Abstract
Fragmin/protamine nanoparticles (F/P NPs) have been used as carriers for the preservation and controlled release of fibroblast growth factor (FGF)-2 and various cytokines in human plasma (HP). This study tested an HP–Dulbecco's modified Eagle's medium (DMEM) gel as a three-dimensional (3D) culture for the expansion of adipose tissue-derived multilineage stromal cells (ASCs) and bone marrow-derived mesenchymal stem cells (BMSCs). The growth of these cells improved in 3D culture using low-concentration HP (2%)–DMEM gel with 0.1 mg/mL F/P NPs and 5 ng/mL FGF-2 without animal serum in comparison to two-dimensional (2D) culture using a low-concentration human serum (2%)–DMEM containing 5 ng/mL FGF-2 on F/P NPs-coated plates. ASCs and BMSCs, which were expanded in the low-concentration HP–DMEM gel with F/P NPs and FGF-2, maintained their multilineage potential for differentiation into adipocytes or osteoblasts similar to the 2D cultured cells. Furthermore, flow cytometric analyses showed that the phenotypic markers which were positive for CD44, CD90, and CD105 (>80%) and negative for CD34 and CD45 (<1%) were well maintained in both 2D and 3D cultures after 7 days. Thus, this 3D culture system in low-concentration HP–DMEM gel with F/P NPs and FGF-2 provided an effective and safe method for the expansion of both cell types without using animal serum.
Key words: cytokine carrier, fragmin/protamine nanoparticles (F/P NPs), plasma-medium gel, three-dimensional (3D) culture
Introduction
Cell-based therapies, namely tissue engineering, require autologous multipotent stem cells such as adipose tissue-derived multilineage stromal cells (ASCs) and bone marrow-derived mesenchymal stem cells (BMSCs). Both cell types possess multipotentiality, because they differentiate in culture1,2 or after implantation in vivo into osteoblasts,3,4 chondrocytes,5,6 adipocytes,7 myotubes,8,9 and neuronal cells.10 Several reports indicate that the transplantation of ASC-cultured constructs into athymic mice stimulates angiogenesis, wound repair, and re-epithelialization significantly better, when compared with fibroblast-cultured constructs.11,12 Thus, the use of ASCs or BMSCs provides a promising solution for tissue engineering strategies.
Most protocols for the proliferation of ASCs and BMSCs include high concentrations (≥10%) of animal serum as a nutritional supplement. Some cell cultures include multiple doses of fetal bovine serum (FBS), which raises concerns over possible infections and immunological reactions due to medium-derived FBS proteins, sialic acid derivatives, and so on. Recently, the persistence of xenogenic proteins in human mesenchymal stem cells (MSCs)13,14 proliferated using FBS was extensively examined. The results indicated that humoral immune responses against FBS proteins were observed in recipients after an intravenous administration of autologous rat MSCs, which had been proliferated using FBS. Most culture methods used for growing human ES cells require animal-derived materials, including FBS and connective tissue cells (feeder layers) from mice. Therefore, patients may experience problems when undergoing autologous cell-based therapies if sera other than an autologous serum are used during the culture of cells in feeder layers. On the other hand, it is difficult to obtain large volumes of autologous serum from a patient for large-scale autologous cell culture.
Human plasma (HP) contains a high concentration of thrombocytes. In the α-granules of platelets, various cytokines and other bioactive proteins augment tissue repair and regeneration processes.15–17 Platelets secrete more than 20 cytokines, including platelet-derived growth factors (PDGFs), fibroblast growth factors (FGFs), hepatocyte growth factor (HGF), transforming growth factors (TGFs), and vascular endothelial growth factors (VEGFs), most of which are known to be heparin binding. Recent studies have suggested that cytokines in HP not only influence the viability of transferred cells but may also play bioactive roles in influencing the proliferation and differentiation of adipocyte precursor cells. Furthermore, the addition of HP to culture medium induces the formation of HP-DMEM gel. Clinical studies have also documented the efficacy and safety of using HP in hard and soft tissue augmentation for stimulating and enhancing native repair and regeneration processes.16,17
In addition to its well-known anticoagulant activity, heparin is associated with cytokines in various biological processes and is involved in cell adhesion, recognition, migration, and regulation of various enzymatic activities.18 Most cytokines found in HP, such as FGFs, HGFs, PDGFs, TGFs, and VEGFs, are immobilized in the extracellular matrix by binding to heparin-like molecules.19,20 Thus, heparin could be useful as a therapeutic agent for various pathological conditions involving these functional proteins.21 However, a high dose of heparin cannot be used because of a high risk of bleeding. In contrast, low-molecular-weight heparin (fragmin) has pharmacological and practical advantages compared with native heparin. The lower protein-binding affinity of fragmin produces a low, stable, and predictable anticoagulant response, thereby preventing the need for laboratory monitoring to adjust the dosage.21,22 On the other hand, protamine is a purified mixture of proteins obtained from fish sperm; it neutralizes heparin and fragmin by forming a stable complex without anticoagulant activity.22,23 Protamine is also clinically used as an antidote for heparin by reversing the anticoagulant activity of heparin after cardiopulmonary bypass and also in cases of heparin-induced bleeding.
In previous studies, we used fragmin as a heparinoid and protamine to produce fragmin/protamine nanoparticles (F/P NPs).24 F/P NPs efficiently bind to tissue culture plates, thereby retaining heparin-binding cytokines. Exogenous and endogenous heparin-binding growth factors bind efficiently to F/P NPs, and their activities are maintained stable. Dermal fibroblast cells (DFCs) and microvascular endothelial cells (MVECs) grow well using low-concentration FBS (2%)–Dulbecco's modified Eagle's medium (DMEM) with 5 ng/mL FGF-2 on F/P NP-coated plates as a two-dimensional (2D) culture.25 BMSCs26 also grow well in low-concentration human serum (HS) (2%)–DMEM with FGF-2 on F/P NP-coated plates. Plasma-medium gel provides a three-dimensional (3D) extracellular matrix for ASCs and BMSCs via the formation of a massive capsule with semipermeable properties that permits the diffusion of medium components into the cells as well as efficient waste product elimination. The aim of this study was to compare the proliferation of ASCs and BMSCs in 3D culture using a low-concentration HP–DMEM gel with F/P NPs and FGF-2, with their previously reported proliferation in the 2D culture.25,26
Materials and Methods
Preparation of F/P NPs
F/P NPs were prepared as previously described.24,27 Briefly, a 10-fold diluted protamine solution (1.0 mg/mL; Mochida Pharmaceutical Co.) in DMEM was added dropwise to a 10-fold diluted fragmin solution (0.64 mg/mL; Kissei Pharmaceutical Co.) with vortexing for ∼2 min. A high yield of F/P NPs was obtained when 300 μL of diluted protamine was mixed with 700 μL of diluted fragmine (ratio, 3:7 v/v). To remove unreacted materials, the mixture was centrifuged at 8000 rpm for 10 min at 4°C using a high-speed centrifuge (MX-300; Tomy). After removing the supernatant, the pellet of NPs was resuspended in 1 mL Dulbecco's modified phosphate-buffered saline (PBS) without Ca2+ and Mg2+. The generated F/P NPs consisted of insoluble round complexes measuring ∼200–1000 nm in diameter (average, ∼450 nm). A yield of more than 5 mg of dry F/P NPs was obtained from 10 mL of the resuspended F/P NPs solution.
For a 2D culture, 24-well tissue culture plates (well area, 1.8 cm2) (Sumitomo Bakelite Corp.) were coated for 3 h at 4°C with 0.3 mL of 0.35 mg/mL F/P NPs in PBS.
Preparation of HP
HP was prepared as previously described.17 Forty milliliters of blood was drawn from volunteers into tubes containing 0.4 mL of 2% sodium citrate. The tubes were centrifuged for 15 min at 1500 rpm (Table-Top Refrigerated Centrifuge 5800, Roter: RS-720; Kubota Corp.) to produce two layers: erythrocytes at the bottom of the tube and the HP layer in the middle and at the top of the tube. HP was frozen at −80°C until required. The platelets in HP were counted using Hematology Analyzer KX-21 (Sysmex Corp.). The platelet concentration in the HP layer was 18.2±3.6×104/μL (n=11).
Enzyme-linked immunosorbent assay to evaluate the F/P NP binding of heparin-binding cytokines in HP
Enzyme-linked immunosorbent assay (ELISA) was performed to detect the heparin-binding cytokines in HP and evaluate the adsorption of these cytokines by F/P NPs. Specific concentrations of F/P NPs were added to DMEM (Life Technologies Oriental) with antibiotics (100 U/mL penicillin G and 100 μg/mL streptomycin) and 2% HP, which had been frozen and thawed several times, followed by incubation for 2 h at 37°C on a rotary shaker. The mixtures were then centrifuged to remove F/P NPs. The levels of each cytokine in the diluted supernatants were measured using highly sensitive colorimetric sandwich ELISA kits (R&D Systems, Inc.) for FGF-2, HGF, keratinocyte growth factor (KGF), VEGF, PDGF-AB, PDGF-BB, and TGF-β1 as per manufacturer's instructions.17
Cell growth assays
Human ASCs and BMSCs were purchased from Takara Bio. Corp. The cells were 2D cultured in 0.5 mL of DMEM with a low concentration of 2% HS (SER019029 from human adults; Biopredic International Corp.) with 5 ng/mL FGF-2 (Fiblast; Kaken Pharmaceutical Corp.) and antibiotics on F/P NP-coated 24-well tissue culture plates incubated in a 5% CO2 incubator at 37°C for 5 days.25 Before a 3D culture using the low-concentration HP–DMEM gel, ∼2.6 mL of HP was prepared from 5 mL of blood drawn from volunteers into tubes containing 0.05 mL of 2% sodium citrate (0.02%), and 0.1 mg/mL of F/P NPs was added to the low-concentration HP–DMEM solution to ensure low-concentration HP–DMEM gelation. For a 3D culture, the cells were suspended in 0.5 mL of low-concentration HP (2%)–DMEM with 0.1 mg/mL F/P NPs and 5 ng/mL FGF-2 on 24-well tissue culture plates incubated in a 5% CO2 incubator at 37°C for 5 days. The cells containing a low concentration of HP-DMEM were gelated within 15 min at 37°C.
After a 3D culture, the HP-DMEM gels were rinsed with PBS, and the cells were released from these gels by treatment with 0.5 mL of trypsin–EDTA solution at 37°C for 10 min. The HP-DMEM gels were completely dissolved by pipetting, and the cell number was counted using a hemocytometer.
Induction of adipocyte and osteoblast differentiation
Adipocyte and osteoblast differentiation was induced by placing the expanded ASCs and BMSCs into each specific medium (defined adipogenic induction medium, defined adipogenic maintenance medium, and defined osteogenic induction medium; Lonza Walkersville, Inc.). For adipocyte differentiation, both cell types were cultured in the defined adipogenic induction medium for 3 days, followed by 3 days of culture in the defined adipogenic maintenance medium. After 3 complete cycles of induction/maintenance, both cell types were cultured for 3 more days in the defined adipogenic maintenance medium. For osteogenic differentiation, both cell types were cultured with the defined osteogenic induction medium for 21 days.
The adipocyte-induced cells were stained with Oil Red O (Wako Pure Chemical Industries, Co. Ltd.). The alkaline phosphatase (ALP) cytochemistry study of osteoblast-induced cells was conducted using the appropriate substrate for ALP (Sigma Fast; Sigma Aldrich GmbH).26
Flow cytometric analyses of 2D- and 3D-cultured ASCs and BMSCs
Cultured ASCs and BMSCs were washed twice with PBS buffer with 2% (v/v) FBS. Aliquots of 1.0×105 cells were incubated with anti-human CD34/phycoerythrin (PE), anti-human CD44/PE, anti-human CD45/fluorescein isothiocyanate (FITC), anti-human CD90/FITC, and anti-human CD105/PE for 30 min at 4°C in the dark. The monoclonal antibodies used for flow cytometry were purchased from BD Biosciences Pharmingen. Isotype control was evaluated using equal aliquots of cells that were not labeled with monoclonal antibodies. Cell populations were analyzed on the EPICSRXL (Beckman Coulter) using the EXPO32 software (Beckman Coulter) and compared with the isotype control.
Statistical analyses
Group means were compared by the unpaired Student's t test using Stat Mate III for Windows (ATMS Co.). p<0.05 was considered statistically significant.
Results
Adsorption of cytokines to F/P NPs
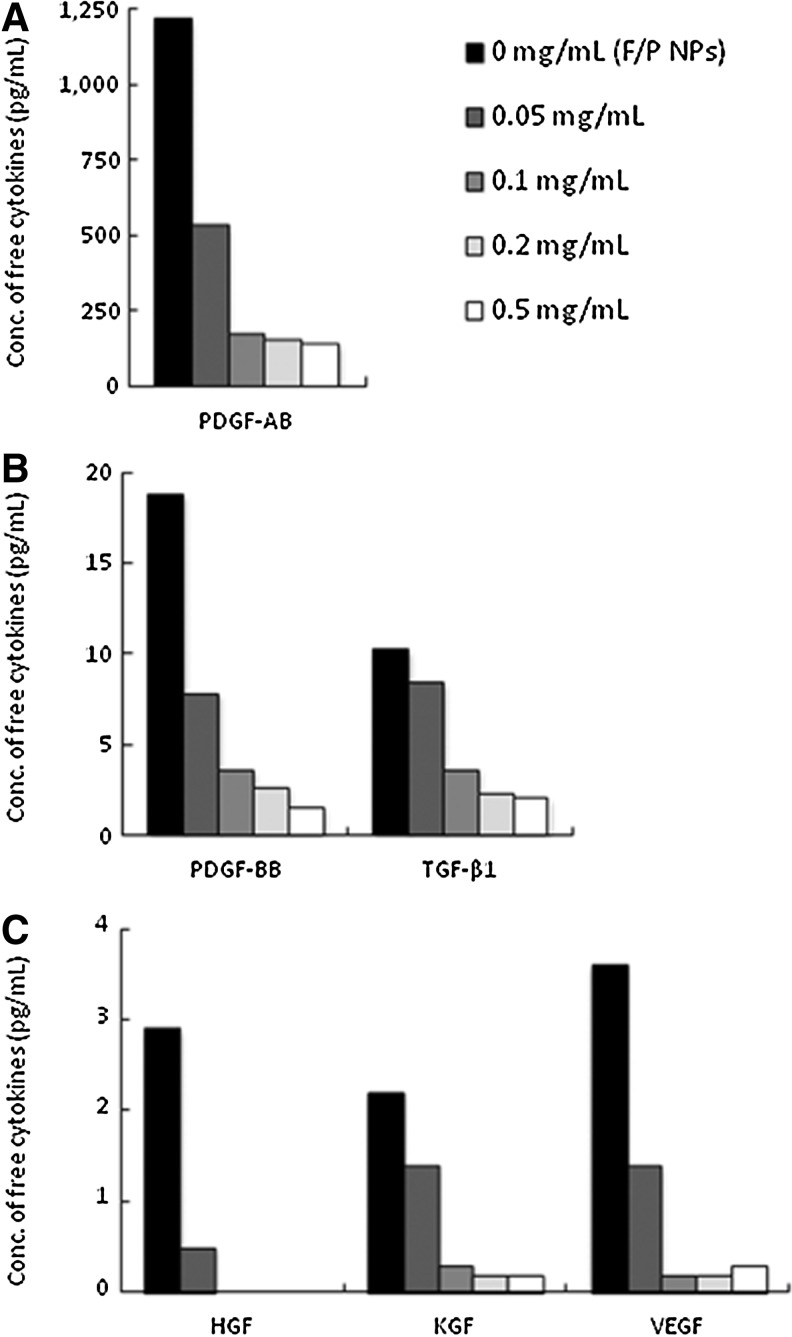
PDGF-AB, PDGF-BB, TGF-β1, HGF, KGF, and VEGF present in HP are known to bind specifically to heparinoids.18–20 The frozen and thawed HP was prepared from 5 mL of blood drawn into tubes containing 0.5 mL of 2% sodium citrate (final, ≥0.2%), and the HP was not gelated in the tube with the low-concentration HP (2%)–DMEM solution. The cytokines in the HP were measured by ELISA, as described earlier. The amounts of PDGF-AB, PDGF-BB, TGF-β1, HGF, KGF, and VEGF in 2% HP are shown in Figure 1. The indicated amounts of F/P NPs were added to 1 mL of low-concentration HP (2%)–DMEM, which was incubated at 37°C for 2 h and centrifuged to separate cytokine-containing F/P NPs. All cytokines in the supernatants decreased significantly in a concentration-dependent manner, indicating that a significant proportion of the cytokines was adsorbed onto F/P NPs (Fig. 1). Approximately 86%, 79%, 68%, 100%, 86%, and 90% of PDGF-AB, PDGF-BB, TGF-β1, HGF, KGF and VEGF in 2% HP, respectively, were bound to 0.1 mg/mL of F/P NPs.
FIG. 1.
Levels of platelet-derived growth factor (PDGF)-AB (A); PDGF-BB and transforming growth factor (TGF)-β1 (B); and hepatocyte growth factor (HGF), keratinocyte growth factor (KGF), and vascular endothelial growth factor (VEGF) (C) in the low-concentration human plasma–Dulbecco's modified Eagle's medium (HP-DMEM) solution. The indicated amounts of fragmin/protamine nanoparticles (F/P NPs) were added to 1 mL of the low-concentration HP–DMEM solution, and the mixtures were incubated at 37°C for 2 h and centrifuged to separate the cytokine-bound F/P NPs.
Growth of ASCs and BMSCs in 3D culture using the low-concentration HP–DMEM gel with F/P MPs and FGF-2
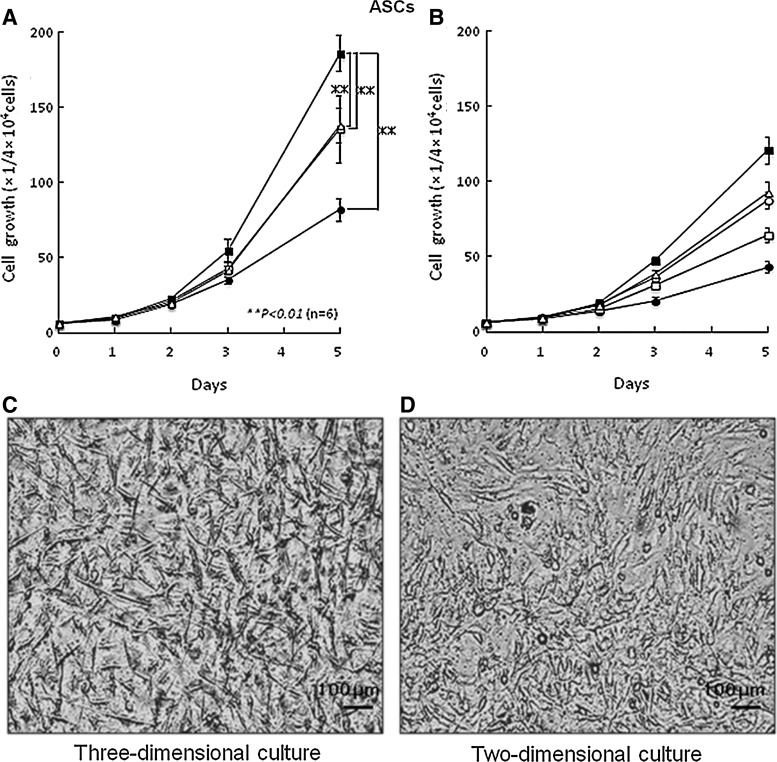
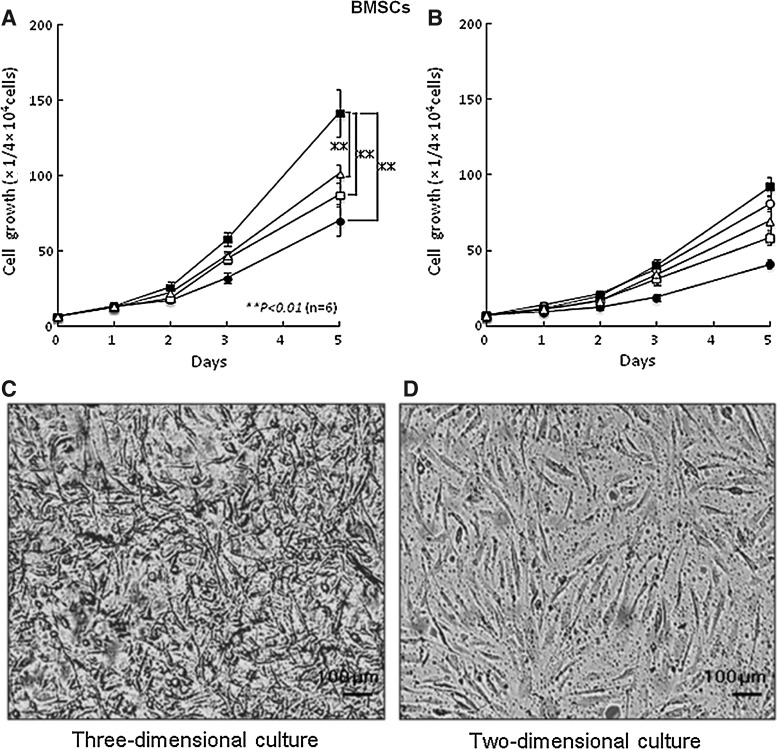
The growth of ASCs and BMSCs in the low-concentration HP (2%)–DMEM gel with F/P NPs (0.1 mg/mL) and FGF-2 (5 ng/mL) as a 3D culture were stimulated with a shorter doubling time of 25 and 26 h, respectively, compared with the low-concentration HP–DMEM gel containing either F/P NPs alone or FGF-2 alone (Fig. 2). Both ASC and BMSC growth were stimulated on F/P NP-coated plates in the low-concentration HS (2%)–DMEM with FGF-2 (5 ng/mL) as a 2D culture with a longer doubling time of ∼28 and 29 h, respectively. The culture medium was gelated in the presence of more than 2% HP, and both cell types grew well in 3D culture conditions with HP-DMEM gel. The yield of cell culture for 5 days in 3D conditions using the low-concentration HP–DMEM gel with F/P NPs and FGF-2 was always ≥50% higher than the yield of cells in the 2D condition using low-concentration HS–DMEM with FGF-2 on F/P NP-coated plates and using high HS (10%)–DMEM on tissue culture plates. In 2D culture, ASCs and BMSCs adhered to the F/P NP-coated plates and noncoated tissue culture plates and exhibited a spindle shape at 5 h, whereas the cells in 3D culture using low-concentration HP–DMEM gel with F/P NPs and FGF-2 exhibited a thin spindle shape and generated a 3D network within 3 days (Figs. 2 and 3).
FIG. 2.
Proliferations of adipose tissue-derived multilineage stromal cells (ASCs) in three-dimensional (3D) and two-dimensional (2D) cultures. (A) ASCs were cultured in a 3D system using a low-concentration HP (2%)–DMEM gel with 0.1 mg/mL F/P NPs and 5 ng/mL fibroblast growth factor (FGF)-2 (■), F/P NPs alone (▵), FGF-2 alone (□), and with no supplement (●). (B) ASCs were cultured in a 2D system using low-concentration human serum (HS) (2%)–DMEM on F/P NP–coated plates (▵) with 5 ng/mL FGF-2 (■), on noncoated plates (●) with FGF-2 alone (□), and high-concentration HS (10%)–DMEM on noncoated plates (○). Data represent mean±SD. (C, D) Photomicrographs of 3D (C) and 2D (D) cultures of ASCs after 3 days of culture to show their morphologies. **The comparison of experimental groups [HP (2%)–DMEM gel containing 0.1 mg/mL F/P NPs and 5 ng/mL FGF-2] in 3D culture with HS (2%)–DMEM on F/P NP–coated plates with FGF-2 and a high concentration of HS (10%)–DMEM on uncoated plates in 2D culture exhibited significant differences with findings of p<0.01 (n=6).
FIG. 3.
Proliferations of bone marrow-derived mesenchymal stem cells (BMSCs) in 3D and 2D cultures. (A) BMSCs were cultured in a 3D system using a low-concentration HP (2%)–DMEM gel with 0.1 mg/mL F/P NPs and 5 ng/mL FGF-2 (■), F/P NPs alone (▵), FGF-2 alone (□), and with no supplement (●). (B) BMSCs were cultured in a 2D system using low-concentration HS (2%)–DMEM on F/P NP–coated plates (▵) with 5 ng/mL FGF-2 (■), on noncoated plates (●) with FGF-2 alone (□), and high-concentration HS (10%)–DMEM on noncoated plates (○). Data represent mean±SD. (C, D) Photomicrographs of 3D (C) and 2D (D) cultures of BMSCs after 3 days of culture to show their morphologies. **The comparison of experimental groups [HP (2%)–DMEM gel containing 0.1 mg/mL F/P NPs and 5 ng/mL FGF-2] in a 3D culture with HS (2%)–DMEM on F/P NP–coated plates with FGF-2 and a high concentration of HS (10%)–DMEM on uncoated plates in 2D culture exhibited significant differences with findings of p<0.01 (n=6).
ASC and BMSC differentiation assays
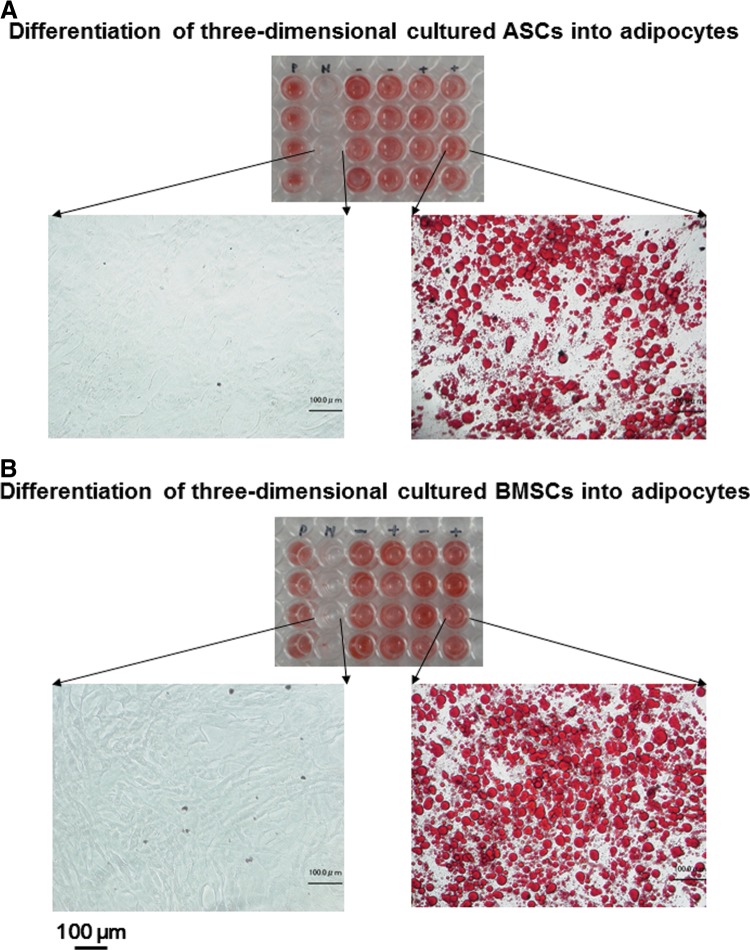
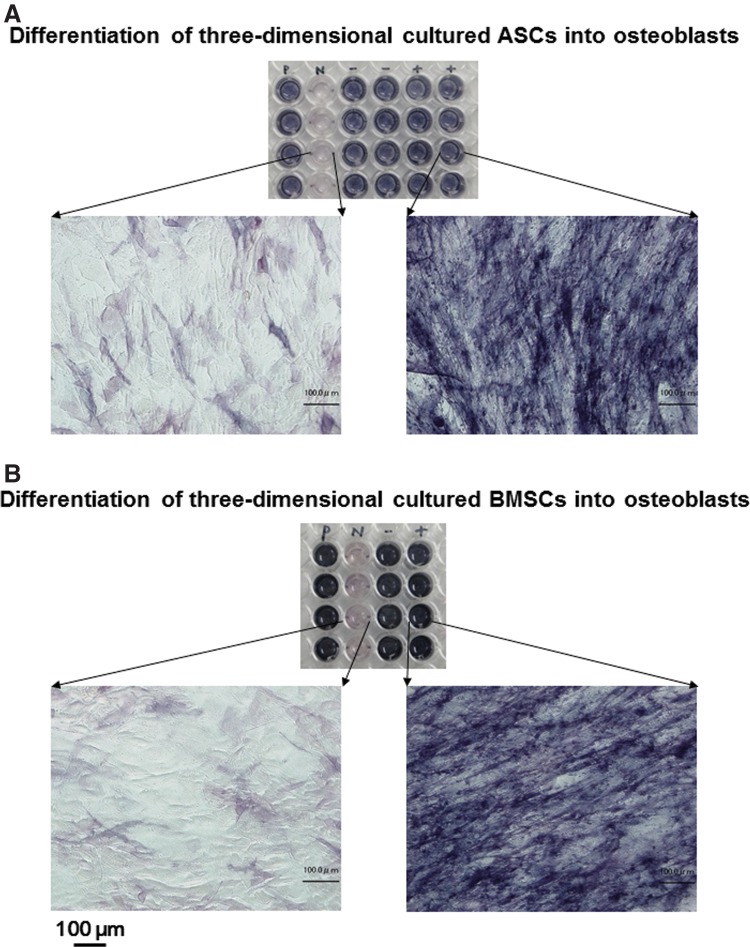
To determine the potential of 3D expanded ASC and BMSC in the low-concentration HP (2%)–DMEM gel with 0.1 mg/mL F/P MPs and 5 ng/mL FGF-2 to form adipogenic and osteogenic phenotype cells, ASCs and BMSCs were cultured in conditions that are specific for each type of differentiation.12,26 For the adipogenic assay, both cell types produced cytoplasmic lipid droplet accumulation and formed adipogenic phenotype cells after cyclic induction using defined adipogenic induction and maintenance media. A histological examination revealed the formation of small lipid droplets in some cultures after three cycles of treatment. The number of lipid droplet-containing cells increased during culture. These lipid droplet-containing cells were stained with Oil Red O after 21 days (Fig. 4). For the osteogenic assay, the expanded ASCs and BMSCs were cultured in a defined osteogenic induction medium. High ALP activity was observed after 21 days (Fig. 5).
FIG. 4.
Potential of proliferated ASCs (A) and BMSCs (B) in the low-concentration HP–DMEM gel with FGF-2 to form adipogenic phenotype cells. Top image of each panel: ASCs and BMSCs proliferated in high-concentration HS (10%)–DMEM without FGF-2 were cultured in adipogenic induction (maintenance) medium (positive [P] control) or control medium (negative [N] control) for 21 days. Both 3D-proliferated cell types in the low-concentration HP–DMEM gel with F/P NPs and FGF-2 were cultured with either adipogenic induction (maintenance) medium on F/P NP–coated (+) or uncoated tissue culture plates (−) for 21 days. Cells were stained with Oil Red O. Lower images of each panel: Magnifications of negative-control and F/P NP–coated plate cultures. Scale bars=100 μm.
FIG. 5.
Potential of proliferated ASCs (A) and BMSCs (B) in the low-concentration HP–DMEM gel with FGF-2 to form osteogenic phenotype cells. Top image of each panel: ASCs and BMSCs proliferated in high-concentration HS (10%) DMEM without FGF-2 were cultured with osteogenic induction medium (positive [P] control) or control medium (negative [N] control) for 21 days. Both 3D-proliferated cell types in the low-concentration HP–DMEM gel with F/P NPs and FGF-2 were cultured with either osteogenic induction medium on F/P NP–coated (+) or uncoated tissue culture plates (−) for 21 days. Cells were stained to detect alkaline phosphatase (ALP) activity using an appropriate substrate for ALP. Lower images of each panel: Magnifications of negative-control and F/P NP–coated plate cultures. Scale bars=100 μm.
Flow cytometric analyses of 2D and 3D cultured ASCs and BMSCs
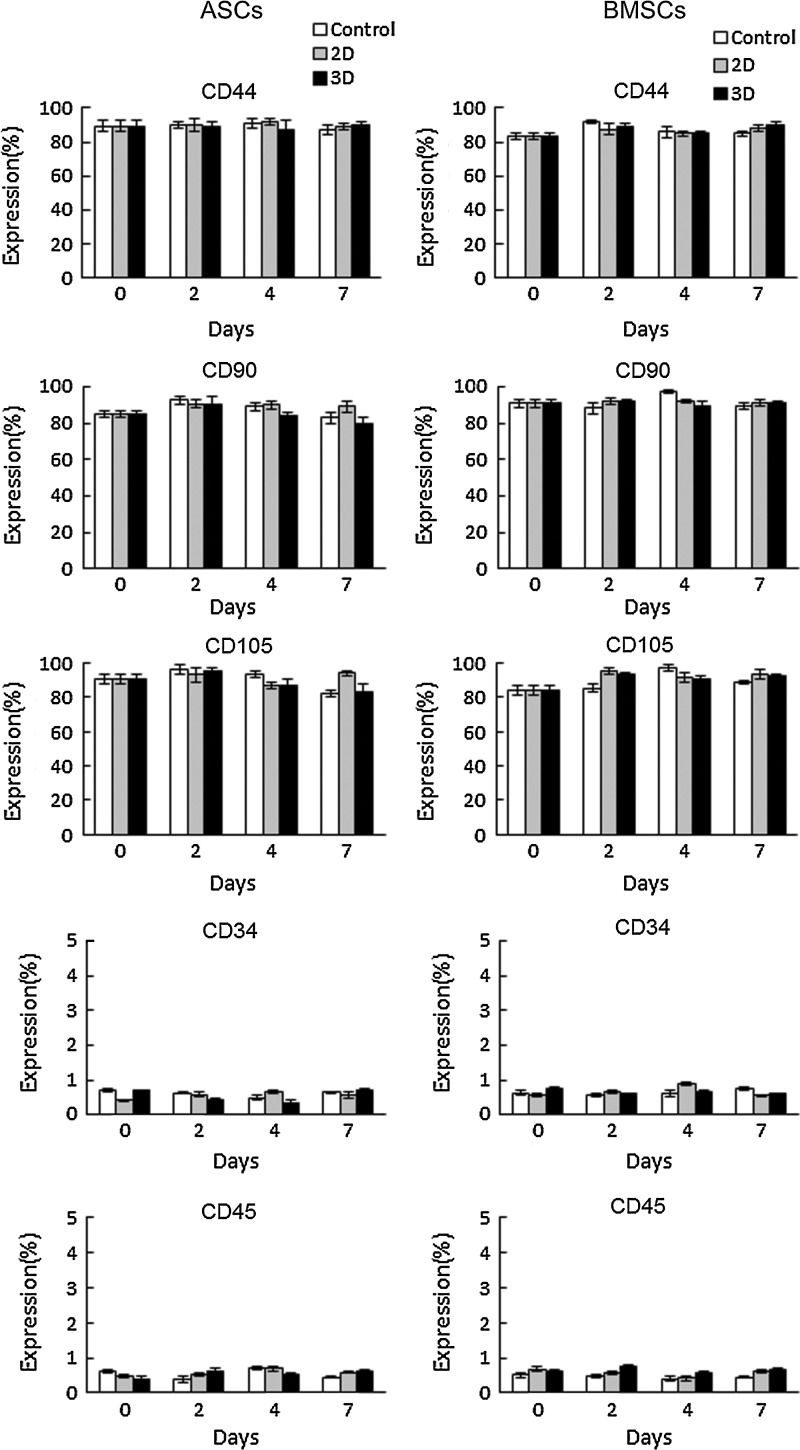
To determine the phenotypic marker expression28 of expanded ASCs and BMSCs in the low-concentration HP (2%)–DMEM gel with 0.1 mg/mL F/P MPs and 5 ng/mL FGF-2, ASCs and BMSCs were cultured in 2D and 3D culture conditions for 0, 2, 4, and 7 days; flow cytometric analyses were then performed (Fig. 6). More than 80% of ASCs and BMSCs cultured in 2D and 3D conditions for the days indicated expressed CD44, CD90, and CD105 markers; while <1% of these cells expressed CD34 and CD45 markers (Fig. 6). These results indicate that the phenotypes of ASCs and BMSCs expanded in 2D and 3D cultures were well maintained during 7-day cultures.
FIG. 6.
The positive ratios of CD44, CD90, CD105, CD34, and CD45 were measured in 2D-, 3D-, and control-cultured ASCs and BMSCs for 0, 2, 4, and 7 days. Data were calculated using identical flow cytometric analyses.
Discussion
Patients may experience problems while undergoing autologous cell-based therapies if sera other than an autologous serum are used during cell culture. In addition, it is difficult to obtain large volumes of autologous serum from a patient. We previously produced F/P NPs as carriers for the controlled release of heparin-binding cytokines such as FGF-2.24 F/P NPs can be stably coated onto plastic and glass surfaces. Our previous study demonstrated the utility of an F/P NP-coating as a heparin-binding cytokine-immobilized substratum to stimulate the growth of BMSCs.26 Furthermore, we reported that DFCs and MVECs could be proliferated in 2D culture using low-concentration HS–DMEM with FGF-2 on F/P NP-coated plates.
In this study, ASCs and BMSCs were grown in 3D culture using the low-concentration HP–DMEM gel with F/P NPs and FGF-2, with a higher growth rate than that in 2D culture, as previously reported.25,26 Furthermore, the phenotypes of both cell types were positive for CD44, CD90, and CD105 (>80%) and negative for CD34 and CD45 (<1%); these were well maintained in 2D and 3D cultures after 7 days. The 3D-proliferated ASCs and BMSCs continued to maintain their multipotent differentiation capacity, that is, they were able to differentiate into adipocytes and osteoblasts. These results demonstrated the optimal proliferation of both cell types using the described 3D culture system in low-concentration HP–DMEM gel with F/P NPs and FGF-2.
We previously reported that F/P NPs measuring 200–1000 nm in diameter (average, ∼450 nm) could be stably coated onto plastic and glass surfaces,25 and that BMSCs could be proliferated in DMEM using low-concentration serum (2% HS or FBS) on FGF-2 immobilized F/P NP-coated plates.26 However, both ASCs and BMSCs did not adhere to or grow well on plates coated with a high density of F/P NPs (data not shown) because of the hydrogel-like property of the coating matrix. The 3D culture system comprised these cells and low-concentration HP–medium gel with F/P NPs and FGF-2; these cells grew better in 3D culture than in 2D culture. Furthermore, the presented 3D culture methods required no animal serum, because the low concentration (2%) of autologous plasma was sufficient.
Platelets are a natural source of cytokines such as PDGFs, TGFs, FGFs, VEGFs, and PDGFs, which are stored in the α-granules of platelets. Platelets are activated by factors such as thrombin or calcium to release these cytokines from the α-granules.15–17 The integration of various cytokines may be useful in tissue engineering strategies to mimic the natural environments of tissue formation.29 Any treatment that aims at mimicking the crucial aspects of the natural biological process should not be limited to the provision of a single cytokine, and multiple cytokines should be released at an optimized ratio in terms of the physiological dose and specific spatiotemporal pattern.29 Our previous results suggested that frozen and thawed platelets appeared to release various cytokines. These released cytokines could be immobilized on F/P NPs. In addition to FGF-2, we previously reported that KGF, VEGF, PDGF, and TGF in PRP could be efficiently immobilized on F/P NPs.17 Conversely, nonheparin-binding cytokines such as epidermal growth factor and insulin-like growth factors were not immobilized on F/P NPs.17 F/P NPs combined with the HP-DMEM gel used in this study may adsorb various heparin-binding growth factors released from platelets and secreted from ASCs or BMSCs within the gels, which are involved in cell proliferation, and the growth factors adsorbed within the HP-DMEM gel may stimulate cell proliferation. Thus, the 3D culture system provides an excellent method for optimizing the proliferation of these cell types.
Fragmin is a low-molecular-weight heparin with a much lower anticoagulant activity than native heparin, allowing it to be administered subcutaneously.21,22 Therefore, fragmin has been used to prepare F/P NPs in some studies. Basic protamine molecules complexed with acidic molecules (fragmin) form polyelectrolyte complexes through ionic interactions. Polypeptides such as FGF-2 may be gradually released from the particles in vivo after binding to F/P NPs (decreasing by half within 5 days).24 In our previous study, no bleeding complications were observed in the animals injected with F/P NPs.12
Heparin and fragmin are known to bind various cytokines, which protect and stimulate their biological activity.18–20 In this study, we showed that F/P NPs could enhance and maintain the biological activities of exogenously added FGF-2 and bioactive substances released from platelet in HP17,25 in the HP-DMEM gel. The 3D culture of ASCs and BMSCs could be significantly stimulated in the low-concentration HP–DMEM gel with F/P NPs and FGF-2, and the growth rates were higher compared with those cells in 2D culture using low-concentration HS–DMEM with FGF-2 on F/P NP-coated plates.
In summary, we established a 3D culture method for the proliferation of ASCs and BMSCs in a low-concentration HP–DMEM gel with F/P NPs and FGF-2. The proliferated ASCs and BMSCs maintained their potential to differentiate into adipocytes and osteoblasts and their phenotypic markers. These results suggest that cells cultured using this method may provide a promising source for cell-based therapies in many clinical fields, particularly those requiring the preparation of a large number of ASCs and BMSCs.
Acknowledgments
This study was partially supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan under grant no. 105800. S.K. was partially supported by a Restart Postdoctoral (RPD) Fellowship from the Japan Society for the Promotion of Science.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger MF. Mackay AM. Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 3.Haynesworth SE. Goshimam J. Goldberg VM, et al. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 4.Rickard DJ. Kassem M. Hefferan TE, et al. Isolation and characterization of osteoblast precurcor cells from bone marrow. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone B. Hering TM. Caplan AI, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 6.Yoo JU. Barthel TS. Nishimura K, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80-A:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Beresford JN. Bennett JH. Devlin C, et al. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 8.Wakitani S. Saito T. Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari G. Cusella-DeAngelis G. Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 10.Woodbury D. Reynoldsk K. Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002;96:908–917. doi: 10.1002/jnr.10365. [DOI] [PubMed] [Google Scholar]
- 11.Nambu M. Ishihara M. Nakamura S, et al. Enhanced healing of mitomycin C-treated wounds in rats using inbred adipose tissue-derived stromal cells within an atelocollagen matrix. Wound Rep Reg. 2007;15:505–510. doi: 10.1111/j.1524-475X.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura S. Kishimoto S. Nakamura S-I, et al. Fragmin/protamine microparticles as cell carriers to enhance viability of adipose-derived stromal cells and their subsequent effect on in vivo neovascularization. J Biomed Mater Res A. 2010;92:1614–1622. doi: 10.1002/jbm.a.32506. [DOI] [PubMed] [Google Scholar]
- 13.Spees JL. Gregory CA. Singh H, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Martin MJ. Muotri A. Gage F, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 15.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Bhanot S. Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002;18:27–33. doi: 10.1055/s-2002-19824. [DOI] [PubMed] [Google Scholar]
- 17.Takikawa M. Nakamura S-I. Nakamura S, et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J Biomed Mater Res. 2011;97B:373–380. doi: 10.1002/jbm.b.31824. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka Y. Kimata K. Adams DH. Modulation of cytokine function by heparan sulfate proteoglycans: sophisticated models for the regulation of cellular responses to cytokines. Proc Assoc Am Physicians. 1998;110:118–125. [PubMed] [Google Scholar]
- 19.Ishihara M. Ono K. Structure and function of heparin and heparan sulfate: heparinoid library and modification of FGF-activities. Trend Glycosci Glycotech. 1998;10:223–233. [Google Scholar]
- 20.Ishihara M. Biosynthesis, structure, and biological activity of basic FGF binding domains of heparan sulfate. Trend Glycosci Glycotech. 1993;5:343–354. [Google Scholar]
- 21.Hirsh J. Warkentin TE. Shaughnessy SG, et al. Heparin and low-molecular-weight heparin, mechanisms of action, phormacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 22.Wolzt M. Weltermann A. Nieszpaur-Los M, et al. Studies on the neutralizing effects of protamine on unfractionated and low molecular weight heparin (FragminR) at the site of activation of the coagulation system in man. Thromb Haemost. 1995;73:439–443. [PubMed] [Google Scholar]
- 23.Pan M. Suarez de Lezo J. Medina A, et al. In-laboratory removal of femoral sheath following protamine administration in patients having intracoronary stent implantation. Am J Cardiol. 1997;80:1336–1338. doi: 10.1016/s0002-9149(97)00676-0. [DOI] [PubMed] [Google Scholar]
- 24.Mori Y. Nakamura S. Kishimoto S, et al. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int J Nanomed. 2010;5:147–155. doi: 10.2147/ijn.s8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishimoto S. Nakamura S. Nakamura S-I, et al. Fragmin/protamine microparticle-coated matrix immobilized cytokines to stimulate various cell proliferations with low serum media. Artif Org. 2009;33:431–438. doi: 10.1111/j.1525-1594.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto S. Hattori H. Nakamura S, et al. Expansion and characterization of human bone marrow-derived mesenchymal stem cells cultured on fragmin/protamine microparticle-coated matrix with fibroblast growth factor-2 in low serum medium. Tissue Eng Part C. 2009;15:523–527. doi: 10.1089/ten.TEC.2008.0492. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S. Kanatani Y. Kishimoto S, et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J Biomed Mater Res. 2009;91A:814–823. doi: 10.1002/jbm.a.32265. [DOI] [PubMed] [Google Scholar]
- 28.Larsen S. Lewis ID. Potential therapeutic applications of mesenchymal stromal cells. Pathology. 2011;43:592–604. doi: 10.1097/PAT.0b013e32834ab72d. [DOI] [PubMed] [Google Scholar]
- 29.Chen FM. Zhang M. Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2001;31:6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]