1. Introduction
Hallucinogens differ in their chemical structures, receptor binding properties, and psychogenic effects in humans (Nichols, 2004; Schindler et al., 2012; Shulgin and Shulgin, 1991; Shulgin and Shulgin, 1997). For instance, the pharmacologist, chemist, and hallucinogen expert, Alexander Shulgin, reported that the phenethylamine hallucinogen, (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), lacksthe “sparkle” ofthe indoleamine, lysergic acid diethylamide (LSD), but “the eyes-closed imagery is excellent…perfect for an artist (Shulgin and Shulgin, 1991).” Another indoleamine, 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), offered Shulgin more auditory hallucination and unfortunately, “slight nausea (Shulgin and Shulgin, 1997).” Despite these differences, it is agreed that hallucinogens exert their particular effects at the serotonin2A (5-HT2A) receptor (Dean, 2003; Nichols, 2004). One major downstream effect of 5-HT2A receptor activation is phosphatidylinositol (PI) hydrolysis. Upon activation, this receptor associates with the Gαq type G-protein, which activates phospholipase C (PLC). PLC splits membrane phosphatidylinsotiol-4,5-bisphosphate into inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 releases sequestered intracellular calcium, and DAG activates protein kinase C. Inhibition of the PLC enzyme has been shown to significantly reduce serotonergic stimulation of PI hydrolysis, both in cells transfected with 5-HT2A receptors (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003) and in antidepressant-stimulated hippocampal and frontocortical tissue prisms (Tyeryar et al., 2008). Furthermore, hallucinogens have been shown to induce PI hydrolysis through agonist activity at 5-HT2A receptors (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003; Pierce and Peroutka, 1988).
Hallucinogens also elicit stereotyped head movements in animals through activation of 5-HT2A receptors (Dave et al., 2002; Gonzalez-Maeso et al., 2007; Schindler et al., 2012; Schreiber et al., 1995). Direct intracortical or intracerebroventricular drug infusion has also been shown to elicit head movements in both rabbits and rats through 5-HT2A receptor activation (Dave et al., 2004; Dave et al., 2007; Willins and Meltzer, 1997). In fact, the ability of various 5-HT2A receptor antagonists to block hallucinogen-elicited mouse head twitches (Peroutka et al., 1981) and rat head shakes (Dursun and Handley, 1996; Schreiber et al., 1995) is strongly correlated to their frontocortical 5-HT2A binding affinity. In addition, both 5-HT2A receptor binding affinity and drug-elicited head twitch behavior in rodents are strongly correlated to the effective hallucinogenic dose of various drugs in humans (Corne and Pickering, 1967; Glennon et al., 1984). These relationships validate the use of animal head movements to model in the effects of hallucinogens in humans.
Despite its use as a model for the hallucinogenic effect and human psychosis, head movement behavior is not thoroughly understood vis à vis PI hydrolysis signaling. Rinaldi-Carmona and colleagues (1993a, 1993b) were the first to demonstrate a relationship between PI hydrolysis and drug-elicited head movements in rodents using the hallucinogen, DOI. This group used chronic in vivo drug treatment to manipulate frontocortical receptor density and PI hydrolysis signaling (Rinaldi-Carmona et al., 1993a; Rinaldi-Carmona et al., 1993b). A more direct method for identifying the biochemical origins of behavior involves the use of an enzyme inhibitor. For instance, intracerebroventricular infusion of the PLC inhibitor, 1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione (U73122), in rodents has been used to demonstrate the role of PI hydrolysis in adrenomedullary outflow (Shimizu et al., 2007), thermal hypernocioception (Galeotti et al., 2006), and anandamide-induced sleep (Murillo-Rodriguez et al., 2001). To our knowledge, PLC inhibition has not been used to investigate drug-elicited head movements.
The goal of the present study was to examine the role of PLC activation/PI hydrolysis in hallucinogen-elicited head movements in the rabbit using the PLC inhibitor, U73122. Hallucinogens representative of the phenethylamine and indoleamine groups (DOI and LSD, respectively) were chosen for this investigation. DOI and LSD were previously shown to require the activation of 5-HT2A receptors to elicit head bobs in rabbits, although their binding properties at frontocortical 5-HT2A receptors differed (Dave et al., 2002; Dave et al., 2007; Schindler et al., 2012). The present study sought to determine whether these two pharmacologically distinct hallucinogens also differed in their use of PI hydrolysis/PLC activation for the elicitation of rabbit head bobs.
2. Results
2.1 In vitro PI hydrolysis
2.1.1 Agonist-stimulated in vitro PI hydrolysis
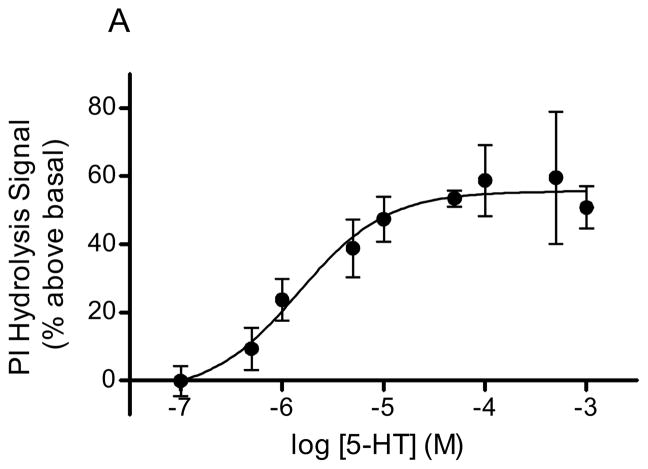
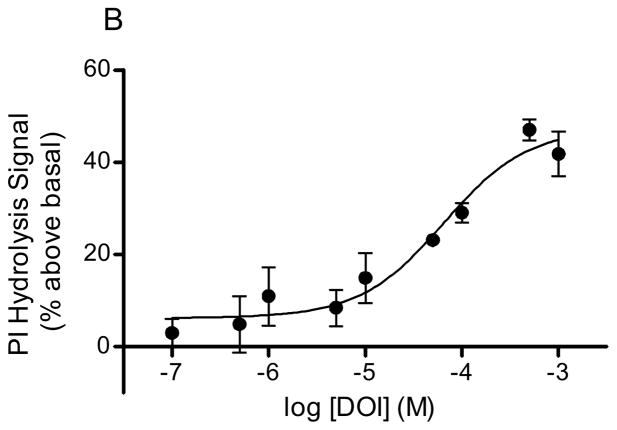
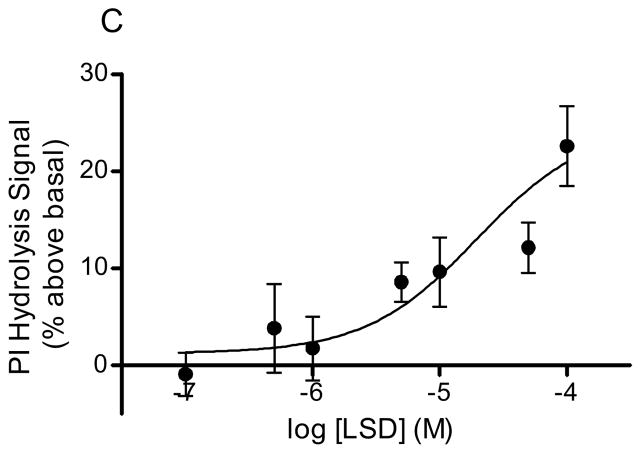
Serotonin, DOI, and LSD stimulated PI hydrolysis in rabbit frontocortical tissue prisms in a concentration dependent manner (Fig 1). The Vmax values for agonist stimulation were as follows: 5-HT, 55.7 ± 3.9% above basal (Fig 1A); DOI, 47.4 ± 4.1% above basal (Fig 1B); LSD, 24.8 ± 5.6% above basal (Fig 1C). The EC50 values for 5-HT and DOI were 1.46 ± 0.9μM and 64.5 ± 30μM, respectively. An accurate EC50 for LSD could not be calculated given the short range of concentrations that produced detectable signals in our assay. At concentrations above 100μM, the LSD signal returned to baseline suggesting a problem with drug solubility or some other factor at higher LSD concentrations.
Figure 1. Hallucinogen-stimulated in vitro PI hydrolysis.
PI hydrolysis was measured in rabbit frontocortical tissue prisms through release of [3H]IP1. The concentration curves for 5-HT (A, n=4), DOI (B, n=3), and LSD (C, n=10) are shown. The maximum concentration of LSD was 100μM, whereas the maximum concentration for 5-HT and DOI was 1mM.
2.1.2 Effects of antagonists on in vitro PI hydrolysis
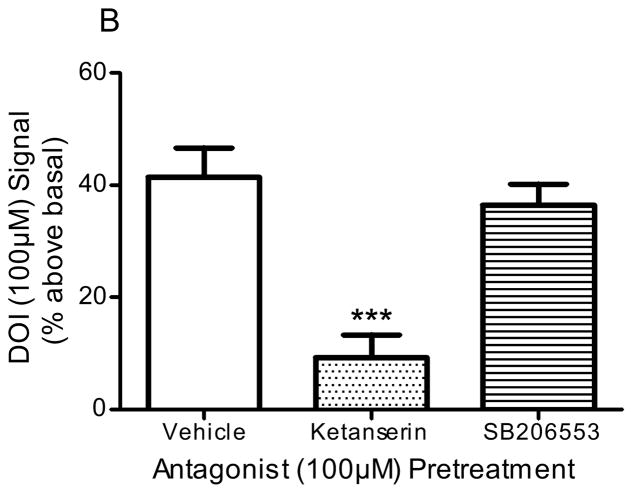
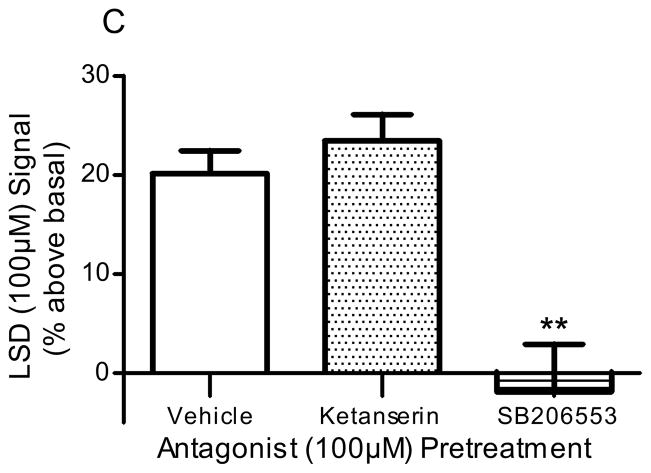
Pre-incubation of frontocortical tissue prisms with the 5-HT2A/2C antagonist, ketanserin (100μM), significantly blocked PI hydrolysis signals stimulated by 5-HT (100μM; p<0.001, F=26.1, ANOVA) and DOI (100μM; p<0.005, F=9.9, ANOVA; Fig 2). Ketanserin reduced the 5-HT-stimulated signal from 51.5 ± 3.0 to 18.5 ± 10.2% above basal (p<0.01, post-hoc Dunnett test; Fig 2A) and the DOI-stimulated signal from 41.5 ± 5.4 to 9.3 ± 4.4% above basal (p<0.001, post hoc Dunnett test; Fig 2B). Ketanserin (100μM) did not significantly alter the PI hydrolysis signal stimulated by LSD (100μM): control, 20.1 ± 2.5% above basal; ketanserin, 23.5 ± 3.8% above basal (p>0.05, post hoc Dunnett test; Fig 2C). Pre-incubation of tissue with the 5-HT2B/2C antagonist, SB206553 (100μM), significantly blocked PI hydrolysis signals stimulated by 5-HT (100μM; p<0.001, F=26.1, ANOVA) and LSD (100μM; p<0.005, F=15.3, ANOVA; Fig 2). SB206553 reduced the 5-HT signal from 51.5 ± 3.0 to 16.5 ± 3.7% above basal (p<0.001, post-hoc Dunnett test; Fig 2A) and the LSD signal from 20.1 ± 2.5 to −1.9 ± 5.9% above basal (p<0.01, post-hoc Dunnett test; Fig 2C). SB206553 (100μM) did not significantly alter the PI hydrolysis signal stimulated by DOI (100μM): control, 41.5 ± 5.4% above basal; SB206553, 36.5 ± 4.2% above basal (100μM; p>0.05, post-hoc Dunnett test; Fig 2B). At the concentration used (100μM), neither antagonist significantly altered the baseline PI hydrolysis signal: control, 0.0 ± 4.0% above basal; ketanserin, −6.1 ± 8.1% above basal; SB206553, −2.2 ± 9.7% above basal (p>0.7, F=0.27, ANOVA).
Figure 2. Effect of antagonists in in vitro PI hydrolysis.
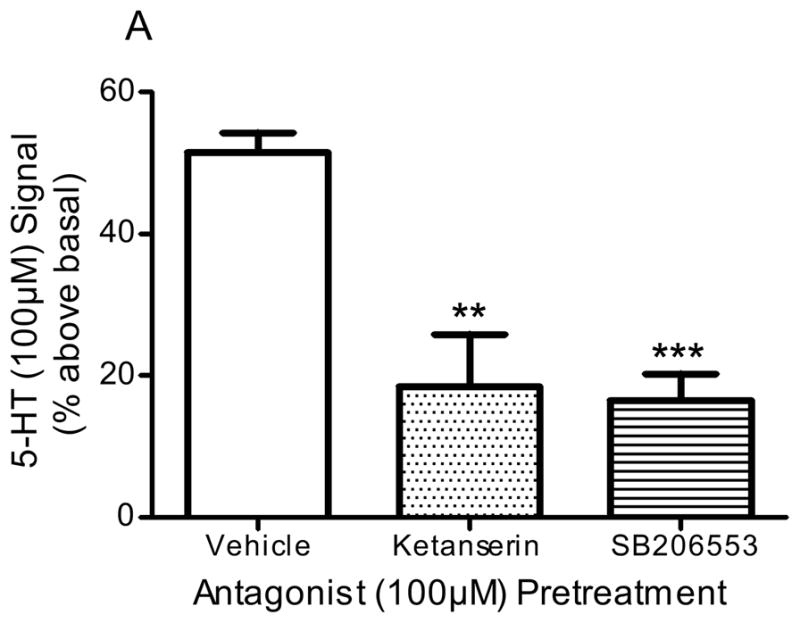
Frontocortical tissue prisms were pre-incubated with ketanserin (100μM), SB206553 (100μM), or vehicle (control) 20 minutes prior to stimulation with 5-HT (A, n=6), DOI (B, control n=10, antagonists n=4), or LSD (C, n=6). **p<0.01, ***p<0.001, post hoc Dunnett test, significantly different from vehicle pre-incubation
2.1.3 Effects of PLC inhibitor on in vitro PI hydrolysis
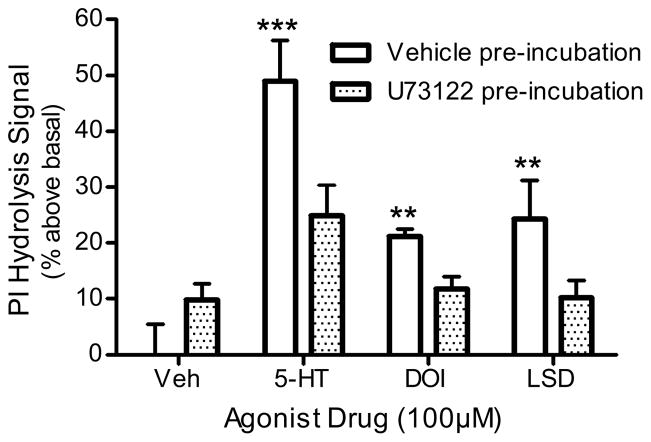
Pre-incubation of frontocortical tissue prisms with the PLC inhibitor, U73122 (100μM), significantly inhibited all agonist (100μM)-stimulated signals (p<0.0001, F=7.8, 2-way ANOVA; Fig 3). For example, 5-HT (100μM) stimulated a signal with vehicle pre-incubation (49.0 ± 8.3% above basal), but not with U73122 pre-incubation (25.0 ± 6.2% above basal; p<0.001, post hoc Bonferroni test). DOI (100μM)-stimulated PI hydrolysis was similarly altered by U73122: control, 21.2 ± 1.6% above basal; U73122, 11.9 ± 2.4% above basal (p<0.01, post hoc Bonferroni test; Fig 3). LSD (100μM)-stimulated PI hydrolysis was also inhibited by U73122: control, 24.4 ± 7.9% above basal; U73122, 10.2 ± 3.6% above basal (p<0.01, post hoc Bonferroni test; Fig 3). U73122 (100μM) alone stimulated a small, but non-significant, PI hydrolysis signal (0.0 ± 6.3 to 9.9 ± 3.3% over basal; p>0.05, post hoc Bonferroni test; Fig 3). The inactive analogue of the PLC inhibitor, U73343 (100μM), did not affect baseline or serotonergic PI hydrolysis (data not shown).
Figure 3. Effect of PLC inhibition in in vitro PI hydrolysis.
Frontocortical tissue prisms were pre-incubated with U73122 (100μM) or vehicle (control) 20 minutes prior to stimulation with 5-HT, DOI, or LSD (n=4). **p<0.01, ***p<0.001, post hoc Bonferroni test, significantly different from vehicle
2.2 In vivo behavior
2.2.1 Head bob response after intracortical hallucinogen infusion
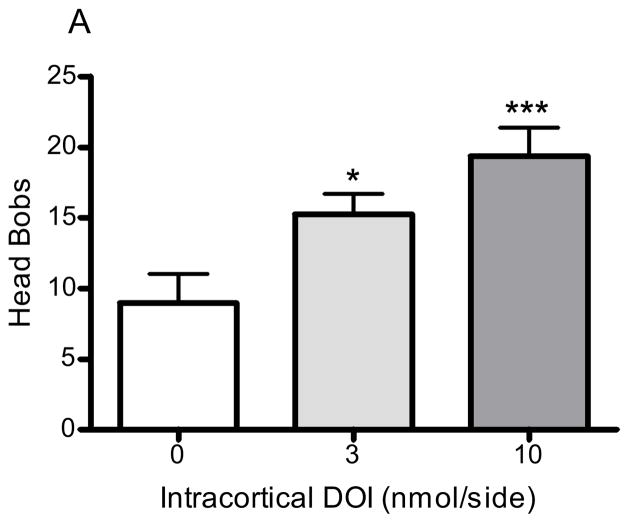
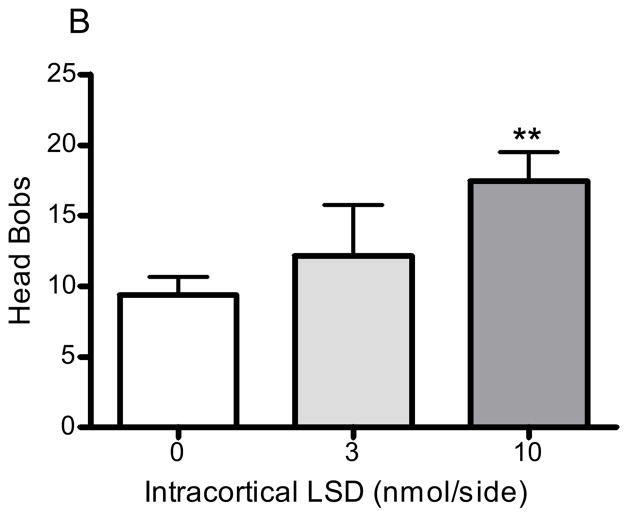
Bilateral infusion of DOI into the medial prefrontal cortex significantly and dose-dependently increased the number of head bobs in rabbits (p<0.005, F=8.1, ANOVA; Fig 4A). DOI 3 and 10nmol/side elicited an average of 15.3 ± 1.5 (p<0.05, post hoc Dunnett test) and 19.4 ±2.1 (p<0.001, post hoc Dunnett test) head bobs, respectively (Fig 4A). LSD also significantly increased the number of head bobs in rabbits (p<0.01, F=5.3, ANOVA; Fig 4B). LSD 3 and 10nmol/side produced an average of 12.2 ± 3.9 (p>0.05, post hoc Dunnett test) and 17.5 ±2.1 (p<0.01, post hoc Dunnett test; Fig 4B) head bobs, respectively. The behavioral response to the two vehicle agents did not significantly differ from one another: DOI vehicle (aCSF), 9.0 ± 2.1 head bobs; LSD vehicle (tartaric acid/NaOH/modified aCSF), 9.4 ± 1.3 head bobs.
Figure 4. Head bobs after intracortical hallucinogen infusion.
DOI (3 and 10nmol/side, n=12; A) or LSD (3 and 10nmol/side, n=16; B) were infused bilaterally into the medial prefrontal cortex at a rate of 0.5μL/min. The vehicle (control) for DOI was aCSF (n=12) and the vehicle (control) for LSD was tartaric acid/NaOH/modified aCSF (n=6; see Methods section 2.2). *p<0.05, **p<0.01, ***p<0.001, post hoc Dunnett test, significantly different from control group
2.2.2 Effects of PLC inhibitor on hallucinogen-elicited head bobs
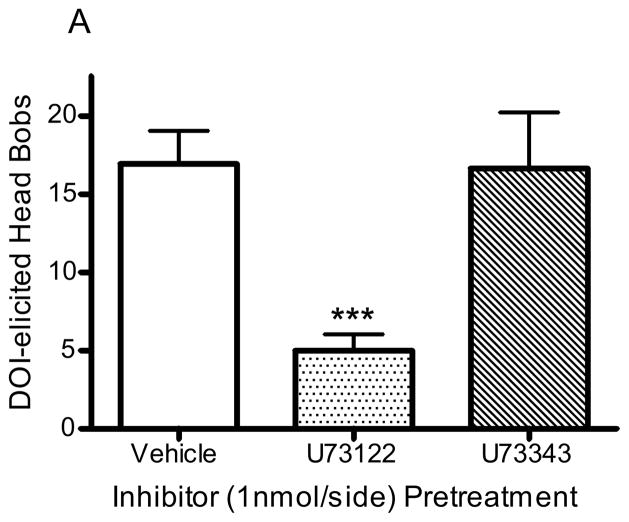
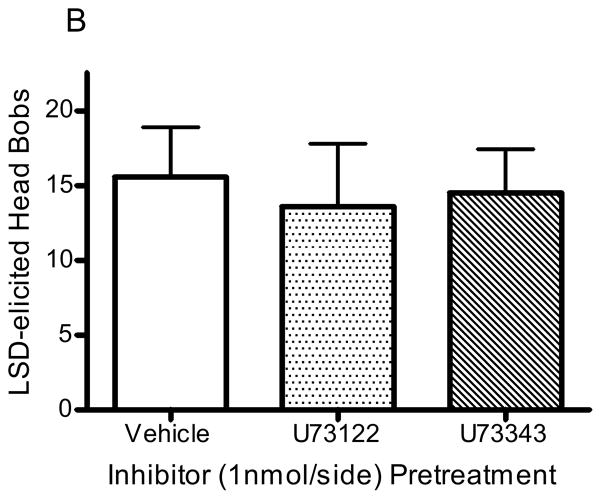
Pretreatment with the PLC inhibitor, U73122 (1nmol/side), significantly reduced the number of head bobs in animals receiving DOI infusion (10nmol/side; p<0.001, F=9.8, ANOVA; Fig 5A); the number of head bobs was reduced from 16.9 ± 2.2 to 5.0 ± 1.1 in animals pretreated with U73122 (1nmol/side; p<0.001, post hoc Dunnett test; Fig 5A). The number of DOI-elicited head bobs was not altered by pretreatment with the inactive analogue, U73343 (1nmol/side; 16.7 ± 3.9 head bobs; p>0.5, post hoc Dunnett test; Fig 5A). In contrast, pretreatment with either U73122 (1nmol/side) or U73343 (1nmol/side) had no significant effect in animals receiving LSD infusion (10nmol/side; p>0.9, F=0.08, ANOVA): control (1% DMF in aCSF), 15.6 ± 3.6 head bobs; U73122, 13.3 ± 4.7 head bobs; U73343, 14.5 ± 3.3 head bobs (Fig 5B). Infusion of enzyme inhibitor alone did not significantly alter baseline responding: vehicle (1% DMF in aCSF), 9.0 ± 0.9 head bobs; U73122, 9.5 ± 2.0 head bobs; U73343, 9.8 ± 1.2 head bobs.
Figure 5. PLC inhibition of head bobs.
Rabbits were infused with U73122 (1nmol/side, n=14), U73343 (1nmol/side, n=12), or vehicle (1% DMF in aCSF, n=4) 15 minutes before infusion with DOI (A) or LSD (B). ***p<0.001, post hoc Dunnett test, significantly different from control group
2.2.3 Verification of cannula placement
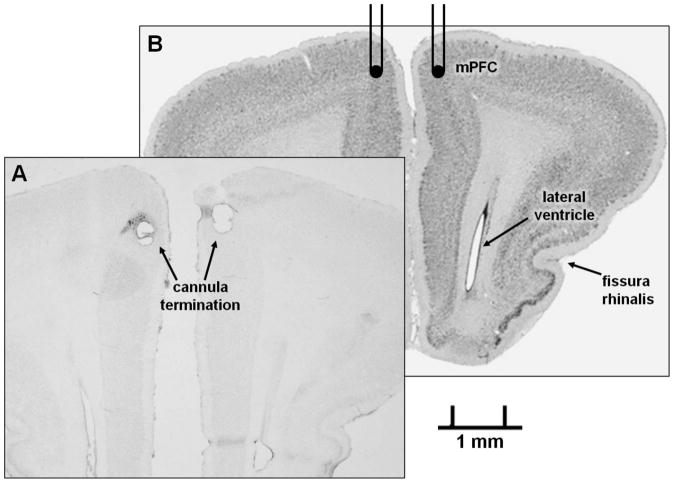
Cannula placement was confirmed by infusion of tryptan blue followed by gross brain dissection. Some animals were perfused at the end of infusion studies for histological confirmation of cannula placement via Cresyl Violet staining. Figure 6 is a representative section showing cannula tracts in the medial prefrontal cortex as described in the Atlas of the Rabbit Brain and Spinal Cord (Shek, 1986). A reference image obtained with permission from the University of Wisconsin and Michigan State University Comparative Mammalian Brain Collections, supported by the US National Science Foundation, is also included (http://www.brainmuseum.org; Oryctolagus cuniculus plate #440).
Figure 6. Surgical placement of cannulae.
After infusion studies were completed, animals were sacrificed by cardiac perfusion and brains sliced and stained with Cresyl Violet for placement verification. A representative image showing the area of cannula termination in the medial prefrontal cortex is shown in panel A. Panel B shows cannula placement in a reference image adapted with permission from http://brainmuseum.org, supported by the US National Science Foundation (Oryctolagus cuniculus plate #440). mPFC denotes medial prefrontal cortex and the bilateral lines represent surgical placement of the cannulae (B).
3. Discussion
3.1 Role of PLC activation/PI hydrolysis in hallucinogen-elicited head bob behavior
In the present study, infusion of PLC inhibitor, U73122, into the mPFC of rabbits completely blocked the head bob response to intracortical DOI, but not LSD, suggesting a role for PLC activation/PI hydrolysis in DOI-, but not LSD-, elicited head bobs. The present use of U73122 to inhibit the rate limiting enzyme in the hydrolysis of membrane phosphatidylinsotiol-4,5-bisphosphate is one of the first to use an enzyme inhibitor in vivo to investigate the role of intracellular signaling in drug-elicited animal head movements (Schmid and Bohn, 2010). This difference observed between DOI and LSD significantly distinguishes the behavioral pharmacology of these two hallucinogens. This work also adds to a growing body of knowledge on the biochemical differences among hallucinogens and related serotonergic compounds (Berg et al., 1998; Edwards et al., 1992; Egan et al., 1998; Frankel and Cunningham, 2002; Gonzalez-Maeso et al., 2007; Kurrasch-Orbaugh et al., 2003; Moya et al., 2007; Pierce and Peroutka, 1988; Schmid et al., 2008; Schmid and Bohn, 2010)
3.2 Halluciongen-stimulated PI hydrolysis
The current study showed that 5-HT, DOI, and LSD all stimulated PI hydrolysis as measured by the release of [3H]inositol phosphate from rabbit frontocortical tissue prisms. Importantly, in this assay, the endogenous agonist, 5-HT, had a potency comparable to those reported in the literature for rat cortical tissue (Edwards et al., 1992; Pierce and Peroutka, 1988; Sanders-Bush et al., 1988). It is known that PI hydrolysis potencies determined in cell-based studies are generally higher than those reported in tissue assays (Berg et al., 1998; Egan et al., 1998; Kurrasch-Orbaugh et al., 2003). In fact, in some tissue studies, EC50 values for certain agonists or tissue types are not reported (Edwards et al., 1992; Pierce and Peroutka, 1988; Rabin and Winter, 1996). In the current study, the EC50 for DOI was lower than values reported in cell-based models (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003), and a potency could not be accurately determined for LSD, despite its recognition as a highly potent compound, both in experimental settings and in human recreational use (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003; Nichols, 2004; Passie et al., 2008; Sanders-Bush et al., 1988). Even with the limitations of the tissue-based assay, all PI hydrolysis signals were blocked by the PLC inhibitor, U73122, which has been used previously to identify a role for PLC activation in DOI-stimulated PI hydrolysis in cells (Berg et al., 1998). The direct implication of PLC activation in LSD-stimulated PI hydrolysis, however, is a new finding. Paradoxically, U73122 also produced a small, but non-significant, PI hydrolysis signal in the current study, a phenomenon described elsewhere (Tyeryar et al., 2008). The authors also recognize that the specificity of U73122 is not limited to the PLC enzyme, though it is an accepted PLC inhibitor, especially when used in conjunction with its analogue, U73343 which does not inhibit PLC activity (Bleasdale and Fisher, 1993).
As is reported in the literature, the maximal PI hydrolysis signal stimulated by DOI in the present study was lower than the maximal signal stimulated by 5-HT, but greater than that stimulated by LSD (Berg et al., 1998; Edwards et al., 1992; Pierce and Peroutka, 1988). LSD’s maximal efficacy in the current study was approximately 25% above the baseline signal, similar to the efficacy reported in both tissue and cell studies (Edwards et al., 1992; Egan et al., 1998; Kurrasch-Orbaugh et al., 2003; Sanders-Bush et al., 1988). Despite these differences in signaling efficacy, the doses of DOI and LSD investigated in the current study (3 and 10nmol/side) elicited approximately the same number of head bobs when infused into the mPFC. Previous reports from our lab also showed that in the full dose-response curves for subcutaneously administered DOI and LSD, the two drugs elicit approximately the same maximal number of head bobs (Dave et al., 2002; Romano et al., 2010; Schindler et al., 2012). Coupled with the previously discussed finding that U73122 blocks DOI-, but not LSD-, elicited head bobs, the difference in PI hydrolysis efficacy between the two drugs points to a discrepancy between intracellular signaling and behavior. Indeed, drug-elicited rat head shaking behavior was previously shown to be independent of a compound’s PI hydrolysis efficacy (Moya et al., 2007). Similarly, drug discrimination studies in rats have shown that a hallucinogen’s stimulus cue does not reflect its PI hydrolysis efficacy (Rabin et al., 2002). Thus, PLC activation/PI hydrolysis may be required for the behavioral effects of some (e.g. DOI), but not all (e.g. LSD), hallucinogenic compounds. Whether this biochemical distinction relates to psychedelic differences among hallucinogens remains to be seen. Clearly, the psychogenic effects of DOI, LSD, and other hallucinogens are complex and cannot be represented by any one behavioral model. Non-hallucinogens elicit animal head movements as well, further limiting the specificity of the model (Lucki et al., 1984; Scarlota et al., 2011; Schmid et al., 2008; Schmid and Bohn, 2010; Schreiber et al., 1995). Animal head movements remain an important tool, however, as decades of work with the model have increased our understanding of ligand-receptor interaction, especially that in the serotonergic system. The most recent studies concurrently investigating head movements and intracellular signaling will help expand the utility of this behavioral model and further illustrate the actions of both hallucinogenic and non-hallucinogenic compounds (Moya et al., 2007; Schmid et al., 2008; Schmid and Bohn, 2010).
3.3 Serotonin receptor subtypes in hallucinogen-stimulated PI hydrolysis
Although U73122 blocked PI hydrolysis stimulated by all agonists investigated in vitro, serotonergic antagonists had differing effects on their signaling. Ketanserin and SB206553 were used to assess 5-HT2A and 5-HT2C receptor involvement, respectively. Though ketanserin also possesses significant 5-HT2C receptor activity, it is 30 times more selective for rabbit frontocortical 5-HT2A receptors (Dave et al., 2002). Furthermore, SB206553 is 750 times more selective for rabbit frontocortical 5-HT2C receptors than 5-HT2A receptors (Dave et al., 2002). The present study showed that both ketanserin and SB206553 inhibited the PI hydrolysis signal stimulated bythe endogenous agonist, 5-HT. In contrast, ketanserin blocked the DOI-, but not LSD-, stimulated signal, whereas SB206553, blocked the LSD-, but not DOI-, stimulated signal. These findings implicate both 5-HT2A and 5-HT2C receptors in the 5-HT-stimulated signal, but it would appear that DOI only signaled through 5-HT2A receptors whereas LSD only signaled through 5-HT2C receptors. This distinctive signaling pattern is unexpected, given that both hallucinogens studied have good affinity for both receptors in rabbit frontal cortex (Schindler et al., 2012). As in the current study, DOI-stimulated PI hydrolysis has previously been blocked by 5-HT2A antagonists in cortical tissue (Edwards et al., 1992; Pierce and Peroutka, 1988). The LSD-stimulated signal was also blocked by ketanserin in cell models, but at 10 to 20 times the LSD concentration (Barker et al., 1991; Egan et al., 1998). One study reported complete blocking ofthe LSD-stimulated PI hydrolysis in rat cortical slices by ketanserin, but the data were not shown (Sanders-Bush et al., 1988). Another group showed that ketanserin failed to affect the LSD signal in rat cortical slices (Pierce and Peroutka, 1988). Importantly, LSD binds 5-HT2A receptors in an irreversible or pseudo-irreversible manner (Berridge and Prince, 1974; Burris and Sanders-Bush, 1992; Schindler et al., 2012). In contrast, ketanserin reversibly binds rabbit frontocortical 5-HT2A receptors (Aloyo et al., 2001; Schindler et al., 2012), allowing for competition by other ligands, such as LSD. Thus, LSD most likely does stimulate PI hydrolysis through 5-HT2A receptors in cortical tissue, but confirming this is hindered by pharmacokinetics. Use of an irreversible or pseudo-irreversible 5-HT2A antagonist, such as ritanserin, should more clearly establish the role of 5-HT2A receptors in LSD-stimulated PI hydrolysis (Leysen et al., 1985; Schindler et al., 2012).
Both LSD and DOI bind frontocortical 5-HT2C receptors in a reversible manner (Schindler et al., 2012). Thus, the present finding that the highly selective 5-HT2C receptor antagonist, SB206553, blocked the LSD-, but not DOI-, stimulated PI hydrolysis signal, clearly points to a role for 5-HT2C receptors in LSD’s effects. Both LSD and the DOI analogue, (±)-1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), were previously shown to stimulate PI hydrolysis through 5-HT2C receptors in 5-HT2C-transfaected cells (Egan et al., 1998) and fibroblasts derived from choroid plexus, a tissue with a dense 5-HT2C receptor concentration (Barker et al., 1991; Burris et al., 1991). The high 5-HT2C receptor density in these models makes for a more sensitive assay than using native frontocortical tissue. Indeed, in rabbit frontocortical tissue, the 5-HT2C receptor density is one third to one half that of 5-HT2A receptor density (Aloyo et al., 2001; Schindler et al., 2012). Thus, 5-HT2C-mediated PI hydrolysis may be difficult to detect in native cortical tissue. As the current findings show, LSD may derive a significant portion of its PI hydrolysis signal in frontal cortex from 5-HT2C receptors, whereas DOI does not. Indeed, the 5-HT2C receptor has been an important focus in the study of hallucinogens, LSD in particular. For instance, LSD stimulated PI hydrolysis in fibroblasts derived from rat choroid plexus, whereas the two non-hallucinogens, (+)-2-bromo-LSD and lisuride, did not generate signals in this model (Burris et al., 1991). These findings, along with those from the present report, maintain a role for 5-HT2C receptors in LSD’s pharmacological mechanism of action. Though the 5-HT2C receptor is not involved in hallucinogen-elicited rabbit head bobs (Dave et al., 2002; Schindler et al., 2012) or rat head shakes (Schreiber et al., 1995), it has been associated with mouse head twitch behavior (Canal et al., 2010; Fantegrossi et al., 2010). Furthermore, in rat drug discrimination studies, both DOI and LSD generalized to the cue of m-chlorophenylpiperazine (mCPP), a hallucinogenic compound with selective 5-HT2C receptor activity (Callahan and Cunningham, 1994). Thus, there is clearly a role for 5-HT2C receptors in the subjective effects of LSD and other hallucinogens, though it may not be manifested in the non-murine head movement model.
3.4 Alternative signaling pathways
The current finding that LSD-elicited head bobs did not require PLC activation suggests that LSD produces its behavioral effects through another signaling pathway. It has been shown, for instance, that LSD, along with DOI and other 5-HT2A agonists, stimulates arachidonic acid (AA) release in cells expressing 5-HT2A receptors (Berg et al., 1998; Kurrasch-Orbaugh et al., 2003; Moya et al., 2007). AA is released from membrane phospholipids primarily through receptor-G-protein coupling followed by phospholipase A2 (PLA2) activation. This intracellular effect is most clearly associated with inflammatory processes, as AA is converted to prostaglandins and leukotrienes, but the significance ofAA in neurotransmission and psychiatric disease is also apparent (Skosnik and Yao, 2003). The AA release pathway shares downstream effects with the PI hydrolysis pathway, such as neurotransmitter release, PKC activation, and gene regulation (Farooqui et al., 1997). Furthermore, PI hydrolysis can indirectly lead to AA production through DAG. Thus, activation of either pathway may conceivably lead to similar effects at the behavioral level. The literature has indeed raised the issue of AA release in stereotyped head movement behavior (Moya et al., 2007; Qu et al., 2005). Specifically, DOI-elicited head twitches in mice were associated with PLA2 activation in brain regions known to have 5-HT2A receptors (Qu et al., 2005). Among various 5-HT2A agonists, however, a drug’s efficacy in stimulating AA release did not correlate directly to its efficacy in eliciting head shakes in rats (Moya et al., 2007). These findings support the consideration of AA release in the mechanism of action of hallucinogens, although further study is required in order to understand the precise contribution of this signaling pathway in their behavioral effects. For example, just as the present study used a PLC enzyme inhibitor in vivo, another group has performed intracranial administration of a PLA2 enzyme inhibitor to demonstrate the importance of PLA2 activation in rat models of short term and long term memory (Schaeffer and Gattaz, 2005). Such a procedure could be replicated in head movement or other behavioral models.
Another intracellular process that has recently been implicated in drug-elicited head movement behavior is β–arrestin signaling. Previously, β–arrestin protein was primarily associated with receptor desensitization and internalization, but β–arrestin was recently found to be essential for 5-HT-elicited mouse head twitches (Schmid et al., 2008; Schmid and Bohn, 2010). Interestingly, β–arrestin interactions were not required for head twitches elicited by the hallucinogens, DOI and 5-MeO-DMT (Schmid et al., 2008; Schmid and Bohn, 2010). The relevance of β–arrestin in LSD’s behavioral effects is not known. The shared chemical structure between 5-HT and LSD might implicate β–arrestin in LSD’s mechanism of action. On the other hand, LSD is a hallucinogen like DOI and 5-MeO-DMT, which might preclude a role for β–arrestin in LSD’s effects. Though β–arrestin and PI hydrolysis pathways also share some common downstream effects, such as ERK1/2 phosphorylation, the relevance of pathway-specific activation by various compounds in the head movement model remains to be determined (Schmid et al., 2008). Further experimentation with β–arrestin interactions, AA release, and other post-receptor processes is essential to refine our understanding of the behavioral effects of hallucinogens and related serotonergic drugs.
3.5 Conclusion
The current study suggests a distinction between DOI and LSD with regards to the role of PLC activation/PI hydrolysis in rabbit head bob behavior. The results of these experiments demonstrate that the two hallucinogens can induce PI hydrolysis in rabbit frontal cortex through PLC activation, but only DOI uses this signaling pathway to elicit head bobs. This pharmacological distinction may begin to address the specific differences in the psychogenic effects of DOI and LSD in humans (Shulgin and Shulgin, 1991; Shulgin and Shulgin, 1997). In conjunction with identifying roles of different receptors, the future study of hallucinogens should consider PI hydrolysis and other downstream effects of 5-HT receptor activation in the mechanism of action of these compounds.
4. Materials and Methods
4.1 Subjects
Adult male New Zealand White rabbits (Covance; Devon, PA), weighing 1.8–2.2kg upon arrival, were housed individually under a standard light-dark cycle of 12 hours with limited access to chow and unlimited access to water in an AAALAC-approved colony maintained at 22 ± 1°C. Rabbits were adapted to the colony room and handled by the experimenter for several days before the initiation of experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
4.2 Drugs and solutions
Serotonin (5-HT)-HCl (FW 212.7), (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-HCl (FW 357.6), lysergic acid diethylamide (LSD base; FW 323.4), pargyline-HCl (FW 195.7), and lithium chloride (LiCl; FW 42.4) were purchased from Sigma-Aldrich (St. Louis, MO). Ketanserin tartrate (FW 554.5) and SB206553-HCl (3,5-dihydro-5-methyl-N-3-pyridinylbenzo[1,2-b:4,5-b′]dipyrrole-1(2H)-carboxamide hydrochloride; FW 328.8) were purchased from Tocris Bioscience (Ellisville, MO). U73122 (1-[6-((17β-3- methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione; FW 464.7) and U73343 (1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-2,5-pyrrolidinedione; FW 466.7) were purchased from Calbiochem (La Jolla, CA). Myo-[2-3H]inositol was purchased from Perkin-Elmer (Boston, MA). Ketamine (100mg/mL), xylazine (20mg/mL), and lidocaine (1% topical solution) were purchased from Henry Schein (Indianapolis, IN). Unless otherwise specified, all other reagents and supplies were purchased from Fisher Scientific (Pittsbugh, PA), Biorad (Richmond, CA), or Sigma-Aldrich (St. Louis, MO). For PI hydrolysis studies: Serotonin and DOI were dissolved and diluted in warm bubbled assay buffer (37°C; 95 O2:5 CO2 gas; 130mM NaCl, 5mM KCl, 25mM NaHCO3, 1.2mM MgSO4, 2mM CaCl2, 10mM dextrose). LSD was dissolved in 2% tartaric acid, neutralized with NaOH, and diluted in warm bubbled assay buffer. Ketanserin was first dissolved in water and then diluted in warm bubbled assay buffer. SB206553 was dissolved in DMSO and diluted in warm bubbled assay buffer for a final DMSO concentration of 0.3%. U73122 and U73343 were dissolved in dimethylformamide (DMF) and diluted into warm bubbled assay buffer for a final DMF concentration of 0.1%. For surgical procedures: Ketamine and xylazine were injected intramuscularly (IM) in their stock form. For intracortical infusions: DOI was dissolved in artificial cerebrospinal fluid (aCSF; 147mM NaCl, 4mM KCl, 0.9mM MgCl2, 2.3mM CaCl2). LSD was dissolved in 2% tartaric acid, neutralized with NaOH, and diluted with a modified aCSF solution that adjusted for the sodium used in neutralization to give a final Na+ concentration of 147mM. U73122 and U73343 were dissolved in DMF and diluted into aCSF for a final DMF concentration of 1%.
4.3 PI hydrolysis
This assay was developed in part based on Berg et al. (Berg et al., 1998). Dissected frontal cortices were cut into 350μm X 350μm prisms using a McIlwain tissue chopper and placed in cold (4°C) Ca2+-free assay buffer bubbled with 95O2: 5 CO2 gas. After three washings to remove debris, the tissue was incubated in bubbled assay buffer with gentle shaking at 37°C for 30 minutes. Myo-[2-3H]inositol (Perkin-Elmer; SA=19.6–21.7Ci/mmol) was purified over Dowex resin (AG-1-X8 100–200 mesh formate form; Biorad) and eluted with deionized H2O. The tissue was then washed in warm bubbled assay buffer containing 2mM Ca2+ and incubated for 90 minutes with myo-[2-3H]inositol at a concentration of 0.5μM. After the labeling incubation, excess radioactivity was removed by washing the tissue four times with warm bubbled assay buffer containing 2mM Ca2+. Next, 25μL of gravity packed tissue was aliquoted into Ca2+- containing assay buffer and incubated with LiCl (10mM) and pargyline (10μM) for 15 minutes. When antagonist or enzyme inhibitor was used, it was added along with LiCl and pargyline and the incubation was extended to 20 minutes. Agonist was then added to make the final assay volume 250μL. Assay tubes were gassed with 95 O2:5 CO2 gas, capped, and incubated for 60 minutes; gassing was repeated every 15 to 20 minutes during the incubation. The reaction was stopped with the addition of 1.5mL of CHCl3 (100)/methanol (200)/HCl (1), followed by a 45 minute repose at room temperature. Phase separation was performed with the addition of 0.5mL of CHCl3 and 0.75mL of water, followed by mixing and then centrifugation for 20 minutes at 200g. A 50μL sample of the organic phase was taken for determination of lipid radioactivity incorporation (via scintillation counting). A 1mL sample of the aqueous phase was diluted with 1mL deionized H2O, applied to columns containing 0.5mL of a Dowex (1) : H2O (1) slurry.
Columns were then washed with 10mL of 5mM myoinositol, 2 × 10mL of sodium tetraborate (5mM)/sodium formate (60mM), and finally [3H]IP1 was eluted with 5mL of ammonium formate (0.2M)/sodium formate (1.0M). The final collection volume was vortexed and sampled for scintillation analysis. Due to variability in the amount of tissue in each assay tube, [3H]IP1 counts were normalized to the organic (membrane) radioactivity: total [3H]IP1/(total [3H]IP1 + total organic radioactivity). Samples of the organic layer were periodically analyzed via thin layer chromatography (TLC) to confirm that the majority of membrane radioactivity was [3H]phosphatidylinositol. The TLCs were performed on silica gel plates (LK6D; Whatman; Clifton, NJ) impregnated with 1.3% potassium oxalate and heat activated at 100°C for 30 minutes. Organic samples were dried under nitrogen (N2) gas, redissolved in 10μL CHCl3 (95) : MeOH (5), and spotted onto individual lanes. Five micrograms of standard PI (L-α-phosphatidylinositol ammonium salt from bovine liver; Sigma-Aldrich) was then applied to all lanes. The plate was dried and then run in a mobile phase containing H2O (8) : CHCl3 (40): MeOH (13): acetic acid (12) : acetone (15). Run-time was approximately 1.5 hours, upon which the plate was removed, dried, and then exposed to iodine (I2) vapors to stain PI. The plate was scraped in 1cm or 0.5cm sections directly into scintillation vials which were counted 24 hours after the addition of scintillation fluid. Radioactivity that co-migrated with authentic PI was taken as [3H]PI.
4.4 Surgical implantation
Animals were anesthetized with a ketamine (60mg/kg)/xylazine (10mg/kg) solution (IM injection) and mounted and secured onto a head-holder. The head was adjusted so that the surface of the skull at bregma was 1.5mm above the surface of the skull at lambda. A double barrel guide cannula was implanted into the medial prefrontal cortex at coordinates 5mm anterior to bregma, 4mm below the surface of the skull at bregma, and 0.75mm to each side of the midline using a Kopf stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). The components used for cannulation were obtained from Plastics One (Roanoke, VA). The double barrel 28-guage obdurators were cut to fit flush with the tip of the 22-guage guide cannula, which extended 11mm below the pedestal. The guide cannula was cemented to three screws drilled into the skull and closed off with a double tip obdurator and screw-on dust cap.
4.5 Intracortical infusion
Animals recovered for one week after surgery as well as between infusions. During each recovery period, dummy and guide cannulae were cleaned carefully one time with ethanol. Additionally, animals were also periodically exposed to the restrainer used for infusions. For infusions, rabbits were comfortably restrained in a Plexiglas restrainer and the dust cap and obdurator removed. Drug was loaded into tubing connected to a Harvard microinjection pump (Instech Laboratories, Plymouth Meeting, PA) and the injector inserted into the guide cannula. The 28-guage injector (Plastics One, Roanoke, VA) was designed in a double fashion with 1.5mm separation and calibrated to extend 1mm beyond the tip of the guide cannula. Drugs were infused bilaterally at a volume of 0.5μL/side at a rate of 0.5μL/min. Hallucinogen (or vehicle) was infused 15 minutes after enzyme inhibitor (or vehicle) infusion.
4.6 Animal behavior
After infusions, animals were placed back in their home cage (with food and water removed) and video recorded for later analysis of head bobs. A head bob is a sequential down-up motion of the head without intervening behaviors (e.g. sniffing, hopping) (Dave et al., 2002). Because drug-elicited head bobs did not differ from vehicle-elicited responses in the first five minutes or beyond 45 minutes after infusion (data not shown), all head bob data are derived from behavior observed between 5 and 45 minutes after infusion.
4.7 Statistical analysis
Data were reported as mean ± standard error of the mean (SEM). Dose response curves were generated using the curve fitting program GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA). The remaining data were analyzed via t-test, analysis of variance (ANOVA), or 2-way ANOVA. Post hoc Dunnett or Bonferroni test were carried out when applicable. Significance for all statistical comparisons was set at p<0.05 using a two-tailed test.
Highlights.
The role of PI hydrolysis in the effects of DOI and LSD was investigated.
Both hallucinogens stimulated PI hydrolysis in rabbit frontocortical tissue prisms.
PI hydrolysis signals were PLC-dependent, but 5-HT receptor involvement differed.
Infusion of hallucinogens into rabbit medial prefrontal cortex elicited head bobs.
Intracortical DOI, but not LSD, elicited head bobs through PLC activation.
Acknowledgments
The authors greatly appreciate the help of Dr. Kelly Berg at the University of Texas Health Science Center of San Antonio and Drs. Joel Horwitz and Alessandro Fatatis at Drexel University College of Medicine in the development of the PI hydrolysis assay. We are also sincerely grateful for the long-standing support from the National Institute of Mental Health through grant #MH16841. Finally, the authors join countless others in remembering and mourning the loss of Dr. John Harvey. He is an irreplaceable thinker, mentor, and friend.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloyo VJ, et al. Selective and divergent regulation of cortical 5-HT(2A) receptors in rabbit. J Pharmacol Exp Ther. 2001;299:1066–72. [PubMed] [Google Scholar]
- Barker EL, Burris KD, Sanders-Bush E. Phosphoinositide hydrolysis linked 5-HT2 receptors in fibroblasts from choroid plexus. Brain Res. 1991;552:330–2. doi: 10.1016/0006-8993(91)90099-h. [DOI] [PubMed] [Google Scholar]
- Berg KA, et al. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Berridge MJ, Prince WT. The nature of the binding between LSD and a 5-HT receptor: a possible explanation for hallucinogenic activity. Br J Pharmacol. 1974;51:269–78. doi: 10.1111/j.1476-5381.1974.tb09657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale JE, Fisher SK. Use of U-73122 as an inhibitor of phospholipase C-dependent processes. Neuroprotocols. 1993;3:125–133. [Google Scholar]
- Burris KD, Breeding M, Sanders-Bush E. (+)Lysergic acid diethylamide, but not its nonhallucinogenic congeners, is a potent serotonin 5HT1C receptor agonist. J Pharmacol Exp Ther. 1991;258:891–6. [PubMed] [Google Scholar]
- Burris KD, Sanders-Bush E. Unsurmountable antagonism of brain 5-hydroxytryptamine2 receptors by (+)-lysergic acid diethylamide and bromo-lysergic acid diethylamide. Mol Pharmacol. 1992;42:826–30. [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. Involvement of 5-HT2C receptors in mediating the discriminative stimulus properties of m-chlorophenylpiperazine (mCPP) Eur J Pharmacol. 1994;257:27–38. doi: 10.1016/0014-2999(94)90690-4. [DOI] [PubMed] [Google Scholar]
- Canal CE, et al. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 2010;209:163–74. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Dave KD, Harvey JA, Aloyo VJ. A novel behavioral model that discriminates between 5-HT2A and 5-HT2C receptor activation. Pharmacol Biochem Behav. 2002;72:371–8. doi: 10.1016/s0091-3057(01)00767-5. [DOI] [PubMed] [Google Scholar]
- Dave KD, et al. Role of central 5-HT2 receptors in mediating head bobs and body shakes in the rabbit. Pharmacol Biochem Behav. 2004;77:623–9. doi: 10.1016/j.pbb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Dave KD, Harvey JA, Aloyo VJ. The time-course for up- and down-regulation ofthe cortical 5-hydroxytryptamine (5-HT)2A receptor density predicts 5-HT2A receptor-mediated behavior in the rabbit. J Pharmacol Exp Ther. 2007;323:327–35. doi: 10.1124/jpet.107.121707. [DOI] [PubMed] [Google Scholar]
- Dean B. The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem. 2003;85:1–13. doi: 10.1046/j.1471-4159.2003.01693.x. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Handley SL. Similarities in the pharmacology of spontaneous and DOI-induced head-shakes suggest 5HT2A receptors are active under physiological conditions. Psychopharmacology (Berl) 1996;128:198–205. doi: 10.1007/s002130050125. [DOI] [PubMed] [Google Scholar]
- Edwards E, Ashby CR, Jr, Wang RY. (+/−)-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane(DOI) and alpha-methyl-5-HT: 5-HT2 receptor agonistic action on phosphatidylinositol metabolism in the rat fronto-cingulate and entorhinal cortex. Neuropharmacology. 1992;31:615–21. doi: 10.1016/0028-3908(92)90139-g. [DOI] [PubMed] [Google Scholar]
- Egan CT, et al. Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology (Berl) 1998;136:409–14. doi: 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, et al. Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther. 2010;335:728–34. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA, et al. Phospholipase A2 and its role in brain tissue. J Neurochem. 1997;69:889–901. doi: 10.1046/j.1471-4159.1997.69030889.x. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Cunningham KA. The hallucinogen d-lysergic acid diethylamide (d-LSD) induces the immediate-early gene c-Fos in rat forebrain. Brain Res. 2002;958:251–60. doi: 10.1016/s0006-8993(02)03548-5. [DOI] [PubMed] [Google Scholar]
- Galeotti N, et al. Signaling pathway of morphine induced acute thermal hyperalgesia in mice. Pain. 2006;123:294–305. doi: 10.1016/j.pain.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–11. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, et al. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther. 2003;304:229–37. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- Leysen JE, et al. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985;27:600–11. [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J Pharmacol Exp Ther. 1984;228:133–9. [PubMed] [Google Scholar]
- Moya PR, et al. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–61. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, et al. Anandamide-induced sleep is blocked by SR141716A, a CB1 receptor antagonist and by U73122, a phospholipase C inhibitor. Neuroreport. 2001;12:2131–6. doi: 10.1097/00001756-200107200-00018. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–81. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Passie T, et al. The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther. 2008;14:295–314. doi: 10.1111/j.1755-5949.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroutka SJ, Lebovitz RM, Snyder SH. Two distinct central serotonin receptors with different physiological functions. Science. 1981;212:827–9. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ. Antagonism of5-hydroxytryptamine2 receptor-mediated phosphatidylinositol turnover by d-lysergic acid diethylamide. J Pharmacol Exp Ther. 1988;247:918–25. [PubMed] [Google Scholar]
- Qu Y, et al. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Winter JC. Effects of ibogaine and noribogaine on phosphoinositide hydrolysis. Brain Res. 1996;731:226–9. doi: 10.1016/0006-8993(96)00688-9. [DOI] [PubMed] [Google Scholar]
- Rabin RA, et al. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 2002;72:29–37. doi: 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, et al. Up-regulation of 5-HT2 receptors in the rat brain by repeated administration of SR 46349B, a selective 5-HT2 receptor antagonist. Eur J Pharmacol. 1993a;246:73–80. doi: 10.1016/0922-4106(93)90012-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, et al. Repeated administration of SR 46349B, a selective 5-hydroxytryptamine2 antagonist, up-regulates 5-hydroxytryptamine2 receptors in mouse brain. Mol Pharmacol. 1993b;43:84–9. [PubMed] [Google Scholar]
- Romano AG, et al. Intrahippocampal LSD accelerates learning and desensitizes the 5-HT(2A) receptor in the rabbit, Romano et al. Psychopharmacology (Berl) 2010;212:441–8. doi: 10.1007/s00213-010-2004-7. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Burris KD, Knoth K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988;246:924–8. [PubMed] [Google Scholar]
- Scarlota LC, Harvey JA, Aloyo VJ. The role of serotonin-2 (5-HT2) and dopamine receptors in the behavioral actions of the 5-HT2A/2C agonist, DOI, and putative 5-HT2C inverse agonist, SR46349B. Psychopharmacology (Berl) 2011;213:393–401. doi: 10.1007/s00213-010-1928-2. [DOI] [PubMed] [Google Scholar]
- Schaeffer EL, Gattaz WF. Inhibition ofcalcium-independent phospholipase A2 activity in rat hippocampus impairs acquisition of short- and long-term memory. Psychopharmacology (Berl) 2005;181:392–400. doi: 10.1007/s00213-005-2256-9. [DOI] [PubMed] [Google Scholar]
- Schindler EA, et al. Serotonergic and dopaminergic distinctions in the behavioral pharmacology of(+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergicacid diethylamide (LSD) Pharmacol Biochem Behav. 2012;101:69–76. doi: 10.1016/j.pbb.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA. 2008;105:1079–84. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ss-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–24. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, et al. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–12. [PubMed] [Google Scholar]
- Shek JW, Wen GY, Wisniewski HM. Altas of the Rabbit Brain and Spinal Cord. Karger; New York: 1986. [Google Scholar]
- Shimizu T, et al. Roles of brain phosphatidylinositol-specific phospholipase C and diacylglycerol lipase in centrally administered histamine-induced adrenomedullary outflow in rats. Eur J Pharmacol. 2007;571:138–44. doi: 10.1016/j.ejphar.2007.05.061. [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Shulgin A. Phenethylamines I Have Known And Loved: A Chemical Love Story. Transform Press; Berkeley: 1991. [Google Scholar]
- Shulgin AT, Shulgin A. Tryptamines I Have Known And Loved: The Continuation. Transform Press; Berkeley: 1997. [Google Scholar]
- Skosnik PD, Yao JK. From membrane phospholipid defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglandins Leukot Essent Fatty Acids. 2003;69:367–84. doi: 10.1016/j.plefa.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Tyeryar KR, Vongtau HO, Undieh AS. Diverse antidepressants increase CDP-diacylglycerol production and phosphatidylinositide resynthesis in depression-relevant regions ofthe rat brain. BMC Neurosci. 2008;9:12. doi: 10.1186/1471-2202-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]