Abstract
The Epstein–Barr virus early antigen (EBV EA) complex consists of multiple proteins with potential significance for diagnosis of EBV-related diseases. In many individuals, detection of antibody to the early antigen (EA) is a sign of active infection, but 20% of healthy people may have this antibody for years. We studied the role of EA immunoglobulin G (IgG) in individuals with atypical antibody responses in the diagnosis of infectious mononucleosis (IM) and in EBV-infected transplant patients. EA IgG was present in 72% of confirmed IM patients. A trend was observed between high viral loads and the presence of EA IgG and between low viral loads and the absence of EA IgG in EBV-associated disease negative liver transplant recipients. Three assays that measure serum EA IgG were compared; enzyme-linked immunosorbent assay (ELISA), chemiluminescent immunoassay (CLIA), and immunoblot assay. The automated CLIA was found to be more accurate than the ELISA when using the immunoblot assay as a “gold standard” assay in the detection of EA IgG. There may be a potential role for EA IgG testing, together with EBV viral load, in the prediction of transplant recipients at risk of EBV-associated disease; however, EA IgG does not play a significant role in the differential diagnosis of EBV infection in immunocompetent individuals.
Key words: early antigen, Epstein–Barr virus, infectious mononucleosis, liver transplant
Introduction
The Epstein–Barr virus early antigen (EBV EA) complex consists of multiple nonstructural proteins with potential significance for diagnosis of EBV-related diseases. At least two forms of early antigen (EA) have been identified on the basis of their distribution in the cell, namely diffuse and restricted.1 The diffuse EA is reported to be expressed during the early lytic phase of virus replication.1 The typical antibody pattern of primary EBV infection is characterized by the presence of immunoglobulin (Ig) M and IgG antibodies to the EBV viral capsid antigen (VCA) and EA and by the absence of Epstein–Barr nuclear antigen (EBNA-1) IgG antibodies.
Primary EBV infection in an immunocompetent adolescent or adult often leads to infectious mononucleosis (IM), a usually self-limited clinical syndrome. However, EBV serology can present with a high degree of variability between individuals, which can complicate serological diagnosis of IM.2–4 This problem can be dealt with through the detection of a combination of EBV serological markers.3 Several reports in the literature suggest that the combination of the three analytes VCA IgM, VCA IgG, and EBNA IgG are generally sufficient for the diagnosis of an acute, past, or no EBV infection.2,3
VCA IgM and VCA IgG in the absence of EBNA-1 IgG are typically found in patients with primary infections.3 In contrast, past infections are typically characterized by the presence of VCA-IgG and EBNA-1 IgG antibodies in the absence of VCA IgM antibodies.3 Primary importance is therefore given to EBNA-1 IgG because a positive result definitely excludes an acute EBV infection.3
Reports on the diagnostic role of EA IgG as a marker for the specific detection of an acute EBV infection are varied in the literature. Several authors suggest that EA IgG is not very useful for the specific detection of acute EBV infection due to the fact that this marker can be present in both acute and reactivated EBV infection.2,3,5 However EBNA-1 IgG is a marker of past infection, and therefore a positive EA IgG result will indicate an acute infection in the absence of EBNA-1 IgG antibodies. Therefore the use of EA IgG testing in conjunction with other EBV serological markers may be useful in the stage-specific diagnosis of EBV infection.
For example, VCA IgM may persist for a long time after acute infection instead of becoming negative at the time of seroconversion of anti-EBNA-1.5 If EBV serology is restricted to the determination of anti-VCA, sera with persistent VCA IgM could be mistaken as indicative of acute infection. In contrast, in some individuals, VCA IgM may be too low for detection or may appear in a delayed fashion during acute EBV infection.5 The addition of EBV EA IgG serology in conjunction with EBNA-1 IgG testing could clarify the diagnosis in both of these atypical serological responses allowing for the accurate diagnosis of an acute, reactivated, or past infection. If EA IgG antibodies are detected in the absence of EBNA-1 IgG antibodies this may indicate an acute EBV infection. The presence of both high EBNA-1 IgG titers and EA IgG may be a sign of EBV reactivation. If EA IgG antibodies are not detected and EBNA-1 IgG antibodies are present, then this may suggest a past infection.
In immunocompetent individuals, EBV reactivation is frequently of short duration and is generally considered to be without clinical relevance.6 In contrast, primary EBV infection is a differential diagnosis in a variety of clinical scenarios.6 Therefore, diagnosing primary versus reactivated EBV infection in patients with suspected IM is of importance.6 Although the addition of EA IgG testing to the traditional EBV diagnostic screen, which typically includes only EBNA IgG, VCA IgM, and VCA IgG, may not be cost effective for routine laboratories, the inclusion of this marker may be important in reference laboratories in order to clarify the diagnosis in atypical cases.
Following primary infection EBV establishes lifelong latency in the B lymphocytes of the host.7 As in the case of other herpes viruses, if host–virus balance is shifted virus reactivation may occur. EBV is transmitted in the saliva, infects B cells in the oropharyngeal epithelium, and enters the lymphoid tissue.7 Following infection of naive B cells in the lymph node, all latent genes are expressed, and the viral latent proteins trigger the B cells to divide and enter the germinal center reaction in the absence of a cognate antigen.8 The transit through the germinal centre results in establishment of a life-long infection of memory B cells. EBV lies latent in these B cells by expressing few immunogenic viral proteins.9 Terminal differentiation to plasma cells results in reactivation of the virus to the lytic cycle, expression of lytic proteins and production of infectious virus.8 In contrast to latently infected B cells, viral genes encoding proteins involved in viral DNA replication and viral particle synthesis are expressed during the lytic cycle.7 The virus can infect B cells within the lymphoid tissue or be shed into the saliva.7
EBV-infected B lymphocytes are initially controlled by natural killer cells and cytotoxic T lymphocytes (CTLs).7 However, the initial CTL response does not remove all of the EBV-infected B cells, permitting the establishment of memory B cells latently infected with EBV.7 When EBV is reactivated from latently infected cells, several viral proteins are expressed and the infected cells are recognized and destroyed by CTLs.7 A balance is therefore established and maintained between viral reactivation and host immune surveillance.7
Transplantation of solid organs has been successful mainly due to the development of immunosuppressive regimens that have reduced the incidence of rejection of the transplanted organ by the recipient.10 However, a major problem associated with the nonspecific nature of immunosuppression is the susceptibility of the recipient to opportunistic infections as well as the increased risk of developing malignancies.11
Primary or reactivated EBV infection in immunocompromised individuals can be associated with life-threatening disorders such as post-transplantation lymphoproliferative disorders (PTLD). Immuncompromised solid organ transplant recipients are at particular risk for the development of EBV-associated PTLD. PTLD is characterized by uncontrolled proliferation of EBV-infected B lymphocytes leading to a wide spectrum of diseases ranging from early hyperplastic lesions to true lymphomas. Primary EBV infection after transplantation is a major risk factor for PTLD development; however, EBV reactivation can also lead to PTLD in transplant recipients.
Early detection of PTLD is important because PTLD tends to be rapidly progressive in immunocompromised patients.12 Early recognition of PTLD may therefore allow for prompt therapy and potentially decrease mortality, thereby improving overall patient management.13 It has been suggested that the use of a serological marker such as EBV EA IgG, in conjunction with EBV viral load, could potentially better predict the risk of developing EBV-induced PTLD.14–17 Given the increasing numbers of all types of transplants and the high mortality rates associated with PTLD in immunocompromised transplant recipients the potential role for EA IgG testing in conjunction with EBV viral load for the early detection of PTLD needs to be determined.
The aims of the study were to investigate the diagnostic value of anti-EA IgG testing in the stage-specific differential diagnosis of EBV infection in immunocompetent individuals with atypical antibody responses. Furthermore, we assessed whether anti-EA IgG detection in conjunction with EBV DNA viral load quantification had better predictive value than EBV DNA alone in the diagnosis of EBV-associated disease in immunosuppressed adult liver transplant recipients since during the study period none of the patients developed PTLD. The performance of three EBV EA IgG serological assays was assessed: an EBV EA IgG enzyme-linked immunosorbent assay (ELISA) and a fully automated indirect chemiluminescence immunoassay (CLIA) were compared with an EBV IgG immunoblot assay.
Patients and Methods
Patients
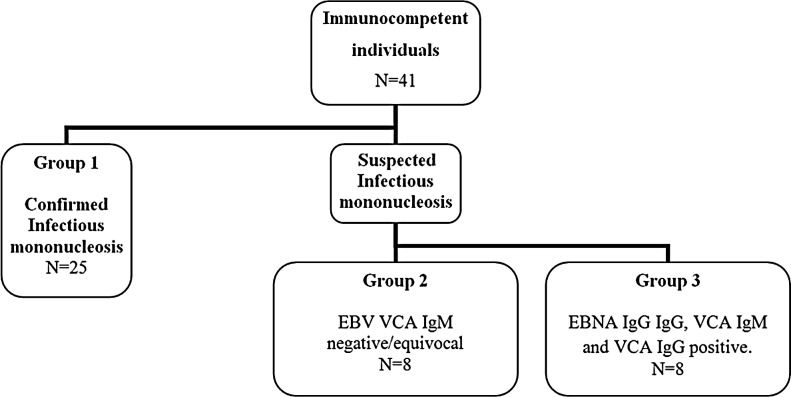
Eighty-five serum or plasma samples from 69 individuals were selected from the archived database at the National Virus Reference Laboratory, Belfield, Ireland. Two patient cohorts were selected on the basis of immune status. Cohort 1 (Fig. 1) consisted of 41 immunocompetent individuals divided into three groups. Group 1 (control group) was composed of 25 individuals with confirmed IM as determined by the presence of VCA IgM and VCA IgG in the absence of EBNA IgG. All of the individuals in this group had typical EBV serological profiles consistent with acute infection. The individuals in Group 2 had clinical symptoms indicative of a mononucleosis-like illness however their VCA IgM antibody results were negative or equivocal. The stage of EBV infection was unclear for the individuals in Group 3 due to the simultaneous presence of VCA IgM, EBNA IgG, and VCA IgG antibodies.
FIG. 1.
Immunocompetent patient selection of Cohort 1 based on EBV serological profiles. EBNA, Epstein–Barr nuclear antigen; EBV, Epstein–Barr virus; VCA, viral capsid antigen.
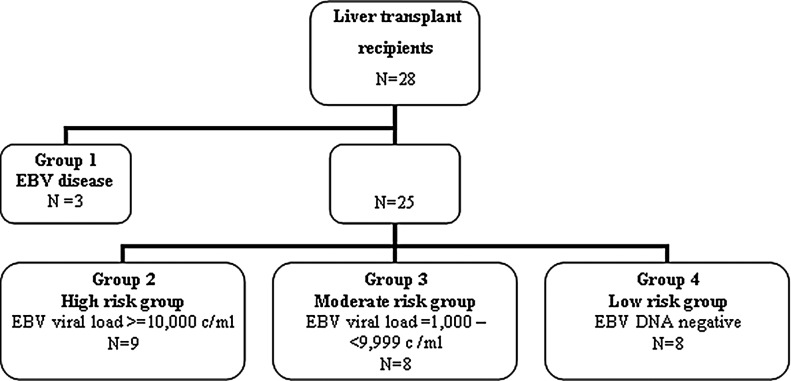
Cohort 2 was composed of 28 immunosuppressed liver transplant recipients (Fig. 2). Five patients were EBV seronegative prior to transplant (18%). Patient samples were preferentially selected within 1 year post-transplant because the risk of developing PTLD has been reported to be greatest in this time frame.18 There were no clinically confirmed cases of PTLD in this cohort; however, three patients were treated for EBV-associated disease (Cohort 2, Group 1). Two of these patients were women, aged 60 and 56 years, with neurological symptoms, and EBV was detected in their cerebrospinal fluid.19 After extensive clinical, radiological, and microbiological analysis, the diagnosis of EBV encephalitis was made because there was no evidence of lymphoproliferation. The third patient was a 55-year-old man that developed a febrile illness, had elevated liver function tests, and high EBV viral loads of 3,807,000 copies/mL. All patients were managed with the reduction of immunosuppression and administration of antiviral therapy.19 Ethical approval for the study was obtained from the Research Ethics Committee of St. Vincent's University Hospital and the Human Research Ethics Committee of University College Dublin.
FIG. 2.
Liver transplant patient selection (Cohort 2) as determined by EBV viral load analysis (copies/mL).
Detection of EA IgG
The detection of EA IgG in patients' samples was carried out using three different specific serological assays. All assays were performed in accordance with the manufacturers' instructions. The EBV EA IgG ELISA (MP Biomedicals Germany GmbH, Eschwege, Germany) is a microtiter plate assay which detects and quantifies specific human IgG against EBV p54 EA. The Liaison® EBV EA IgG CLIA (DiaSorin S.p.A., Saluggia [VC], Italy) is a two-step immunoluminometric sandwich assay using directly coated magnetic particles for the detection of EBV EA IgG against the p54 EA. The recomBlot EBV IgG (Mikrogen, Molekularbiologische Entwicklungs GmbH, Neuried, Germany) is a qualitative immunoblot assay that detects IgG against EBV membrane antigen, VCA, EBNA, and EA (p54 and p138). All samples were tested for EA IgG using the ELISA and CLIA methods. One patient's sample from Cohort 2, Group 3 was unsuitable for testing on the immunoblot assay; therefore, the total number of patients tested in this group was seven.
Determination of EBV DNA viral load in whole blood
DNA was extracted from 200 μL of whole blood samples using the Qiagen DNA mini-kit (Qiagen, Crawley, United Kingdom) and eluted in 100 μL according to the manufacturer's instructions. Amplification and quantification was performed using the Light Cycler EBV Quantification kit (Roche Diagnostics GmbH, Roche Applied Science, Mannheim, Germany) according to manufacturer's protocol. EBV DNA viral loads are expressed as copies per milliliter.
Statistical analysis
The results from the EBV EA IgG ELISA, CLIA, and immunoblot assays were compared using McNemars test. This nonparametric test compares proportions arising from matched pairs (i.e., different tests performed on the same patients). The sensitivity, specificity, positive predictive value, and the negative predictive value of the ELISA and CLIA assays were calculated in relation to the immunoblot assay. The 95% confidence limits were calculated for the standard error of the proportions.
Results
EA IgG detection in immunocompetent individuals and immunosuppressed liver transplant patients
Table 1 shows that in the IM-positive Group 1, 72% of individuals tested positive for EA IgG. EA IgG was detected in two patients in Group 2 (patients 56 and 58). EA IgG, VCA IgM, and EBNA IgG antibodies were not detected in the initial sample from patient 56. However, a second sample taken 2 months later was positive for EA IgG, VCA IgG, and IgM antibodies and weakly positive for EBNA IgG. These results were reported as being “consistent with recent EBV infection.” Patient 58 was also reported as having a “recent EBV infection” due to a positive VCA IgG, negative VCA IgM, and negative EBNA IgG antibody result in addition to clinical details of a “recent positive monospot test and mononucleosis-like illness.”
Table 1.
Detection of Early Antigen IgG Using the Immunoblot Assay
| Patients | n | EA IgG positive |
|---|---|---|
| Cohort 1: Immunocompetent group | 41 | |
| Group 1 (confirmed IM) | 25 | 18 (72%) |
| Group 2 (VCA IgM equivocal/negative) | 8 | 2 (25%) |
| Group 3 (VCA IgM+/IgG+/EBNA IgG+) | 8 | 5 (63%) |
| Cohort 2: Immunosuppressed group | 27 | |
| Group 1 (EBV-associated disease) | 3 | 1 (33%) |
| Group 2 (high risk: EBV DNA ≥10,000 copies/mL) | 9 | 5 (56%) |
| Group 3 (moderate risk: EBV DNA 1000–9999 copies/mL) | 7a | 3 (43%) |
| Group 4 (low risk: EBV DNA negative) | 8 | 1 (13%) |
One patient's sample was unsuitable for testing on the immunoblot assay and therefore the total number of patients tested using the immunoblot assay in this group was 7.
EA, early antigen; EBNA, Epstein–Barr nuclear antigen; EBV, Epstein–Barr virus; Ig, immunoglobulin; IM, infectious mononucleosis; VCA, viral capsid antigen.
All eight individuals in Group 3 were positive for EBNA IgG, VCA IgG, and IgM antibodies. EA IgG was detected in five patients. A report comment was sent out stating “it is probable that EBV infection occurred more than 3 months ago; however, if this patient is immunocompromised, and EBV reactivation is suspected, an EDTA whole blood should be collected for EBV DNA testing to document the current EBV status.” However, no further samples were received from these patients. Acute infection was ruled out due to the presence of EBNA IgG antibodies.
Serum from one of the three patients with EBV-associated disease, Group 1 Cohort 2, tested positive for EA IgG antibodies (Table 1). This patient was EBV seronegative prior to transplant while the other two patients were EBV seropositive. Only one of the EA IgG–positive patients from both Group 2 and Group 3 was EBV seronegative pretransplant. In Group 4, only one patient tested positive for EA IgG. All patients in this group were EBV seropositive before transplant.
Comparison of three assays for the detection of EA IgG in serum
Table 2 shows the results from 68 patients tested for EA IgG using the ELISA and CLIA methods. Tables 3 and 4 show that the CLIA and immunoblot produced more comparable results (p=0.782). However, there was a significant difference between the results of the ELISA and the immunoblot EA IgG assays (p=0.0003) and the results of the CLIA and ELISA assays (p=0.00027). Comparisons with the immunoblot results showed that the CLIA is more sensitive (77%) than the ELISA (50%) but that the CLIA is less specific (42%) than the ELISA (100%) (Tables 5 and 6). Although the negative predictive value and efficiency of the two assays were comparable (CLIA 45%, ELISA 48%), the positive predictive value of the ELISA (100%) was greater than the CLIA assay (74%).
Table 2.
Comparison Between Detection of Early Antigen IgG Using the ELISA and CLIA Screening Methods
| CLIA positive | CLIA negative | Total | |
|---|---|---|---|
| ELISA positive | 12 | 1 | 13 |
| ELISA negative | 16 | 39 | 55 |
| Total | 28 | 40 | 68a |
Total patient number for the method comparison analysis is 68 because two samples from patient 56 (56a and 56b) gave conflicting results; therefore, this patient was not included.
CLIA, chemiluminescent immunoassay; ELISA, enzyme-linked immunosorbent assay.
Table 3.
Comparison Between Detection of Early Antigen IgG Using the Immunoblot and CLIA Screening Methods
| Immunoblot positive | Immunoblot negative | Total | |
|---|---|---|---|
| CLIA positive | 20 | 7 | 27 |
| CLIA negative | 6 | 5 | 11 |
| Total | 26 | 12 | 38 |
Table 4.
Statistical Evaluation of the ELISA and CLIA Methods Using the Immunoblot Assay as the Reference Test
| p value | McNemars test resulta | Resultb | |
|---|---|---|---|
| ELISA and immunoblot (p54) | 0.0003 | Significant | Fail to accept |
| CLIA and immunoblot (p54) | 0.782 | Not significant | Accept |
The difference between the results reported between the two screening assays.
Null hypothesis: ELISA/CLIA produce similar results to the immunoblot assay.
Table 5.
Comparison Between Detection of Early Antigen IgG Using the Immunoblot and ELISA Screening Methods
| Immunoblot positive | Immunoblot negative | Total | |
|---|---|---|---|
| ELISA positive | 13 | 0 | 13 |
| ELISA negative | 13 | 12 | 25 |
| Total | 26 | 12 | 38 |
Table 6.
Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value, and Overall Efficiency Calculations for ELISA, CLIA, and Immunoblot Assay, and 95% Confidence Limits for the Standard Error of the Proportions
| Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV | Efficiency | |
|---|---|---|---|---|---|
| ELISA and Immunoblot | 50% (31–69) | 100% (1) | 100% | 48% | 66% |
| CLIA and Immunoblot | 77% (61–93) | 42% (14–70) | 74% | 45% | 66% |
CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Discussion
In agreement with literature reports that the prevalence rate of EA antibodies in immunocompetent individuals with acute primary IM is between 60% and 80%, our cohort showed 72% positivity.2,20 In the patients with atypical serology, Cohort 1, Groups 2 and 3, we found that the inclusion of EA IgG testing to the typical testing algorithm, involving VCA IgG, VCA IgM, and EBNA IgG, generated results that were consistent with the diagnosis reported from using these three markers alone. The simultaneous presence of VCA IgM, EBNA IgG, VCA IgG, and EA IgG antibodies in four patients in Group 3 could possibly indicate a reactivation of EBV infection. However, the use of EA IgG testing in the diagnosis of acute EBV infection must be interpreted with caution because EA IgG antibodies are not always present in every individual with acute IM and can remain detectable for years after the initial EBV infection in 20%–30% of individuals.6,20 Therefore, the entire clinical picture must be taken into consideration when making a diagnosis. Although a further sample was requested for EBV DNA testing from these patients, none were received. The analysis of specific EBV IgG avidity facilitates more accurate estimations on the exact dates of infection, since avidity rises increasingly during the course of infection, and may therefore be a preferential alternative to EA IgG testing.3,6
In immunocompromised transplant recipients, the prediction of individuals at high risk of EBV-associated PTLD development is of paramount importance in order to implement therapeutic interventions as early as possible.7,21 Due to the growing number of solid organ transplantations and the high mortality rates associated with PTLD, the identification of these patients is a key concern.2,22–24 The potential role of EA IgG testing, in conjunction with viral load testing, is therefore an important area of investigation.
The results of the present study demonstrated that in a small number of patients, the presence of EA IgG was proportional to EBV DNA viral load (Table 1). In a published study that directly correlated EA titers with EBV viral load and PTLD development, all PTLD-negative, high-risk patients were EA positive and all low-risk patients were EA negative.14 All PTLD-positive individuals in the same study had high EBV DNA viral loads and low EA titers. Therefore, it was concluded that transplant patients with high EBV viral loads and negative EA antibody results were at risk of PTLD development. Although the results from the present study are similar to those of Carpentier and colleagues,14 not all of the high-risk patients in the present study were positive for EA IgG, and one patient in the low-risk group was positive for EA IgG. Our findings of the absence of EA IgG in some patients with high viral loads indicates that the use of EA IgG testing in conjunction with viral load may possibly increase the positive predictive value of viral load testing. A similar conclusion was reached in a study on pediatric solid organ transplant recipients16; however, the limitation of our study was that no patients developed clinically confirmed PTLD. Given that primary EBV infection after transplantation is a major risk factor for PTLD development,14 the present study was also limited by the fact that only 5 of the 28 patients (18%) in this cohort were EBV seronegative prior to transplantation. Other factors, such as the type and/or severity of the patients' immunosuppressive treatment and/or co-infection with cytomegalovirus are also significant risk factors in the development of PTLD.11,17,25,26 Furthermore, it is known that up to 10% of PTLD cases are EBV negative.
Traditionally the indirect immunofluorescence assay is the “gold standard” for serological diagnosis of EBV infection.3,4,15,27 There is good agreement between the immunofluorescence assay and the immunoblot methods.15,27,28 Therefore, the immunoblot assay was used as a reference point in the current study. Our findings demonstrated a highly significant difference between the ELISA and CLIA methods. This finding was unexpected since both assays detect IgG to the EBV p54 EA. Comparisons with the immunoblot results showed that the CLIA is a more sensitive but less specific assay. The poor sensitivity of the ELISA assay leading to false-negative results has been previously reported.15,28 Furthermore, it has been reported that immunoassay interference in certain assays carried out on the Liaison analyzer, can lead to false-positive results.29 This may explain the higher proportion of false-positive results, and hence the lower positive predictive value and poor specificity of the CLIA method. This assay interference can be partially prevented by the addition of chemical blocking reagents, such as polyvinylpyrrolidone and polyvinyl alcohol, to the assay buffers.29 Studies are currently underway to examine this further.
Acknowledgments
The authors would like to thank Joanne Moran (National Virus Reference Library) for help with statistical analysis of data. The authors also thank the National Virus Reference Laboratory for funding.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bhaduri-McIntosh S. Landry ML. Nikiforow Rotenberg M, et al. Serum IgA antibodies to Epstein–Barr virus (EBV) early lytic antigens are present in primary EBV infection. J Infect Dis. 2007;195:483–492. doi: 10.1086/510916. [DOI] [PubMed] [Google Scholar]
- 2.Hess RD. Routine Epstein–Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J Clin Microbiol. 2004;42:3381–3387. doi: 10.1128/JCM.42.8.3381-3387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer G. The rational basis for efficient Epstein–Barr virus (EBV) serology. Clin Lab. 1995;41:623–634. [Google Scholar]
- 4.Feng Z. Li Z. Sui B, et al. Serological diagnosis of infectious mononucleosis by chemiluminescent immunoassay using capsid antigen p18 of Epstein–Barr virus. Clin Chim Acta. 2005;354:77–82. doi: 10.1016/j.cccn.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Aalto SM. Linnavuori K. Peltola H, et al. Immunoreactivation of Epstein–Barr virus due to cytomegalovirus primary infection. J Med Virol. 1998;56:186–191. [PubMed] [Google Scholar]
- 6.Nystad TW. Myrmel H. Prevalence of primary versus reactivated Epstein–Barr virus infection in patients with VCA IgG-, VCA IgM- and EBNA-1-antibodies and suspected infectious mononucleosis. J Clin Virol. 2007;38:292–297. doi: 10.1016/j.jcv.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JI. Bollard CM. Khanna R. Pittaluga S. Current understanding of the role of Epstein–Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leuk Lymphoma. 2008;49:27–34. doi: 10.1080/10428190802311417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochford R. Cannon MJ. Moormann AM. Endemic Burkitt's lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3:182–187. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 9.Young LS. Dawson CW. Eliopoulos AG. The expression and function of Epstein–Barr virus encoded latent genes. Mol Pathol. 2000;53:238–247. doi: 10.1136/mp.53.5.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A. Nalesnik M. Reyes J, et al. Posttransplant lymphoproliferative disorders in liver transplantation: a 20-year experience. Ann Surg. 2001;236:429–436. doi: 10.1097/00000658-200210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loren AW. Porter DL. Stadtmauer EA. Tsai DE. Post-transplant lymphoproliferative disorder: a review. Bone Marrow Transpl. 2003;31:145–155. doi: 10.1038/sj.bmt.1703806. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino Y. Kimura H. Tanaka N, et al. Prospective monitoring of the Epstein Barr virus DNA by real-time quantitative polymerase chain reaction after allogenic stem cell transplantation. Brit J Haematol. 2001;115:105–111. doi: 10.1046/j.1365-2141.2001.03087.x. [DOI] [PubMed] [Google Scholar]
- 13.Leblond V. Choquet S. Lymphoproliferative disorders after liver transplantation. J Hepatol. 2004;40:728–735. doi: 10.1016/j.jhep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Carpentier L. Tapiero B. Alvarez F, et al. Epstein–Barr virus (EBV) early-antigen serologic testing in conjunction with peripheral blood EBV DNA load as a marker for risk of posttransplantation lymphoproliferative disease. J Infect Dis. 2003;188:1853–1864. doi: 10.1086/379834. [DOI] [PubMed] [Google Scholar]
- 15.Gartner BC. Fischinger JM. Roemer K, et al. Evaluation of a recombinant line blot for diagnosis of Epstein–Barr virus compared with ELISA, using immunofluorescence as reference method. J Virol Methods. 2001;93:89–96. doi: 10.1016/s0166-0934(00)00301-3. [DOI] [PubMed] [Google Scholar]
- 16.Leruez-Ville M. Talbotec C. Iserin F, et al. Epstein–Barr virus (EBV) early-antigen antibodies and EBV DNA load in blood, in posttransplantation lymphoproliferative disease. J Infect Dis. 2004;190:1524–1525. doi: 10.1086/423896. author reply 1525–1526. [DOI] [PubMed] [Google Scholar]
- 17.Gulley ML. Tang W. Laboratory assays for Epstein–Barr virus–related disease. J Mol Diag. 2008;10:279–292. doi: 10.2353/jmoldx.2008.080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker A. Bowles K. Bradley JA, et al. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients: BCSH and BTS Guidelines. Brit J Haematol. 2010;149:675–692. doi: 10.1111/j.1365-2141.2010.08161.x. [DOI] [PubMed] [Google Scholar]
- 19.Schaffer K. Hassan J. Staines A, et al. Surveillance of EBV viral loads in adult liver transplantation: association with age, gender, time post-transplant and transplant indication. Liver Transpl. 2011;17:1420–1426. doi: 10.1002/lt.22406. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) National Center for Infectious Diseases. Epstein–Barr Virus and Infectious Mononucleosis. www.cdc.gov/ncidod/diseases/ebv.html. May 16, 2006. www.cdc.gov/ncidod/diseases/ebv.html
- 21.Meerbach A. Wutzler P. Hafer R, et al. Monitoring of Epstein–Barr virus load after hematopoietic stem cell transplantation for early intervention in post-transplant lymphoproliferative disease. J Med Virol. 2008;80:441–454. doi: 10.1002/jmv.21096. [DOI] [PubMed] [Google Scholar]
- 22.Allen U. Hebert D. Petric M, et al. Utility of semiquantitative polymerase chain reaction for Epstein–Barr virus to measure virus load in pediatric organ transplant recipients with and without posttransplant lymphoproliferative disease. Clin Infect Dis. 2001;33:145–150. doi: 10.1086/321806. [DOI] [PubMed] [Google Scholar]
- 23.Baldanti F. Grossi P. Furione M, et al. High levels of Epstein–Barr virus DNA in blood of solid-organ transplant recipients and their value in predicting posttransplant lymphoproliferative disorders. J Clin Microbiol. 2000;38:613–619. doi: 10.1128/jcm.38.2.613-619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens SJ. Verschuuren EA. Pronk I, et al. Frequent monitoring of Epstein–Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165–1171. doi: 10.1182/blood.v97.5.1165. [DOI] [PubMed] [Google Scholar]
- 25.Davis JE. Sherritt MA. Bharadwaj M, et al. Determining virological, serological and immunological parameters of EBV infection in the development of PTLD. Int Immunol. 2004;16:983–989. doi: 10.1093/intimm/dxh099. [DOI] [PubMed] [Google Scholar]
- 26.Tanner JE. Alfieri C. The Epstein–Barr virus and post-transplant lymphoproliferative disease: interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transpl Infect Dis. 2001;3:60–69. doi: 10.1034/j.1399-3062.2001.003002060.x. [DOI] [PubMed] [Google Scholar]
- 27.Buisson M. Fleurent B. Mak M, et al. Novel immunoblot assay using four recombinant antigens for diagnosis of Epstein–Barr virus primary infection and reactivation. J Clin Microbiol. 1999;37:2709–2714. doi: 10.1128/jcm.37.8.2709-2714.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gartner BC. Schafer H. Marggraff K, et al. Evaluation of use of Epstein–Barr viral load in patients after allogeneic stem cell transplantation to diagnose and monitor posttransplant lymphoproliferative disease. J Clin Microbiol. 2002;40:351–358. doi: 10.1128/JCM.40.2.351-358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berth M. Bosmans E. Prevention of assay interference in infectious-disease serology tests done on the Liaison® platform. Clin Vaccine Immunol. 2008;15:891–892. doi: 10.1128/CVI.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]