Abstract
Human pluripotent stem cell (hPSC)-derived cell therapy requires production of therapeutic cells in large quantity, which starts from thawing the cryopreserved cells from a working cell bank or a master cell bank. An optimal cryopreservation and thaw process determines the efficiency of hPSC expansion and plays a significant role in the subsequent lineage-specific differentiation. However, cryopreservation in hPSC bioprocessing has been a challenge due to the unique growth requirements of hPSC, the sensitivity to cryoinjury, and the unscalable cryopreservation procedures commonly used in the laboratory. Tremendous progress has been made to identify the regulatory pathways regulating hPSC responses during cryopreservation and the development of small molecule interventions that effectively improves the efficiency of cryopreservation. The adaption of these methods in current good manufacturing practices (cGMP)-compliant cryopreservation processes not only improves cell survival, but also their therapeutic potency. This review summarizes the advances in these areas and discusses the technical requirements in the development of cGMP-compliant hPSC cryopreservation process.
Key words: cell banking, cryopreservation, human pluripotent stem cell
Introduction
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), can be expanded indefinitely and differentiated into any cell type in three-germ layers.1–3 These unique characteristics make hPSCs attractive as the cell source for tissue engineering, regenerative medicine, and drug screening. Recently, hESC-derived oligodendrocyte progenitor cells (OPC) and hESC-derived retinal pigment epithelial cells have been investigated in Phase I/II clinical trials.4–6 More clinical trials are expected for treatment of other diseases, such as amyotrophic lateral sclerosis and diabetes.7 A critical requirement for hPSC's clinical applications is the supply of a large quantity of hPSC-derived therapeutic cells. Production of these therapeutic cells starts from thawing cells from qualified cell banks, such as the master cell bank (MCB) or the working cell bank (WCB), under current good manufacturing practices (cGMP). The quality of the cell bank plays an important role in hPSC bioprocessing as it impacts post-thaw hPSC expansion, the efficiency of the subsequent lineage-specific differentiation, and process reproducibility. A robust cryopreservation and thaw process in generating MCB and WCB thus becomes the crucial first steps that ensure the quality of hPSC-based cell production.
Cryopreservation has been a routine practice for hematopoietic stem cells (HSCs) for bone marrow transplantation and cord blood banking since 1990s.8 Other adult stem cell banks, especially mesenchymal stem cells (MSCs), are also being established for clinical application.9 Besides the increased flexibility and cell availability in laboratory practices, cryopreservation has been an important bioprocessing step to create large-scale cell banks that provide uniform cell populations for clinical study. Different from adult stem cell banking, the unlimited proliferation capacity of hPSCs enables the banking scale in the order of greater than 109 cells.10 However, maintaining hPSC survival after cryopreservation and thaw has been a significant challenge, and thus the main focus of research efforts. The traditional cryopreservation and thaw methods led to the diminishing Oct-4 expression and poor hPSC survival.11 Due to the high sensitivity to cryoinjury, various groups have been working on optimizing the protocols to efficiently cryopreserve hPSCs with different formulations and procedures.12 Although cell viability post-thaw has been significantly improved, the feasibility of these methods for large-scale banking has not been adequately addressed. Some methods based on vitrification may only be suitable for laboratory practices, while the scalability of other methods, such as slow cooling, needs to be further improved for large-scale cell banking.
This review started from cryopreservation principles and adult stem cell banking that are currently used in clinical practice. The recent progress of hPSC cryopreservation bioprocess was then summarized with an emphasis on slow cooling methods due to their potential in large-scale application. The regulatory mechanisms of apoptosis, reaction oxygen species (ROS), and cell–cell and cell–matrix interaction on hPSC viability and recovery were then discussed to highlight the role of small molecule intervention in improving cryopreservation efficiency. Finally, the current status and challenges of large-scale hPSC banking under cGMP are discussed, and the importance of integrated cell expansion and cryopreservation process in cGMP cell banking is emphasized.
Cryopreservation Principles
During cryopreservation and thaw, the main cause of cell death usually is not the long-term storage at low temperature, but rather the process that the cells travel across −15°C to −60°C, which happens once during cooling and once during warming.13,14 During slow cooling, ice formed in the extracellular environment causes an increase of solution osmolality. To compensate the osmolality imbalance, the intracellular water passes through cell membrane to the extracellular solution, causing cellular dehydration, which may damage cell membrane and organelles. During rapid cooling, however, cellular damages can be resulted from intracellular ice formation due to insufficient water outflow and super cooling. As both events are detrimental to the cells, the cell cryopreservation requires an optimal cooling rate and the presence of cryoprotectant (CPA), which reduces cell shrinkage during dehydration and inhibits intracellular ice formation until the intracellular water achieves a “glassy state.”15 The typical CPAs are low molecular weight organic compounds that effectively penetrate into the cells and prevent the intracellular ice formation, which include glycerol, dimethyl sulfoxide (DMSO), ethylene glycol (EG), and propylene glycol (PG).16 During warming, a rapid warming rate is preferred to prevent ice recrystallization and the effective removal of CPA is required to minimize the cytotoxicity.
Although the cooling mechanism is universal for all types of cells, cell type-dependent responses have long been observed due to variations in several attributes: (1) cell surface to volume ratio or cell size; (2) cell membrane permeability to water and CPA and Arrhenius activation energy that affects the temperature dependence of cell membrane permeability; (3) osmotic limit of cells.13 In general, a slower cooling rate is necessary as cell size increases to avoid intracellular freezing, because the fractional water loss during cooling is proportional to the cell surface to volume ratio. For ESCs (10–15 μm), the optimal cooling rate is usually less than 3°C/min. In addition, the osmotically inactive cell volume (Vb), which is occupied by inert materials with no osmotic pressure, such as fats and proteins, is cell type-dependent. ESCs have high nucleus-to-cytoplasm ratio and Vb was found to be 49.8% of isosmotic cell volume, compared to 32% for cord blood CD34+ cells and 20.5% for bone marrow HSC.17–19 The considerable variation in Vb is another factor that influences the cooling rate. The cell membrane permeability, including hydraulic conductivity and CPA permeability coefficient, is a function of temperature and can be used to predict the cell water change during cryopreservation in two-parameter mass transport model.20 However, the main limitation of this type of theoretical analysis is the significant variation in permeability and osmotic limit among different cell types, donors, and CPA formulations. For the emerging cell populations, such as hPSC, there is still lack of quantitative information and analysis for cryobiological parameters and the cryopreservation is mainly based on the empirical data and experimental optimization.
Clinical application of adult stem cells over the last decades has accumulated valuable knowledge for optimizing the cryopreservation process and provides valuable experience and prospects for future hPSC clinical application. In the following sections, the current status of adult stem cell cryopreservation will be briefly summarized followed by the discussion of the advancements and challenges in large-scale hPSC cryopreservation.
Cryopreservation of Adult Stem Cells
Cryopreservation has played an important role for HSC collection and clinical transplantation. For example, more than 250,000 human leukocyte antigen-typed cord blood units are stored in public banks worldwide.21 With the optimal cooling rate and cryopreservation formulation, cryopreservation of HSCs has become a routine practice in clinics.21 Recently, bone marrow-derived MSCs have emerged as an important cell source for cell therapy and tissue regeneration.22 The standard MSC cyopresevation solution is 10% DMSO plus 90% fetal bovine serum (FBS) with the recovery efficiency around 80–90% after cryopreservation.23 With good cell recovery, the focus of adult stem cell cryopreservation has been on clinical safety. The two major research areas are (1) elimination of animal serum in cell processing and storage; (2) reducing the toxic DMSO level or developing its nontoxic replacement. Serum residue after washing can trigger adverse reactions in patients and DMSO residue has side effects, including headache, nausea, and vomiting.9 To reduce animal serum, a defined serum-free freezing solution has been developed, where FBS was replaced by serum albumin.23 Recently, an animal product-free formulation CryoStor has been used for adult stem cell cryopreservation with good recovery.24,25 In an effort to reduce DMSO, high molecular weight polymers, such as polyvinylpyrrolidone (PVP) and hydroxyethyl starch (HES) have been included in CPAs.26,27 Recently, ectoine, a natural osmoprotectant from micro-organisms, has been tested as an alternative CPA to replace DMSO in a serum-free cryomedium.28 The clinical scale cryopreservation of adult stem cells usually requires large cryocontainers (i.e., scale up) rather than hundreds of cryo units (i.e., scale out).29 The cryo unit for cord blood was around 15–30 mL and 150 mL for bone marrow, so cryobags in the range of 50–250 mL are sufficient for individual patients and typically used in the banks.10,30,31 Compared to unassociated adult stem cells, a unique aspect of hPSC is the intimate cell–cell and cell–matrix interactions, which possess new challenges in preserving hPSC viability and therapeutic potency post-cryopreservation.
Cryopreservation of hPSCs
hPSCs usually grow as colonies of highly associated adherent cells, which require tight gap junctions and adhesion to extracellular matrix (ECM)-coated surface. hPSCs are sensitive to traditional cryopreservation and thaw method in large part due to the disruption of this cellular organization. In addition to necrosis caused by ice crystallization or osmotic shock, several other cryopreservation-induced events that affect the hPSC survival include activated apoptosis, disruption of cell–cell and cell–matrix adhesions, and the elevated ROS production.
Induction of apoptosis and anoikis
Apoptosis was found to be the major cause of cell loss after hESC cryopreservation rather than necrosis.32 Apoptosis may not happen immediately post-thaw, but 12–24 h later. The main inducers of apoptosis during hESC cryopreservation include caspase activation and cytokine interaction. Analysis revealed that progression of apoptosis coincides with upregulation of caspase-8 and -10 genes, which activate caspase-3 and lead to apoptosis.33 Caspase-8 and -10, components of mitogen-activated protein kinase family, are activated through the transforming growth factor (TGF)-β and interleukin (IL)-1β pathways, in which TGF-β and its receptor ACVR1C along with IL-1β and its receptor IL-1R are upregulated following cryopreservation.33 Because of the roles of TGF-β and IL-1β in initiating the apoptotic events,34,35 Rho-associated kinase (ROCK) inhibitor Y-27632, which suppresses the caspase activation through IL-1β/IL-1R and TGF-β/ACVR1C interactions, has been used to reduce the apoptosis.33,36 Other apoptosis inhibitors, including caspase inhibitor Z-VAD-VMK and p53 inhibitor pifithrin-μ have also been shown to improve hPSC cryopreservation.37,38
Cell detachment and dissociation during hPSC cryopreservation also contribute to cell death through a process known as “anoikis,” meaning “homelessness,” which is a subtype of apoptosis induced by the loss of cell adhesion or inappropriate cell adhesion.39 In the cascade of cellular events leading to anoikis, Bcl-2 family, caspase-3, and Fas signaling play the key roles.40 For example, loss of anchorage to ECM leads to an increase in Fas expression, which then activates caspase activity.40 While, in general, the adherence on ECM proteins prevents hPSC to enter anoikis, addition of a growth factor, such as the basic fibroblast growth factor (bFGF), has been found to prevent anoikis by activation of extracellular signal-regulated kinase and inhibition of Bcl-2-interacting mediator of cell death and the caspase-ROCK1-myosin signaling.41 Anoikis can be avoided by preserving ECM attachment and intact cell–cell interactions during cryopreservation. Anoikis induced by detachment and dissociation of hPSC can also be protected by ROCK inhibitor, which disrupts extracellular cues that would normally induce apoptosis and increases the cell–cell interactions through modulating cadherins and gap junctions to enable the cell reaggregation.42
Disruption of cell–cell adhesion and cell–matrix adhesion
Recent investigations indicate that disruptions of E-cadherin and F-actin through the G13 signaling pathway and functional cell-to-cell gap junctions, such as connexin 43 and connexin 45 caused hESC-death after freeze–thawing.43–45 E-cadherin was found to be essential for hESC survival because E-cadherin-mediated cell–cell adhesion regulates Rho activity and enhances cell attachment.46 As such, small molecules that target Rho-ROCK signaling and stabilize E-cadherin, such as Thiazovivin and Tyrintegin have been used to promote cell survival by enhancing cell–ECM adhesion, activating integrin signaling, and synergizing with growth factors, such as bFGF and insulin-like growth factor.46 hPSCs treated by ROCK inhibitor Y27632 clustered rapidly and maintained E-cadherin and F-actin distribution, improving the recovery.45 Similarly, preserving endogenous ECM during cryopreservation has also been shown to improve cell survival by maintaining cell–ECM and cell–cell adhesion, supporting the strategy of in situ hPSC cryopreservation.47
Production of ROS
ROS is typically resulted from the transfer of one, two, or three electrons to O2 to form, respectively, a superoxide radical (O2−), hydrogen peroxide (H2O2), or a hydroxyl radical (HO−) in mitochondria.48 Cell cryopreservation is associated with elevated intracellular ROS as a result of mitochondrial leakage and damage.49 The high levels of O2− and H2O2 after cryopreservation lead to the release of cytochrome C from mitochondria to cytosol that activates caspase activity. Production of HO− can also be induced via Fenton reaction (Fe/H2O2) as a consequence of elevated iron release from ferritin by NADPH or from iron–sulfur clusters by O2− under cold temperature.50,51 Since the scavengers for the free radicals and oxidative stress became less active at low temperature, the elevated ROS is more potent for inducing apoptosis. The approach to reduce the oxidative stress and prevent ROS production is to add antioxidants and radical scavengers in the CPAs, such as glutathione, mannitol, vitamin E, or its analog Trolox, and polyethylene glycol (PEG).38,52,53 The intracellular preservation solutions that contain these compounds have been shown to prevent injury from free radicals and improve the storage of islet cells, MDCK cells, and hepatocytes.53–55 Adding glutathione to the CPA and the post-thaw solution also improved the survival of mouse ESC.56
Unknown events that impact the lineage-specific differentiation
To date, the main efforts in hPSC cryopreservation have focused on improving hPSC survival and the maintenance of pluripotency markers, but the impact of different freeze–thawing methods on hPSC lineage-specific differentiation has been inadequately investigated.57 In the development of hESC-derived OPC and cardiomyocyte production process at Geron, the author (Y.L.) has observed a large variation of differentiation potential from different hESC banks, although they all demonstrated comparable survival and pluripotency (unpublished results). One hypothesis is that cryopreservation and thaw generate selective pressure that influences the lineage-specific propensity during hPSC differentiation. Although the prediction and detection of cryopreservation-induced differentiation preference have been challenging, elucidating the mechanism involved in the lineage-specific differentiation post-cryopreservation beyond the cell survival is crucial in refining hPSC cryopreservation methods.
Methods of hPSC Cryopreservation
With the goal of preserving cell viability and multilineage potential, strategies that reduce ROS production and preserve cell–cell and cell–matrix contacts during cryopreservation have been actively pursued together with the traditional cryobiological parameters. In general, there are two types of cryopreservation method: vitrification and slow cooling.58
Vitrification
Vitrification has been extensively applied in the cryopreservation of embryos for animal reproduction, including sheep, mouse, and bovine.59–61 Some early studies have suggested that vitrification could also be beneficial for the cryopreservation of human blastocysts,62 the stage at which hESCs were derived. In the subsequent studies, vitrification has been shown to be effective for hESC cryopreservation with significantly improved recovery compared to the slow cooling method (100% vs. 16% colony recovery).63,64 The initial vitrification method was performed in open pulled straw, which drew small volume of cell clusters resuspended in viscous CPA (20% DMSO, 20% EG, and sucrose). The straw was then quickly immersed into the liquid nitrogen (N2) to achieve the vitrification and avoid intracellular ice formation. The thawing of vitrified hESCs was also very rapid to avoid ice recrystallization. Table 1 summarizes the modifications that have been made for ease of operation, fast processing, and safe clinical applications.
Table 1.
Summary of Different Vitrification Methods for Human Pluripotent Stem Cell Cryopreservation
| Method description | Cryoprotectant | hESC/hiPSC | Colony recovery | References |
|---|---|---|---|---|
| Open pulled straw vitrification | 20% DMSO, 20% EG, sucrose, SR medium | HES-1, HES-2 | All colonies recovered | Reubinoff et al.;63 |
| H1 | Li et al.64 | |||
| Closed sealed straw, xeno-free vitrification | 20% DMSO, 20% EG, sucrose, HSA | HES-2, HES-3, HES-4 | 75–88% | Richards et al.65 |
| Bulk vitrification | 20% DMSO, 20% EG, sucrose, SR medium | α-ES-C | 95–99% of frozen clumps | Li et al.67 |
| Modified bulk vitrification | 20% DMSO, 20% EG, sucrose, SR medium | β-HES-2, α-ES-C | 95–99% of frozen clumps | Li et al.68 |
| DMSO-free, xeno-free vitrification | 40% EG, 10% PEG, Euro-Collins solution | hiPSC 253G4 | 30% | Nishigaki et al.66 |
| Surface vitrification | 20% DMSO, 20% EG, sucrose, SR medium | H1 | 184% vital residual area at 24 h | Beier et al.;70 Heng et al.69 |
hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell; DMSO, dimethyl sulfoxide; EG, ethylene glycol; SR, serum replacement; HES, hydroxyethyl starch; HSA, human serum albumin; PEG, polyethylene glycol.
Improvements to avoid contamination
Closed-straw vitrification, where the straw was closed by heat-sealing after the cells were taken, has been developed to avoid direct contact with liquid N2 as occurred in the open pull straw vitrification.65 In addition, human serum albumin has been used to replace FBS to achieve serum-free cryopreservation. Toward xeno-free formulation, a DMSO-free vitrification solution using 40% EG, 10% PEG, and Eurocollins solution (dextrose and salts) has been tested for hPSC cryopreservation.66 This solution improved the recovery rate to about 30% compared to 18–19% with the DMSO and serum replacement medium. Although the cooling rate was about −150°C/min in this study, it was sufficient to achieve vitrification.
Improvement of process efficiency
A bulk vitrification method has been developed using a cell strainer to hold hESC clusters instead of straw.67 In this method, each cell strainer was able to hold about 100–150 clumps compared to 5–10 clumps in one open pulled straw. Later, this bulk vitrification method was modified using the custom-made vitrification cryovials.68 The stainless-steel mesh with 70-μm mesh size was assembled to the upper half of the cryovial. hESC clumps were transferred to the modified cryovial with the vitrification solution and immersed into liquid N2.
Adherent cell vitrification
Vitrification of adherent cells was proposed to achieve rapid cooling and long-term storage in liquid N2 by using the cell culture plates with detachable culture wells.69 Recently, a surface vitrification method has been developed to vitrify the adherent cell colonies.70 The cells were immobilized on modified Thermanox® coverslips with feeder cells before the vitrification, and the vitrified colonies showed better vital residual area compared with slow frozen colonies (89% vs. 51%).
Low scalability
The inherent problems of vitrification methods include the difficulty to scale up and the possibility of contamination in liquid nitrogen. Vitrification is a tedious and operator-dependent process, and requires strict timing with a very low capacity. The bulk vitrification method has increased the processing capacity with about 100–150 cell clumps loaded in each cryocarrier. However, the cryocarriers need to be individually handled, which is a significant limit in large-scale cell banking. Additionally, direct exposure to the liquid phase of liquid nitrogen for rapid cooling and long-term storage raises the concern of pathogen transmission. Closed-straw vitrification has been developed to address this issue, but this method is operator-dependent and the heat-sealing process is not scalable.
Slow cooling
Despite the poor initial performance, advances in slow cooling of hPSC have improved its scalability. Cryobiological parameters and CPAs have been extensively studied for freezing hPSC clusters and the new strategies to freeze hPSC as either adherent or single cells have also improved the recovery and efficiency. Currently hPSCs can be frozen in three different organizations: cell clusters, adherent cells, and single cells (Table 2).
Table 2.
Summary of Different Slow Cooling Methods for Human Pluripotent Stem Cell Cryopreservation
| Method description | Cryoprotectant | hESC/hiPSC | Recovery | References |
|---|---|---|---|---|
| Frozen as cell clumps | ||||
| Cooling program and seeding | 10% DMSO, 25% FBS in DMEM | H1 | 79%, 55% | Ware et al.;71 Yang et al.72 |
| Synthetic serum and step-wise equilibrium plus seeding | 10% DMSO, 90% SR, 2 M DMSO, SR medium | H9, CHA-hES3, VAL-3, VAL-5, H9 | 51% relative survival; 41–68% | Lee et al.;73 Valbuena et al.74 |
| Adding EG as cryoprotectant | 5% DMSO, 10% EG, 50% FBS | SNUhES-3 | 30% | Ha et al.75 |
| Xeno-free formulation with polymer | DMSO, dextrose, polymer (not disclosed) | HS293, HS306 | About 90%a | Holm et al.78 |
| Formulation with polymer | 5% DMSO, 5% HES | VUB01, H1, H9, 181, UGent2 | 45.5–168.2% recovery ratio | T'joen et al.77 |
| Frozen as adherent cells | ||||
| hESCs grown on matrigel-coated well plate | 10% DMSO, FBS, conditioned medium | H1 and H9 | About 80% recovered colony | Ji et al.47 |
| hESCs grown on clinical cell culture cassette | 10% DMSO, 90% FBS | Shef 4, Shef 5, Shef 6, Shef 7 | Proliferation ratio 8–195 | Amps et al.79 |
| hESCs grown on matrigel-coated microcarriers | 10% DMSO, 30% FBS, 60% conditioned medium | H1 and H9 | 1.5–1.9-fold compared to free colonies | Nie et al.80 |
| Alginate-encapsulated hESC immobilized on microcarriers | 10% DMSO, 90% SR, 5 μM ROCKi | SCED™ 461 | 71% at day 1 | Serra et al.82 |
| Frozen as dissociated single cells | ||||
| Dissociated hESCs on feeders or feeder-free | 10% DMSO, 90% SR | CA1, CA2, H1, H9 | About 50%b | Li et al.83,87 |
| Dissociated hESCs on feeders | 10% DMSO, 90% SR medium, 10 μM ROCKi | HS207, HS401 | About 50%a | Martin-Ibanez et al.85 |
| Dissociated hESCs/hiPSCs in feeder-free culture | 10% DMSO, 90% FBS, 10 μM ROCKi; 20% DMSO, 80% SR medium, 10 μM ROCKi | Royan H5, H6, hiPSC1, hiPSC4; H9, BG01V, hiPSC | About 85%a; Use number of colonies | Mollamohammadi et al.;88 Claassen et al.89 |
| Dissociated hESCs, reduced DMSO | 7.5% DMSO, 2.5% PEG, 90% SR medium | HUES2 | About 80%a | Xu et al.38 |
| Dissociated hiPSCs, DMSO-free | 10% EG, 90% SR medium, 10 μM ROCKi | hiPSC derived from hESC-fibroblasts | 63%c | Katkov et al.91 |
| Dissociated hESCs, alternative to ROCKi | 10% DMSO, 90% FBS, 100 μM Pinacidil | Shef 4, Shef 5, Shef 7, H7 | About 85%a | Barbaric et al.90 |
Viability determined by Trypan blue immediately post-thaw.
Viability determined by PI/Annexin staining.
Viability determined by 7AAD staining.
The methods using PI/Annexin or 7AAD staining are more sensitive than Trypan blue method.
FBS, fetal bovine serum; DMEM, Dulbecco's modified Eagle's medium; PI, propidium iodide.
Cryopreservation of hPSCs as cell clusters
In general, hPSCs are not amenable to be passaged and cryopreserved as a single-cell suspension because failure to maintain cell–cell contact usually leads to significant cell death and spontaneous differentiation.12 The cells are usually harvested by mechanical scraping and cryopreserved as cell clusters in the slow cooling process. Since the initial attempt to freeze hESC clusters by the slow cooling method, various improvements have been made with emphasis on two aspects: (1) cryobiological variables, including the seeding process, the cooling rate, and step-wise loading and unloading CPA; (2) CPA formulations.
Seeding, a process to introduce ice crystals in a super cool solution, causes quick formation of extracellular ice that minimizes the detrimental effect of phase change from liquid to ice in the course of cooling and preserves adhesive ECM. Seeding should be performed at the temperature above spontaneous intracellular ice formation, which is in the range between −7°C and −12°C. Introducing seeding during hESC cryopreservation has achieved about 80% survival rate.71 A similar study has been performed to optimize the cooling rate with seeding at −10°C and the optimal cooling rate at 0.5°C/min.72 As another cryobiological variable, step-wise CPA addition can minimize the mechanical damage due to osmotic shock.73 More than 50% hESC recovery has been observed with a two-step equilibration compared to 20% using one-step equilibration. A four-step CPA addition has also been tested with the improved post-thaw survival comparable to the vitrification method.74
CPA formulation has been extensively studied in hPSC cryopreservation during the past 10 years. Similar to adult stem cell cryopreservation, eliminating DMSO and animal serum without compromising cell recovery become two major tasks. To reduce DMSO level, EG was tested and the most favorable combination was found to be 5% DMSO, 5% EG, and 50% FBS.75 Disaccharide trehalose has also been added into the formulation and showed beneficial effect in the elution buffer and freezing medium.76 However, the beneficial effect of trehalose was small in a separate study and only observed when serum concentration exceeded 50%.47 Adding nonpenetrating polymers accelerates the vitrification of extracellular solution by reducing the water diffusion out of cells and preventing extracellular ice propagation.16 High molecular weight HES has recently been tested in hESC cryopreservation and a combination of 5% DMSO and 5% HES was found to be optimal.77 To eliminate animal serum, a commercially available xeno-free cryopreservation solution STEM-CELLBANKER, containing a high molecular weight polymer (not disclosed), was used for hPSC cryopreservation.78 More than 90% cells remained alive after thawing compared with 50% for control condition.
Cryopreservation of hPSCs as adherent cells
Because anoikis can be minimized when cell–cell gap junctions and cell–ECM interactions are preserved, cryopresevation of adherent hESC colonies has been investigated.47 The hESCs were cryopreserved on a tissue culture plate directly with a thin layer of CPA covering the cell layer. The post-thaw survival was significantly improved compared to the cell clusters frozen in a suspension (80% vs. 2% colony recovery). Another in situ cryopreservation of hESCs has been performed in a gas permeable culture cassette.79 A range of 8–115-fold higher post-thaw proliferation ratio was achieved compared to a tissue culture flask. However, freezing hESCs in the tissue culture plate or cassette is not feasible for future scale up. To address this issue, the method of freezing adherent hPSC has been adapted on microcarriers to increase the scalability.80 The recovery of the microcarriers with cells, however, was still performed on the tissue culture surface with mouse embryonic fibroblasts. The future development of this method should allow the direct recovery of hESC-microcarrier constructs in a suspension, which has been demonstrated for bone marrow MSC.81 Cells grown on microcarriers have also been encapsulated by alginate and cryopreserved in comparison with encapsulated cell suspension.82 Similarly, hESCs immobilized on microcarriers had high viability and cell recovery compared to the suspended cells.
Cryopreservation of hPSCs as single cells
hPSC cryopreservation as single cells has become possible in the presence of ROCK inhibitor. Addition of ROCK inhibitor Y27632 in the post-thaw medium has been shown to increase hESC survival from 5% to 53%, because Y27632 enhances cell–cell adhesion and cell aggregation by modulating gap junctions, thereby blocking the pathway to apoptosis.83,84 The mechanism that ROCK inhibitor improved cell survival may not only be due to blocking the apoptotic pathway, but also the ability to avoid anoikis.42 Cryopreservation of single cells has been further optimized by adding Y27632 in the preservation solution or Matrigel™ in addition to the post-thaw medium.85,86 The effect of Y27632 on feeder-free culture is similar to feeder culture both for hESCs and hiPSCs.87–89 However, adding Y27632 during cryopreservation did not change the survival of CHiPS-A cells, which may be due to the effect of the solution STEM-CELLBANKER or a cell line-dependent response.78 An alternative chemical Pinacidil has recently been found to have similar effect compared to Y-27632.90 Since Y-27632 has a higher cost and is associated with patent issues, Pinacidil has been suggested to be more suitable for commercial applications.
The CPA of single hPSC has been optimized at reduced DMSO concentration. A combination of 7.5% DMSO and 2.5% PEG has improved hESC recovery by 30% compared to 10% DMSO alone.38 Introducing PEG in the preservation solution could inhibit ROS production and reduce apoptosis. The presence of Y27632 and p53 inhibitor in the subsequent culture medium further improved cell recovery. Another study completely replaced DMSO by EG during hiPSC cryopreservation because DMSO was found substantially more toxic to hPSC than EG.91 In this study, similar recovery of freezing dissociated single cells and adherent cells with EG has been reported.
Large Scale hPSC Banking Under cGMP
For therapeutic cell production, large-scale hPSC banks provide the starting materials for a new bank (in the case of MCB) or the differentiated therapeutic cell products (in the case of WCB). A general MCB or WCB should contain at least 300–400 vials with each vial containing 5–10×106 cells. In practice, the banking process requires the capacity to handle up to 4×109 cells in a single day, in which the prolonged exposure to CPA must be minimized. This scale out approach requires efficient cell processing, where the vitrification methods appear unfeasible. The alternative approach of hESC lyophilization for long-term storage has been proposed, but still requires proof-of-concept data.10 To date, the slow cooling method for dissociated single cells appears to be the best choice for making MCB and/or WCB. With the development of bioreactor systems and the integration of cryopreservation and hPSC expansion, freezing adherent cells on microcarriers or as aggregates is another important development that has the potential to address the limitations for large-scale cryopreservation.92,93 The further development of microcarrier or aggregate-based cell preservation depends on understanding the role of cell–cell and cell–matrix interactions and the impact of spatial temperature and cryogen gradients on cell properties.
Different from laboratory protocols, a production process should be a well-defined procedure with controlled inputs, outputs, and operational parameters that ensure reproducibility and regulatory compliance.94 For large cGMP banking, the process should be simple, robust, and easily performed by different operators. However, many current cryopreservation methods have been developed using a variety of hPSC lines with large variations in procedure, making it difficult to evaluate their feasibility for MCB/WCB production. In future development, both process efficiency and regulatory compliance should be considered and implemented in the early stage of development. At a minimum, a desired process for cGMP hPSC banking must meet the following criteria: (1) Cell recovery and expansion post-thaw is fast enough to achieve ∼4×109 cells within five passages; (2) Xeno-free formulation is preferred with a simple procedure capable of processing a large number of cells in one operational day; (3) The thawed cells maintain pluripotency; (4) The thawed cells maintain the ability to produce high purity of the targeted cell product; (5) vial-to-vial variability is minimized for process consistency.
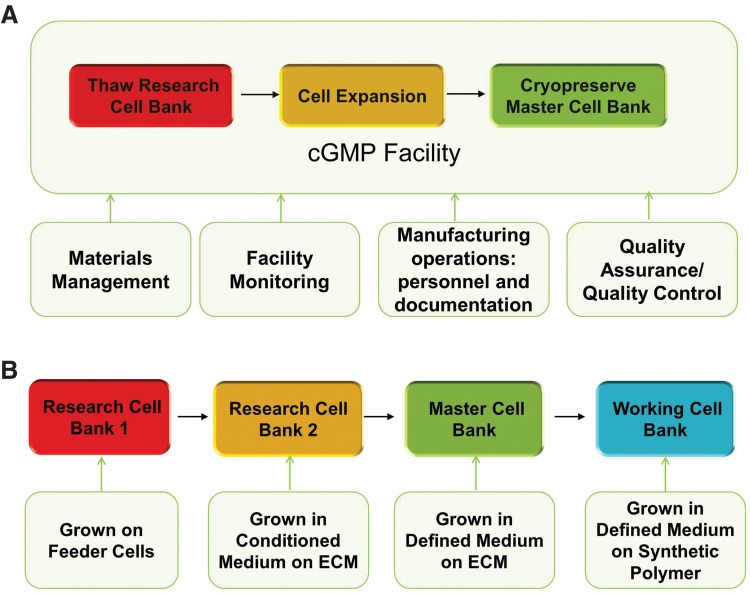
Successful production of MCB/WCB under cGMP requires the integration of expansion and cryopreservation process. On the cryopreservation day, all the expanded cells need be harvested, formulated, and distributed into hundreds of freezing containers, such as cryovials, which are frozen in the controlled-rate freezer with a traceable record. To make this operation successful, thawing, expansion, and final cryopreservation process need to be streamlined and validated for cGMP production. Well-trained personnel, the detailed documentation, such as batch production record, the qualified raw materials, and the well-monitored cGMP facility are required to ensure the successful MCB/WCB production.95 Quality assurance and quality control also need to be implemented to meet the regulatory requirement for cell bank release. Figure 1A illustrates the overall process flow for cell banking, demonstrating the need for process integration by various functional groups. Integrated cell expansion and cryopreservation process would improve efficiency and facilitate automation.82
FIG. 1.
(A) Illustration of bioprocess for hPSC MCB manufacturing. The cells are thawed from the research cell bank, expanded, and cryopreserved as MCB in current good manufacturing practices facility. This operation requires the cooperation of materials management, facility monitoring, personnel training, and documentation, quality control, and quality assurance. (B) Example of different hPSC banks expanded in different growth conditions, including feeder-culture and feeder-free cultures in a conditioned medium on ECM, a defined medium on ECM, and a defined medium on a synthetic polymer. The cells are thawed from the research cell bank to manufacture MCB and the cells are thawed from MCB to make WCB. Thus, the cells in WCB may have been histologically cultured in different growth conditions. hPSC, human pluripotent stem cell; MCB, master cell bank; WCB, working cell bank; ECM, extracellular matrix.
Due to the rapid progress in hPSC expansion, MCB/WCB are expected to be thawed and expanded in different culture conditions, which could potentially impact cell properties post-cryopreservation (Fig. 1B). From the feeder cell culture to feeder-free culture, from the conditioned medium to the defined medium with known essential components, such as the E8 medium,96–98 from the undefined ECM to the synthetic polymerase substrate,99 the research cell bank made years back may need to be thawed into a completely different culture system to make cGMP banks.100 Recent advances of hPSC expansion on microcarriers and as aggregates also require the compatible cryopreservation process with the culture process.82,92,101 During the culture process transition from bank to bank, care needs to be taken for validating the banking process and maintaining the bank history. To address these issues, a bridging or compatibility study is required to use different MCB/WCB for therapeutic cell production. In particular, the impact of expansion and cryopreservation process on lineage-specific differentiation is critical and needs to be addressed before clinical applications.
To date, the primary focus of hPSC characterization postcryopreservation remains on the pluripotency rather than the lineage-specific differentiation. Cryopreservation and thaw procedure may lead to a selection process and affect the differentiation capacity to the desired lineage even when they maintain the pluripotent markers. Thus, the capacity for lineage-specific differentiation is another set of criteria for choosing the cell banking process in clinical application. While studies have led to the development of ROCK inhibitor with significantly improved cell survival and maintenance of pluripotency, mechanistic insight is required to develop scalable and GMP-compatible strategy that better preserves hPSC's capacity for lineage-specific differentiation. In addition to optimizing traditional cryobiological and process parameters, identifying the regulatory pathways associated with cryopreservation will play a critical role to improve process efficiency. Although the focus of this article is the cryopreservation of undifferentiated hPSC banks, cryopreservation of hPSC-derived cells for final product storage is another important task in hPSC therapy.102
Conclusions
Significant progress has been made in hPSC cryopreservation during recent years. Strategies to reduce ROS production and apoptosis and to preserve cell–cell and cell–matrix adhesions have played important roles to improve the large-scale hPSC banking process. Experience in hPSC cryopreservation as cell clusters, adherent cells, and single cells using the slow cooling method suggests its suitability for large-scale bioprocessing. To translate the protocols to industrial bioprocess, detailed characterization of cryopreservation and thaw process using lineage-specific differentiation as output criteria will be a necessary next step. Identification of novel markers and assays to predict the ability of MCB/WCB to produce high purity of therapeutic cell products will also play an important role in improving process efficiency and ensuring quality.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thomson JA. Itskovitz-Eldor J. Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Yu J. Vodyanik MA. Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K. Tanabe K. Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Bretzner F. Gilbert F. Baylis F, et al. Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell. 2011;8:468–475. doi: 10.1016/j.stem.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Wirth E., 3rd Lebkowski JS. Lebacqz K. Response to Frederic Bretzner et al. “Target populations for first-in-human embryonic stem cell research in spinal cord injury”. Cell Stem Cell. 2011;8:476–478. doi: 10.1016/j.stem.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz SD. Hubschman JP. Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 7.Serra M. Brito C. Correia C, et al. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012;30:350–359. doi: 10.1016/j.tibtech.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Berz D. McCormack EM. Winer ES, et al. Cryopreservation of hematopoietic stem cells. Am J Hematol. 2007;82:463–472. doi: 10.1002/ajh.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thirumala S. Goebel WS. Woods EJ. Clinical grade adult stem cell banking. Organogenesis. 2009;5:143–154. doi: 10.4161/org.5.3.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coopman K. Large-scale compatible methods for the preservation of human embryonic stem cells: current perspectives. Biotechnol Prog. 2011;27:1511–1521. doi: 10.1002/btpr.680. [DOI] [PubMed] [Google Scholar]
- 11.Katkov , II Kim MS. Bajpai R, et al. Cryopreservation by slow cooling with DMSO diminished production of Oct-4 pluripotency marker in human embryonic stem cells. Cryobiology. 2006;53:194–205. doi: 10.1016/j.cryobiol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Hunt CJ. Cryopreservation of human stem cells for clinical application: a review. Transfus Med Hemother. 2011;38:107–123. doi: 10.1159/000326623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 14.Mazur P. Stopping biological time. The freezing of living cells. Ann NY Acad Sci. 1988;541:514–531. doi: 10.1111/j.1749-6632.1988.tb22288.x. [DOI] [PubMed] [Google Scholar]
- 15.Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Lett. 2004;25:375–388. [PubMed] [Google Scholar]
- 16.Meryman HT. Cryopreservation of living cells: principles and practice. Transfusion. 2007;47:935–945. doi: 10.1111/j.1537-2995.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 17.Kashuba Benson CM. Benson JD. Critser JK. An improved cryopreservation method for a mouse embryonic stem cell line. Cryobiology. 2008;56:120–130. doi: 10.1016/j.cryobiol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt CJ. Armitage SE. Pegg DE. Cryopreservation of umbilical cord blood: 1. Osmotically inactive volume, hydraulic conductivity and permeability of CD34(+) cells to dimethyl sulphoxide. Cryobiology. 2003;46:61–75. doi: 10.1016/s0011-2240(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 19.Gao DY. Chang Q. Liu C, et al. Fundamental cryobiology of human hematopoietic progenitor cells. I: osmotic characteristics and volume distribution. Cryobiology. 1998;36:40–48. doi: 10.1006/cryo.1997.2060. [DOI] [PubMed] [Google Scholar]
- 20.Katkov , II A two-parameter model of cell membrane permeability for multisolute systems. Cryobiology. 2000;40:64–83. doi: 10.1006/cryo.1999.2226. [DOI] [PubMed] [Google Scholar]
- 21.Solves P. Mirabet V. Perales A, et al. Banking strategies for improving the hematopoietic stem cell content of umbilical cord blood units for transplantation. Curr Stem Cell Res Ther. 2008;3:79–84. doi: 10.2174/157488808784223096. [DOI] [PubMed] [Google Scholar]
- 22.Salem HK. Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y. Xu X. Ma X, et al. Cryopreservation of human bone marrow-derived mesenchymal stem cells with reduced dimethylsulfoxide and well-defined freezing solutions. Biotechnol Prog. 2010;26:1635–1643. doi: 10.1002/btpr.464. [DOI] [PubMed] [Google Scholar]
- 24.Ginis I. Grinblat B. Shirvan MH. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods. 2012;18:453–463. doi: 10.1089/ten.TEC.2011.0395. [DOI] [PubMed] [Google Scholar]
- 25.Nicoud IB. Clarke DM. Taber G, et al. Cryopreservation of umbilical cord blood with a novel freezing solution that mimics intracellular ionic composition. Transfusion. 2012 doi: 10.1111/j.1537-2995.2011.03547.x. [DOI] [PubMed] [Google Scholar]
- 26.Luo K. Wu G. Wang Q, et al. Effect of dimethylsulfoxide and hydroxyethyl starch in the preservation of fractionated human marrow cells. Cryobiology. 1994;31:349–354. doi: 10.1006/cryo.1994.1042. [DOI] [PubMed] [Google Scholar]
- 27.Thirumala S. Wu X. Gimble JM, et al. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng Part C Methods. 2010;16:783–792. doi: 10.1089/ten.TEC.2009.0552. [DOI] [PubMed] [Google Scholar]
- 28.Grein TA. Freimark D. Weber C, et al. Alternatives to dimethylsulfoxide for serum-free cryopreservation of human mesenchymal stem cells. Int J Artif Organs. 2010;33:370–380. [PubMed] [Google Scholar]
- 29.Kirouac DC. Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369–381. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Heidemann R. Lunse S. Tran D, et al. Characterization of cell-banking parameters for the cryopreservation of mammalian cell lines in 100-mL cryobags. Biotechnol Prog. 2010;26:1154–1163. doi: 10.1002/btpr.427. [DOI] [PubMed] [Google Scholar]
- 31.Fleming KK. Hubel A. Cryopreservation of hematopoietic and non-hematopoietic stem cells. Transfus Apher Sci. 2006;34:309–315. doi: 10.1016/j.transci.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Heng BC. Ye CP. Liu H, et al. Loss of viability during freeze-thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. J Biomed Sci. 2006;13:433–445. doi: 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- 33.Ichikawa H. Nakata N. Abo Y, et al. Gene pathway analysis of the mechanism by which the Rho-associated kinase inhibitor Y-27632 inhibits apoptosis in isolated thawed human embryonic stem cells. Cryobiology. 2012;64:12–22. doi: 10.1016/j.cryobiol.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Fortunato SJ. Menon R. IL-1 beta is a better inducer of apoptosis in human fetal membranes than IL-6. Placenta. 2003;24:922–928. doi: 10.1016/s0143-4004(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim SG. Jong HS. Kim TY, et al. Transforming growth factor-beta 1 induces apoptosis through Fas ligand-independent activation of the Fas death pathway in human gastric SNU-620 carcinoma cells. Mol Biol Cell. 2004;15:420–434. doi: 10.1091/mbc.E03-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SY. Kim EY. Cui XS, et al. Increase in DNA fragmentation and apoptosis-related gene expression in frozen-thawed bovine blastocysts. Zygote. 2006;14:125–131. doi: 10.1017/S0967199406003649. [DOI] [PubMed] [Google Scholar]
- 37.Heng BC. Clement MV. Cao T. Caspase inhibitor Z-VAD-FMK enhances the freeze-thaw survival rate of human embryonic stem cells. Biosci Rep. 2007;27:257–264. doi: 10.1007/s10540-007-9051-2. [DOI] [PubMed] [Google Scholar]
- 38.Xu X. Cowley S. Flaim CJ, et al. Enhancement of cell recovery for dissociated human embryonic stem cells after cryopreservation. Biotechnol Prog. 2010;26:781–788. doi: 10.1002/btpr.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taddei ML. Giannoni E. Fiaschi T, et al. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380–393. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 40.Grossmann J. Molecular mechanisms of “detachment-induced apoptosis–anoikis”. Apoptosis. 2002;7:247–260. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- 41.Wang X. Lin G. Martins-Taylor K, et al. Inhibition of caspase-mediated anoikis is critical for basic fibroblast growth factor-sustained culture of human pluripotent stem cells. J Biol Chem. 2009;284:34054–34064. doi: 10.1074/jbc.M109.052290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krawetz RJ. Li X. Rancourt DE. Human embryonic stem cells: caught between a ROCK inhibitor and a hard place. Bioessays. 2009;31:336–343. doi: 10.1002/bies.200800157. [DOI] [PubMed] [Google Scholar]
- 43.Wong RC. Dottori M. Koh KL, et al. Gap junctions modulate apoptosis and colony growth of human embryonic stem cells maintained in a serum-free system. Biochem Biophys Res Commun. 2006;344:181–188. doi: 10.1016/j.bbrc.2006.03.127. [DOI] [PubMed] [Google Scholar]
- 44.Wong RC. Pebay A. Nguyen LT, et al. Presence of functional gap junctions in human embryonic stem cells. Stem Cells. 2004;22:883–889. doi: 10.1634/stemcells.22-6-883. [DOI] [PubMed] [Google Scholar]
- 45.Ichikawa H. Yoshie S. Shirasawa S, et al. Freeze-thawing single human embryonic stem cells induce e-cadherin and actin filament network disruption via g13 signaling. Cryo Lett. 2011;32:516–524. [PubMed] [Google Scholar]
- 46.Xu Y. Zhu X. Hahm HS, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci USA. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji L. de Pablo JJ. Palecek SP. Cryopreservation of adherent human embryonic stem cells. Biotechnol Bioeng. 2004;88:299–312. doi: 10.1002/bit.20243. [DOI] [PubMed] [Google Scholar]
- 48.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 49.Xu X. Cowley S. Flaim CJ, et al. The roles of apoptotic pathways in the low recovery rate after cryopreservation of dissociated human embryonic stem cells. Biotechnol Prog. 2010;26:827–837. doi: 10.1002/btpr.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rauen U. de Groot H. Cold-induced release of reactive oxygen species as a decisive mediator of hypothermia injury to cultured liver cells. Free Radic Biol Med. 1998;24:1316–1323. doi: 10.1016/s0891-5849(97)00456-5. [DOI] [PubMed] [Google Scholar]
- 51.Rauen U. de Groot H. Mammalian cell injury induced by hypothermia—the emerging role for reactive oxygen species. Biol Chem. 2002;383:477–488. doi: 10.1515/BC.2002.050. [DOI] [PubMed] [Google Scholar]
- 52.Mathew AJ. Hollister WR. Addona T, et al. Vitamin E and EDTA improve the efficacy of hypothermosol-implication of apoptosis. In Vitro Mol Toxicol. 1999;12:163–172. [PubMed] [Google Scholar]
- 53.Ostrowska A. Gu K. Bode DC, et al. Hypothermic storage of isolated human hepatocytes: a comparison between University of Wisconsin solution and a hypothermosol platform. Arch Toxicol. 2009;83:493–502. doi: 10.1007/s00204-009-0419-x. [DOI] [PubMed] [Google Scholar]
- 54.Lakey JR. Rajotte RV. Fedorow CA, et al. Islet cryopreservation using intracellular preservation solutions. Cell Transplant. 2001;10:583–589. [PubMed] [Google Scholar]
- 55.Baust JM. Vogel MJ. Van Buskirk R, et al. A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transplant. 2001;10:561–571. [PubMed] [Google Scholar]
- 56.Kim GA. Lee ST. Ahn JY, et al. Improved viability of freeze-thawed embryonic stem cells after exposure to glutathione. Fertil Steril. 2010;94:2409–2412. doi: 10.1016/j.fertnstert.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter MK. Frey-Vasconcells J. Rao MS. Developing safe therapies from human pluripotent stem cells. Nat Biotechnol. 2009;27:606–613. doi: 10.1038/nbt0709-606. [DOI] [PubMed] [Google Scholar]
- 58.Heng BC. Kuleshova LL. Bested SM, et al. The cryopreservation of human embryonic stem cells. Biotechnol Appl Biochem. 2005;41:97–104. doi: 10.1042/BA20040161. [DOI] [PubMed] [Google Scholar]
- 59.Ali J. Shelton JN. Successful vitrification of day-6 sheep embryos. J Reprod Fertil. 1993;99:65–70. doi: 10.1530/jrf.0.0990065. [DOI] [PubMed] [Google Scholar]
- 60.Ali J. Shelton JN. Vitrification of preimplantation stages of mouse embryos. J Reprod Fertil. 1993;98:459–465. doi: 10.1530/jrf.0.0980459. [DOI] [PubMed] [Google Scholar]
- 61.Vajta G. Holm P. Kuwayama M, et al. Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev. 1998;51:53–58. doi: 10.1002/(SICI)1098-2795(199809)51:1<53::AID-MRD6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 62.Yokota Y. Sato S. Yokota M, et al. Successful pregnancy following blastocyst vitrification: case report. Hum Reprod. 2000;15:1802–1803. doi: 10.1093/humrep/15.8.1802. [DOI] [PubMed] [Google Scholar]
- 63.Reubinoff BE. Pera MF. Vajta G, et al. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- 64.Li Y. Tan JC. Li LS. Comparison of three methods for cryopreservation of human embryonic stem cells. Fertil Steril. 2010;93:999–1005. doi: 10.1016/j.fertnstert.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 65.Richards M. Fong CY. Tan S, et al. An efficient and safe xeno-free cryopreservation method for the storage of human embryonic stem cells. Stem Cells. 2004;22:779–789. doi: 10.1634/stemcells.22-5-779. [DOI] [PubMed] [Google Scholar]
- 66.Nishigaki T. Teramura Y. Nasu A, et al. Highly efficient cryopreservation of human induced pluripotent stem cells using a dimethyl sulfoxide-free solution. Int J Dev Biol. 2011;55:305–311. doi: 10.1387/ijdb.103145tn. [DOI] [PubMed] [Google Scholar]
- 67.Li T. Zhou C. Liu C, et al. Bulk vitrification of human embryonic stem cells. Hum Reprod. 2008;23:358–364. doi: 10.1093/humrep/dem386. [DOI] [PubMed] [Google Scholar]
- 68.Li T. Mai Q. Gao J, et al. Cryopreservation of human embryonic stem cells with a new bulk vitrification method. Biol Reprod. 2010;82:848–853. doi: 10.1095/biolreprod.109.080713. [DOI] [PubMed] [Google Scholar]
- 69.Heng BC. Bested SM. Chan SH, et al. A proposed design for the cryopreservation of intact and adherent human embryonic stem cell colonies. In Vitro Cell Dev Biol Anim. 2005;41:77–79. doi: 10.1290/04090651.1. [DOI] [PubMed] [Google Scholar]
- 70.Beier AF. Schulz JC. Dorr D, et al. Effective surface-based cryopreservation of human embryonic stem cells by vitrification. Cryobiology. 2011;63:175–185. doi: 10.1016/j.cryobiol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Ware CB. Nelson AM. Blau CA. Controlled-rate freezing of human ES cells. Biotechniques. 2005;38:879–880. doi: 10.2144/05386ST01. , 882–873. [DOI] [PubMed] [Google Scholar]
- 72.Yang PF. Hua TC. Wu J, et al. Cryopreservation of human embryonic stem cells: a protocol by programmed cooling. Cryo Lett. 2006;27:361–368. [PubMed] [Google Scholar]
- 73.Lee JY. Lee JE. Kim DK, et al. High concentration of synthetic serum, stepwise equilibration and slow cooling as an efficient technique for large-scale cryopreservation of human embryonic stem cells. Fertil Steril. 2010;93:976–985. doi: 10.1016/j.fertnstert.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 74.Valbuena D. Sanchez-Luengo S. Galan A, et al. Efficient method for slow cryopreservation of human embryonic stem cells in xeno-free conditions. Reprod Biomed Online. 2008;17:127–135. doi: 10.1016/s1472-6483(10)60302-1. [DOI] [PubMed] [Google Scholar]
- 75.Ha SY. Jee BC. Suh CS, et al. Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum Reprod. 2005;20:1779–1785. doi: 10.1093/humrep/deh854. [DOI] [PubMed] [Google Scholar]
- 76.Wu CF. Tsung HC. Zhang WJ, et al. Improved cryopreservation of human embryonic stem cells with trehalose. Reprod Biomed Online. 2005;11:733–739. doi: 10.1016/s1472-6483(10)61692-6. [DOI] [PubMed] [Google Scholar]
- 77.T'Joen V. De Grande L. Declercq H, et al. An efficient, economical slow-freezing method for large-scale human embryonic stem cell banking. Stem Cells Dev. 2012;21:721–728. doi: 10.1089/scd.2011.0192. [DOI] [PubMed] [Google Scholar]
- 78.Holm F. Strom S. Inzunza J, et al. An effective serum- and xeno-free chemically defined freezing procedure for human embryonic and induced pluripotent stem cells. Hum Reprod. 2010;25:1271–1279. doi: 10.1093/humrep/deq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amps KJ. Jones M. Baker D, et al. In situ cryopreservation of human embryonic stem cells in gas-permeable membrane culture cassettes for high post-thaw yield and good manufacturing practice. Cryobiology. 2010;60:344–350. doi: 10.1016/j.cryobiol.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Nie Y. Bergendahl V. Hei DJ, et al. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog. 2009;25:20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lippens E. Cornelissen M. Slow cooling cryopreservation of cell-microcarrier constructs. Cells Tissues Organs. 2010;192:177–186. doi: 10.1159/000313419. [DOI] [PubMed] [Google Scholar]
- 82.Serra M. Correia C. Malpique R, et al. Microencapsulation technology: a powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS One. 2011;6:e23212. doi: 10.1371/journal.pone.0023212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X. Meng G. Krawetz R, et al. The ROCK inhibitor Y-27632 enhances the survival rate of human embryonic stem cells following cryopreservation. Stem Cells Dev. 2008;17:1079–1085. doi: 10.1089/scd.2007.0247. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe K. Ueno M. Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 85.Martin-Ibanez R. Unger C. Stromberg A, et al. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23:2744–2754. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- 86.Baharvand H. Salekdeh GH. Taei A, et al. An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat Protoc. 2010;5:588–594. doi: 10.1038/nprot.2009.247. [DOI] [PubMed] [Google Scholar]
- 87.Li X. Krawetz R. Liu S, et al. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24:580–589. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]
- 88.Mollamohammadi S. Taei A. Pakzad M, et al. A simple and efficient cryopreservation method for feeder-free dissociated human induced pluripotent stem cells and human embryonic stem cells. Hum Reprod. 2009;24:2468–2476. doi: 10.1093/humrep/dep244. [DOI] [PubMed] [Google Scholar]
- 89.Claassen DA. Desler MM. Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barbaric I. Jones M. Buchner K, et al. Pinacidil enhances survival of cryopreserved human embryonic stem cells. Cryobiology. 2011;63:298–305. doi: 10.1016/j.cryobiol.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Katkov II. Kan NG. Cimadamore F, et al. DMSO-free programmed cryopreservation of fully dissociated and adherent human induced pluripotent stem cells. Stem Cells Int. 2011;2011:981606. doi: 10.4061/2011/981606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zweigerdt R. Olmer R. Singh H, et al. Scalable expansion of human pluripotent stem cells in suspension culture. Nat Protoc. 2011;6:689–700. doi: 10.1038/nprot.2011.318. [DOI] [PubMed] [Google Scholar]
- 93.Oh SK. Chen AK. Mok Y, et al. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009;2:219–230. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 94.Sensebe L. Bourin P. Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther. 2011;22:19–26. doi: 10.1089/hum.2010.197. [DOI] [PubMed] [Google Scholar]
- 95.Brunette E. Li Y. Yeganeh E, et al. Production of human embryonic stem cell master cell bank for therapeutic use using serum-free non-conditioned medium. 3rd Annual Meeting of ISSCR; 2005 Jun 23–25;; San Francisco, CA. Poster Number 641. [Google Scholar]
- 96.Chen G. Gulbranson DR. Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y. Powell S. Brunette E, et al. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng. 2005;91:688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 98.Xu C. Inokuma MS. Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 99.Melkoumian Z. Weber JL. Weber DM, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 100.Ausubel LJ. Lopez PM. Couture LA. GMP scale-up and banking of pluripotent stem cells for cellular therapy applications. Methods Mol Biol. 2011;767:147–159. doi: 10.1007/978-1-61779-201-4_11. [DOI] [PubMed] [Google Scholar]
- 101.Chen VC. Couture SM. Ye J, et al. Scalable GMP compliant suspension culture system for human ES cells. Stem Cell Res. 2012;8:388–402. doi: 10.1016/j.scr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Xu C. Police S. Hassanipour M, et al. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen Med. 2011;6:53–66. doi: 10.2217/rme.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]