Abstract
DNA-cross-linked polyacrylamide hydrogels (DNA gels) are dynamic mechanical substrates. The addition of DNA oligomers can either increase or decrease the crosslinker density to modulate mechanical properties. These DNA-responsive gels show promise as substrates for cell culture and tissue-engineering applications, since the gels allow time-dependent mechanical modulation. Previously, we reported that fibroblasts plated on DNA gels responded to modulation in elasticity via an increase or decrease in crosslinker density. To better characterize fibroblast mechanical signals, changes in stress and elastic modulus of DNA gels were measured over time as crosslinker density altered. In a previous study, we observed that as crosslinker density decreased, stress was generated, and elasticity changed over time; however, we had not evaluated stress and elastic modulus measurements of DNA gels as crosslinker density increased. Here, we completed this set of fibroblast studies by reporting stress and elastic modulus measurements over time as the crosslinker density increased. We found that the stress generated and the elastic modulus alterations were correlated. Hence, it seemed impossible to separate the effect of stress from the effect of modulus changes for fibroblasts plated on DNA gels. Yet, previous results and controls revealed that stress contributed to fibroblast behavior.
Key words: compliance, elasticity, extracellular matrix, force generation, stiffness, stress
Introduction
DNA-crosslinked polyacrylamide hydrogels (DNA gels) are tunable hydrogels developed by our group to mimic the mechanical properties of the dynamic in vivo microenvironment.1–6 It has been shown that mechanical properties are altered as a function of time via the addition of DNA oligomers. Lin et al.5 and Previtera et al.7 demonstrated the ability of DNA gels to compress or expand with an increase or decrease in crosslinker density, respectively. Jiang et al.2 and Previtera et al.7 found that fibroblast behavior was direction dependent and dynamic dependent. However, previous quantification of DNA gel compressive stress and its effects on fibroblast behavior as crosslinker density increased was not evaluated.2 To quantify stress generated by DNA gels with increasing crosslinker density, a force transducer and proof-of-concept experiment was performed to assess DNA gel mechanical properties to understand their effect on fibroblast behavior.2 We also correlated the stresses generated with alterations in elasticity for DNA gels. This study completed a series of experiments quantifying the effects of DNA gel mechanics on cell behavior and demonstrated that mechanical properties of DNA gels can be used to manipulate fibroblast behavior for tissue-engineering purposes.
Materials and Methods
DNA gel preparation
DNA gels were prepared as previously described.1,2,4,5 Two different sets of gels were made: (1) SA1 and SA2 each with a length of 14 bases and L3 (crosslinker) with a length of 40 bases (D-14),5 and (2) SA1 and SA2, each with a length of 20 bases and L2 (crosslinker) with a length of 40 bases (D-20).1
Elastic modulus measurements
A 70% crosslinked D-14 gel (70% D-14 gel; n=4) was modulated to a 100% crosslinked gel (100% gel) by increasing crosslinker density with the addition of 30% L3. Elastic modulus measurements were performed at room temperature (RT) at specific time points over 24 h using a bead test,8 and elastic modulus alterations were calculated as previously described.8
Force transducer method
Stress measurements were set up and performed as previously described.7 Briefly, samples of 50% D-14 (n=3) and 50% D-20 (n=2) gels were prepared as described above. Then, 225 μL of sample was pipetted into a 20 mm×5 mm×5 mm chamber between two porous blocks. Buffer was added into the chamber, and the DNA gel was swelled for 24 h. One of the blocks was removed after gel swelling, and the force transducer was introduced into the gel and thisblock. Next, 50% of L3 or L2 was added into the chamber containing 50% D-14 or 50% D-20 gels, respectively. It should be noted that diffusion was specifically chosen as the method of DNA delivery to perform cellular study comparisons.2,3 Time-lapse images of the force transducer deflection were taken every 2 h for 24 h. Force was calculated as previously described.7
Polydimethylsiloxane method
A 2-mm-thick layer of polydimethylsiloxane (PDMS; Sylgard 184, Dow Corning Corporation) was prepared by mixing the silicone elastomer base and the curing agent in a 40:1 ratio (Fig. 1A, n=2). Two PDMS strips of 20 mm×10 mm were separately placed on a precleaned microslide (Gold Seal). The two PDMS-microslides were placed approximately 100-μm apart in a plate (PDMS slit). Optical glue (Norland Optical Adhesive 73) was applied to the top surface of the PDMS, and a swollen 50% D-20 gel was placed on the gap between the two PDMS blocks. The device was exposed to UV light to secure the gel. Fifty percent of L2 DNA was pipetted on to the top surface of the DNA gel, and the gel was allowed to compress for 24 h. Time-lapse images of PDMS slit as the DNA gel compressed were taken every 2 h for 24 h.
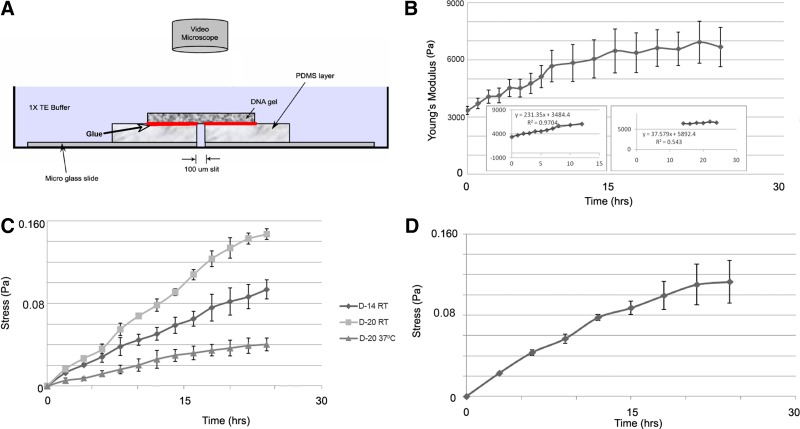
FIG. 1.
(A) PDMS methodology schematic. DNA gel pulled on PDMS blocks when stress was generated. Red indicates optical glue. (B) Time-lapse of elasticity changes for DNA gels with increasing crosslinker density. D-14 gel elasticity increased when crosslinker density increased from 70% to 100%. Inset: The trendine equations and R2 values for the first (left) and last (right) 12 h of the experiment. (C) Force transducer measurements for D-14 and D-20 gels. These gels generated stress when crosslinker density increased from 50% to 100% at RT and 37°C. (D) PDMS measurements for D-20 gels. D-20 gels generated stress when crosslinker density increased from 50% to 100% at RT. Mean±standard error of the mean. PDMS, polydimethylsiloxane; RT, room temperature.
The following assumptions were made when calculations were performed: the bottom layer was assumed to be stationary; the PDMS block was assumed to have linear elasticity, and the DNA gel and PDMS block were assumed to have perfect bonding. The contact area between the gel and the PDMS block (A), and the thickness of the PDMS block (h) were measured. The deflection (δ) of the PDMS was monitored with respect to time and the shear strain was computed as:
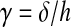 |
(1) |
Since the shear modulus (G) of the PDMS was known to be equal to about 16 kPa at RT,9 the force applied by the hydrogel (F) per unit area (stress) was calculated as:
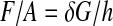 |
(2) |
Results and Discussion
First, the temporal modulus sensed by cells on dynamic substrates was quantified. Elastic modulus alterations were measured for 24 h as crosslinker density increased. Twenty-four hours after the addition of 30% crosslinker, Young's modulus increased to 6680±1020 Pa for D-14 gels (Fig. 1B). In the first 12 h, the elastic modulus almost doubled from 3350±210 Pa to 6050±1010 Pa (mean±standard error of the mean) with a rate of 231.35 Pa/h (Fig. 1B, inset, left). During the next 12 h, the increase in elastic modulus was negligible, measuring from 6050±1010 Pa to 6680±1020 Pa with a rate of 37.56 Pa/h (Fig. 1B, inset, right).
To quantify stress generated by mechanically modulating gels, a force transducer system was developed and validated based on the beam equation.7 As time progressed, the stress generated increased for both D-14 and D-20 gels when 50% additional crosslinker was added at RT (Fig. 1C). At RT, the amount of stress generated in D-20 and D-14 gels after 24 h was 0.147±0.0052 Pa and 0.0935±0.0091 Pa, respectively. After 24 h, D-20 gels generated ∼1.5× more stress compared to D-14 gels. These data suggested that D-20 gels became more tightly packed as crosslinker density increased, compared to D-14 gels, to generate a larger amount of stress. These forces are less than those seen for softening DNA gels (∼0.5 Pa),7 likely due to the energy needed to form bonds.
To mimic the cellular and incubator environment, stress generation experiments were performed at 37°C by increasing the D-20 gel crosslinker density. The stress generated after 24 h of adding crosslinker was 0.0403±0.06 Pa, which is 3.5× lower than at RT (Fig. 1C). Therefore, at higher temperatures, gel elastic modulus decreased because the DNA gel was relatively loosely cross-linked from hydrogen bond dissociation.
As predicted, gel compression upon an increase in crosslinker density was analogous to the linear thermal expansion of a metal rod upon heating. When a metal rod is heated, the force generated is proportional to the modulus of elasticity, change in temperature, and the area of cross-section of the rod. In a similar way, the contraction of the DNA gel was dependent on the modulus of elasticity of the gel, change in the crosslinker density, and area of cross-section of the gel.
The next goal was to establish whether stress generated by the modulating DNA gel was capable of applying stress onto cells. Twenty-four hours after an additional 50% crosslinker was added to 50% D-20, the length of the PDMS slit decreased and 0.113±0.0211 Pa of stress was generated (Fig. 1D). These data provided a proof of concept that gels with increasing crosslinker density have the ability to generate stress on cells. Furthermore, these measurements were on the same order of magnitude as force transducer measurements. Therefore, both methodologies accurately quantified stress generated by soft, elastic substrates.
These mechanical experiments model the DNA gel's ability to generate stress as elasticity alters over time. How do these mechanical models correlate to the mechanical alterations sensed by fibroblasts? The mechanical changes of the DNA gel are diffusion dependant. In mechanical studies, the majority of changes in a >1-mm-thick DNA gel occurred about 15 h after administration of an additional crosslinker. In fibroblast studies,2 cell behavior on 400–700-μm-thick DNA gels was observed 2 days after administration of additional crosslinker. Fibroblasts sensed changes in DNA gel mechanical properties at a faster rate than mechanical changes seen in mechanical models here, because gels3 in cell experiments were thinner (400–700 μm) than gels in the mechanical studies (>1 mm). Therefore, fibroblasts theoretically have over 24 h to respond to the final changes in gel mechanical properties. However, it is still questionable whether fibroblasts are in a final or transient morphological state after 2 days. To evaluate the transition state, fibroblast studies would have to extend beyond 2 days.
Furthermore, questions are raised concerning the morphological state of the cells within the first 24 h of DNA gel mechanical alterations, because cellular behavior on dynamic substrates is time dependent. For example, stem cell behavior is directed by timing of mechanical cues.10–12 First, stem cell mechanosensitivity is limited by the stage of differentiation.10 A preosteoblast cell line is mechanosensitivity as opposed to undifferentiated bone marrow stem cells. However, predifferentiation of bone marrow stem cells into preosteoblast cells results in equivalent mechanosensitivity to the preosteoblast cell line. Secondly, stem cell fate is dependent on the culture period.11 Substrate stiffening later in culture differentiated adipogenic cells, whereas substrate stiffening earlier in culture differentiated osteogenic cells. Thirdly, a dynamic increase in elastic modulus changed protein levels in human mesenchymal stem cells grown in a 3D environment.12 These three studies demonstrate that temporal modulation of substrate mechanical properties can direct cell behavior. Hence, results seen 2 days after modulating DNA gel mechanical properties may not represent fibroblast behavior 1 day or 5 days after modulating DNA gels. Therefore, the timing of modulating fibroblast cultures in vitro still needs to be investigated to determine the fibroblast threshold of mechanosensitivity. These experiments will demonstrate if fibroblast morphology can be time controlled for tissue-engineering purposes.
Lastly, the stress generated by modulating DNA gels correlated to alterations in elastic modulus. The Young's moduli of 100% crosslinked D-20 and D-14 gels were measured at 10,400 Pa1 and 6680 Pa (Fig. 1B), respectively. The ratio of Young's modulus to stress for a 100% cross-linked gel was always found to be a constant, 7×104 (±1×103). This constant suggests that the stress generated and the elastic modulus alterations are interdependent, and it is impossible to directly evaluate the independent effects of stress generated or stiffness changes on fibroblast morphology with DNA gels.
However, based on previous literature and control DNA experiments, we hypothesized that stress was responsible for altering fibroblast morphology. Jiang et al.2 showed that rat and mouse fibroblasts grown on 50% DNA gels that increased to 80% cross-linked became smaller and more polarized. Stress was the main mechanical property responsible for these changes, because modulus does not have a similar affect. In fact, fibroblasts become larger as substrate modulus increases,13 and fibroblast morphology is unchanged when comparing 80% and 100% control DNA gels, that is, DNA gels that do not change crosslinker density.2 In addition, other studies have shown that compressed fibroblasts become polarized.14 We can conclude that stress was a dominant mechanical property that influenced fibroblast morphology on DNA gels.
The stress-dominant hypothesis was DNA-condition-specific, because results from Jiang et al.'s study2 were dependent on other factors such as DNA gel starting stiffness, magnitude of crosslinker density increase, and fibroblast species. Rat fibroblasts grown on 80% DNA gels did not exhibit morphological changes when the crosslinker density was increased to 100%. Rat fibroblasts grown on control 80% gels were larger and less polarized than fibroblasts grown on control 100% gels. The combination of changes in DNA gel modulus, which makes the cells less polarized, and DNA gel stress, which makes the cells more polarized, resulted in no change in fibroblast morphology likely due to a cancellation effect. Therefore, unlike fibroblasts grown on 50% DNA gels that had an increase in crosslinker density to 80%, fibroblasts grown on 80% DNA gels that had an increase in crosslinker density to 100% are equally affected by both compression and elasticity changes.
Conclusion
Here, we reported the stresses generated and elastic modulus altered in DNA-cross-linked gels with increasing crosslinker density. Both stress and the modulus increased simultaneously as the crosslinker density increased. This correlated response prevents independent evaluation of stress and elastic modulus changes on fibroblasts.2 Yet, we hypothesize that compression forces were contributing to fibroblast morphology when plated on stiffening DNA gels based on control studies and previous reports.
Acknowledgments
This study was supported by the grants from the New Jersey Commission on Spinal Cord Research (Grant # 05-3041-SCR-E-0). The authors also thank Dr. Devendra Verma for his scientific guidance during this project.
Author Disclosure Statement
No competing financial interests.
References
- 1.Jiang FX. Yurke B. Firestein BL, et al. Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann Biomed Eng. 2008;36:1565–1579. doi: 10.1007/s10439-008-9530-z. [DOI] [PubMed] [Google Scholar]
- 2.Jiang FX. Yurke B. Schloss RS, et al. The relationship between fibroblast growth and the dynamic stiffnesses of a DNA crosslinked hydrogel. Biomaterials. 2010;31:1199–1212. doi: 10.1016/j.biomaterials.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 3.Jiang FX. Yurke B. Schloss RS, et al. Effect of dynamic stiffness of the substrates on neurite outgrowth by using a DNA-crosslinked hydrogel. Tissue Eng Part A. 2010;16:1873–1889. doi: 10.1089/ten.TEA.2009.0574. [DOI] [PubMed] [Google Scholar]
- 4.Lin DC. Yurke B. Langrana NA. Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. J Biomech Eng. 2004;126:104. doi: 10.1115/1.1645529. [DOI] [PubMed] [Google Scholar]
- 5.Lin DC. Yurke B. Langrana NA. Inducing reversible stiffness changes in DNA-crosslinked gels. J Mater Res. 2005;20:1456–1464. [Google Scholar]
- 6.Lin DC. Yurke B. Langrana NA. Use of rigid spherical inclusions in Young's moduli determination: application to DNA-crosslinked gels. J Biomech Eng. 2005;127:571. doi: 10.1115/1.1933981. [DOI] [PubMed] [Google Scholar]
- 7.Previtera ML. Trout KL. Verma D, et al. Fibroblast morphology on dynamic softening of hydrogels. Ann Biomed Eng. 2012;40:1061–1072. doi: 10.1007/s10439-011-0483-2. [DOI] [PubMed] [Google Scholar]
- 8.Chippada U. Yurke B. Langrana NA. Simultaneous determination of Young's modulus, shear modulus, and Poisson's ratio of soft hydrogels. J Mater Res. 2010;25:545–555. [Google Scholar]
- 9.Lötters JC. Olthuis W. Veltink PH, et al. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J Micromech Microeng. 1997;7:145. [Google Scholar]
- 10.Hsiong SX. Carampin P. Kong HJ, et al. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85:145–156. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- 11.Guvendiren M. Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 12.Marklein RA. Soranno DE. Burdick JA. Magnitude and presentation of mechanical signals influence adult stem cell behavior in 3-dimensional macroporous hydrogels. Soft Matter. 2012;8:8113–8120. [Google Scholar]
- 13.Yeung T. Georges PC. Flanagan LA, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 14.Thoumine O. Ott A. Time scale dependent viscoelastic and contractile regimes in fibroblasts probed by microplate manipulation. J Cell Sci. 1997;110:2109–2116. doi: 10.1242/jcs.110.17.2109. [DOI] [PubMed] [Google Scholar]