Abstract
Animal models for cancer therapy are invaluable for preclinical testing of potential cancer treatments; however, therapies tested in such models often fail to translate into clinical settings. Therefore, a better preclinical model for cancer treatment testing is needed. Here we demonstrate that an immunodeficient line of pigs can host and support the growth of xenografted human tumors and has the potential to be an effective animal model for cancer therapy. Wild-type and immunodeficient pigs were injected subcutaneously in the left ear with human melanoma cells (A375SM cells) and in the right ear with human pancreatic carcinoma cells (PANC-1). All immunodeficient pigs developed tumors that were verified by histology and immunohistochemistry. Nonaffected littermates did not develop tumors. Immunodeficient pigs, which do not reject xenografted human tumors, have the potential to become an extremely useful animal model for cancer therapy because of their similarity in size, anatomy, and physiology to humans.
Key words: immunodeficient swine, large-animal cancer model, melanoma, pancreatic carcinoma, xenografts
Background
Preclinical research on animal models is essential in developing and evaluating cancer therapeutics.1 Syngeneic, xenograft, and genetically engineered mouse models have been developed to study cancer and cancer drug development.2 Mouse xenograft models are used extensively in preclinical studies because of their relatively good correlation with human clinical data, as compared to other animal models.3–5 However, studies on these mouse models often fail to accurately predict the response to and the effect of anticancer agents in human patients.3–5 Ninety percent of new anticancer drugs that showed antitumor efficacy in mouse-based preclinical studies failed in human clinical studies.4,5 Several methods of overcoming this shortfall have been proposed, including genetically engineered transgenic mouse models and orthotopic xenograft models,6,7 but these have yet to demonstrate significant improvements in translatability.6 Thus, there is a tremendous demand for more sophisticated animal models, which may improve the translation efficiency from preclinical to clinical studies.
Pigs are large animals with similar anatomy and physiology to humans and have been used in many research areas.8 The higher sequence homology of pigs with human xenobiotic receptors may allow more accurate prediction of pharmacodynamic and pharmacokinetic properties of drugs compared with mice.9 Several attempts have been made to establish porcine tumor models in pigs as a treatment model for human cancers, for example, investigating spontaneous myelogenous leukemias,10 developing transplantable hematologic tumors,11 and genetically inducing tumorigenesis.12
A xenograft model of human tumors in pigs would be an excellent model. Xenograft models of human tumors are often used in severe combined immunodeficiency (SCID) mice, which have severe lymphopenia due to defects in a DNA-dependent protein kinase gene that prevents variable–diversity–joining [V(D)J] gene region recombination.13 The severe lymphopenia prevents SCID mice from rejecting human tumors. SCID-associated severe lymphopenia is also known in other species, notably humans. Several genetic defects have been identified in humans as causing SCID, including defects in adenylate kinase 2, adenosine deaminase, purine nucleoside phosphorylase, interleukin (IL)-2 receptor γ, Janus kinase 3, and the IL-7 receptor.14 We recently identified pigs that are severely immunocompromised (SCID-like pigs).15 Yorkshire pigs bred for increased feed efficiency were noted to exhibit SCID-like symptoms. Further analysis of these pigs showed extremely decreased levels of lymphocytes in circulation and significantly atrophied thymus and lymph nodes. The mode of inheritance appears to be simple autosomal recessive, although the actual mutation remains to be elucidated. In the present study, we show evidence that these pigs can be used as human xenograft tumor models. As proof of concept, human melanoma cells (A375SM, amelanotic melanoma) and human pancreatic carcinoma cells (PANC-1) were transplanted subcutaneously into immunodeficient pigs, and the tumor-forming ability of the neoplastic cells was evaluated.
Methods
Reagents and cells
PANC-1 cells and A375SM cells were purchased from ATCC (Manassas, VA). Dulbecco's Modified Eagle's Medium (DMEM) and penicillin (10,000 units/mL)/streptomycin (10,000 μg/mL) were purchased from Life Technologies (Grand Island, NY). Fetal bovine serum (FBS), hematoxylin, eosin, Tris–hydrochloride, and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma-Aldrich (St. Louis, MO). Antimitochondrial clone 113-1 (mouse anti-human mitochondrial antibody) was purchased from Millipore (Temecula, CA). Bond Polymer Refine Red detection kit, Bond Primary Antibody Diluent, and alkaline phosphatase-conjugated Poly-AP anti-mouse antibody were purchased from Leica Microsystems (Buffalo Grove, IL). Fatal-plus (pentobarbital sodium) was obtained from Vortech Pharmaceuticals (Dearborn, MI). Banamine (flunixin meglumine) was obtained from Merck Animal Health/Intervet (Summit, NJ). Excenel (ceftiofur HCl) was obtained from Pfizer Animal Health (New York, NY). Second Bite Medicated feed with Tiamulin (35 g/ton) and chlortetracycline (400 g/ton) was purchased from Key Feeds, Fourth and Pomeroy Associates (Clay Center, KS).
Pig care
Six littermate pigs (6 weeks of age, two male and four female) were obtained from Iowa State University from a boar and a sow that have been identified as carriers of the immunodeficiency gene. Pigs were identified at 2 weeks of age as likely to be immunodeficient or immunocompetent based on lymphocyte counts: values were 1.08, 1.22, and 1.81×103 lymphocytes/μL for pigs expected to be immunodeficient (n=3, one male, two female) and 3.08, 4.13, and 5.18×103 lymphocytes/μL for presumed immunocompetent pigs (n=3, one male, two female). Pigs were transported to the Kansas State University at 6 weeks of age. Pigs were housed in a clean environment in raised pens upon arrival; however, neither the previous housing nor transportation was aseptic. Two days after arrival, blood samples were collected from the pigs to confirm the status of each pig by immunophenotyping. After confirmation of the immune status, the immunodeficient pigs were separated from the immunocompetent pigs in two different rooms in a clean environment in raised pens. Pigs were kept on a medicated diet and monitored daily for health status; ceftiofur HCl (2.2 mg/kg) and flunixin meglumine (1.1 mg/kg) were administered intramuscularly as indicated by veterinary consultation.
Xenograft tumor injection
A375SM cells and PANC-1 cells were cultured in the DMEM with 10% FBS and 1% penicillin/streptomycin. PANC-1 and A375SM cells were lifted, counted, and concentrated to 40 million cells/mL in PBS. Pigs were anesthetized by administration of isoflurane gas vaporized into oxygen (1–5%) and delivered via a face mask. 100 μL (4 million cells) of the PANC-1 cell suspension was injected subcutaneously into the right ear by tenting the skin of the ear near the base; 100 μL (4 million cells) of the A375SM cell suspension was injected subcutaneously into the left ear by tenting the skin of the ear near the base. After injecting, the pigs were removed from anesthesia and observed until reaching sternal recumbency.
After tumor injection, the pigs were monitored daily for tumor growth. Both right and left ears were visually inspected and palpated to determine presence of tumors daily. Once tumors were identified, calipers were used to measure the tumor size.
Histopathology and immunohistochemistry
Immunocompromised pigs were monitored for signs of respiratory disease, which often occurs because of the immunocompromised status. When a serious disease presented, the pigs were euthanized using Fatal-plus (days 6, 14, and 22). Unaffected littermates were euthanized with the last immunocompromised pig at day 22. At euthanasia, ear tissue was collected and fixed in 10% buffered formalin, processed routinely for sectioning, and then stained with hematoxylin and eosin (H&E). H&E sections were evaluated for histological evidence of tumors. For immunohistochemical analysis, unstained paraffin-embedded tissue was probed with anti-human mitochondrial antibody. Tissues were stained using the Leica Bond-Max automatic stainer (Leica Microsystems) with the Bond Polymer Refine Red detection kit. Tissues were pretreated for 20 min with Tris–EDTA (pH 9.0) for antigen retrieval. The primary antibody was diluted 1:100 using Bond Primary Antibody Diluent. Tissues were then stained with the primary antibody for 15 min followed by a secondary antibody (Powervision Poly-AP Anti-Mouse) for 25 min. Antibody-probed tissues were then counterstained with hematoxylin. Antibody-probed sections were evaluated for positive staining, indicating the presence of human cells.
Results
Pig observations
No visible tumor growth was noted at day 6 when the first immunodeficient pig (pig 1) was euthanized. On day 13, a small, firm, very slightly raised, elongated white mass was identified visually and by palpation on the left ear (amelanotic melanoma) of pig 2, but was too small to measure with the caliper. Pig 2 was euthanized on day 14. On day 14, a similar small, firm, raised, elongated white mass was identified on the left ear of pig 3, but was too small to measure with the caliper. On day 20, gross photographs were taken of the mass (Fig. 1). On day 22, pig 3 was euthanized. At postmortem examination, the tumor in the left ear (melanoma) was dissected free from the skin and measured 10.3×5.5 mm. No grossly discernible tumors were observed in the right ears (pancreatic carcinoma) of any of the immunodeficient pigs. No grossly discernible tumors were observed in either ear in the wild-type pigs.
FIG. 1.
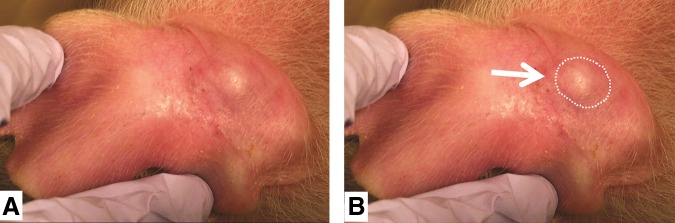
Antemortem visual evidence of tumor growth in pig 3 (day 20). (A) Photograph indicates a large growth on the left ear. (B) Same photograph as in (A), with the growth outlined for visual reference.
Histopathology and immunohistochemistry
Histology and immunohistochemistry revealed the presence of tumors in all injection sites for both tumors in SCID-like pigs. Pig 1 showed small tumors in both the right ear (Fig. 2A) and the left ear (Fig. 3A) histologically, indicating the lack of rejection of both the PANC-1 and the ASM375 cells. These tumors were verified by strong positive cytoplasmic staining with anti-human mitochondrial antibody (Figs. 2B, C and 3B, C). Pig 2 also showed tumors in both the right ear (Fig. 2D) and the left ear (Fig. 3D) histologically, which were verified by positive immunostaining (Figs. 2E, F and 3E, F). Pig 3 also showed tumors in both the right ear and the left ear that were substantially larger than the tumors of pigs 1 and 2. These tumors were also identified histologically and verified by positive staining with anti-human mitochondrial antibody (Figs. 2G–I and 3G–I).
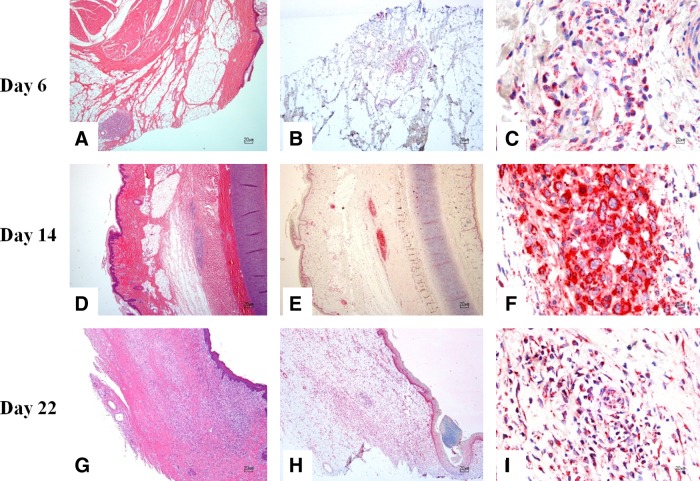
FIG. 2.
Right ear with pancreatic carcinoma cells from pigs euthanized at day 6, 14, and 22 post-transplantation. (A) There is a focal, well-demarcated, unencapsulated neoplasm composed of nests and packets of neoplastic cells within the subcutis. (B, C) Strong positivity to anti-human mitochondrial antibody is evident within the cytoplasm of neoplastic cells. (D) Within the subcutis and skeletal muscle, there is an unencapsulated, moderately demarcated, and mildly infiltrative neoplasm composed of nests and packets of neoplastic cells. (E, F) Strong positivity to anti-human mitochondrial antibody is evident within the cytoplasm of neoplastic cells. (G) Within the dermis, subcutis, and skeletal muscle, there is an unencapsulated, poorly demarcated, and infiltrative neoplasm composed of nests and packets of neoplastic cells. (H, I) Strong positivity to anti-human mitochondrial antibody is evident within the cytoplasm of neoplastic cells. (A, D, G) H&E stain; (B, C, E, F, H, I) anti-human mitochondrial antibody immunohistochemistry. H&E, hematoxylin and eosin.
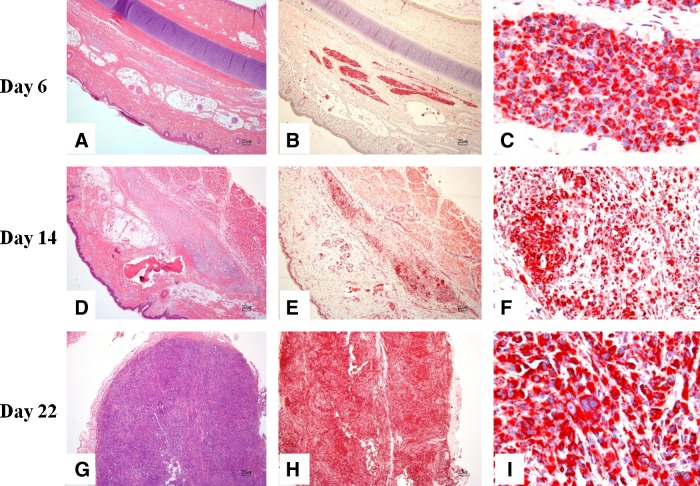
FIG. 3.
Left ear with melanoma cells from pigs euthanized at day 6, 14, and 22 post-transplantation. (A) Within the deep dermis, subcutis, and skeletal muscle, there is an unencapsulated and infiltrative neoplasm composed of nests and short streams of neoplastic cells. (B, C) Strong positivity to anti-human mitochondrial antibody is evident within the cytoplasm of neoplastic cells. (D) Within the deep dermis and subcutis, there is an unencapsulated and infiltrative neoplasm composed of nests and short streams of neoplastic cells within a moderate amount of fibrovascular stroma. (E, F) Strong positivity to anti-human mitochondrial antibody is evident within the cytoplasm of neoplastic cells. (G) Within the deep dermis and subcutis, there is an unencapsulated, moderately demarcated, and infiltrative neoplasm composed of nests and short streams of neoplastic cells within a moderate amount of fibrovascular stroma. (H, I) Strong positivity to anti-human mitochondrial antibody is evident within the cytoplasm of neoplastic cells. (A, D, G) H&E stain; (B, C, E, F, H, I) anti-human mitochondrial antibody immunohistochemistry.
All six tumor sites showed characteristic histologic features of malignant neoplasia, including bizarre and atypical mitotic figures and prominent anisocytosis and anisokaryosis (Fig. 4). Full histopathological descriptions of the tumors of each immunodeficient pig are found in the Supplementary Data. No tumors were identified histologically in the ears of wild-type pigs (Fig. 5), consistent with expected rejection of human origin cells by pigs with intact immune systems.
FIG. 4.
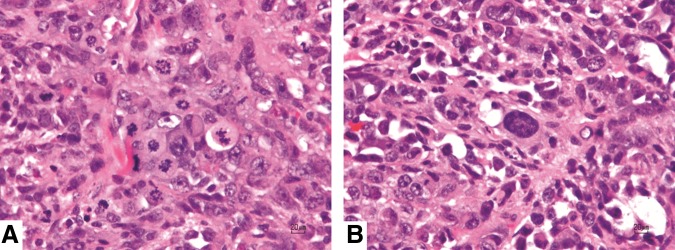
Photomicrographs of the left ear of pig 3 demonstrating histologic features of neoplasia. (A) Note multiple mitotic figures. (B) Note significant cellular and nuclear pleomorphism. H&E stain.
FIG. 5.
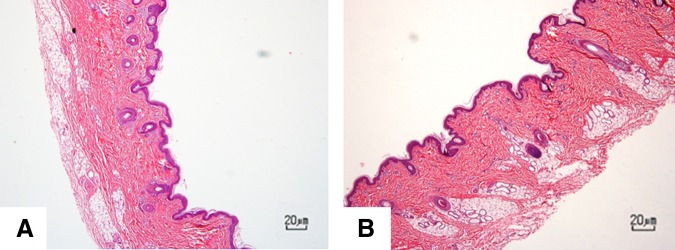
Right ear (A) and left ear (B) from control pigs at day 22. No tumors were identified at the site of injection of human-origin neoplastic cells. H&E stain.
Discussion
A large-animal model of human tumors that closely mimics the size, anatomy, and physiology of humans would be of great value to cancer research. Here we have demonstrated that a naturally occurring immunodeficient line of pigs is capable of hosting xenograft human cells and developing active human tumors. It is possible that immunodeficient pigs fail to reject human xenograft tumors due to their low levels of lymphocytes, but immunodeficient pigs do not fail to produce all lymphocytes. Preliminary results indicate that the lymphocytes present in immunodeficient pigs do not express CD3, CD4, CD8, CD21, or CD79a. This may indicate that the remaining cells are natural killer (NK) cells that may not be functional due to lack of T-cell-associated cytokine stimulation. Therefore, there may be no functional lymphocytes that would be able to reject the human tumor xenograft. Further studies are being done on the lymphocyte profiles in the immunodeficient pigs.
The melanoma model described here is orthotopic; the subcutaneous model of pancreatic adenocarcinoma, however, is not orthotopic. For this study, the proof of concept that immunodeficient pigs would not reject tumors was demonstrated using an easily monitored tumor location. In the future, orthotopic models of pancreatic cancer may be explored in these pigs to determine their suitability for investigating this devastating human cancer.
One of the major problems with current animal models for human tumors is the low translatability to clinical settings.4 This is often due to the limited similarity in anatomy and physiology of common tumor models such as SCID mice.3,4 Because pig anatomy and physiology are very similar to that of humans, the immunodeficient pig tumor model could be used for testing multiple types of cancer therapy, including chemotherapy, radiotherapy, and surgical reduction, with more realistic results. Therefore, this model has the potential to be a valuable human cancer model for preclinical cancer research, with a high rate of translatability to the clinical settings.
The usefulness of this model may not be limited to cancer therapy. Since the immunodeficient pigs do not reject xenografts, they may be a useful model for other disease states as well. For example, human liver cell xenografts could be grown in pigs and then infected with hepatitis B or C for testing various antiviral medications. Similarly, the pigs could be reconstituted with primitive human hematopoietic stem cells (e.g., cord blood) to generate a pig chimera with a human immune system, a useful model for studies such as human immunodeficiency virus antiviral therapies. Thus, a pig model that does not reject human xenografts is a unique animal model with potential uses in a variety of preclinical applications for human health.
Supplementary Material
Acknowledgments
The authors thank the Kansas Agricultural Experiment Station and the Johnson Cancer Center for financial support. The researchers also thank Iowa State University for providing the pigs for this study. The authors also thank the Comparative Medicine Group at the Kansas State University for facilitating these studies.
Author Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Rothenberg ML. Carbone DP. Johnson DH. Improving the evaluation of new cancer treatments: challenges and opportunities. Nat Rev Cancer. 2003;3:303–309. doi: 10.1038/nrc1047. [DOI] [PubMed] [Google Scholar]
- 2.Richmand A. Su Y. Mouse xenograft models vs gem models for human cancer therapeutics. Dis Models Mech. 2008;1:78–82. doi: 10.1242/dmm.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelland LR. “Of mice and men”: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer. 2004;40:827–836. doi: 10.1016/j.ejca.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Liu M. Hicklin D. Human tumor xenograft efficacy models. In: Teicher BA, editor. Tumor Models in Cancer Research. Springer; New York: 2010. pp. 99–124. [Google Scholar]
- 5.Sausville EA. Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–3354. doi: 10.1158/0008-5472.CAN-05-3627. [DOI] [PubMed] [Google Scholar]
- 6.Garber K. Realistic rodents? Debate grows over new mouse models of cancer. J Natl Cancer Inst. 2006;98:1176–1178. doi: 10.1093/jnci/djj381. [DOI] [PubMed] [Google Scholar]
- 7.Sharpless NE. DePinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 8.Schook L. Swine in biomedical research: creating the building blocks of animal models. Anim Biotechnol. 2005;16:183–190. doi: 10.1080/10495390500265034. [DOI] [PubMed] [Google Scholar]
- 9.Swanson KS. Mazur MJ. Vashisht K, et al. Genomics and clinical medicine: rationale for creating and effectively evaluating animal models. Ex Biol Med. 2004;229:866–875. doi: 10.1177/153537020422900902. [DOI] [PubMed] [Google Scholar]
- 10.Duran-Struuck R. Cho PS. Teague AGS, et al. Myelogenous leukemia in adult inbred mhc defined miniature swine: a model for human myeloid leukemias. Vet Immunol Immunopathol. 2010;135:243–256. doi: 10.1016/j.vetimm.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho PS. Lo DP. Wikiel KJ, et al. Establishment of transplantable porcine tumor cell lines derived from mhc-inbred miniature swine. Blood. 2007;110:3996–4004. doi: 10.1182/blood-2007-02-074450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adam SJ. Rund LA. Kuzmuk KN, et al. Genetic induction of tumorigenesis in swine. Oncogene. 2007;26:1038–1045. doi: 10.1038/sj.onc.1209892. [DOI] [PubMed] [Google Scholar]
- 13.Finnie NJ. Gottlieb TM. Blunt T, et al. DNA-dependent protein kinase defects are linked to deficiencies in DNA repair and v(d)j recombination. Philos Trans R Soc Lond Ser B Biol Sci. 1996;351:173–179. doi: 10.1098/rstb.1996.0014. [DOI] [PubMed] [Google Scholar]
- 14.Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol. 2010;125:S182–S194. doi: 10.1016/j.jaci.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 15.Cino Ozuna A. Rowland R. Nietfeld J, et al. Lymphoid hypoplasia and absence of a specific antibody response in pigs: a suspected primary immunodeficiency disorder. Vet Pathol (in revision) 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.