Abstract
Therapeutic strategies that involve the manipulation of the host’s immune system are gaining momentum in cancer research. Antigen-loaded nanocarriers are capable of being actively taken up by antigen presenting cells (APCs) and have shown promising potential in cancer immunotherapy by initiating a strong immunostimulatory cascade that results in potent antigen-specific immune responses against the cancer. Such carrier systems offer versatility in that they can simultaneously co-deliver adjuvants with the antigens to enhance APC activation and maturation. Furthermore, modifying the surface properties of these nanocarriers affords active targeting properties to APCs and/or enhanced accumulation in solid tumors. Here we review some recent advances in these colloidal and particulate nanoscale systems designed for cancer immunotherapy and the potential for these systems to translate into clinical cancer vaccines.
Keywords: Cancer Immunotherapy, Polymeric Nanoparticles, Liposomes, Colloidal Nanocarriers, Tumor Targeting
1. INTRODUCTION
Cancer encompasses a heterogeneous array of malignant diseases that are characterized by the unregulated proliferation of aberrant cells. Despite significant advances made in screening and treatments over the past five decades, cancer is still the second leading cause of mortality in the United States, with 1 in 4 deaths attributed to it (1). In fact, it was expected that in the United States alone nearly 1.5 million new cases of cancer would be diagnosed in 2009 and that there would be over 500,000 cancer-related deaths. Currently applied and well established treatments for cancer include chemotherapy, radiotherapy and surgery. These treatments have proven to be variably effective depending on the type of cancer. Chemotherapy has the disadvantage of indiscriminately targeting proliferating cells, thus resulting in killing of both tumor and healthy cells. The major limitation of radiotherapy and surgery is that these procedures fail to combat metastases. Hence, the need for more efficacious and less harmful cancer therapies still exists.
Tumor vaccines and immunotherapy are an attractive alternative, or addition, to conventional cancer treatments and their study has increased significantly in the past two decades (2–3). The idea behind these focuses primarily on manipulating the patient’s own immune system to recognize and destroy cancer cells. Significant advantages to these approaches are their ability to: 1) induce specific killing of tumor cells, with minimal detriment to healthy, non-tumor cells, 2) systemically stimulate anti-tumor immune responses that can target primary and secondary metastases, and 3) result in immunological memory that would provide long-term protection against possible future tumor recurrences (4–8).
The use of nanoparticulate pharmaceutical carriers to enhance the efficacy of therapeutic agents is being increasingly investigated and many such carriers have been successfully developed to date (9–14). In tumor immunotherapy, the primary cargo of nanocarriers will usually be peptides from, or DNA encoding, tumor-associated antigens (TAAs). TAAs are proteins inappropriately or aberrantly expressed by tumor cells but not generally found in normal tissue. It is now widely accepted that most tumors express TAAs and it has been demonstrated through multiple animal tumor studies that the immune system can be triggered to recognize these TAAs as non-self and thereby affect a specific anti-tumor response. From an immunotherapeutic perspective, it would be desirable to develop novel carriers, carrying TAAs, that can either actively or passively target professional APCs, known as dendritic cells (DCs), resulting in the generation of a strong tumor-specific cytotoxic CD8+ T lymphocyte (CTL) response. The targeting and consequent activation of DCs is of particular importance as DCs are potent initiators of immune responses. In order to achieve a potent anti-tumor CTL response, it is necessary to activate DCs, such that they generate a pro-inflammatory (Th1) response. Such a response involves the generation of IFN-gamma producing T lymphocytes. Additionally, nanocarriers can be designed to target the tumor itself resulting in a site-specific accumulation of the TAA and/or adjuvants and provide a controlled release for development of long-term antigenic memory (15). Nanoscale carriers have the potential for addressing all the above mentioned goals due to their physico-chemical properties and ease of modification to improve tumor targetability (16–27). Nanocarriers offer unique advantages over the administration of the soluble form of the antigen. These include: 1) protection of the drug/antigen/adjuvant from premature enzymatic and proteolytic degradation, 2) enhanced absorption of the drug/antigen/adjuvant into targeted tumor tissue either by the EPR effect or via active targeting with the use of ligands, and 3) ability to control the pharmacokinetic and drug/antigen/adjuvant tissue distribution profile and enhance cellular uptake by DCs to trigger a strong immunostimulatory cascade. Furthermore, these nanoscale carriers offer the unique advantage of multi-component loading which is of considerable significance particularly in immunotherapy where simultaneous delivery of antigens, immunoadjuvants and targeting ligands is optimal (11, 15, 28). Additionally, due to their large surface area, these nanocarriers can be surface functionalized with relative ease. The smaller size affords a large surface to volume ratio, thus increasing the efficiency of reaction kinetics and multiple surface derivatizations. The fabrication of such multifunctional nanocarriers with controlled properties often require the conjugation of proteins, peptides, polymers, cell-penetrating moieties, reporter groups and other functional and targeting ligands to the carrier surface. This modification is usually simple and in most cases proceeds via a non-covalent hydrophobic interaction or by covalent conjugation of proteins and peptides onto the nanocarrier surface (14, 29–32). Thus, the simplicity of design and use coupled with multifunctionality makes nanoparticulates a versatile and attractive carrier system for tumor vaccines and immunotherapy. Here, we discuss recent progress on the use of a variety of different classes of nanocarriers in immunotherapeutic applications. These include liposomes, viruses, particles prepared from biodegradable or natural polymers and inorganic particles.
2. LIPOSOMES
Liposomes are spherical unilamellar/multilamellar lipid vesicles usually made from one or more phospholipid bilayers and containing an aqueous center (17, 33–36). They have many uses including drug delivery for cancer, and vaccination with antigens or DNA. Liposomes can be used as carriers for vaccine delivery where antigenic stimuli are: 1) encapsulated in the core, 2) embedded in the bilayer or 3) adsorbed or engrafted to the outer surface. In addition to antigenic stimuli, liposomes can also be designed to carry adjuvants that will stimulate the innate arm of the immune response and enhance anti-tumor immunity since it is generally recognized that conventional liposomes have only modest or no intrinsic immunoadjuvant properties. Many variations of liposomes have been studied with respect to their vaccine potential. Since the primary target of these vaccines is usually dendritic cells (DCs) the design of liposomes is therefore based on their ability to activate, and/or deliver antigen to, the DC. Liposomes carrying immunogenic peptides are capable of fusing with the membranes of DCs or being pinocytosed. Some of the protein is processed via the cytoplasmic MHC class I pathway while some is processed though the endosome/lysosome MHC class II pathway. Below we have subcategorized different liposomes based on their chemical properties, however, these groups are not always mutually exclusive.
2.1 Conventional liposomes
Conventional liposomes are composed of neutral lipids and phosphatidylcholine and are relatively non-toxic. However, they suffer from having a short systemic half-life as they are readily cleared by the reticular endothelial system (RES) (37). This is a definite drawback if trying to target the tumor directly with the liposomal cargo (e.g chemotherapeutic drugs or cytokines to induce DC tumor infiltration). However, targeting the macrophages of the RES in spleen and liver does have therapeutic potential. For example, conventional liposomes harboring a muramyl dipeptide derivative (ImmTher®) promoted macrophage tumoricidal activity against Ewing’s sarcoma and was shown to have anti-tumor activity in phase I trials against lung and liver colorectal metastases (38). One of the most advanced liposome carriers, in terms of clinical trials, is BLP25 (or Stimuvax®) which comprises conventional liposomes, Lipid A adjuvant and a MUC1 peptide (a 25-mer)(39). MUC1 is a mucinous transmembrane glycoprotein that becomes overexpressed in many types of cancer, particularly adenocarcinomas (reviewed by (40)). In addition to overexpression, this protein becomes aberrantly glycosylated resulting in the exposure of normally cryptic epitopes. It is the exposure of the protein core of MUC1 that is being exploited by this vaccine. BLP25 is a lipopeptide comprising 25 amino acids representing the exposed core of MUC1. This 25-mer has been palmitoylated to facilitate insertion into the liposome. The liposome itself is a combination of cholesterol, dimyristoyl phosphatidylglycerol, dipalmitoyl phosphatidylcholine and the immunoadjuvant monophosphoryl Lipid A (MPL). MPL is a much less toxic derivative of a lipopolysaccharide that is capable of stimulating APCs through TLR-4 (41–42). Preclinical studies revealed the vaccine’s capacity for inducing human cytotoxic T lymphocyte (CTL) responses (43). Phase I and II clinical trials have revealed low toxicity and enhanced survival of patients with an advanced form of non-small cell lung cancer (NSCLC, stage III). Administration involved repeated weekly doses delivered subcutaneously (44). A randomized phase III study using BLP25 is currently underway.
2.2 Stealth liposomes
Stealth liposomes are liposomes that have been sterically stabilized rendering them less readily opsonized and removed by the mononuclear phagocytes of the RES (37). This steric alteration can be performed using polyethylene glycol (PEG). PEG-modification of lipids has been shown to increase circulation time of liposomes and improve the CD8+ T lymphocyte response to antigen (45–46). In an attempt to fabricate a liposomal formulation with an increased circulatory half-life for tumor immunotherapy, Altin and co-workers prepared stealth liposomes from a mixture of disteroyl phosphocholine (DSPC), cholesterol and disteroyl phosphoethanolamine (DSPE) with surface grafted PEG 750 (47). Furthermore, two peptides derived from high-mobility box (HMGB1) protein were independently engrafted onto the liposomal surface to function as DC-targeting ligands that also induced activation and maturation of DCs. Mice were vaccinated intravenously with these stealth liposomes which were also formulated to encapsulate ovalbumin (OVA) and were subsequently shown to induce OVA-specific IFN-γ producing CD8+ T lymphocytes. Prophylactic or therapeutic anti-tumor potency was not investigated with these HMGB-OVA-liposomes. An earlier study by the same group showed that vaccination of mice intravenously with DC-targeting stealth liposomes (encapsulating OVA +/− LPS or IFN-γ) were capable of generating an antitumor response that protected against lung metastases by OVA-expressing B16 melanoma cells (48). Apart from demonstrating the effectiveness of using stealth liposomes these studies showed that both targeting the DC and concomitantly providing danger signals with antigen are crucial in generating a tumor-specific CTL response and, consequently, tumor protection.
2.3 Cationic liposomes
Cationic lipids are another class of lipids that have received considerable attention from a cancer immunotherapy standpoint. Cationic lipids are amphiphilic molecules that consist of a charged head connected to a hydrophobic anchor via a carbon skeleton. The most common cationic lipids that are used in liposomes include 1,2-Dioleoyl-3-trimethylammoniumpropane (DOTAP), Dimethyldioctadecylammonium (DDAB) and N,N-dioleyl-N,N-dimethyl ammonium chloride (DODAC). Cationic liposomes, when compared to anionic or neutral liposomes, have been shown to possess a greater efficiency at: 1) being internalized within DCs and macrophages, and 2) inducing CTL responses in vivo (reviewed by (45)).
Cationic liposomes-DNA-complexes (CLDC) comprise cationic lipids complexed with DNA and/or the TLR-9 agonist, CpG. CLDCs were shown to have anti-tumor activity in various mouse tumor models by inducing both the innate and adaptive immune responses (49). To further enhance their tumor vaccine potential, the addition of tumor-associated antigens (TAAs) may be included and/or the inclusion of DNA encoding immunostimulatory proteins. The co-encapsulation of OVA antigen and CpG in cationic liposomes was shown to increase the immune response to OVA when compared to administration of unencapsulated OVA and CpG (50). In a separate study, it was shown that co-administration of OVA with liposome-encapsulated CpG resulted in efficient uptake by DCs and macrophages and preferential localization to draining lymph nodes resulting in the generation of OVA-specific CTL and in vivo anti-tumor immunity (51). This group went on to show that the vaccination procedure was also effective when the syngeneic TAA, TRP-2, was used as the immunogen, instead of xenogeneic OVA, resulting in increased resistance to pulmonary metastasis by B16 melanoma cells. Here, the liposomes used were neutral at physiological pH but contained an ionizable amino lipid 1,2-Dioleoyl-3-dimethylammonium propane (DODAP) that facilitated encapsulation of CpG. An example of CLDCs being used in clinical trials is Allovectin-7®, a cationic liposome combined with plasmid DNA encoding HLA-B7 and beta-2 microglobulin. This was given intratumorally to patients with late stage melanoma in a phase II clinical trial and yielded a 9% overall response rate (complete and partial remissions) (52). When a dose increase was made in a subsequent phase II clinical trial there was an 11.8% overall response rate and phase III studies are underway (reviewed by (53)).
2.4 Archaeosomes
Archaeosomes are liposomes fabricated to comprise the unique glycerolipids of Archaea. These lipids possess an ether-linked isoprenoid phytanyl core which engenders membrane stability, thereby promoting potent immune memory. Additionally, the variable head domains of these glycerolipids have potent and unique APC-stimulating properties (reviewed by (54)). Preparation of archaeosomes is similar to liposomes in that the archaeal lipids are extracted using chloroform/methanol/water from frozen-thawed Archeae (55). Total polar lipids are precipitated using cold acetone and resuspended and stored in chloroform/methanol. Following dessication, hydration using water or phosphate buffered saline with the antigen(s) to be encapsulated results in multilamellar archaeosomes in the size range of 100 – 150 nm. These archaeosomes are very stable when stored in suspension. Although rapidly cleared upon intravenous or oral administration, archaeosomes form a prolonged depot when injected subcutaneously and are consequently capable of promoting both Th1 and Th2 responses with long memories (56). The type of immune response appears to be dependent upon the strain of Archeae used as the source of lipids for the archaeosome. An inverse relationship has been reported where those archaeosomes that promote a strong CTL response are only modest at evoking an antibody response and vice versa (57). This phenomenon seems to be at least partially due to the relative amounts of the archaeal lipid, caldarchaeol, that each strain possesses. As a result the strain of Archeae known as Methanobrevibacter smithii has gained most attention regarding cancer immunotherapy since it is favorably rich in cardarchaeol and the archaeosomes made from it are potent activators of CTLs (57). In murine tumor models, using Ova as the encapsulated antigen, it has been shown that prophylactic vaccinations with archaoasomes increased survival times through the activation of antigen-specific CD8+ T lymphocytes (57–58). M. smithii –derived archaeosomes harboring antigen have been shown to be capable of cross-presentation, a phenomenon which conventional liposomes generally lack (59). Immune protection was also induced in a murine melanoma study where TAAs, TRP-2 or MART-1, were used (57). In therapeutic studies using the Ova-expressing EG.7 tumor, increased survival was shown for mice injected twice post-tumor challenge with archaeosomes carrying Ova (57–58). These studies also observed the intrinsic capacity of archaeosomes to recruit NK cells to the tumor site. Studies using archaeosomes in clinical cancer trials have not been attempted as yet. However, given the aforementioned findings, and that archaeosomes have been shown to be biocompatible, they appear to have the necessary credentials for further evaluation (60).
2.5 pH sensitive liposomes
Liposomes can be designed such that they are pH sensitive rendering them more capable of delivering peptides that get processed and presented through the endogenous/cytosolic MHC class I pathway, while, in comparison, pH insensitive liposomes tend to be processed through the MHC class II pathway (61–62). The mechanism by which the former occurs is proposed to involve the liposome breaking up due to the low pH within the endosome and subsequently fusing with the endosome membrane resulting in spillage of some of the liposome contents into the cytoplasm (reviewed by (63)). The most commonly used lipid to fabricate such pH sensitive liposomes typically involves a blend of phosphatidylethanolamine (PE) or its derivatives with compounds containing an acidic group (e.g. carboxylic group) or cholesterol hemisuccinate or oleic acid lipids. Although stable liposomes are formed at physiological pH, acidification triggers protonation of the carboxylic groups of the amphiphiles, reducing their stabilizing effect, leading to destabilization of liposomes under these conditions. pH sensitive liposomes have significant potential for immunotherapeutic applications but further studies evaluating their potential and limitations in vivo are necessary.
3. VIRUSES/VIRUS-LIKE PARTICLES/VIROSOMES
3.1 Viruses
Viruses are infectious agents (15 – 400 nm in size) that encapsulate their genome in a protein coat which may or may not be enclosed in host cell-derived lipid bilayers. The use of live attenuated viruses as vaccines has proven extremely successful in controlling and, in the case of small pox, eradicating, numerous diseases (reviewed by (64)). However, the situation with cancer is more problematic in that the majority of cancers (> 80%) are not virally generated and the immune response mounted against viral components of the vaccine are often undesirably potent and at the expense of the cargo. In order to trigger a long-term adaptive CTL-mediated anti-tumor response it is likely that repetitive doses of immunogen are required (65). Should such a regime be necessary then first generation viral vectors, at least, would not be suitable. As a result very few viruses have been used to date in clinical cancer trials. Those that have been used include phase II studies comparing vaccination alone with combined vaccination and chemotherapy on patients with metastatic androgen-independent prostate cancer (66–67). The vaccination procedure employed a prime/boost regime that involved priming with recombinant vaccinia virus vector containing 4 transgenes that included PSA, a known TAA, and three T cell co-stimulatory molecules, B7.1, ICAM-1 and LFA-3. The boost was performed using recombinant fowlpox virus vector containing the same 4 transgenes. The results of these studies indicated improved survival times for those patients with less aggressive disease. Another vaccine currently used in clinical trials is TroVax® (MVA-5T4), a modified vaccinia Ankara virus (MVA) encoding the TAA 5T4 (reviewed by (68)). 5T4 is a cell surface oncofetal antigen expressed in the placenta and rarely in healthy adult tissues, however it is highly expressed by a number of adenocarcinomas. MVA is highly attenuated, replication deficient, nonpathogenic and has a good safety profile. Preclinical murine tumor studies using a syngeneic colon carcinoma cell line expressing human 5T4 showed that vaccination with TroVax® intraperitoneally was effective both prophylactically and therapeutically (69). Interestingly, the antitumor effect was dependent on CD4+ T lymphocytes and primarily mediated by antibodies rather than CTLs. Phase I and II studies are in progress in colorectal, metastatic renal and hormone refractory prostate cancer. Thus far, the indications are that this vaccine, while not significantly improving overall survival, was safe and promoted the production of 5T4 specific CTLs (68).
Adenoviral (Ad) vectors are one of the most efficient methods for gene delivery in vivo for vaccine applications. However, as for viral vectors in general, Ad vector use has been limited due to its activation of unwanted cellular, humoral and innate immune responses. Ad vectors are particularly efficient at stimulating innate immunity through both TLR-dependent and independent mechanisms leading to morbidly high levels of type I IFN-α and other inflammatory cytokines (reviewed by (70)). Additionally, due to the prevalence of adenovirus infections, a high proportion of the population already have neutralizing antibodies to the more commonly used Ad vectors such as Ad5 and Ad2. Nevertheless, there are significant advantages to using Ad vectors should these disadvantages be overcome. These include the ability of adenoviruses, as opposed to retroviruses, to infect both dividing and non-dividing cells without integrating into the genome of the host cell. Adenoviruses enter cells through ubiquitously expressed cellular receptors and are endocytosed in a clathrin-dependent fashion. They then escape the endosome and inject the genome through nuclear pore complexes into the nucleus where it can be stably maintained and expressed. Another advantage of recombinant Ad vectors is that they can be grown to high yields under conditions suitable for manufacture for clinical use.
To overcome the potential for neutralizing antibodies rendering the vaccine ineffective alternative rarer human serotypes of adenovirus, or even non-human Ad vectors, may be favorable. It was recently reported that the use of a second generation chimpanzee Ad vector (ChAd) carrying DNA encoding for TAAs was able to break tolerance in TAA-transgenic mice resulting in anti-tumor activity that led to significant levels of protection (71). Specifically, one of the TAAs studied was Her-2/neu oncoprotein, a tyrosine kinase receptor overexpressed on several human cancers and associated with poor prognosis. Mice transgenic for rat Neu oncoprotein, and resultantly developing spontaneous mammary tumors, were shown to have significantly increased survival when administered with ChAd-rat neu. It was also shown that the vaccinations resulted in TAA-specific IFN-γ-producing CTLs.
3.2 Virus-like particles (VLPs)
VLPs are self-assembling non-infectious spheres comprising a viral coat but lacking the viral genome (reviewed by (72)). They have natural immunogenic properties making them a favorable option for vaccine strategies. Their safety profile is superior to that of attenuated viruses since they are non-infectious and do not replicate. VLPs can be safely, efficiently and often inexpensively manufactured for therapeutic use in a number of expression systems, including yeast, green plants (e.g. Tobacco mosaic virus) and attenuated gut flora (72). VLPs are capable of generating strong adaptive immune responses without the requirement of an additional adjuvant. Although their specific mechanism of action will vary depending upon the virus that VLPs are derived from, it is generally accepted that they are readily taken up by DCs via macro-pinocytosis and endocytosis and consequently trigger both the innate and adaptive arms of the immune response (IR). VLPs can stimulate IRs by both the MHC class-I and MHC class-II pathways (reviewed by (73)). It has been suggested that enhanced MHC class-I presentation occurs when VLPs possess envelop proteins that can enhance uptake via receptor dependent fusion (74).
One notable VLP-based vaccine to have been licensed in the last decade is Gardisil®, a quadrivalent vaccine against human papilloma virus (HPV). HPV is a primary cause of cervical cancer which is responsible for over a quarter of a million deaths worldwide per year. Gardisil® is made from a mixture of 4 recombinant HPV type-specific VLPs comprising the L1 major capsid proteins of HPV 6, 11, 16, and 18 which are synthesised in the yeast Saccharomyces cerevisiae (75). Both phase II and III (randomized, double blind, placebo controlled) studies involving susceptible females revealed the vaccine to be highly protective (75–76).
VLPs by their nature are likely to be limited to prophylactic use for cancers of viral origin (< 20 % of total cancers). Although the potential for them to form sustained and effective neutralizing antibodies has been well documented, their capacity to induce strong cellular-based effective anti-tumor responses has not been thoroughly addressed.
3.3 Virosomes
Virosomes are unilamellar lipid envelops, derived from viruses, that retain the viral fusogenic and antigenic properties but lack the viral genome. To date only two virosome vaccines have been licensed. These are both immunopotentiating reconstituted influenza virosomes (IRIVs) which protect against hepatitis A (Epaxal®) or influenza (Inflexal®). IRIVs are considered to be one of the more promising virosomes with regard to immunotherapy of cancers (reviewed by (77)). They are approximately 150 nm in diameter and comprise a combination of natural and synthetic phospholipids along with influenza surface glycoproteins (78). A vital component of IRIVs is the influenza-derived hemagglutinin. Hemagglutinin has a dual function, each of which is carried out by a different subunit. The first subunit is responsible for attaching to the APC via sialic acid residues, while the second subunit promotes fusion of the endocytosed virosome within the endosome resulting in the spillage of virosomal contents into the cytoplasm (78). IRIVs are also capable of upregulating co-stimulatory molecules such as CD80 and CD86 on the surface of DCs. In a preclinical study, CTL responses to a melanoma TAA (Melan-A/Mart127–35) were induced by IRIVs loaded with the TAA. It was shown that the CTL response was both dependent on hemagglutinin-mediated delivery of antigen to the cytoplasm as well as antigen–independent activation of a CD4+ T helper response (79). In a separate study, an IRIV vaccination protocol based on those used for Hepatitis A and Influenza has recently undergone a phase I trial in patients with breast cancer (80). Here, IRIVs were generated and the TAA, Her2/neu, was coupled to phosphatidylethanolamine and hemagglutinin and integrated into the virosome during reconstitution. The patients (n = 10) used in this study had hormone receptor positive metastatic breast cancer and were receiving concomitant hormone therapy but no chemotherapy. Three intramuscular vaccinations were administered and the primary and secondary endpoints of this phase I study, which were safety and immune responsiveness respectively, were both met. In terms of immune responsiveness, it was established that anti-Her-2/neu antibodies were generated in 8/10 patients. Generation of a CTL-mediated response was not presented, although it was demonstrated that the vaccinations resulted in a significant lowering in the percentage of CD4+CD25+FoxP3+ T lymphocytes (T regs).
A major disadvantage of the more conventional virosomes, such as the IRIVs mentioned above, is the limited loading capacity for antigenic peptides (81). It was demonstrated that the fusion of epitope-loaded conventional liposomes with IRIVs generated chimeric IRIVs (CIRIVs) that possessed a 30-fold greater loading capacity. The immunoadjuvant properties of the IRIV were retained and the CIRIVs could generate TAA-specific CTL responses in vitro (82). These chimeric carriers were capable of being stably stored at 4°C for at least 1 month. CIRIVs represent a promising mode of generating anti-tumor immune responses to TAAs and future clinical studies are anticipated.
Another of the virosomes to receive attention recently is the Sendai virosomes. These are vesicular nanoparticles that are reconstituted from Sendai viral envelops. Similar to IRIVs, Sendai virosomes express sialic acid binding proteins which allow for APC attachment. In addition, Sendai virosomes possess a fusion protein that promotes the fusion of the virosome with the plasma membrane resulting in release of the virosomal contents into the cytoplasm.
4. SYNTHETIC BIODEGRADABLE DELIVERY SYSTEMS
4.1 Poly(D,L-lactic-co-glycolic acid (PLGA) and polylactide (PLA)
Poly(D,L-lactic-co-glycolic acid (PLGA (AKA:PLG)) and polylactide (PLA) are biocompatible and biodegradable polymers that have FDA approval for human use. PLGA and PLA particles have shown great potential for protein, peptide and DNA delivery over the last two decades (83–85). These particles are fabricated using techniques such as emulsification/solvent evaporation (86–88). Several studies have shown that careful control over the formulation parameters such as surfactant concentration and stirring speed can be used to optimize loading levels and particle sizes (28, 31, 89–92). The main advantages of using PLGA/PLA particles, from a cancer vaccine/immunotherapeutic perspective, is that they provide a non-toxic, protective vehicle for co-delivery of antigens and adjuvant that can be released over a sustained period of time. In addition, these particles are efficiently internalized by APCs as they possess comparable dimensions to the pathogens that the immune system has evolved to combat (93). Particulate antigens in the size range of 0.1 – 10 μm can induce CTL responses through MHC class I cross-presentation of antigen via the phagosome-cytosol pathway of antigen presentation (94). The mechanism of cross-presentation is unknown, but appears to be independent of the chemical nature of the particle (95). Nevertheless, it has been specifically demonstrated that PLGA and PLA particles are capable of promoting cross-presentation of antigen in APCs (96–97).
4.2 Poly(D,L-lactic-co-glycolic acid (PLGA)
The use of biodegradable particles in tumor immunotherapy has primarily involved PLGA particles and they are therefore the primary focus here. PLGA particles were originally used for parenteral drug delivery due to: 1) their capacity to protect their cargo from degradation and 2) their ability to prolong the release of their cargo. Early animal studies with PLGA vaccinations showed that a single subcutaneous injection of OVA entrapped in, rather than surface-attached to, PLGA particles (1.5 μm in size) was a superior immunogen than soluble OVA (98). Specifically, the mice vaccinated with OVA-PLGA particles had enhanced levels of OVA-specific IgG which remained high for 1 year. These results were iterated by a separate group using OVA entrapped in PLGA particles of a larger average size (3.69 μm) (99). This group also showed that only one subcutaneous administration was necessary to generate a maximal OVA-specific antibody response and that the PLGA-OVA particles were more effective at inducing responses than complete Freund’s adjuvant (CFA) combined with soluble OVA. Similar results were obtained by a different group using bovine serum albumin as the immunogen entrapped in PLGA particles (1 μm) (100). Thus it is evident that PLGA particles possess an intrinsic adjuvant effect which has been largely attributed to: 1) the ability of the particle to protect the immunogen from rapid degradation and clearance, 2) the release profile of the protein where sustained release of the immunogen occurs for lengthy periods (> 30 days) and 3) the efficiency with which the PLGA particles are taken up by DCs. In addition, it may be that empty PLGA particles themselves have modest, yet significant, adjuvant properties, as it was demonstrated that they are capable of upregulating a costimulatory molecule (CD80) on in vitro cultured bone marrow-derived murine DCs (101).
Preclinical studies showed that PLGA particles (350 – 450 nm) are efficiently taken up by murine DCs in vitro (102). The same study went on to demonstrate that the TLR4 ligand, monophosphoryl lipid A (MPLA), was significantly better at inducing maturation of DCs in vitro when provided in PLGA particles rather than in soluble form. In addition, whilst MPLA alone had little effect on cytokine production by DCs, the complexation of MPLA with PLGA resulted in the production of high levels of proinflammatory cytokines (IL-6, IL-12 and TNF-alpha) while IL-4 and IL-13 expression remained low. We have evaluated the immune potency of PLGA particles (~2.4 μm in diameter) complexed with tumor lysate proteins (from the B16 murine melanoma) and CpG in an in vivo mouse tumor model (103). We have shown that intraperitoneal vaccination with the aforementioned particles were capable of protecting against subsequent tumor challenge, but only if T regs were concomitantly diminished through low dose cyclophosphomide or anti-CD25 treatment. The vaccine was shown to be effective prophylactically (103). We hypothesize that diminishing Tregs in the therapeutic model would also be advantageous as was the case prophylactically. Another group has shown that mice vaccinated subcutaneously (with 2 boosts) with PLGA particles encapsulating both a TAA peptide (TRP-2180–188) and a TLR-4 ligand (7-acyl lipid A) could control tumor growth through the induction of TRP-2-specific CTLs. Furthermore, it was demonstrated that within the tumor microenvironment there was an upregulation of Th1 cytokines (IL-6, IL-12, IFN-γ, TNFα) and a down-regulation of the pro-angiogenic vascular endothelium growth factor (VEGF) (104).
4.3 PLA (polylactic acid)
PLA is a homopolymer that is more crystalline than PLGA. Unlike PLGA, PLA has been less intensively investigated with respect to tumor immunotherapy. A recent study showed that PLA particles (150 nm) encapsulating OVA were not capable of inducing an OVA-specific antibody response greater than soluble OVA, when administered transcutaneously or subcutaneously (105). These results are in stark contrast to those obtained with PLGA-OVA particles mentioned above (98–99). Two possible explanations for this discrepancy are: 1) the higher antigen-retention capacity inherent to PLA resulting in sub-optimal immunostimulation or 2) the lower particle sizes used in the PLA study were less effective at targeting DCs.
One group used PLA microparticles (~1.59 μm in diameter) admixed with a HLA-A2.1 restricted Her2/neu CTL epitope to vaccinate HLA-A2.1 transgenic mice (96). The type of immune response evoked was assessed by in vitro stimulation of the spleen cells, from the immunized mice, with Her2/neu peptide. The proliferative response was significantly greater when compared to spleen cells from mice vaccinated with CFA plus the tumor epitope. In addition, it was shown through ELISA and ELISPOT assays that the PLA-peptide admix was capable of generating enhanced numbers of IFN-γ-producing lymphocytes while IL-4 production remained low. Such a Th1-biased immune response suggests a tumor protective role for this vaccine that requires further follow-up studies.
A novel tumor therapy approach was recently reported using PLA microparticles to which were bound antibodies that independently recognized CD40 and RNEU (106). CD40 is a DC surface protein that, upon ligation, triggers DC maturation and activation, while RNEU is the growth factor receptor expressed by the rat her2/neu oncogene. Mice were challenged with syngeneic TUBO cells (mouse mammary tumor cell line expressing RNEU) and subsequently treated intratumorally with the PLA microparticles displaying antibodies to CD40 and RNEU. This treatment resulted in bringing the host DCs into close association with the tumor, triggering the production of proinflammatory cytokines, and triggering an antitumor-specific CTL response that resulted in tumor rejection. The authors state that this strategy could feasibly use PLGA or gold nanorods interchangeably with PLA.
The paucity of data published on PLA particle based tumor vaccines makes its potential in cancer immunotherapy difficult to assess. The fact that PLGA has received considerably more attention than PLA may reflect a lack of promising, and therefore published, data when PLA carriers were used. More published studies are required, in particular, studies that directly compare PLGA and PLA as tumor vaccines.
PLGA particles were heralded as the future vaccination method for a wide variety of diseases due to many of the properties mentioned above, particularly because of their burst and slow release kinetics (107–108). Such a release profile meant the potential for single injection vaccines as opposed to multiple injections currently used. However, PLGA has proven problematic in that protein degradation can occur for some proteins during the encapsulation process (107). The fabrication of PLGA/PLA carriers often requires the use of organic solvents, heat and high speed mechanical agitation. Proteins are highly susceptible to denaturation upon exposure to any of the aforesaid conditions and may result in loss of antigenic epitope recognition thereby failing to trigger an immune response. These concerns can be addressed by a more careful fabrication design and stringent control of formulation process parameters including volume of organic solvents, assessing the extent of residual solvent content and use of protein stabilizing additives including various sugars and polysaccharides. Possibly another major stumbling block is the financial consideration of upscaling of production for commercial use and also the clean-up procedure to ensure aseptic and uncontaminated delivery (108).
4.4 Acid degradable hydrogels
Acid degradable hydrogels afford an advantage over many other biodegradable particulate carriers, such as PLGA, in that they strongly disrupt endosomes, allowing for larger cytoplasmic antigen delivery. Standley and co-workers designed these hydrogels so they are stable at physiological pH, but degrade at the acidic pH of endosomes (pH 5) (109). Upon acid degradation the hydrogels generate a large number of molecules which increases the osmotic pressure gradient across the endosomal membrane. This causes a rapid influx of water into the endosome and results in its disruption and the subsequent release of the endosomal contents into the cytoplasm. This group went on to report the use of such acid degradable hydrogel particles for tumor immunotherapy using OVA as a model antigen (109). These particles were synthesized by copolymerizing an acid-degradable cross-linker and a 3′ methacrylamide monomer, in the presence of OVA, using an inverse emulsion polymerization technique. In order to improve the suspendability and syringability for parenteral administration, these particles were modified by surface conjugation with a long hydrophilic oligoethylene glycol layer. The modified particles possessed enhanced colloidal properties due to greater hydration and steric stabilization. Vaccination of mice with particles encapsulating OVA resulted in increased survival rates in mice subsequently challenged with OVA-expressing EG.7 tumor cells, as compared to vaccination with soluble OVA alone. In follow-up studies by this research group, an attempt was made to improve the immunogenicity of the carrier by co-encapsulation of CpG and OVA (110–111). The 3′ methacrylamide monomer was modified and CpG was covalently conjugated to the monomer prior to the co-polymerization step. The final particles exhibited a high antigen loading efficiency of 70% with high acidic degradability. This group confirmed that the particles encapsulating OVA and CpG were phagocytosed by a murine DC cell line, RAW 309, in vitro. In addition, murine immunization studies demonstrated potent stimulation of OVA-specific CD8+ T lymphocytes (110). It was subsequently demonstrated that the same particle immunization protocol in mice resulted in strong OVA-specific CTL activation that coincided with enhanced survival after challenge with OVA-expressing B16 melanoma tumor cells (111). Compared to this group’s first study (109), this latter report showed that the inclusion of CpG significantly enhanced antigen-specific responses and tumor protection. The therapeutic potency of these acid degradable hydrogels in clinical studies has yet to be determined.
5. GELATIN BASED NANOPARTICLES
Interest in natural polymers like gelatin for cancer immunotherapy has increased over the last five years owing to its biodegradability, biocompatibility and safety (112). Previous clinical studies have proven the capability of gelatin at preserving the bioactivity of the therapeutic agent to be delivered in vivo. Gelatin is easy to cross-link and can be sterilized using a wide range of chemical and heat sterilization methods. The material itself, unlike several other natural polymers, is available in a pure form. In addition, gelatin is pyrogen-free and possesses low immunogenicity.
As a protein-based product, gelatin possesses several functional groups which are available for covalent modifications such as: 1) TAA or immunoglobulin (Ig) binding, 2) active tumor targeting and 3) PEGylation for resistance to opsonization. In addition, simple surface charge modifications can be made to gelatin by the conjugation of cationic moieties to the surface. Gelatin nanoparticles carrying an antigen of interest can be generated using a complex coacervation technique using water miscible solvents like acetone and alcohols, or salts like sodium sulfate, followed by further cross-linking. However, the broad molecular weight distribution of commercial gelatin, ranging between 20 – 1500 kDa, limits reproducible particle preparation and can cause aggregation issues when using this technique. To mitigate these problems, a two-step desolvation technique for fabricating gelatin nanoparticles was developed which results in the production of stable gelatin nanoparticles (113). In this method, gelatin is first dissolved in water under constant stirring and heating, then sedimented with acetone. The supernatant, containing mainly lower molecular weight dissolved gelatin fractions, is removed and the sediment is redissolved and resedimented at a pH of 2.5 to yield positively charged gelatin that can be further used to fabricate nanoparticles.
Recently, the use of gelatin nanoparticles for delivery of antigens and/or adjuvants to DCs was investigated. Coester and co-workers fabricated gelatin nanoparticles to quantify uptake of these particles by murine DCs (114). The gelatin nanoparticles were prepared by a two-step desolvation process using acetone, and trimethylrhodamine (TMR)-dextran was loaded into these particles by complex coacervation technique using glutaraldehyde as the cross-linking agent. This method yielded particles with a mean size of 245 nm and the loading efficiency for TMR-dextran was 70%. These TMR-dextran-loaded particles were shown to be phagocytosed by ~90% of CD11c+ murine DCs which led to significant DC maturation. Following this initial report, the same group produced cationized gelatin particles (Figure 1) to enhance delivery of immunostimulatory CpG to DCs (115). In vitro, murine DCs phagocytosed the particles, which resulted in upregulation of MHC class II and CD86, as well as production of pro-inflammatory cytokines. Intravenous injection of CpG-loaded gelatin nanoparticles into mice caused increased serum levels of pro-inflammatory cytokines, IL-6 and IL-12. Human plasmacytoid DCs also phagocytosed, and were stimulated by, CpG-loaded gelatin nanoparticles. This stimulation was greater than that elicited by soluble CpG. Interestingly, loading of CpG into particles preferentially activated human plasmacytoid DCs while simultaneously inhibiting B-cell activation. This discrepancy demonstrates the favorable targeting of these particles to DCs.
Figure 1.

Scanning electron micrograph of cationized gelatin nanoparticles. Reprinted with permission from (139) © Springer Inc. (2008).
Bourquin and colleagues built upon their previous findings regarding gelatin nanoparticles loaded with CpG (114–115) by showing that specific and protective anti-tumor immune responses could be induced in mice immunized with OVA and CpG-loaded cationized gelatin nanoparticles (115). Cationic particles were used since they are recognized as being favorably phagocytosed by DCs and macrophages when compared to neutral or negatively charged particles. In this study, the gelatin nanoparticles were surface modified using cholamine hydrochloride to render a net positive charge. CpG was then physically adsorbed to the positively charged particles at a payload of 5 – 10% w/w of the nanoparticles so that they remained stable under physiological conditions and simultaneously retained a positive charge. This group directly compared immunizations that co-delivered OVA and CpG in particulate form to those that delivered particulated antigen with soluble CpG. Strikingly, co-particulated vaccines induced augmented antigen-specific CD8+ T lymphocyte responses and enhanced protective responses, upon tumor challenge (FIGURE 2, Bourquin et al 2008). Furthermore, by delivering CpG in particulate form, the non-specific and systemic inflammatory responses, normally elicited by soluble CpG, were completely abrogated.
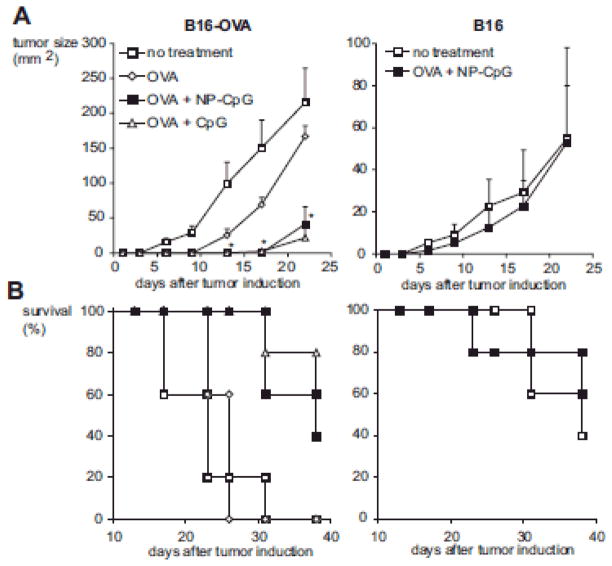
Figure 2.
CpG-loaded nanoparticles elicit an OVA-specific antitumor response. In brief, B16-OVA or B16 tumors were implanted s.c. in C57BL/6 mice after four immunizations with 50 μg OVA and 100 μg free CpG or NP-bound CpG (n = 5). A, Immunization with OVA and NP-CpG significantly reduced growth of B16-OVA tumors compared to untreated mice (p < 0.03 at all time points from day 6) or to mice treated with OVA alone 0.02 from day 13). No effect of immunization was seen in the wild-type B16 tumors (n.s. at all time points). B, In mice with B16-OVA tumors, immunization with OVA and NP-CpG increased survival times compared with untreated mice (p = 0.009) or to mice treated with OVA alone (p = 0.003). No effect of immunization on survival was seen in the wild-type B16 tumors. Similar results were obtained in two independent experiments. Reprinted with permission from (139) © Springer Inc. (2008).
Despite the potential that gelatin nanoparticles show for delivering cancer vaccines in animal tumor models, no clinical trials appear to be currently underway to address their therapeutic potential. Future examination of the ability of gelatin nanoparticle delivery systems to break tolerance to known TAAs will be of interest and might dictate whether these particles progress to clinical cancer vaccine trials.
6. NANOEMULSIONS
Nanoemulsions are colloidal dispersions of nanosized droplets of oil and water stabilized with a surface active film. They are thermodynamically stable systems typically in the size range of 20 – 200 nm. The nanometer size range afford increased kinetic stability against sedimentation and creaming, which are problems commonly associated with conventional emulsions. In recent years, considerable effort has been directed towards developing nanoemulsions as vaccine carriers. Some of these have recently been described for the mucosal route vaccination against infectious diseases, such as Hepatitis B (116), HIV (117), and influenza (118). Nanoemulsions offer the versatility of delivering macromolecules, like antigenic proteins and peptides, either locally or systemically, resulting in potent humoral and cellular antigen-specific immune responses.
In terms of vaccine delivery, nanoemulsions possess several advantages. These include long circulatory times and increased cellular uptake by APCs. Nanoemulsions can efficiently encapsulate antigen resulting in protection against enzymatic degradation and providing a sustained release of the antigen upon lipolysis of the continuous phase forming the emulsion. A number of techniques can be used to prepare nanoemulsions, however high energy emulsification methods are most favorable for fabrication of nanoemulsions for vaccine carriers due to the ease of industrial scale up and preparation. High pressure homogenizers or ultrasonic emulsification under magnetic power produces very homogenous dispersions with high stability in relatively short periods of time.
Because of the promise this technology holds for producing stable, safe and efficacious vaccines, it has been recently assessed in cancer vaccine formulations. Two groups reported that nanoemulsion vaccine delivery of tumor-specific antigens, in mice, induced strong tumor-targeted antibody and CTL responses, which ultimately conferred protection against tumor growth (119–123). Firstly, Shi and colleagues developed a vaccine that aimed to co-deliver immunostimulatory CpG and a previously identified gastric cancer specific antigen, MG7 (119, 124–125). They fabricated their nanoemulsion carrier by a vacuum high shear ultrasonic emulsification device with high payloads of 70% and 93% for the antigen and the CpG respectively. The nanoemulsion was formed using a magnetic ultrasound method with the aqueous phase consisting of 0.8% surfactant mixture of Span-80 and Tween-80 whereas the oil phase consisted of the same ratio of surfactant in soybean oil combined with antigen and CpG solubilized in PEG2000. The oil phase was homogenized using a vacuum high shear emulsification device at a pressure of 0.7 KPa with a final size reduction being performed using an ultrasound generator operating at a frequency of 40 KHz. It was observed that mice immunized with MG7 and CpG co-encapsulated in these nanoemulsions showed significant inhibition of tumor growth after challenge with MG7-expressing carcinoma cells. The tumor protection directly correlated with MG7-specific antibody and IFN-γ production. Not unexpectedly, co-delivery of CpG and MG7 antigen augmented the MG7-specific response as compared to nanoemulsion vaccines that contained the peptide antigen alone.
A second group, Wei and colleagues, have been successful in developing melanoma-targeted nanoemulsion vaccines by encapsulating the TAAs, MAGE-1 and/or MAGE-3 (120–123), using a similar technique as described above. In addition to the TAAs, their formulations contained heat shock protein 70 (HSP70) and Staphylococcal enterotoxins A (SEA), which are proposed to augment induction of tumor-specific immunity. A magnetic ultrasound method was used to incorporate the components to fabricate a water/oil emulsion. The system consisted of the protein in a pluronic 88 solution forming the dispersed phase while the soybean oil forming the continuous phase used Span-20 as the emulsion stabilizer. The nanoemulsion formed under high pressure homogenization pressure of 0.7 kPa yielded a droplet size of 20 nm with an entrapment efficiency of 91%. This melanoma vaccine carrier exhibited high stability over a period of six months with no evidence of creaming or sedimentation under shelf storage conditions. In mice studies, this group demonstrated that delivery of MAGE-1/HSP70/SEA encapsulated within a nanoemulsion significantly enhanced tumor-specific responses and protection, as compared to non-encapsulated delivery. Furthermore, direct comparison of various routes of administration (i.e., intravenous, intraperitoneal, subcutaneous or peroral) revealed no difference in the efficacy of their nanoemulsion vaccine formulations (122–123). After vaccination, mice responded with increased levels of MAGE-1-specific antibody production, as well as increased tumor cell lysis in vitro and inhibited tumor growth upon challenge in vivo (FIGURE 3, Ge et al 2009).
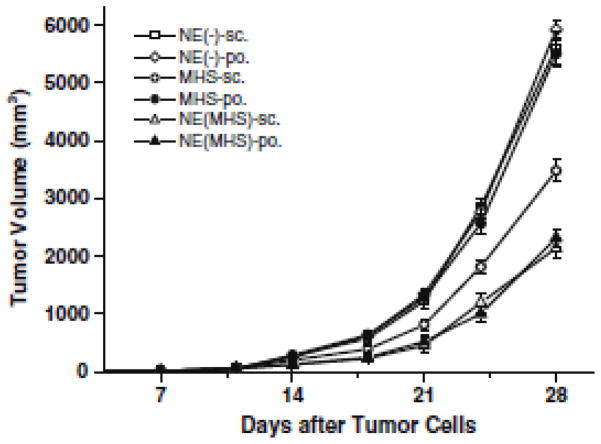
Figure 3.
The immunotherapy effect of challenged B16-MAGE-1 melanoma with the tumor vaccine. Mice were sc. inoculated with B16-MAGE-1 tumor cells (1 x 105 cells/mouse, respectively). Seven days later, they were randomly divided into six groups (n = 6 mice/group) and vaccinated as described in the Fig. 2. Data presented are mean ± SEM. Vaccination with NE (MHS), whether via sc. route or po. route, signicantly delayed tumor growth compared with vaccination using MHS or NE (−), and there were no statistical differences between NE (MHS)-sc. and NE (MHS)-po. group at the observation points. Reprinted with permission from (145) © Springer Inc. (2008).
Currently, pre-clinical and phase I trials with nanoemulsion vaccine formulations have only targeted Hepatitis B (116) and seasonal influenza (Data presented at the 2008 ICAAC/IDSA meeting in Washington, DC). Promising tumor protection data from animal studies and demonstrated safety and stability of nanoemulsion formulations, provide a platform upon which clinical trials for cancer patients should be considered.
7. AMPHIPHILIC PARTICLES
Another interesting area in the use of nanoscopic systems for tumor immunotherapy is the use of amphiphilic block-graft co-polymers as antigen/protein carriers using the biodegradable polymer poly (γ-glutamic acid) (γ-PGA). γ-PGA is produced by several Bacillus species as an extracellular polymer. γ-PGA is frequently referred to as a pseudo-amino acid with the glutamate repeat units in γ-PGA containing linkages between the α-amino and γ-carboxylic acid functional groups. γ-PGA is entirely biodegradable and non-toxic to humans. These are degraded in vivo by γ-glutamyl transpeptidase which is widely distributed in humans and catalyzes the hydrolysis of the polymer to its constituent amino acids. These self-assembled amphiphilic nanocarriers typically possess a hydrophobic corona and are commonly referred to as core-corona type polymeric particles. The hydrophobic microdomains of these self-aggregates can be used for encapsulating proteins.
The use of biodegradable γ-PGA nanoparticles was recently shown to be effective for delivery of protein antigen (126–127). Nakagawa and co-workers developed such a self-assembly system using γ-PGA in which the L-phenylalanine ester (PAE) was introduced as a hydrophobic residue on the α-position carboxylic acid groups of the γ-PGA in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) (128). The antigenic protein of interest, OVA, was encapsulated within this carrier using an electrostatic interaction mechanism. These particles entrapping OVA exhibited a mean size of 250 nm with a 60% protein loading efficiency. The authors further showed a controlled release of the entrapped antigenic protein for a period of 30 days. Preliminary studies with these nanoparticles indicated their potential for use as cancer vaccines. Specifically, these particles were efficiently phagocytosed by DCs in vitro, which induced maturation, as evidenced by cytokine production and up-regulation of co-stimulatory molecules (129). Furthermore, the signaling pathways involved in the γ-PGA nanoparticle-induced maturation of DCs were found to be MyD88-dependent, and ultimately resulted in NF-κB activation. In vivo, mice immunized with OVA-loaded γ-PGA nanoparticles responded with strong OVA-specific T- and B-lymphocyte responses, as measured by lysis of OVA-expressing target cells, production of IFN-γ by OVA-restimulated splenocytes, and production of IgG anti-OVA (FIGURE 4, Uto et al 2007). This group demonstrated that mice previously immunized with γ-PGA nanoparticles with immobilized bacterial antigen on the surface were significantly protected after an in vivo challenge with a lethal dose of Listeria monocytogenes, a model for CD8+ T lymphocyte-mediated protection against intracellular pathogens. These findings were followed up by a brief study that further demonstrated the effective delivery of peptide antigens and induction of CD8+ T lymphocytes responses when the γ-PGA nanoparticulated peptides were targeted to the ER (130).
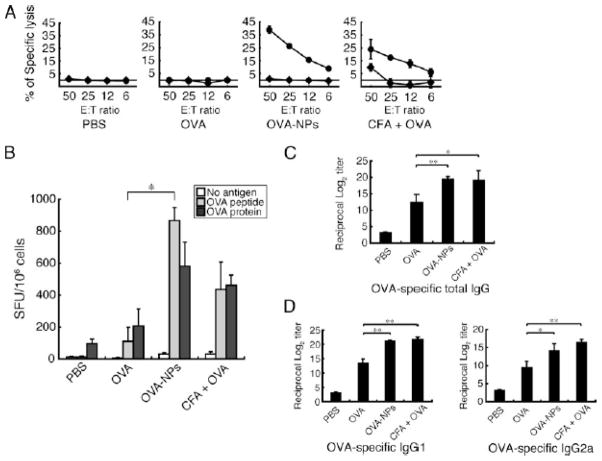
Figure 4.
Induction of Ag-specific cellular and humoral immune responses by OVA-NPs. Mice were immunized with either PBS, OVA, OVA-NPs, or CFA plus OVA through their footpads. A, Spleen cells were restimulated with the OVA257–264 peptide and IL-2. The spleen cells were examined for their cytolytic activity to peptide-treated or untreated EL4 target cells at various E:T ratios by a standard 51Cr-releasing assay. The experiments were performed in triplicate, and data are expressed as mean ± SD. The results are a representative of three separate experiments. The difference in specific lysis between the OVA-NPs group and the CFA plus OVA group is statistically significant (p < 0.05) at an E:T ratio of 50. B, Spleen cells were restimulated with the OVA257–264 peptide or OVA protein. IFN-γ producing T cells were counted and expressed as the spot forming unit (SFU) per one million cells. Data represent mean ± SD for three to four separate experiments. *,p < 0.05. C and D, Sera were tested for their Ab titers of OVA-specific IgG and its subclasses as determined by ELISA. Data represent mean ± SD of endpoint titers for three to four separate experiments. *, p < 0.05, **, p < 0.005. Reprinted with permission from (154) © The American Association of Immunologists Inc. (2007).
Tumor protection studies employing γ-PGA nanoparticles as cancer vaccines have followed these initial reports. Yoshikawa and colleagues first reported that this system could be used to deliver antigenic vaccines that target APCs and promote the MHC class I presentation pathway, ultimately conferring significant tumor protection in models using OVA-expressing tumors (128, 131) (FIGURE 5, Yoshikawa et al 2008). Based on their findings, they reported that preliminary clinical trials were planned for the near future. Most recently, γ-PGA nanoparticles loaded with a known TAA, EphA2, were used to vaccinate mice to determine the level of protection induced upon EphA2-expressing tumor cell challenge (132). For this study, tumor cells were injected into the livers of mice to simulate a model of tumor metastasis. Mice vaccinated with EphA2-γ-PGA nanoparticles exhibited enhanced EphA2-specific CD8+ lymphocyte activation, target cell lysis, and decreased overall liver size as a measure of tumor protection. Importantly, immunization and induction of responses against the liver tumors did not result in liver pathology or any toxic effects on liver or kidney function, indicating this system is safe and a good candidate for clinical applications. Based on these findings this group also reported that they were currently preparing their γ-PGA nanoparticle vaccine formulations for clinical testing.
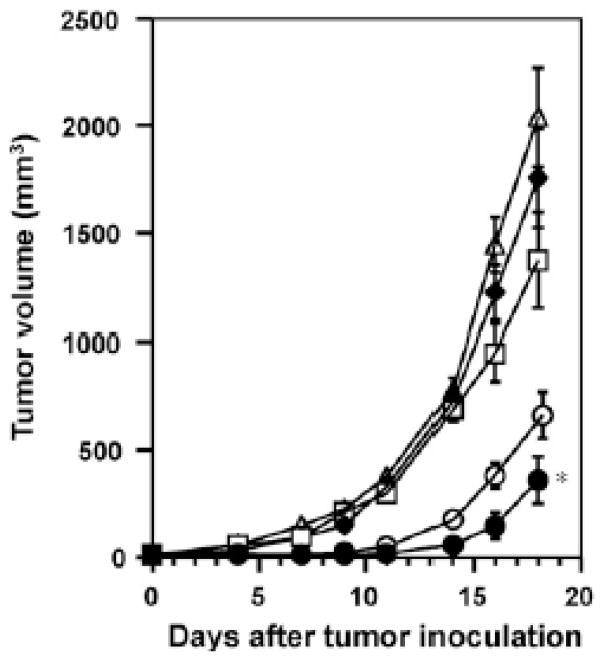
Figure 5.
Anti-tumor effect elicited by immunization with γ-PGA NP/OVA. C57BL/6 mice were immunized subcutaneously with γ-PGA NP/OVA (●; 100 μg OVA, ○; 10 μg OVA), CFA/OVA (□ 100 μg OVA), OVA solution (◆; 100 μg OVA), or PBS (△). Ten days later, 106 E.G7-OVA cells were inoculated intradermally into the flank of each mouse; then the tumor volume was monitored. Each point represents the mean ± S.E. from 5 to 9 mice. Statistical significance of γ-PGA NP/OVA (100 μg OVA) vs. CFA/OVA on day 18 was determined by Student’s t-test (*P < 0.05). Reprinted with permission from (156) © Elsevier Inc. (2008).
4.4 Polyalkylcyanoacrylate (PBCA)
Malignant brain tumors, or gliomas, are the second leading cause of cancer-related deaths in children under the age of 20, in men under the age of 40, and in women under the age of 20 (133). In 2009, it was expected that approximately 20,000 new cases of malignant brain tumors would be diagnosed. Recent advances in glioma-targeted immunotherapies have focused heavily on inhibition of transforming growth factor-beta (TGF-β), a cytokine that can be produced by tumor cells that benefits tumor development via its impact on immunosuppression, metastasis, angiogenesis, and proliferation (134–137). Multiple studies, carried out primarily in rats, have shown that prognosis is improved in glioma-challenged animals when TGF-β antisense oligonucleotides are delivered alone or in conjunction with tumor vaccines. A key issue, however, is that targeting the brain tumor cells by delivery of such antisense oligonucleotides can be limited by their ability to efficiently cross the blood brain barrier (BBB). The use of certain nanoparticle delivery systems, which can be fabricated in a size range less than 100 nm, bypasses these limitations, resulting in successful crossing of the BBB (138–139).
Polyalkylcyanoacrylate (PBCA) polymers have been recently garnering interest in the area of tumor immunotherapy. The interest stems from previous studies carried out using PBCA particles loaded with doxorubicin (140–143). The particles were successful at tumor targeting and enhanced targeting of doxorubicin to the brain. Their properties including high tensile strength, biodegradability, biocompatibility and drug compatibility have further added to the interest in these polymeric substrates in the preparation of injectable delivery systems. PBCA is typically prepared from (iso)butylcyanoacrylate monomers by emulsion anionic polymerization in a dextran 70 containing acidic solution. Nanoparticles made from this polymer are biodegradable and display relatively low toxicity (LD50 = 242 mg/kg in rats). The degradation products are butanol and cyanoacrylic acid that are removable from the body. Unmodified PBCA nanoparticles lack stealth properties and are rapidly cleared from the blood stream and hence modification to yield a hydrophilic surface is often essential to render longer circulatory times for these particles particularly for active brain targeting. One way of modifying these particles is by the adsorption of polysorbate-80 onto the surface of these nanoparticles. As a mechanism of action, the hypothesis is that these polysorbate-80 coated particles were transported across the BBB via endocytosis by the brain capillary endothelial cells triggered by serum protein, APO E.
Most recently, a study performed by Schneider and colleagues addressed whether delivery of TGF-β antisense oligonucleotides using biodegradable polybutyl cyanoacrylate nanoparticles, coated with Polysorbate 80, would enhance gene delivery and tumor therapy in a rat glioblastoma model (144). The nanoparticles were prepared by a cationic polymerization followed by complexation with the AON. Polysorbate (Tween)-80 was further coated onto the surface of these particles. The nanoparticles exhibited a high ODN binding efficiency of 93.4%. They utilized a strategy based on previous work documenting increased delivery of drugs across the BBB when encapsulated within such nanoparticles (145–151). First, gene delivery to brain tissue after injection of their Polysorbate 80-coated nanoparticles was found to be successful, resulting in significant reporter gene expression (144). Importantly, they were able to achieve the gene delivery when the nanoparticles were administered via both intravenous and intraperitoneal routes, showing their ability to cross the blood brain barrier in their system. Furthermore, delivery of both their reporter gene and TGF-β antisense oligonucleotides via nanoparticles resulted in preferential uptake by tumor cells in the brain, with little to no uptake by non-tumor tissue. Increased survival time of tumor-challenged rats was only observed when TGF-β antisense oligonucleotides nanoparticles were co-delivered with a tumor cell vaccine. Survival outcomes directly correlated to the number of activated T cells (CD25+) found in peripheral blood. Of note is the minimal protection conferred by administration of TGF-β antisense oligonucleotides nanoparticles alone, suggesting that this treatment is most beneficial when used in conjunction with a vaccine regimen aimed at inducing tumor-specific T cell activation.
In 2000, the first phase I and II clinical trials involving direct intratumoral delivery of a TGF-β antisense compound (AP 12009) in high-grade glioma patients were undertaken (136, 152). Direct injection into brain tumor tissue was chosen to avoid the issue of limited accessibility of these molecules across the BBB. Results from AP 12009 treated patients were promising, with median survival times increasing significantly beyond those seen with conventional chemotherapy and very little toxicity observed from the treatments. Currently, a phase IIb clinical trial involving AP 12009 treatment of recurrent or refractory high-grade glioma is ongoing. To date, no clinical studies have been undertaken to examine the efficacy of systemic delivery of AP 12009 or any other TGF-β antisense oligonucleotides via a nanoparticulate system as described by Schneider and colleagues (144). Such systems could provide a powerful alternative to direct intratumoral injections.
8. ADDITIONAL NANOPARTICLE DELIVERY SYSTEMS
8.1 Gold nanoparticles
Targeting TAAs to solid tumors using metallic nanoclusters has been an area of sustained interest for several years now. Colloidal gold (Au), a sol comprised of nanoparticles of Au, has been used as a therapeutic for the treatment of cancer as well as an indicator for immunodiagnostics. Colloidal gold nanoparticles conjugated with functional biomolecules present a versatile platform for tumor immunotherapy research. These non-biodegradable, gold-based nanoparticles have been studied primarily in two forms for cancer therapy: immunonanoshells (153–154) and carbohydrate-based glyconanoparticles (155).
Ojeda and co-workers fabricated a gold nanoparticulate system with self-assembled monolayers of carbohydrate antigens (glyconanoparticles, GLN) as a potential carrier for cancer vaccines (155). GLNs were constructed by the reduction of gold salt in the presence of thiol functionalized neoglycon-conjugates. These GLNs are highly stable to glycolytic enzymes and release the carbohydrates, which are T cell dependent antigens, in a controlled manner for stimulation of the immune system. Examination of these particles was limited and only addressed the feasibility of their production, with no in vitro or in vivo tumor studies carried out to date. This group demonstrated that tumor oligosaccharides were successfully incorporated into various gold glyconanoparticles formulations, but the potential clinical applications of this system remains to be elucidated.
Another class of colloidal gold carriers, known as immunonanoshells, has potential as a mediator of passive immunotherapy of tumors. Gold nanoshells are reported to accumulate preferentially in tumor tissue due to the EPR effect. Upon absorption of near infrared (NIR) light by gold nanoshells heat is generated which causes ablation of tumor cells. This process is termed photothermal cancer therapy and phase I clinical trials using NIR-absorbing gold nanoshells are reported to be ongoing (156). The development of immunonanoshells came about as a way to enhance delivery of these particles to tumor sites via the incorporation of tumor surface antigen-specific antibodies onto the outer shell (153–154). Hence, immunonanoshells constitute a form of passive cancer immunotherapy since their ultimate tumor targeting and destruction mechanism does not involve the induction of tumor-specific T or B lymphocyte responses. One research group successfully incorporated monoclonal antibodies against Her2/neu or the interleukin-13 receptor-alpha-2 (a receptor commonly overexpressed in gliomas) into immunonanoshells (153–154). Both were evaluated in vitro and were found to be tumor cell specific with non-targeted cells exhibiting no cell death after NIR light treatment. Incorporation of immunonanoshells into clinical trials has not yet been reported.
8.2 Magnetite nanoparticles
Magnetite nanoparticles were first described for hyperthermia cancer therapy, or “heat immunotherapy”, a little over a decade ago (157–158). Since then these nanoparticles have repeatedly been demonstrated to induce significant tumor regression via induced heat shock protein (HSP) expression, which ultimately leads to enhanced MHC class I-dependent TAA presentation and the development of anti-tumor T lymphocyte-mediated immunity (159–169). This effect has been studied in several animal tumor models using magnetite nanoparticles alone, and in combination with additional immunotherapies or chemotherapies, such as immunoliposomes (164, 170) and intratumoral cytokine or DC injection (159, 165–166).
Recently, preliminary phase I and II clinical trials focusing on a melanoma-targeting chemo-thermo-immunotherapy regimen began in Japan, with remission for more than 24 months observed in 2 of the 4 patients enrolled (169, 171). However, an important limitation of this system to consider is that in all cases the particles were delivered via intratumoral injection. This route of delivery would exclude it as a treatment for those patients with inaccessible tumors, or for whom surgery poses a serious risk. The research group that has primarily studied this system previously achieved successful targeting of magnetite particles to Her2/neu-overexpressing breast cancer cells in vitro (164) and in vivo (170) by combining them with anti-Her2/neu immunoliposomes (164), but systemic delivery was not examined. This promising therapy might be more universally applicable in the clinical setting if it could be modified for systemic delivery with preferential targeting of tumor cells.
One additional important consideration is that inorganic particles, gold and magnetite, may not provide advantages over other types of nanoparticles for systemic targeting of individual cancer cells because they are not biodegradable or small enough to be cleared easily, resulting in potential accumulation in the body, which may cause long-term toxicity.
9. CONCLUSION
Nanocarrier systems for delivering tumor immunotherapy are primarily aimed at generating a strong adaptive cytotoxic anti-tumor response that is systemic. With some novel exceptions the majority of nanocarriers reviewed here were designed to achieve this goal by specifically or passively targeting dendritic cells (DCs) such that: 1) the tumor antigens delivered by these nanocarriers are at least partially presented in the context of MHC class I (i.e. cross-presented) and thus, a trigger for antigen-specific cytotoxic CD8+ lymphocytes is provided, and: 2) the delivery of a second signal (“danger signal”) is achieved usually in the form of various intrinsic or co-encapsulated adjuvants, which result in activation and maturation of the targeted DCs. The majority of the treatment modalities have demonstrated promising preclinical results in terms of prophylactic and/or therapeutic tumor models. Some have progressed as far as phase I,II and III clinical trials that demonstrate the safety and potential therapeutic benefits of these nanocarrier systems. It is difficult at this early stage to prognosticate as to which systems are most likely to progress as established vehicles for cancer immunotherapy. Nevertheless, aside from the empirical therapeutic benefits, other strongly determining factors are likely to be biocompatibility, the ease and cost of manufacture and storage, along with a nanocarriers malleability with regard to design features and routes of administration.
Table 1.
Advantages, disadvantages and indications for various types of nanocarriers.
| Nanocarrier | Advantages | Disadvantages | Latest Progress |
|---|---|---|---|
| Conventional liposomes |
|
|
|
| Cationic liposomes-DNA complexes (CLDC) |
|
|
|
| Stealth liposomes |
|
||
| Archeosomes |
|
||
| Fusogenic liposomes |
|
||
| Viruses |
|
|
|
| Adenovirus |
|
||
| Virus-like particles |
|
|
|
| Virosomes |
|
||
| Chimeric virosomes |
|
|
|
| PLGA |
|
Acknowledgments
We gratefully acknowledge support from the American Cancer Society (RSG-09-015-01-CDD), the National Cancer Institute at the National Institutes of Health (1R21CA13345-01/1R21CA128414-01A2/UI Mayo Clinic Lymphoma SPORE), and the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation. C. Lemke acknowledges support from the PhRMA foundation for a post-doctoral fellowship and Y. Krishnamachari acknowledges support from a Guillory Fellowship.
References
- 1.Cancer Facts & Figures 2009. American Cancer Society; Atlanta: 2009. [Google Scholar]
- 2.Krishnamachari Y, Salem AK. Innovative strategies for co-delivering antigens and CpG oligonucleotides. Advanced Drug Delivery Reviews. 2009;61:205–217. doi: 10.1016/j.addr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goforth R, Salem AK, Zhu XY, Miles S, Zhang XQ, Lee JH, Sandler AD. Immune stimulatory antigen loaded particles combined with depletion of regulatory T-cells induce potent tumor specific immunity in a mouse model of melanoma. Cancer Immunology Immunotherapy. 2009;58:517–530. doi: 10.1007/s00262-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattman JN, Greenberg PD. Cancer immunotherapy: A treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 5.Diebold SS, Kaisho T, Hemmi H, Akira S, Sousa CRE. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 6.Melief CJM. Cancer - Immune pact with the enemy. Nature. 2007;450:803–804. doi: 10.1038/nature06363. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 8.Williams N. An immune boost to the war on cancer. Science. 1996;272:28–30. doi: 10.1126/science.272.5258.28. [DOI] [PubMed] [Google Scholar]
- 9.Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu A, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salem AK, Searson PC, Leong KW. Multifunctional nanorods for gene delivery. Nature Materials. 2003;2:668–671. doi: 10.1038/nmat974. [DOI] [PubMed] [Google Scholar]
- 11.Salem AK, Hung CF, Kim TW, Wu TC, Searson PC, Leong KW. Multi-component nanorods for vaccination applications. Nanotechnology. 2005;16:484–487. [Google Scholar]
- 12.Pearce ME, Melanko JB, Salem AK. Multifunctional nanorods for biomedical applications. Pharmaceutical Research. 2007;24:2335–2352. doi: 10.1007/s11095-007-9380-7. [DOI] [PubMed] [Google Scholar]
- 13.Intra J, Salem AK. Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. Journal of Controlled Release. 2008;130:129–138. doi: 10.1016/j.jconrel.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce ME, Mai HQ, Lee N, Larsen SC, Salem AK. Silicalite nanoparticles that promote transgene expression. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/17/175103. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XQ, Dahle CE, Weiner GJ, Salem AK. A comparative study of the antigen-specific immune response induced by co-delivery of CpG ODN and antigen using fusion molecules or biodegradable microparticles. Journal of Pharmaceutical Sciences. 2007;96:3283–3292. doi: 10.1002/jps.20978. [DOI] [PubMed] [Google Scholar]
- 16.Torchilin VP. Multifunctional nanocarriers. Advanced Drug Delivery Reviews. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Torchilin VP. Micellar nanocarriers: Pharmaceutical perspectives. Pharmaceutical Research. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 18.Torchilin VP. Nanocarriers. Pharmaceutical Research. 2007;24:2333–2334. doi: 10.1007/s11095-007-9463-5. [DOI] [PubMed] [Google Scholar]
- 19.Torchilin VP. Multifunctional nanocarriers for delivery of drugs, genes, and diagnosticals in the body. Febs Journal. 2009;276:85–85. [Google Scholar]
- 20.Couvreur P, Vauthier C. Nanotechnology: Intelligent design to treat complex disease. Pharmaceutical Research. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 21.Hartig SM, Greene RR, Dikov MM, Prokop A, Davidson JM. Multifunctional nanoparticulate polyelectrolyte complexes. Pharmaceutical Research. 2007;24:2353–2369. doi: 10.1007/s11095-007-9459-1. [DOI] [PubMed] [Google Scholar]
- 22.Staples M, Daniel K, Cima MJ, Langer R. Application of micro- and nano-electromechanical devices to drug delivery. Pharmaceutical Research. 2006;23:847–863. doi: 10.1007/s11095-006-9906-4. [DOI] [PubMed] [Google Scholar]
- 23.Sutton D, Nasongkla N, Blanco E, Gao JM. Functionalized micellar systems for cancer targeted drug delivery. Pharmaceutical Research. 2007;24:1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 24.van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharmaceutical Research. 2007;24:1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhang SF, Uludag H. Nanoparticulate Systems for Growth Factor Delivery. Pharmaceutical Research. 2009;26:1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 26.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. Journal of Controlled Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Jabr-Milane L, van Vlerken L, Devalapally H, Shenoy D, Komareddy S, Bhavsar M, Amiji M. Multi-functional nanocarriers for targeted delivery of drugs and genes. Journal of Controlled Release. 2008;130:121–128. doi: 10.1016/j.jconrel.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XQ, Dahle CE, Baman NK, Rich N, Weiner GJ, Salem AK. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. Journal of Immunotherapy. 2007;30:469–478. doi: 10.1097/CJI.0b013e31802fd8c6. [DOI] [PubMed] [Google Scholar]
- 29.Intra J, Salem AK. Fabrication, Characterization and In Vitro Evaluation of Poly(D,L-Lactide-co-Glycolide) Microparticles Loaded With Polyamidoamine-Plasmid DNA Dendriplexes for Applications in Nonviral Gene Delivery. Journal of Pharmaceutical Sciences. 2010;99:368–384. doi: 10.1002/jps.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XQ, Intra J, Salem AK. Comparative study of poly (lactic-co-glycolic acid)-poly ethyleneimine-plasmid DNA microparticles prepared using double emulsion methods. Journal of Microencapsulation. 2008;25:1–12. doi: 10.1080/02652040701659347. [DOI] [PubMed] [Google Scholar]
- 31.Abbas AO, Donovan MD, Salem AK. Formulating poly(lactide-co-glycolide) particles for plasmid DNA delivery. Journal of Pharmaceutical Sciences. 2008;97:2448–2461. doi: 10.1002/jps.21215. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XQ, Intra J, Salem AK. Conjugation of polyamidoamine dendrimers on biodegradable microparticles for nonviral gene delivery. Bioconjugate Chemistry. 2007;18:2068–2076. doi: 10.1021/bc070116l. [DOI] [PubMed] [Google Scholar]
- 33.Alper J. Drug delivery - Breaching the membrane. Science. 2002;296:838–839. doi: 10.1126/science.296.5569.838. [DOI] [PubMed] [Google Scholar]
- 34.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 35.Lasic DD. Doxorubicin in sterically stabilized liposomes. Nature. 1996;380:561–562. doi: 10.1038/380561a0. [DOI] [PubMed] [Google Scholar]
- 36.Szoka F. Molecular biology - The art of assembly. Science. 2008;319:578–579. doi: 10.1126/science.1154253. [DOI] [PubMed] [Google Scholar]
- 37.Frank MM. The reticuloendothelial system and bloodstream clearance. J Lab Clin Med. 1993;122:487–488. [PubMed] [Google Scholar]
- 38.Worth LL, Jia SF, An T, Kleinerman ES. ImmTher, a lipophilic disaccharide derivative of muramyl dipeptide, up-regulates specific monocyte cytokine genes and activates monocyte-mediated tumoricidal activity. Cancer Immunol Immunother. 1999;48:312–320. doi: 10.1007/s002620050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sangha R, North S. L-BLP25: a MUC1-targeted peptide vaccine therapy in prostate cancer. Expert Opin Biol Ther. 2007;7:1723–1730. doi: 10.1517/14712598.7.11.1723. [DOI] [PubMed] [Google Scholar]
- 40.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–293. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 41.Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol. 2000;165:5780–5787. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- 42.De Becker G, Moulin V, Pajak B, Bruck C, Francotte M, Thiriart C, Urbain J, Moser M. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int Immunol. 2000;12:807–815. doi: 10.1093/intimm/12.6.807. [DOI] [PubMed] [Google Scholar]
- 43.Agrawal B, Krantz MJ, Reddish MA, Longenecker BM. Rapid induction of primary human CD4+ and CD8+ T cell responses against cancer-associated MUC1 peptide epitopes. Int Immunol. 1998;10:1907–1916. doi: 10.1093/intimm/10.12.1907. [DOI] [PubMed] [Google Scholar]
- 44.Sanghaand C, Butts R. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res. 2007;13:s4652–4654. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]
- 45.Chikhand MP, Schutze-Redelmeier G. Liposomal delivery of CTL epitopes to dendritic cells. Biosci Rep. 2002;22:339–353. doi: 10.1023/a:1020151025412. [DOI] [PubMed] [Google Scholar]
- 46.Ignatius R, Mahnke K, Rivera M, Hong K, Isdell F, Steinman RM, Pope M, Stamatatos L. Presentation of proteins encapsulated in sterically stabilized liposomes by dendritic cells initiates CD8(+) T-cell responses in vivo. Blood. 2000;96:3505–3513. [PubMed] [Google Scholar]
- 47.Faham A, Bennett D, Altin JG. Liposomal Ag engrafted with peptides of sequence derived from HMGB1 induce potent Ag-specific and anti-tumour immunity. Vaccine. 2009;27:5846–5854. doi: 10.1016/j.vaccine.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 48.van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 2004;64:4357–4365. doi: 10.1158/0008-5472.CAN-04-0138. [DOI] [PubMed] [Google Scholar]
- 49.Rudginsky S, Siders W, Ingram L, Marshall J, Scheule R, Kaplan J. Antitumor activity of cationic lipid complexed with immunostimulatory DNA. Mol Ther. 2001;4:347–355. doi: 10.1006/mthe.2001.0463. [DOI] [PubMed] [Google Scholar]
- 50.Gursel I, Gursel M, Ishii KJ, Klinman DM. Sterically stabilized cationic liposomes improve the uptake and immunostimulatory activity of CpG oligonucleotides. J Immunol. 2001;167:3324–3328. doi: 10.4049/jimmunol.167.6.3324. [DOI] [PubMed] [Google Scholar]
- 51.de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, Yuan N, Hope M, Cullis P, Tam Y. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother. 2007;56:1251–1264. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez R, Hutchins L, Nemunaitis J, Atkins M, Schwarzenberger PO. Phase 2 trial of Allovectin-7 in advanced metastatic melanoma. Melanoma Res. 2006;16:521–526. doi: 10.1097/01.cmr.0000232299.44902.41. [DOI] [PubMed] [Google Scholar]
- 53.Bedikian AY, Del Vecchio M. Allovectin-7 therapy in metastatic melanoma. Expert Opin Biol Ther. 2008;8:839–844. doi: 10.1517/14712598.8.6.839. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan L, Sprott GD. Archaeosome adjuvants: immunological capabilities and mechanism(s) of action. Vaccine. 2008;26:2043–2055. doi: 10.1016/j.vaccine.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 55.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 56.Omri A, Makabi-Panzu B, Agnew BJ, Sprott GD, Patel GB. Influence of coenzyme Q10 on tissue distribution of archaeosomes, and pegylated archaeosomes, administered to mice by oral and intravenous routes. J Drug Target. 2000;7:383–392. doi: 10.3109/10611869909085521. [DOI] [PubMed] [Google Scholar]
- 57.Krishnan L, Dennis Sprott G. Archaeosomes as self-adjuvanting delivery systems for cancer vaccines. J Drug Target. 2003;11:515–524. doi: 10.1080/10611860410001670044. [DOI] [PubMed] [Google Scholar]
- 58.Krishnan L, Sad S, Patel GB, Sprott GD. Archaeosomes induce enhanced cytotoxic T lymphocyte responses to entrapped soluble protein in the absence of interleukin 12 and protect against tumor challenge. Cancer Res. 2003;63:2526–2534. [PubMed] [Google Scholar]
- 59.Krishnan L, Sad S, Patel GB, Sprott GD. Archaeosomes induce long-term CD8+ cytotoxic T cell response to entrapped soluble protein by the exogenous cytosolic pathway, in the absence of CD4+ T cell help. J Immunol. 2000;165:5177–5185. doi: 10.4049/jimmunol.165.9.5177. [DOI] [PubMed] [Google Scholar]
- 60.Patel GB, Omri A, Deschatelets L, Sprott GD. Safety of archaeosome adjuvants evaluated in a mouse model. J Liposome Res. 2002;12:353–372. doi: 10.1081/lpr-120016712. [DOI] [PubMed] [Google Scholar]
- 61.Reddy R, Zhou F, Huang L, Carbone F, Bevan M, Rouse BT. pH sensitive liposomes provide an efficient means of sensitizing target cells to class I restricted CTL recognition of a soluble protein. J Immunol Methods. 1991;141:157–163. doi: 10.1016/0022-1759(91)90142-3. [DOI] [PubMed] [Google Scholar]
- 62.Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- 63.Alving CR, Koulchin V, Glenn GM, Rao M. Liposomes as carriers of peptide antigens: induction of antibodies and cytotoxic T lymphocytes to conjugated and unconjugated peptides. Immunol Rev. 1995;145:5–31. doi: 10.1111/j.1600-065x.1995.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 64.Plotkin SLPaSA. A short history of vaccines. In: Orenstein SAPaWA., editor. Vaccines. WB Saunders Company; Philadelphia: 2004. pp. 1–15. [Google Scholar]
- 65.Speiser DE, Miranda R, Zakarian A, Bachmann MF, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel RM, Ohashi PS. Self antigens expressed by solid tumors Do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arlen PM, Pazdur M, Skarupa L, Rauckhorst M, Gulley JL. A randomized phase II study of docetaxel alone or in combination with PANVAC-V (vaccinia) and PANVAC-F (fowlpox) in patients with metastatic breast cancer (NCI 05-C-0229) Clin Breast Cancer. 2006;7:176–179. doi: 10.3816/CBC.2006.n.032. [DOI] [PubMed] [Google Scholar]
- 67.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, Cereda V, Vergati M, Steinberg SM, Halabi S, Jones E, Chen C, Parnes H, Wright JJ, Dahut WL, Schlom J. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amato RJ. 5T4-modified vaccinia Ankara: progress in tumor-associated antigen-based immunotherapy. Expert Opin Biol Ther. 2010;10:281–287. doi: 10.1517/14712590903586213. [DOI] [PubMed] [Google Scholar]
- 69.Harrop R, Ryan MG, Myers KA, Redchenko I, Kingsman SM, Carroll MW. Active treatment of murine tumors with a highly attenuated vaccinia virus expressing the tumor associated antigen 5T4 (TroVax) is CD4+ T cell dependent and antibody mediated. Cancer Immunol Immunother. 2006;55:1081–1090. doi: 10.1007/s00262-005-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang X, Yang Y. Innate immune recognition of viruses and viral vectors. Hum Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peruzzi D, Dharmapuri S, Cirillo A, Bruni BE, Nicosia A, Cortese R, Colloca S, Ciliberto G, La Monica N, Aurisicchio L. A novel chimpanzee serotype-based adenoviral vector as delivery tool for cancer vaccines. Vaccine. 2009;27:1293–1300. doi: 10.1016/j.vaccine.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ludwig C, Wagner R. Virus-like particles-universal molecular toolboxes. Curr Opin Biotechnol. 2007;18:537–545. doi: 10.1016/j.copbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deml L, Speth C, Dierich MP, Wolf H, Wagner R. Recombinant HIV-1 Pr55gag virus-like particles: potent stimulators of innate and acquired immune responses. Mol Immunol. 2005;42:259–277. doi: 10.1016/j.molimm.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 74.Marsac D, Loirat D, Petit C, Schwartz O, Michel ML. Enhanced presentation of major histocompatibility complex class I-restricted human immunodeficiency virus type 1 (HIV-1) Gag-specific epitopes after DNA immunization with vectors coding for vesicular stomatitis virus glycoprotein-pseudotyped HIV-1 Gag particles. J Virol. 2002;76:7544–7553. doi: 10.1128/JVI.76.15.7544-7553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, Skjeldestad FE, Olsson SE, Steinwall M, Brown DR, Kurman RJ, Ronnett BM, Stoler MH, Ferenczy A, Harper DM, Tamms GM, Yu J, Lupinacci L, Railkar R, Taddeo FJ, Jansen KU, Esser MT, Sings HL, Saah AJ, Barr E. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 76.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 77.Adamina M, Guller U, Bracci L, Heberer M, Spagnoli GC, Schumacher R. Clinical applications of virosomes in cancer immunotherapy. Expert Opin Biol Ther. 2006;6:1113–1121. doi: 10.1517/14712598.6.11.1113. [DOI] [PubMed] [Google Scholar]
- 78.Zurbriggen R. Immunostimulating reconstituted influenza virosomes. Vaccine. 2003;21:921–924. doi: 10.1016/s0264-410x(02)00541-8. [DOI] [PubMed] [Google Scholar]
- 79.Schumacher R, Adamina M, Zurbriggen R, Bolli M, Padovan E, Zajac P, Heberer M, Spagnoli GC. Influenza virosomes enhance class I restricted CTL induction through CD4+ T cell activation. Vaccine. 2004;22:714–723. doi: 10.1016/j.vaccine.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 80.Wiedermann U, Wiltschke C, Jasinska J, Kundi M, Zurbriggen R, Garner-Spitzer E, Bartsch R, Steger G, Pehamberger H, Scheiner O, Zielinski CC. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a phase I study. Breast Cancer Res Treat. 2010;119:673–683. doi: 10.1007/s10549-009-0666-9. [DOI] [PubMed] [Google Scholar]
- 81.Amacker M, Engler O, Kammer AR, Vadrucci S, Oberholzer D, Cerny A, Zurbriggen R. Peptide-loaded chimeric influenza virosomes for efficient in vivo induction of cytotoxic T cells. Int Immunol. 2005;17:695–704. doi: 10.1093/intimm/dxh249. [DOI] [PubMed] [Google Scholar]
- 82.Schumacher R, Amacker M, Neuhaus D, Rosenthal R, Groeper C, Heberer M, Spagnoli GC, Zurbriggen R, Adamina M. Efficient induction of tumoricidal cytotoxic T lymphocytes by HLA-A0201 restricted, melanoma associated, L(27)Melan-A/MART-1(26–35) peptide encapsulated into virosomes in vitro. Vaccine. 2005;23:5572–5582. doi: 10.1016/j.vaccine.2005.07.099. [DOI] [PubMed] [Google Scholar]
- 83.Gupta RK, Chang AC, Siber GR. Biodegradable polymer microspheres as vaccine adjuvants and delivery systems. Modulation of the Immune Response to Vaccine Antigens. 1998;92:63–78. [PubMed] [Google Scholar]
- 84.Hedley ML, Curley J, Urban R. Microspheres containing plasmid-encoded antigens elicit cytotoxic T-cell responses. Nature Medicine. 1998;4:365–368. doi: 10.1038/nm0398-365. [DOI] [PubMed] [Google Scholar]
- 85.Helson R, Olszewska W, Singh M, Megede JZ, Melero JA, Hagan DO, Openshaw PJM. Polylactide-co-glycolide (PLG) microparticles modify the immune response to DNA vaccination. Vaccine. 2008;26:753–761. doi: 10.1016/j.vaccine.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Lassalleand ML, Ferreira V. PLA nano- and microparticles for drug delivery: An overview of the methods of preparation. Macromolecular Bioscience. 2007;7:767–783. doi: 10.1002/mabi.200700022. [DOI] [PubMed] [Google Scholar]
- 87.Krishnamachari Y, Madan P, Lin SS. Development of pH- and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon. International Journal of Pharmaceutics. 2007;338:238–247. doi: 10.1016/j.ijpharm.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 88.Kumar PS, Ramakrishna S, Saini TR, Diwan PV. Influence of microencapsulation method and peptide loading on formulation of poly(lactide-co-glycolide) insulin nanoparticles. Pharmazie. 2006;61:613–617. [PubMed] [Google Scholar]
- 89.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. Journal of Controlled Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Pean JM, Venier-Julienne MC, Boury F, Menei P, Denizot B, Benoit JP. NGF release from poly(D,L-lactide-co-glycolide) microspheres. Effect of some formulation parameters on encapsulated NGF stability. Journal of Controlled Release. 1998;56:175–187. doi: 10.1016/s0168-3659(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 91.Raghuvanshi RS, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. International Journal of Pharmaceutics. 2002;245:109–121. doi: 10.1016/s0378-5173(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 92.Román BS, Irache JM, Gómez S, Tsapis N, Gamazo C, Espuelas MS. Co-encapsulation of an antigen and CpG oligonucleotides into PLGA microparticles by TROMS technology. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70:98–108. doi: 10.1016/j.ejpb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 93.Singh M, O’Hagan DT. Recent advances in vaccine adjuvants. Pharmaceutical Research. 2002;19:715–728. doi: 10.1023/a:1016104910582. [DOI] [PubMed] [Google Scholar]
- 94.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 95.Waeckerle-Men Y, Groettrup M. PLGA microspheres for improved antigen delivery to dendritic cells as cellular vaccines. Adv Drug Deliv Rev. 2005;57:475–482. doi: 10.1016/j.addr.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 96.Nikou KN, Stivaktakis N, Avgoustakis K, Sotiropoulou PA, Perez SA, Baxevanis CN, Papamichail M, Leondiadis L. A HER-2/neu peptide admixed with PLA microspheres induces a Th1-biased immune response in mice. Biochim Biophys Acta. 2005;1725:182–189. doi: 10.1016/j.bbagen.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Men Y, Audran R, Thomasin C, Eberl G, Demotz S, Merkle HP, Gander B, Corradin G. MHC class I- and class II-restricted processing and presentation of microencapsulated antigens. Vaccine. 1999;17:1047–1056. doi: 10.1016/s0264-410x(98)00321-1. [DOI] [PubMed] [Google Scholar]
- 98.O’Hagan DT, Jeffery H, Davis SS. Long-term antibody responses in mice following subcutaneous immunization with ovalbumin entrapped in biodegradable microparticles. Vaccine. 1993;11:965–969. doi: 10.1016/0264-410x(93)90387-d. [DOI] [PubMed] [Google Scholar]
- 99.Uchida T, Martin S, Foster TP, Wardley RC, Grimm S. Dose and load studies for subcutaneous and oral delivery of poly(lactide-co-glycolide) microspheres containing ovalbumin. Pharm Res. 1994;11:1009–1015. doi: 10.1023/a:1018987404751. [DOI] [PubMed] [Google Scholar]
- 100.Igartua M, Hernandez RM, Esquisabel A, Gascon AR, Calvo MB, Pedraz JL. Enhanced immune response after subcutaneous and oral immunization with biodegradable PLGA microspheres. J Control Release. 1998;56:63–73. doi: 10.1016/s0168-3659(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 101.Zhang XQ, Dahle CE, Baman NK, Rich N, Weiner GJ, Salem AK. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. J Immunother. 2007;30:469–478. doi: 10.1097/CJI.0b013e31802fd8c6. [DOI] [PubMed] [Google Scholar]
- 102.Elamanchili P, Lutsiak CM, Hamdy S, Diwan M, Samuel J. “Pathogen-mimicking” nanoparticles for vaccine delivery to dendritic cells. J Immunother. 2007;30:378–395. doi: 10.1097/CJI.0b013e31802cf3e3. [DOI] [PubMed] [Google Scholar]
- 103.Goforth R, Salem AK, Zhu X, Miles S, Zhang XQ, Lee JH, Sandler AD. Immune stimulatory antigen loaded particles combined with depletion of regulatory T-cells induce potent tumor specific immunity in a mouse model of melanoma. Cancer Immunol Immunother. 2009;58:517–530. doi: 10.1007/s00262-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26:5046–5057. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 105.Mattheolabakis G, Lagoumintzis G, Panagi Z, Papadimitriou E, Partidos CD, Avgoustakis K. Transcutaneous delivery of a nanoencapsulated antigen: induction of immune responses. Int J Pharm. 2010;385:187–193. doi: 10.1016/j.ijpharm.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 106.Dominguez AL, Lustgarten J. Targeting the tumor microenvironment with anti-neu/anti-CD40 conjugated nanoparticles for the induction of antitumor immune responses. Vaccine. 2010;28:1383–1390. doi: 10.1016/j.vaccine.2009.10.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Hagan DT, Singh M, Ulmer JB. Microparticle-based technologies for vaccines. Methods. 2006;40:10–19. doi: 10.1016/j.ymeth.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 108.Johansen P, Martinez Gomez JM, Gander B. Development of synthetic biodegradable microparticulate vaccines: a roller coaster story. Expert Rev Vaccines. 2007;6:471–474. doi: 10.1586/14760584.6.4.471. [DOI] [PubMed] [Google Scholar]
- 109.Standley SM, Kwon YJ, Murthy N, Kunisawa J, Shastri N, Guillaudeu SJ, Lau L, Frechet JM. Acid-degradable particles for protein-based vaccines: enhanced survival rate for tumor-challenged mice using ovalbumin model. Bioconjug Chem. 2004;15:1281–1288. doi: 10.1021/bc049956f. [DOI] [PubMed] [Google Scholar]
- 110.Cohen JA, Beaudette TT, Tseng WW, Bachelder EM, Mende I, Engleman EG, Frechet JM. T-cell activation by antigen-loaded pH-sensitive hydrogel particles in vivo: the effect of particle size. Bioconjug Chem. 2009;20:111–119. doi: 10.1021/bc800338n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beaudette TT, Bachelder EM, Cohen JA, Obermeyer AC, Broaders KE, Frechet JM, Kang ES, Mende I, Tseng WW, Davidson MG, Engleman EG. In vivo studies on the effect of co-encapsulation of CpG DNA and antigen in acid-degradable microparticle vaccines. Mol Pharm. 2009;6:1160–1169. doi: 10.1021/mp900038e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kauland M, Amiji G. Long-circulating poly(ethylene glycol)-modified gelatin nanoparticles for intracellular delivery. Pharm Res. 2002;19:1061–1067. doi: 10.1023/a:1016486910719. [DOI] [PubMed] [Google Scholar]
- 113.Kreuter J. Nanoparticulate systems in drug delivery and targeting. J Drug Target. 1995;3:171–173. doi: 10.3109/10611869509015940. [DOI] [PubMed] [Google Scholar]
- 114.Coester C, Nayyar P, Samuel J. In vitro uptake of gelatin nanoparticles by murine dendritic cells and their intracellular localisation. Eur J Pharm Biopharm. 2006;62:306–314. doi: 10.1016/j.ejpb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 115.Bourquin C, Anz D, Zwiorek K, Lanz AL, Fuchs S, Weigel S, Wurzenberger C, von der Borch P, Golic M, Moder S, Winter G, Coester C, Endres S. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J Immunol. 2008;181:2990–2998. doi: 10.4049/jimmunol.181.5.2990. [DOI] [PubMed] [Google Scholar]
- 116.Makidon PE, Bielinska AU, Nigavekar SS, Janczak KW, Knowlton J, Scott AJ, Mank N, Cao Z, Rathinavelu S, Beer MR, Wilkinson JE, Blanco LP, Landers JJ, Baker JR., Jr Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS One. 2008;3:e2954. doi: 10.1371/journal.pone.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bielinska AU, Janczak KW, Landers JJ, Markovitz DM, Montefiori DC, Baker JR., Jr Nasal immunization with a recombinant HIV gp120 and nanoemulsion adjuvant produces Th1 polarized responses and neutralizing antibodies to primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2008;24:271–281. doi: 10.1089/aid.2007.0148. [DOI] [PubMed] [Google Scholar]
- 118.Huang MH, Huang CY, Lin SC, Chen JH, Ku CC, Chou AH, Liu SJ, Chen HW, Chong P, Leng CH. Enhancement of potent antibody and T-cell responses by a single-dose, novel nanoemulsion-formulated pandemic influenza vaccine. Microbes Infect. 2009;11:654–660. doi: 10.1016/j.micinf.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 119.Shi R, Hong L, Wu D, Ning X, Chen Y, Lin T, Fan D, Wu K. Enhanced immune response to gastric cancer specific antigen Peptide by coencapsulation with CpG oligodeoxynucleotides in nanoemulsion. Cancer Biol Ther. 2005;4:218–224. doi: 10.4161/cbt.4.2.1472. [DOI] [PubMed] [Google Scholar]
- 120.Ge W, Sui YF, Wu DC, Sun YJ, Chen GS, Li ZS, Si SY, Hu PZ, Huang Y, Zhang XM. MAGE-1/Heat shock protein 70/MAGE-3 fusion protein vaccine in nanoemulsion enhances cellular and humoral immune responses to MAGE-1 or MAGE-3 in vivo. Cancer Immunol Immunother. 2006;55:841–849. doi: 10.1007/s00262-005-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ge W, Sun YJ, Li Y, Zhang SH, Zhang XM, Huang Y, Hu PZ, Sui YF. Anti-tumor immune responses of nanoemulsion-encapsulated MHS vaccine. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008;24:457–460. [PubMed] [Google Scholar]
- 122.Ge W, Hu PZ, Huang Y, Wang XM, Zhang XM, Sun YJ, Li ZS, Si SY, Sui YF. The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following different administration routes. Oncol Rep. 2009;22:915–920. doi: 10.3892/or_00000517. [DOI] [PubMed] [Google Scholar]
- 123.Ge W, Li Y, Li ZS, Zhang SH, Sun YJ, Hu PZ, Wang XM, Huang Y, Si SY, Zhang XM, Sui YF. The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following peroral administration route. Cancer Immunol Immunother. 2009;58:201–208. doi: 10.1007/s00262-008-0539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hang QL, Ding J, Gong AC, Yu ZC, Qiao TD, Chen BJ, Zhang XY, Fan DM. Screening of bioactive peptide that mimic the epitope of gastric cancer associated antigen. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:308–310. [PubMed] [Google Scholar]
- 125.Xu L, Jin BQ, Fan DM. Selection and identification of mimic epitopes for gastric cancer-associated antigen MG7 Ag. Mol Cancer Ther. 2003;2:301–306. doi: 10.4161/cbt.2.3.386. [DOI] [PubMed] [Google Scholar]
- 126.Akagi T, Kaneko T, Kida T, Akashi M. Preparation and characterization of biodegradable nanoparticles based on poly(gamma-glutamic acid) with l-phenylalanine as a protein carrier. J Control Release. 2005;108:226–236. doi: 10.1016/j.jconrel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 127.Akagi T, Higashi M, Kaneko T, Kida T, Akashi M. In vitro enzymatic degradation of nanoparticles prepared from hydrophobically-modified poly(gamma-glutamic acid) Macromol Biosci. 2005;5:598–602. doi: 10.1002/mabi.200500036. [DOI] [PubMed] [Google Scholar]
- 128.Yoshikawa T, Okada N, Oda A, Matsuo K, Mukai Y, Yoshioka Y, Akagi T, Akashi M, Nakagawa S. Development of amphiphilic gamma-PGA-nanoparticle based tumor vaccine: potential of the nanoparticulate cytosolic protein delivery carrier. Biochem Biophys Res Commun. 2008;366:408–413. doi: 10.1016/j.bbrc.2007.11.153. [DOI] [PubMed] [Google Scholar]
- 129.Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, Baba M. Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol. 2007;178:2979–2986. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]
- 130.Matsuo K, Yoshikawa T, Oda A, Akagi T, Akashi M, Mukai Y, Yoshioka Y, Okada N, Nakagawa S. Efficient generation of antigen-specific cellular immunity by vaccination with poly(gamma-glutamic acid) nanoparticles entrapping endoplasmic reticulum-targeted peptides. Biochem Biophys Res Commun. 2007;362:1069–1072. doi: 10.1016/j.bbrc.2007.08.112. [DOI] [PubMed] [Google Scholar]
- 131.Yoshikawa T, Okada N, Oda A, Matsuo K, Kayamuro H, Ishii Y, Yoshinaga T, Akagi T, Akashi M, Nakagawa S. Nanoparticles built by self-assembly of amphiphilic gamma-PGA can deliver antigens to antigen-presenting cells with high efficiency: a new tumor-vaccine carrier for eliciting effector T cells. Vaccine. 2008;26:1303–1313. doi: 10.1016/j.vaccine.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 132.Yamaguchi S, Tatsumi T, Takehara T, Sasakawa A, Yamamoto M, Kohga K, Miyagi T, Kanto T, Hiramastu N, Akagi T, Akashi M, Hayashi N. EphA2-derived peptide vaccine with amphiphilic poly(gamma-glutamic acid) nanoparticles elicits an anti-tumor effect against mouse liver tumor. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Facts & Statistics, 2009. American Brain Tumor Association; Des Plaines, IL: 2009. [Google Scholar]
- 134.Fakhrai H, Dorigo O, Shawler DL, Lin H, Mercola D, Black KL, Royston I, Sobol RE. Eradication of established intracranial rat gliomas by transforming growth factor beta antisense gene therapy. Proc Natl Acad Sci U S A. 1996;93:2909–2914. doi: 10.1073/pnas.93.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maggard M, Meng L, Ke B, Allen R, Devgan L, Imagawa DK. Antisense TGF-beta2 immunotherapy for hepatocellular carcinoma: treatment in a rat tumor model. Ann Surg Oncol. 2001;8:32–37. doi: 10.1007/s10434-001-0032-6. [DOI] [PubMed] [Google Scholar]
- 136.Schlingensiepen KH, Schlingensiepen R, Steinbrecher A, Hau P, Bogdahn U, Fischer-Blass B, Jachimczak P. Targeted tumor therapy with the TGF-beta 2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129–139. doi: 10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 137.Liu Y, Wang Q, Kleinschmidt-DeMasters BK, Franzusoff A, Ng KY, Lillehei KO. TGF-beta2 inhibition augments the effect of tumor vaccine and improves the survival of animals with pre-established brain tumors. J Neurooncol. 2007;81:149–162. doi: 10.1007/s11060-006-9222-1. [DOI] [PubMed] [Google Scholar]
- 138.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 139.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm. 2002;28:1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- 140.Vauthier C, Dubernet C, Fattal E, Pinto-Alphandary H, Couvreur P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Advanced Drug Delivery Reviews. 2003;55:519–548. doi: 10.1016/s0169-409x(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 141.Yang SC, Ge HX, Hu Y, Jiang XQ, Yang CZ. Doxorubicin-loaded poly(butylcyanoacrylate) nanoparticles produced by emulsifier-free emulsion polymerization. Journal of Applied Polymer Science. 2000;78:517–526. [Google Scholar]
- 142.Soma CE, Dubernet C, Bentolila D, Benita S, Couvreur P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials. 2000;21:1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 143.Soma CE, Dubernet C, Barratt G, Nemati F, Appel M, Benita S, Couvreur P. Ability of doxorubicin-loaded nanoparticles to overcome multidrug resistance of tumor cells after their capture by macrophages. Pharmaceutical Research. 1999;16:1710–1716. doi: 10.1023/a:1018902031370. [DOI] [PubMed] [Google Scholar]
- 144.Schneider T, Becker A, Ringe K, Reinhold A, Firsching R, Sabel BA. Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J Neuroimmunol. 2008;195:21–27. doi: 10.1016/j.jneuroim.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 145.Alyautdin RN, Tezikov EB, Ramge P, Kharkevich DA, Begley DJ, Kreuter J. Significant entry of tubocurarine into the brain of rats by adsorption to polysorbate 80-coated polybutylcyanoacrylate nanoparticles: an in situ brain perfusion study. J Microencapsul. 1998;15:67–74. doi: 10.3109/02652049809006836. [DOI] [PubMed] [Google Scholar]
- 146.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674:171–174. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 147.Schroderand BA, Sabel U. Nanoparticles, a drug carrier system to pass the blood-brain barrier, permit central analgesic effects of i.v. dalargin injections. Brain Res. 1996;710:121–124. doi: 10.1016/0006-8993(95)01375-x. [DOI] [PubMed] [Google Scholar]
- 148.Kreuter J, Petrov VE, Kharkevich DA, Alyautdin RN. Influence of the type of surfactant on the analgesic effects in duced by the peptide dalargin after its delivery across the blood-brain barrier using surfactant-coated nanoparticles. Journal of Controlled Release. 1997;49:81–87. [Google Scholar]
- 149.Schroeder U, Sommerfeld P, Ulrich S, Sabel BA. Nanoparticle technology for delivery of drugs across the blood-brain barrier. J Pharm Sci. 1998;87:1305–1307. doi: 10.1021/js980084y. [DOI] [PubMed] [Google Scholar]
- 150.Schroeder U, Sommerfeld P, Sabel BA. Efficacy of oral dalargin-loaded nanoparticle delivery across the blood-brain barrier. Peptides. 1998;19:777–780. doi: 10.1016/s0196-9781(97)00474-9. [DOI] [PubMed] [Google Scholar]
- 151.Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16:1564–1569. doi: 10.1023/a:1018983904537. [DOI] [PubMed] [Google Scholar]
- 152.Schlingensiepen KH, Fischer-Blass B, Schmaus S, Ludwig S. Antisense therapeutics for tumor treatment: the TGF-beta2 inhibitor AP 12009 in clinical development against malignant tumors. Recent Results Cancer Res. 2008;177:137–150. doi: 10.1007/978-3-540-71279-4_16. [DOI] [PubMed] [Google Scholar]
- 153.Lowery AR, Gobin AM, Day ES, Halas NJ, West JL. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomedicine. 2006;1:149–154. doi: 10.2147/nano.2006.1.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bernardi RJ, Lowery AR, Thompson PA, Blaney SM, West JL. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: an in vitro evaluation using human cell lines. J Neurooncol. 2008;86:165–172. doi: 10.1007/s11060-007-9467-3. [DOI] [PubMed] [Google Scholar]
- 155.Ojeda R, de Paz JL, Barrientos AG, Martin-Lomas M, Penades S. Preparation of multifunctional glyconanoparticles as a platform for potential carbohydrate-based anticancer vaccines. Carbohydr Res. 2007;342:448–459. doi: 10.1016/j.carres.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 156.Day ES, Morton JG, West JL. Nanoparticles for thermal cancer therapy. J Biomech Eng. 2009;131:074001. doi: 10.1115/1.3156800. [DOI] [PubMed] [Google Scholar]
- 157.Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: in vitro study. Jpn J Cancer Res. 1996;87:1179–1183. doi: 10.1111/j.1349-7006.1996.tb03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: ex vivo study. Jpn J Cancer Res. 1997;88:630–632. doi: 10.1111/j.1349-7006.1997.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, Saida T, Kobayashi T. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci. 2003;94:308–313. doi: 10.1111/j.1349-7006.2003.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J Biosci Bioeng. 2003;96:364–369. doi: 10.1016/S1389-1723(03)90138-1. [DOI] [PubMed] [Google Scholar]
- 161.Ito A, Shinkai M, Honda H, Yoshikawa K, Saga S, Wakabayashi T, Yoshida J, Kobayashi T. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol Immunother. 2003;52:80–88. doi: 10.1007/s00262-002-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ito A, Matsuoka F, Honda H, Kobayashi T. Heat shock protein 70 gene therapy combined with hyperthermia using magnetic nanoparticles. Cancer Gene Ther. 2003;10:918–925. doi: 10.1038/sj.cgt.7700648. [DOI] [PubMed] [Google Scholar]
- 163.Ito A, Matsuoka F, Honda H, Kobayashi T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother. 2004;53:26–32. doi: 10.1007/s00262-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ito A, Kuga Y, Honda H, Kikkawa H, Horiuchi A, Watanabe Y, Kobayashi T. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett. 2004;212:167–175. doi: 10.1016/j.canlet.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 165.Tanaka K, Ito A, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Heat immunotherapy using magnetic nanoparticles and dendritic cells for T-lymphoma. J Biosci Bioeng. 2005;100:112–115. doi: 10.1263/jbb.100.112. [DOI] [PubMed] [Google Scholar]
- 166.Tanaka K, Ito A, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int J Cancer. 2005;116:624–633. doi: 10.1002/ijc.21061. [DOI] [PubMed] [Google Scholar]
- 167.Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunol Immunother. 2006;55:320–328. doi: 10.1007/s00262-005-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ito A, Kobayashi T, Honda H. Heat immunotherapy with heat shock protein expression by hyperthermia using magnetite nanoparticles. Ann Cancer Res Therap. 2007;15:27–34. [Google Scholar]
- 169.Takada T, Yamashita T, Sato M, Sato A, Ono I, Tamura Y, Sato N, Miyamoto A, Ito A, Honda H, Wakamatsu K, Ito S, Jimbow K. Growth inhibition of re-challenge B16 melanoma transplant by conjugates of melanogenesis substrate and magnetite nanoparticles as the basis for developing melanoma-targeted chemo-thermo-immunotherapy. J Biomed Biotechnol. 2009;2009:457936. doi: 10.1155/2009/457936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kikumori T, Kobayashi T, Sawaki M, Imai T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res Treat. 2009;113:435–441. doi: 10.1007/s10549-008-9948-x. [DOI] [PubMed] [Google Scholar]
- 171.Jimbow K, Takada T, Sato M. Melanin biology and translational research strategy; melanogenesis and nanomedicine as the basis for melanoma-targeted DDS and chemothermo-immunotherapy. Pigment Cell and melanoma Research. 2008;21:243. [Google Scholar]
- 172.Dow SW, Fradkin LG, Liggitt DH, Willson AP, Heath TD, Potter TA. Lipid-DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. Journal of Immunology. 1999;163:1552–1561. [PubMed] [Google Scholar]
- 173.Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Levy E, Krzakowski M, Hess D, Tartour E, Chenard MP, Limacher JM, Bizouarne N, Acres B, Halluard C, Velu T. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008;3:735–744. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 174.Dharmapuri S, Peruzzi D, Aurisicchio L. Engineered adenovirus serotypes for overcoming anti-vector immunity. Expert Opin Biol Ther. 2009;9:1279–1287. doi: 10.1517/14712590903187053. [DOI] [PubMed] [Google Scholar]
- 175.Foged C, Sundblad A, Hovgaard L. Targeting vaccines to dendritic cells. Pharm Res. 2002;19:229–238. doi: 10.1023/a:1014474414097. [DOI] [PubMed] [Google Scholar]