biol. 1993;13(9):5907–5917. doi: 10.1128/mcb.13.9.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak TW, Saunders ME. The immune response : basic and clinical principles. New York: Elsevier/Academic; 2005. [Google Scholar]
- Martineau AR, Wilkinson KA, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178(11):7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- Martineau AR, Wilkinson RJ, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176(2):208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, et al. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008 doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcos MM, Gabr AA, et al. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137(5):157–164. [PubMed] [Google Scholar]
- Muhe L, Lulseged S, et al. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349(9068):1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- Nursyam EW, Amin Z, et al. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38(1):3–5. [PubMed] [Google Scholar]
- Papapoulos SE, Clemens TL, et al. 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1(8117):627–630. doi: 10.1016/s0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- Papi A, Bellettato CM, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- Penna G, Roncari A, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- Pichler J, Gerstmayr M, et al. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52(1):12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Piemonti L, Monti P, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164(9):4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- Ponchon G, Kennan AL, et al. “Activation” of vitamin D by the liver. J Clin Invest. 1969;48(11):2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, et al. 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. J Immunol. 1986;136(8):2734–2740. [PubMed] [Google Scholar]
- Rehman PK. Sub-clinical rickets and recurrent infection. J Trop Pediatr. 1994;40(1):58. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- Reichel H, Koeffler HP, et al. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab. 1987;65(6):1201–1209. doi: 10.1210/jcem-65-6-1201. [DOI] [PubMed] [Google Scholar]
- Reichel H, Koeffler HP, et al. Synthesis in vitro of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by interferon-gamma-stimulated normal human bone marrow and alveolar macrophages. J Biol Chem. 1987;262(23):10931–10937. [PubMed] [Google Scholar]
- Ren S, Nguyen L, et al. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem. 2005;280(21):20604–20611. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- Robinson DS. Regulatory T cells and asthma. Clin Exp Allergy. 2009;39(9):1314–1323. doi: 10.1111/j.1365-2222.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- Rook GA, Steele J, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Roth DE, Jones AB, et al. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J Infect Dis. 2008;197(5):676–680. doi: 10.1086/527488. [DOI] [PubMed] [Google Scholar]
- Roth DE, Soto G, et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis. 2004;190(5):920–927. doi: 10.1086/423212. [DOI] [PubMed] [Google Scholar]
- Searing DA, Zhang Y, et al. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125(5):995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8(3):285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- Sly LM, Lopez M, et al. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276(38):35482–35493. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168(3):1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- Sutherland ER, Goleva E, et al. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181(7):699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AL, MacDonald PN. “Vitamin D: more than a bone-a-fide” hormone. Mol Endocrinol. 20030;17(5):777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- Upham JW. The role of dendritic cells in immune regulation and allergic airway inflammation. Respirology. 2003;8(2):140–148. doi: 10.1046/j.1440-1843.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Ustianowski A, Shaffer R, et al. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J Infect. 2005;50(5):432–437. doi: 10.1016/j.jinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Wayse V, Yousafzai A, et al. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58(4):563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- Wejse C, V, Gomes F, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76(9):3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RJ, Llewelyn M, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. The Lancet. 2000;355(9204):618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- Xystrakis E, Kusumakar S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116(1):146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xystrakis E, Urry Z, et al. Regulatory T cell therapy as individualized medicine for asthma and allergy. Curr Opin Allergy Clin Immunol. 2007;7(6):535–541. doi: 10.1097/ACI.0b013e3282f14d7c. [DOI] [PubMed] [Google Scholar]
- ZEHNDER D, BLAND R, et al. Expression of 25-Hydroxyvitamin D 3-1{alpha}-Hydroxylase in the Human Kidney. J Am Soc Nephrol. 1999;10(12):2465–2473. doi: 10.1681/ASN.V10122465. [DOI] [PubMed] [Google Scholar]