Abstract
Human mesenchymal stem cells (hMSCs) are highly desirable cells for bone engineering due to the inherent multipotent nature of the cells. Unfortunately, there is a high degree of variability, as primary hMSC cultures quickly undergo replicative senescence with loss of proliferative potential as they are continually propagated in cell culture. We sought to reduce the variability of these cells by insertion and expression of human telomerase reverse transcriptase (TERT) to immortalize the cell line. hMSCs were transduced with a lentivirus containing the human TERT gene. The resulting cell line has been propagated through more than 70 population-doubling level (PDL) to date and continues to grow exhibiting the characteristic fibroblastic hMSC phenotype. Expression of TERT mRNA and protein activity was confirmed in the TERT-transduced cells. Mock-transduced hMSCs had almost undetectable levels of TERT mRNA and protein activity and lost proliferation potential at PDL 14. The enhanced growth capacity of the hMSC TERT cells was due to increased cell proliferation and reduced cellular senescence rather than due to inhibition of apoptosis. The multipotent nature of the TERT cells was confirmed by differentiation toward the osteoblastic and adipogenic lineages in vitro. Osteoblastic differentiation was confirmed by both expression of alkaline phosphate and mineral deposition visualized by Alizarin Red staining. Adipogenic differentiation was confirmed by production of lipid droplets, which were detected by Oil Red-O staining. In summary, we have generated a stable hMSC line that can be continually propagated and retains both osteoblastic and adipogenic differentiation potential.
Key words: adipogenic differentiation, human mesenchymal stem cell, osteoblastic differentiation, TERT expression
Introduction
Mesenchymal stem cells (MSCs) have the potential to differentiate into a number of cell types, sparking a great interest in their use in regenerative medicine for cartilage and bone. MSCs have been isolated from bone marrow stromal cells (BMSC) and are present in a very low frequency, whereas only 0.001% to 0.01% of nucleated cells in BMSC are actually MSCs.1 As a stem cell source, human MSCs (hMSCs) are well studied and have been shown to differentiate toward osteogenic, chondrogenic, adipogenic, and myogenic lineages.2,3 Unfortunately, hMSCs undergo replicative senescence4,5 with prolonged growth in cell culture, further limiting the availability of hMSCs that could be used in clinical applications. Thus, continuous passage of hMSCs in cell culture resembles an aging process with the later-passage cells being more differentiated than early-passage cells until the cells cease dividing altogether.4 Rapidly dividing cells undergo about 20–40 population doublings before succumbing to cell senescence, resulting in a shorter life span than slower growing cells.6 Replicative senescence (ageing) is associated with the loss of telomeric DNA with each cell division due to incomplete replication of the telomeric ends.7 Telomeric loss results in a variety of consequences, including inhibition of the mitotic clock, genotoxic damage due to accumulation of free radicals, and chromosomal rearrangement, which can trigger a DNA damage response and ultimately leading to senescence and cell apoptosis.8–10
The length of the telomeric ends is controlled by expression of the telomerase reverse transcriptase (TERT) gene.11 Somatic cells usually have low or undetectable levels of telomerase. Decreased TERT activity correlates with increased differentiation and passage of MSCs in cell culture. Cancer cells have been shown to have increased levels of telomerase activity.12 Transgenic mice constitutively expressing TERT have a higher incidence of cancers, although the tumorigenicity is due to loss of tumor suppressor genes and not specifically due to expression of TERT.13 Constitutive expression of TERT has been shown to prevent senescence, increase proliferation, yet maintain differentiation ability in hMSCs.14–16 We report here the generation of an hMSC line expressing TERT that exhibits enhanced cell proliferation and stability in cell culture and retains the ability to differentiate toward both the osteogenic and adipogenic lineages.
Materials and Methods
Cell culture
hMSCs, hMSC complete growth medium (HMSCGM), and growth supplements were obtained from Lonza, Inc. (Allendale, NJ). hMSCs used in this study were from a 32-year-old Caucasian man (Lonza Lot # 4F0591). Cells were subcultured at 60%–70% confluence at 37°C under 95% air/5% CO2 atmosphere, and infected with TERT-expressing lentivirus (LV) at passage 4. For differentiation to the osteoblastic lineage, hMSCs were treated every 3–4 days with an osteogenic medium (OGM) consisting of HMSCGM (Lonza PT-3001) supplemented with 50 mM ascorbic acid-2-phosphate, 10 mM β-glycerophosphate, and 10−7 M dexamethasone (Sigma-Aldrich, St. Louis, MO). For differentiation to adipocytes, hMSCs were treated with three cycles of an hMSC adipogenic induction medium (AIM; Lonza PT-3102B containing h-insulin, l-glutamine, MCGS, dexamethasone, indomethacin, 3-isobuty-l-methyl-xanthine [IBMX], and GA-1000) for 3 days and an hMSC adipogenic maintenance medium for 3 days (AMM; Lonza PT-3102A containing h-insulin, l-glutamine, MCGS, and GA-1000), and then further cultured in the AMM until analysis.
Generation of telomerase-expressing LV
Plasmid pBABE-puro-hTERT (#1771) containing the TERT gene17 was obtained from Addgene (Cambridge, MA) and the TERT gene isolated on a 3.5-kB EcoRI/SalI fragment. pLVX-IRES-Hyg (Clontech, Mountain View, CA) was restricted with SpeI. The EcoRI/SalI/SpeI 5′-overhangs were end-filled using Klenow to generate blunt ends for cloning. The blunt EcoRI-TERT-SalI fragment was ligated into the blunt SpeI-pLVX-IRES-Hyg vector and transformed into NEB 5-alpha- (New England Biolabs, Ipswich, MA) competent Escherichia coli selecting for ampicillin resistance. Transformants were screened for correct insertion/orientation of the TERT fragment by restriction analysis. In this construct (pLVX-TERT-IRES-Hyg), TERT is expressed as a bicistronic transcript with the hygromycin (hyg) gene allowing hyg resistance to be used as an indicator of transduction efficiency and as a marker for selection.
TERT-expressing LV was generated after transfection of pLVX-TERT-IRES-Hyg into HEK293 cells (American Type Culture Collection, Manassas, VA) using the Lenti-X HTX Packaging system (Clontech). Briefly, 5×106 HEK293 cells were plated in one 10-cm dish in a tetracycline-free medium and transfected the next day with pLVX-TERT-IRES-Hyg and the Lenti-X HT Packaging Mix for 4 h using Xfect Polymer (Clontech). After 4 h, the medium was replaced with 10 mL tetracycline-free medium. Infectious LV was collected after 72 h, centrifuged 10 min at 500 g, and stored in aliquots at −80°C. LV obtained was at a high titer and did not need to be concentrated further.
Transduction of hMSCs with TERT-expressing LV
hMSCs (passage 4) were plated in six-well dishes (10,000 cells/cm2; 2 mL HMSCGM medium/well) and allowed to attach overnight. Two cycles of LV transduction were used. LV TERT was diluted 1:2 with HMSCGM, and polybrene (Sigma-Aldrich) was added (24 μg/mL). One milliliter of this dilution was added drop-wise to the 2 mL already in the wells. This gave a final concentration of 8 μg/mL polybrene in each well. The plate was centrifuged (1200 g; 32°C) for 1 h and then incubated (37°C) for an additional 5 h before changing the medium (2 mL HMSCGM) overnight. The next day, the LV TERT transduction was repeated using a fresh dilution of virus on the same wells. The transduction medium was replaced with HMSCGM containing 25 μg/mL hyg (Sigma-Aldrich). Mock-transfected wells containing no virus (with polybrene) were treated with hyg as controls. After 6 days, LV TERT hyg-resistant wells were passaged into T25 flasks with hyg. Mock-transfected wells with hyg were dead. After 1 week, the cells were passaged into T75 flasks without hyg to begin cell characterization and were designated hMSCs (mock-transfected) and hMSC TERT (TERT-expressing) cells.
Analysis of cell culture growth characteristics
hMSC and hMSC TERT cells were passage into two T75 flasks each at 5000 cells/cm2 without hyg for 1 week and then harvested and counted. Similar cultures were combined and passaged again into two T75 flasks. Using cumulative counts, the population-doubling level (PDL) was calculated at each passage as follows:18 PDL(n/n−1)=log (Nf/N0)/log 2, where n=passage number, Nf=final number of cells, and N0=number of cells seeded at passage. After each passage, PDL(n/n−1) was added to the previous PDL(n) to get the new PDL(n+1). Serial passage levels were expressed as PDL or as the passage number as Px(y), where x is the passage number after transduction; and y is the passage number from the original primary culture. At each passage, samples were taken for RNA analysis using the Qiagen RNeasy Miniprep kit (Qiagen, Valencia, CA) and telomerase activity using the TeloTAGGG telomerase polymerase chain reaction (PCR) ELISA (Roche Applied Science, Indianapolis, IN). Cell proliferation was studied using the Cell Proliferation ELISA and BrdU (colometric) Assay (Roche Applied Science); cell senescence using the Senescence Cells Histochemical Staining Kit (Sigma-Aldrich); and apoptosis using the TiterTACS™ apoptosis detection kit from R&D Systems, Inc. (Minneapolis, MN).
Quantitative real-time reverse transcription–PCR
Gene sequences for the osteogenic marker alkaline phosphatase (ALP), the telomerase (TERT), the 18S ribosomal RNA (18S rRNA), and the tumor suppressor genes p51, p53, and p16INK4a, and klotho were designed using the National Center for Biotechnology (NCBI) gene database (Table 1). Relative mRNA levels were quantitated by real-time reverse transcription (RT)–PCR using the Opticon continuous fluorescence system (Bio-Rad Laboratories, Hercules, CA) and the SYBR Green RT-PCR kit (Qiagen). Reactions were performed in triplicate: reverse transcription at 50°C for 30 min, 95°C for 15 min; followed by 40 cycles of 94°C for 15 sec (denaturing), 60°C for 30 sec (annealing), and 72°C for 30 sec (extension). Relative changes in gene expression were calculated in relation to 18S levels and the reference time point using the 2−ΔΔC(T) method.19
Table 1.
Primer Sequences Used in Quantitative Real-Time Reverse Transcription–Polymerase Chain Reaction
| Gene of interest | Oligonucleotide sequence | GenBank ID |
|---|---|---|
| ALP sense | 5′-AAGCCGGTGCCTGGGTGGCCAT-3′ | NM_000478 |
| ALP antisense | 5′-ACAGGAGAGTCGCTTCAGAG-3′ | |
| TERT sense | 5′-CTACGGCGACATGGAGAAC-3′ | NM_198253.2 |
| TERT antisense | 5′-GACACTTCAGCCGCAAGAC-3′ | |
| 18S rRNA sense | 5′-CCGCAGGTTCACCTACTG-3′ | NR_003286.2 |
| 18S rRNA antisense | 5′-CGGGTCATAAGCTTGCCTG-3′ | |
| KLOTHO sense | 5′-GTGGCCTGGCACGGCTACG-3′ | NM_004795.3 |
| KLOTHO antisense | 5′-GCCAGACTTTGGCATGAGCCAGG-3′ | |
| p21 sense | 5′-AGAGGAGGCGCCATGTCAGAACCG-3′ | NM_000389.4 |
| p21 antisense | 5′-GCATCACAGTCGCGGCTCAGC-3′ | |
| p53 sense | 5′-TAAGCGAGCACTGCCCAACAACA-3′ | NM_000546.4 |
| p53 antisense | 5′-GCTCTCGGAACATCTCGAAGCGC-3′ | |
| p16INK4a sense | 5′-GTGGACCTGGCTGAGGAG-3′ | NM_058195.3 |
| p16INK4a antisense | 5′-CTTTCAATCGGGGATGTCTG-3′ |
ALP, alkaline phosphatase; TERT, telomerase reverse transcriptase; 18S rRNA, 18S ribosomal RNA; p21, cyclin-dependent kinase inhibitor 1A; p53, tumor protein 53.
Chemotaxis assays
Chemotaxis assays were performed as previously described.20 Cells (2.5×104 in 100 μL) were plated in triplicate onto Costar transwell membranes in the serum-free HMSCGM with 0.1% bovine serum albumin. Lower chambers contained HMSCGM with 1% fetal bovine serum (FBS). Transwells were incubated at 37°C (4 h), fixed with 10% neutral buffered formalin, and stained with hematoxylin. Migrating cells were counted (400×) on a Nikon Eclipse E400 microscope with five fields analyzed per membrane.
Osteogenic and adipogenic differentiation
hMSCs and hMSC TERT cells were plated (5000 cell/cm2) in HMSCGM in triplicate in 48-well plates and allowed to attach overnight before treatment with OGM. Total RNA was isolated at 0, 3, 7, 10, and 14 days. Differentiation was monitored by mRNA expression of the osteoblastic marker gene ALP and 18S rRNA as a housekeeping marker. For mineralization analysis, cells were plated (10,000 cell/cm2) in 96-well plates, treated with HMSCGM or OGM for 4 weeks, and stained with Alizarin Red. For adipogenic analysis, cells were seeded in 96-well plates (5000 cell/cm2), treated with three cycles of AIM/AMM (3 days each/cycle), and then cultured in the AMM. Lipid droplets were visualized with Oil Red-O staining 3 weeks after adipogenic induction.
Statistics
Data are reported as the mean value±SD. Values were analyzed by nonparametric one-way analysis of variance with Bonferroni post hoc test analysis and by Student's t-test using GraphPad Prism 5.04 (GraphPad Software, San Diego, CA). A value of p<0.05 was considered significant.
Results
Construction of LV
To address the problem of variability in hMSC populations, we sought to develop a cell line of hMSCs that expresses TERT, and therefore may have the potential for continued growth in cell culture. The human TERT gene was subcloned into pLVX-IRES-Hyg (Clontech) and is expressed as a bicistronic transcript with the hyg-resistant gene, allowing hyg to be used as an indicator of transduction efficiency and as a marker for selection. The bicistronic construction of this vector ensures that hyg-resistant cells must also be expressing the TERT gene.
Phenotype of cells
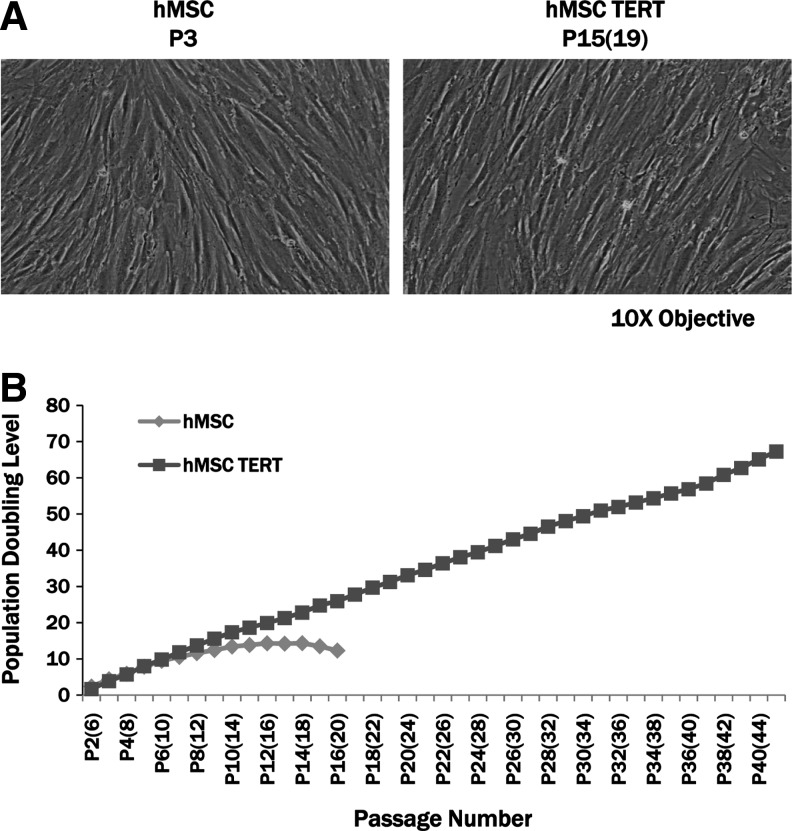
Early-passage hMSCs have a characteristic fibroblast morphology. Figure 1A shows passage P3 hMSCs detailing the long fibroblast phenotype and the characteristic alignment of the cells as the culture becomes confluent. Later-passage [PDL 25; P15(19)] hMSC TERT cells still retain the fibroblastic phenotype of younger-passage hMSCs.
FIG. 1.
Phenotype and growth characteristics of human mesenchymal stem cell (hMSCs) and hMSC telomerase reverse transcriptase (TERT) cells. (A) Late-passage [P15(19)] hMSC TERT cells still exhibit the characteristic fibroblastic phenotype of early-passage (P3) hMSCs. (B) Mock-transduced hMSCs and hMSC TERT cells were serial passaged in cell culture, and the population-doubling level (PDL) was calculated at each passage number. At early passages [P2(6)–P7(11)], the PDLs are similar for the two cell lines, but after P8(12), hMSCs proliferate at a slower rate and ceased growth at P16(20) with a maximum PDL of 14. hMSC TERT cells continually proliferate even after PDL 70 [P40(44)].
Analysis of culture growth characteristics
Both the hMSC and hMSC TERT cell lines were propagated through serial passage in T75 flasks to assess the ability of the cells to continue to grow in cell culture in the absence of hyg. At early passages, the hMSCs and hMSC TERT cells grew at similar rates, but at PDL 11 [passage P6(10)], the cell yield of the hMSCs decreased steadily and reached a minimum between P13(17) and P16(20) at ∼2×105 cell/flask (Fig. 1B). In contrast, hMSC TERT cells produced between 9×105 and 1.5×106 cells/flask throughout the time course of the study. The hMSC TERT cell line has produced a cumulative total of over 7.8×107 cells and shows no signs of diminished proliferation even after 70 PDLs [P40(44); Fig. 1B], whereas the mock-transduced hMSC line ceased proliferating at 1.5×107 cells after 14 PDLs [P16(20)].
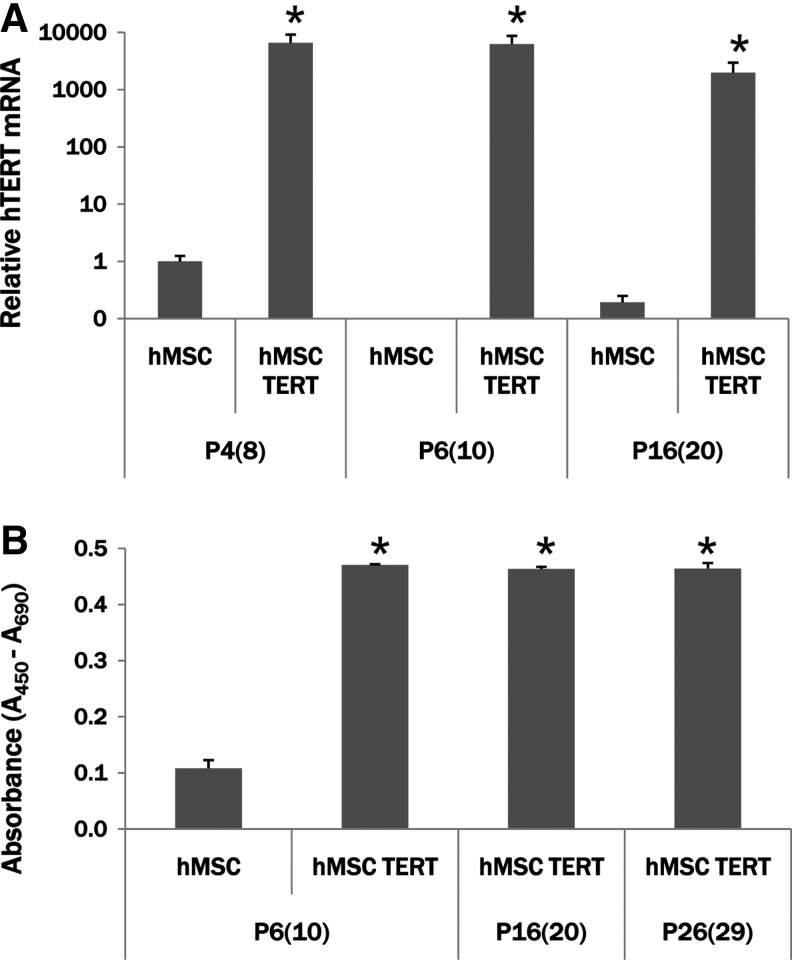
Analysis of TERT expression and activity in hMSC TERT cells
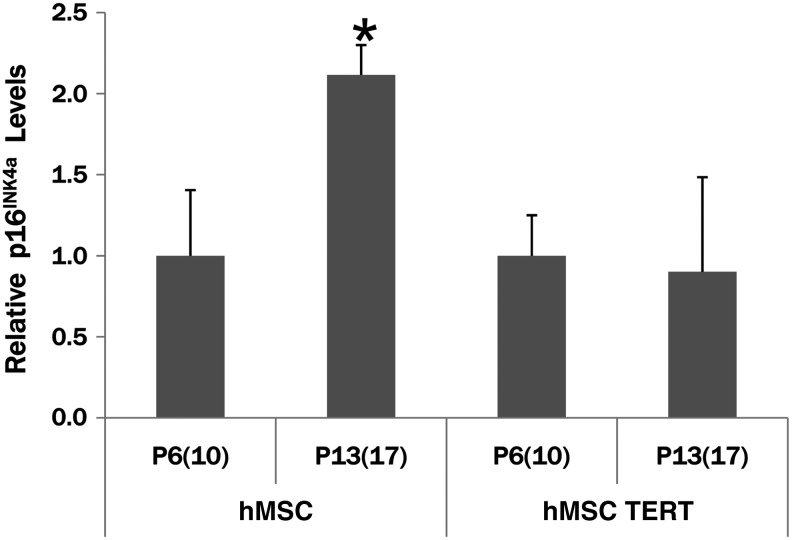
RNA isolated at various passages throughout the time course was analyzed by real time RT-PCR to confirm TERT expression. hMSCs [PDL 6; P4(8)] have a low level of detectable TERT mRNA (Fig. 2A), whereas P4(8) hMSC TERT cells have over 6400× the level of TERT mRNA as hMSCs. TERT mRNA in hMSCs decreases to almost undetectable levels at P6(10) and P16(20). Even though the level of TERT mRNA in the hMSC TERT cells decreases slightly, there is still significantly higher expression than hMSC levels at P4(8). Telomerase enzyme activity was also measured at various points in comparison to early-passage hMSCs (Fig. 2B). At PDL 10 [P6(10)], hMSC TERT cells have significantly higher levels of telomerase activity in comparison to hMSCs at the same passage. The high level of telomerase activity was sustained at later passages [P16(20) and P26(29)]. mRNA levels of the cell cycle regulator p16INK4a significantly increased at P13(17) in hMSCs, but not in TERT cells (Fig. 3). Tumor suppressor genes p21, p53, and klotho were also quantitated, but were not significantly different comparing hMSC and hMSC TERT cultures at these passages (data not shown).
FIG. 2.
TERT expression in hMSCs and hMSC TERT cells. (A) Relative changes in TERT mRNA expression were determined by real-time reverse transcription (RT)–polymerase chain reaction (PCR) and expressed in relation to 18S levels, and the reference point [mock-transduced hMSC P4(8) levels] using the 2−ΔΔC(T) method. hMSCs have a low, but detectable, level of TERT mRNA expression at P4(8), but with continued passage TERT expression quickly decreases to nearly undetectable levels even by early-passage P6(10). hMSC TERT cells have significantly high levels of TERT mRNA at all passages, even late passage P16(20). *p<0.05 compared to hMSC levels at each passage. (B) TERT enzymatic activity was also measure at both early [P6(10)] and late [P16(20) and P26(30)] passages. There are significantly higher levels of TERT activity in hMSC TERT cell extracts than in hMSC extracts throughout the time course of the study. *p<0.05 compared to hMSC levels at P6(10).
FIG. 3.
Expression of p16INK4a cell cycle regulator in hMSCs and hMSC TERT cells. mRNA levels were quantitated by real-time RT-PCR and normalized to expression levels at P6(10) for each cell line. p16INK4a levels are significantly increased in hMSCs at later passages [P13(17)], indicating aging of the culture. Levels in hMSC TERT cell lines are unchanged. *p<0.05 compared to P6(10) levels for each cell line.
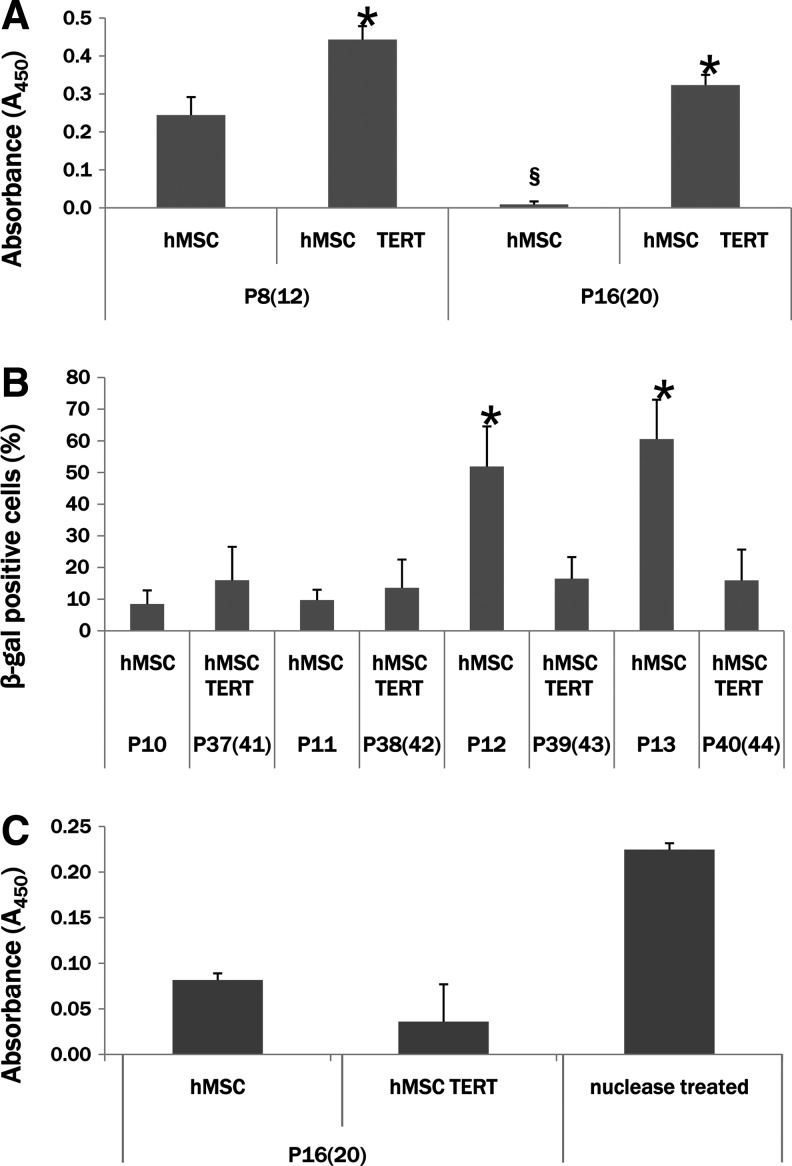
Cell proliferation, senescence, and apoptosis
To determine the nature of the enhanced growth capacity of hMSC TERT cells, we examined cell proliferation, cellular senescence, and apoptosis at various passages. Cell proliferation was assayed at both early [P8(12)] and late [P16(20)] passages. hMSC TERT cells exhibited a significantly higher rate of cell proliferation after 4 days in culture in comparison to hMSCs at a similar passage (Fig. 4A). Cell proliferation at late passage in the hMSCs was significantly reduced from that seen in early passage hMSCs, whereas cell proliferation in hMSC TERT cells was only slightly reduced when comparing early- and late-passage cells.
FIG. 4.
Enhanced growth characteristics of hMSC TERT cells due to increased cell proliferation and decreased cellular senescence. (A) Cellular proliferation was assayed by measurement of BrdU incorporation during DNA replication, indicating that at both early [P8(12)] and late [P16(20)] passage, the proliferative rate of hMSC TERT cells is significantly higher than that of hMSCs (mock-transduced). *p<0.05 compared to hMSC levels at each passage. The proliferative rate of hMSCs decreases significantly from P8(12) to P16(20), whereas the rate of proliferation of hMSC TERT cells decreases slightly but insignificantly over the same passages. §p<0.05 compared to hMSC levels at P8(12). (B) Cellular senescence was measured by the histochemical stain for β-galactosidase activity. There is a significant increase in β-galactosidase-positive cells in hMSCs after passage P12, whereas the percentage of positive hMSC TERT cells did not increase even after P40(44). *p<0.05 compared to hMSC levels at passage P10. (C) Apoptosis potential was determined by colorimetric determination of DNA fragmentation in hMSCs or hMSC TERT cells. There was no significant difference in the level of apoptosis, although the trend was for lower apoptosis in hMSC TERT cells in comparison to mock-transduced hMSCs.
Cell senescence was assayed using β-galactosidase activity as a marker at four consecutive passages. The percentage of cells exhibiting β-galactosidase staining was unchanged in hMSC TERT cells even up to P40(44), whereas β-galactosidase activity was not significantly different in hMSCs at P10 and P11, but significantly increased at passages P12 and P13, representing an increase in cell senescence in hMSCs after only 12 passages (Fig. 4B).
DNA fragmentation as a measure of apoptosis was analyzed in late-passage [P16(20)] cells. Although the apoptosis level was lower in hMSC TERT cultures in comparison to hMSC cultures, the results were not significantly different (Fig. 4C). Taken together, these results indicate that the enhanced growth characteristics of hMSC TERT cells are due to increased cell proliferation and inhibition of cellular senescence.
hMSCs have the ability to chemotax toward several attractants. To test if later-passage hMSC TERT cells retain this ability, chemotaxis assays were performed to compare late-passage hMSCs (P8–P10) and hMSC TERT cells [P11(15)–P12(16)]. hMSCs show a low level of chemotaxis toward a medium containing 1% FBS, whereas hMSC TERT cells exhibit a significantly higher level of chemotaxis at even later passages (Fig. 5).
FIG. 5.
Chemotaxis toward a medium containing 1% fetal bovine serum (FBS). Data are expressed as a chemotactic index, defined as the number of cells migrating in response to the medium (1% FBS) divided by the number of cells migrating in the negative control (−). hMSC TERT cells [P12(16)] exhibit significantly higher levels of chemotaxis than hMSCs at earlier PDL (P8). *p<0.05 compared to negative control.
Differentiation capability of hMSC TERT cells
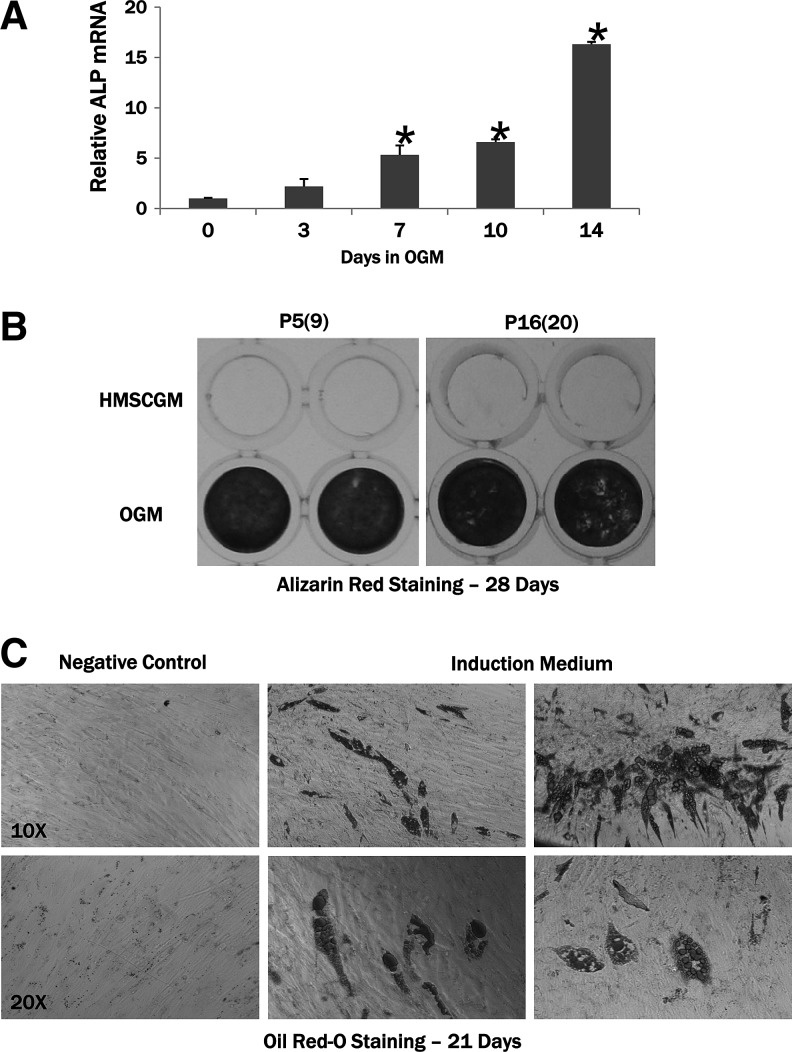
To test the capacity of the hMSC TERT cells to undergo differentiation toward the osteogenic lineage, cells were treated for 14 days with OGM. OGM treatment of late-passage [P16(20)] hMSC TERT cells significantly induced mRNA expression of the early chondro-/osteogenic marker alkaline phosphatase (hALP) beginning at 7 days and throughout the 14-day time course of the experiment (Fig. 6A). This is consistent with our previous results for hMSC osteogenic differentiation.21 hMSC TERT cells from early and late passages were also treated for 4 weeks in OGM and then analyzed for mineral deposition using Alizarin Red stain (Fig. 6B). Alizarin Red-stained wells were only present in wells treated with OGM, indicating that the cells reached the late stage of osteoblastic differentiation and could deposit mineral. Late-passage [P22(26)] hMSC TERT cells were also treated with AIM to confirm the ability of these cells to differentiate toward the adipogenic lineage (Fig. 6C). Oil Red-O staining confirmed the presence of lipid deposits in those wells treated with AIM and absent in those treated with AMM.
FIG. 6.
Differentiation of hMSC TERT cells toward the osteoblastic and adipogenic lineages. hMSC TERT cells were treated with an osteogenic medium (OGM) for 14 or 28 days. (A) Relative changes in alkaline phosphatase (ALP) expression were determined by real-time RT-PCR. ALP mRNA expression is significantly upregulated at 7, 10, and 14 days. *p<0.05 compared to hMSC TERT levels at day 0. (B) Alizarin Red staining of early- [P5(9)] and late- [P16(20)] passage cells demonstrates that the hMSC TERT cells can undergo terminal differentiation, resulting in the deposition of mineral after OGM treatment for 28 days. (C) Oil Red-O staining of lipid droplets confirms that the hMSC TERT cells can also undergo adipogenic differentiation after induction for 21 days.
Discussion
hMSCs have the potential to be useful in a variety of reconstructive engineering capacities due to the inherent multipotent capacity of the cells to differentiate into osteogenic, adipogenic, and chondrogenic lineages.2,3 A major drawback to using hMSCs is that they are only present in low numbers in the bone marrow,1 and although they propagate readily in cell culture, serial passage of the cells ultimately leads to replicative senescence limiting the number of available cells that could be used in a clinical setting.4,5 Replicative senescence in hMSCs resembles an aging process with each consecutive passage becoming slightly more differentiated. Problems arise when trying to use the cells to study developmental processes as the cultures are continually changing (aging), necessitating the need to use multiple donors to do repetitive experiments. To address the problem of replicative senescence, we sought to constitutively express the TERT gene in hMSCs, thereby immortalizing the culture, and hopefully lessening the variability of the cultures due to aging in vitro. The TERT gene was inserted into pLVX-IRES-Hyg (Clontech) and is expressed as a bicistronic transcript with the hygromycin (hyg)-resistant gene. Even though the hMSC TERT cell line is resistant to hyg, the cultures were grown without the addition of hyg for two reasons. First, to assess the stability of the TERT expression without selective pressure, and second, since cell growth of hMSC TERT cells was slower in the presence of hyg (data not shown), we wanted to be sure that we were assessing the effect of TERT expression and not the effect of hyg in comparison to the hyg-sensitive hMSCs. This cloning strategy (hyg resistance with TERT expression) was undertaken with the knowledge that Clontech also produces another vector with puromycin (puro) resistance (pLVX-Puro) that could be used to express other inserted genes selecting for both hyg and puro, thus generating immortalized cell lines expressing both TERT and another gene of interest.
hMSC TERT cells have been serial-passaged to over 70 population doublings and still exhibit the characteristic fibroblastic cell phenotype typical of early-passage hMSCs. TERT expression is stable without the need for continued hyg selection. Previous studies have shown that constitutive expression of TERT by itself does not generate malignant conditions22; although, TERT expression has been associated with cancer.12 We also looked at expression of the tumor suppressor genes p16INK4a, cyclin-dependent kinase inhibitor p21 (Waf-1/Cip-1), p53, and klotho. The expression of these tumor suppressor genes in the hMSC TERT cell line is not significantly different than expression in early-passage hMSCs. Additionally, both p16INK4a and klotho have been characterized as genes associated with aging.23,24 p16INK4a is frequently induced as cells age and inhibits stem cell proliferation and regenerative potential,24 and was induced in later passages of hMSCs, but not induced in the hMSC TERT cell line. Klotho-deficient and klotho-null mice exhibit a premature aging phenotype, whereas overexpression of klotho has been shown to extend the lifespan of such mice by 20%–30%.23 While it was surmised that TERT expression may increase klotho expression, we did not see any changes in klotho gene expression. TERT expression does not significantly alter expression levels of these tumor suppressor genes, and therefore the hMSC TERT cells do not appear to be neoplastic, although this would have to be verified in vivo. The multipotent nature of hMSC TERT cells is demonstrated with differentiation toward the osteogenic and adipogenic lineages. Osteogenic differentiation leads to both ALP mRNA induction and mineralization as we have reported previously in hMSC cultures,21 and adipogenic differentiation leads to cells filled with lipid droplets.
In summary, we have generated a hMSC line that is hyg resistant, does not exhibit replicative senescence typical of primary hMSCs, and retains the ability to chemotax and differentiate toward the osteogenic and adipogenic lineages. The hMSC TERT cell line is ideal for both basic and applied tissue engineering studies of bone development and repair due to the reduced variability offered by the enhanced growth potential in cell culture over that of hMSC primary cultures.
Acknowledgment
This work was supported by a Veterans' Affairs National Merit Review Grant awarded to D.T.Y.
Author Disclosure Statement
The authors have no financial interest in the products mentioned in the article.
References
- 1.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF. Mackay AM. Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P. Robey PG. Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenderup K. Justesen J. Clausen C, et al. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Metz T. Verdonk G. In vitro aging of human bone marrow derived stromal cells. Mech Ageing Dev. 1981;16:81–89. doi: 10.1016/0047-6374(81)90035-x. [DOI] [PubMed] [Google Scholar]
- 6.Bruder SP. Jaiswal N. Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls C. Li H. Wang JQ, et al. Molecular regulation of telomerase activity in aging. Protein Cell. 2011;2:726–738. doi: 10.1007/s13238-011-1093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayouaz A. Raynaud C. Heride C, et al. Telomeres: hallmarks of radiosensitivity. Biochimie. 2008;90:60–72. doi: 10.1016/j.biochi.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Rochette PJ. Brash DE. Human telomeres are hypersensitive to UV-induced DNA Damage and refractory to repair. PLoS Genet. 2010;6:e1000926. doi: 10.1371/journal.pgen.1000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greider CW. Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 12.Shay JW. Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 13.Tomás-Loba A. Flores I. Fernández-Marcos PJ, et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Böker W. Yin Z. Drosse I, et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J Cell Mol Med. 2008;12:1347–1359. doi: 10.1111/j.1582-4934.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakahara H. Misawa H. Hayashi T, et al. Bone repair by transplantation of hTERT-immortalized human mesenchymal stem cells in mice. Transplantation. 2009;88:346–353. doi: 10.1097/TP.0b013e3181ae5ba2. [DOI] [PubMed] [Google Scholar]
- 16.Simonsen JL. Rosada C. Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 17.Counter CM. Hahn WC. Wei W, et al. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andley UP. Rhim JS. Chylack LT, Jr, et al. Propagation and immortalization of human lens epithelial cells in culture. Invest Ophthalmol Vis Sci. 1994;35:3094–3102. [PubMed] [Google Scholar]
- 19.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 1(−ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Makhijani NS. Bischoff DS. Yamaguchi DT. Regulation of proliferation and migration in retinoic acid treated C3H10T1/2 cells by TGF-β isoforms. J. Cell Physiol. 2005;202:304–313. doi: 10.1002/jcp.20128. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff DS. Zhu JH. Makhijani NS, et al. Angiogenic CXC chemokine expression during differentiation of human mesenchymal stem cells towards the osteoblastic lineage. J Cell Biochem. 2008;103:812–824. doi: 10.1002/jcb.21450. [DOI] [PubMed] [Google Scholar]
- 22.Harley CB. Telomerase is not an oncogene. Oncogene. 2002;21:494–502. doi: 10.1038/sj.onc.1205076. [DOI] [PubMed] [Google Scholar]
- 23.Kuro-O M. Klotho and aging. Biochim Biophys Acta. 2009;1790:1049–1058. doi: 10.1016/j.bbagen.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamurthy J. Ramsey MR. Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]