Abstract
Biodegradable nanofibrous scaffolds serving as an extracellular matrix substitute have been shown to be applicable for cartilage tissue engineering. However, a key challenge in using nanofibrous scaffolds for tissue engineering is that the small pore size limits the infiltration of cells, which may result in uneven cell distribution throughout the scaffold. This study describes an effective method of chondrocyte loading into nanofibrous scaffolds, which combines cell seeding, mixing, and centrifugation to form homogeneous, packed cell-nanofiber composites (CNCs). When the effects of different growth factors are compared, CNCs cultured in medium containing a combination of insulin-like growth factor-I and transforming growth factor-β1 express the highest mRNA levels of collagen type II and aggrecan. Radiolabeling analyses confirm the effect on collagen and sulfated-glycosaminoglycans (sGAG) production. Histology reveals chondrocytes with typical morphology embedded in lacuna–like space throughout the entire structure of the CNC. Upon culturing using a rotary wall vessel bioreactor, CNCs develop into a smooth, glossy cartilage-like tissue, compared to a rough-surface tissue when maintained in a static environment. Bioreactor-grown cartilage constructs produce more total collagen and sGAG, resulting in greater gain in net tissue weight, as well as express cartilage-associated genes, including collagen types II and IX, cartilage oligomeric matrix protein, and aggrecan. In addition, dynamic culture enhances the mechanical property of the engineered cartilage. Taken together, these results indicate the applicability of nanofibrous scaffolds, combined with efficient cell loading and bioreactor technology, for cell-based cartilage tissue engineering.
Introduction
Tissue engineering presents a promising approach to cartilage repair and regeneration. The basic concept of cartilage tissue engineering requires the culturing of cells (differentiated chondrocytes or differentiating chondro-progenitor cells) in three-dimensional biomaterial scaffolds to produce cartilage in vitro or in vivo. The ideal scenario for effective cartilage regeneration is that the seeded cells reside in a biomaterial scaffold environment similar to that in vivo, thus allowing for the natural production and deposition of cartilage-specific extracellular matrix (ECM), leading ultimately to formation of functional cartilage. Cell-cell and cell-matrix interactions are critical in eliciting biological signals in this process. For example, higher density cell seeding, such as in micromass1 and cell pellet cultures2, provide the necessary cell-cell interactions to support chondrogenic differentiation of mesenchymal chondro-progenitor cells.
Biomaterial scaffolds offer exciting biomimetic systems for the ECM to provide physical protection as well as establish cell-matrix interactions with residing cells. In a scaffold culture, cell-matrix interactions is critically dependent on cell distribution that is partially determined by the original cell seeding density3. It has been a challenge, however, to seed cells evenly in a three-dimensional, porous scaffold4. For example, the most commonly used cell seeding technique, gravity cell seeding, usually results in a vertical cell density gradient within the scaffold5. Alternative methods, such as the use of spinning flasks6 or vacuum chamber7, showed improved cell seeding, and more uniform cell and matrix distribution in the construct. Homogeneous cell distribution in the construct is a crucial step for uniform tissue formation during tissue engineering.
Electrospun nanofibrous scaffolds have demonstrated success in supporting the maintenance of chondrocyte phenotype8 and chondrogenic induction of mesenchymal stem cells9, and thus hold great promise for cartilage tissue engineering applications. However, the biggest challenge with using nanofibrous scaffolds is the intrinsically small size of the pores formed by the fibers, which limits infiltration and migration of the seeded cells, and affects cell distribution in the scaffold. In this study, using electrospun poly(L-lactic) acid (PLLA), we have modified the cellular construct fabrication method by adopting a cell pelleting procedure (see Fig. 1A) to introduce cells into the nanofibrous scaffold. Specifically, bovine chondrocytes are mixed with electrospun PLLA nanofibers and subjected to centrifugation to create a packed cell-nanofiber composite (CNC). This novel method overcomes the barrier of small pore size of the nanofibrous scaffold, resulting in optimized cell distribution within the CNC.
Figure 1.
(A) Schematic illustrating the construction of a cell-nanofiber composite (CNC) for cartilage tissue engineering. Details are described in Materials and Methods. (B) H&E low-magnification histology of the cross-section of a representative CNC construct 1 day after cell seeding. Cells (arrows) were seen dispersed among nanofibers and distributed across the entire thickness of the construct. Bar = 500 μm. (C and D) H&E high-magnification histology of representative cortical region of a CNC viewed under (C) Hoffman modulation contrast and (D) phase contrast, showing presence of populations of distinct cells packed within the nanofibers (arrow). Bar = 25 μm. (E and F) Macroscopic morphology of CNC cultured for 42 days. (E) Side view showing a thick, homogeneous, cartilage tissue-like CNC with an irregular edge. (F) Top view showing a compact, circular cartilaginous tissue with a rough and glossy surface. Scale in 1 mm divisions.
In the absence of blood supply, growing a large piece of cartilage-like construct in vitro is critically dependent on efficient diffusion and transport of nutrients. Various designs of bioreactors with improved medium circulation have been used to culture scaffold-based cartilage constructs10,11. Among these, the rotary wall vessel (RWV) bioreactor particularly has been demonstrated to enhance the production of cartilage matrix in different scaffold cultures12,13. In RWV bioreactors, engineered constructs encounter a simulated micro-gravity environment because its free-fall is counteracted by the upward force of medium rotation14,15. Also, the rotation of culture medium exerts mechanical stimulus on the cells within the construct, and enhances the exchange of nutrients, oxygen, and metabolic wastes. All these factors contribute to an improved culture environment for in vitro tissue engineering using RWV bioreactors compared to conventional culture systems, such as flasks and Petri dishes16. In this study, the RWV was used to enhance the growth of CNC based cartilage constructs and these “dynamic” cultures were compared to “static” cultures maintained continuously as a cell pellet in a 50-mL conical centrifuge tube. In addition, we applied insulin-like growth factor-I (IGF-I) and transforming growth factor-β1 (TGF-β1), two growth factors that have previously been shown to regulate chondrocyte activity11, to enhance cartilage development. The CNC-based engineered cartilage was analyzed on the basis of macroscopic and histological evaluation of construct and cell morphologies, reverse transcription-polymerase chain reaction (RT-PCR) for quantification of cartilage-specific ECM genes, biochemical analysis of cartilaginous matrix production, and mechanical testing of material properties.
Materials and methods
Fabrication of CNC
PLLA (MW = 50,000) nanofibrous scaffolds were produced by electrospinning as described previously17. Primary bovine chondrocytes were isolated from 4-6 month old calves using a previously published protocol18. Cells were cultured in 150-cm2 tissue culture flasks containing chondrocyte growth medium and maintained at 37°C in a humidified, 5% CO2 atmosphere. The chondrocyte growth medium was composed of Dulbecco's Modified Eagle's Medium (DMEM), 10% (v/v) fetal bovine serum (FBS), 1% (v/v) Minimum Essential Medium (MEM) vitamin solution, 50 μg/mL L-ascorbic acid 2-phosphate, and antibiotics (50 μg/mL of streptomycin, 50 IU of penicillin/mL). Cell culture medium was replaced every 3 days. Cells obtained at passages 1 to 2 were used in this study.
The procedure of making CNCs is depicted in Fig. 1A. Briefly, 1 × 107 harvested chondrocytes were mixed with 30 mg of sterilized PLLA nanofibers in a 50 mL conical tube containing 2 mL of 10% FBS containing medium, and cultured for 2 hours. Samples were centrifuged for 5 min at 1,000 × g to create a cell-packed CNC.
Culture of CNCs
To analyze the effects of growth factors, CNCs maintained in 50-mL conical tubes were cultured in a chondrogenic, serum-free medium composed of DMEM, containing 100 nM dexamethasone, 50 μm/mL ascorbate 2-phosphate, 100 μg/mL sodium pyruvate, 40 μg/mL proline, antibiotics, and ITS-plus Premix diluted 1:100, and supplemented with either 50 ng/mL of IGF-I, 10 ng/ml of TGF-β1, combined IGF-I and TGF-β1, or no growth factor as control. Culture medium was changed every 3 days.
To analyze the effects of culture conditions, CNCs were transferred, after 3 days of static culture in conical tubes, to an RWV bioreactor (Synthecon, Houston, TX) for dynamic culture for an additional 42 days, in comparison to static cultures of CNCs maintained in the conical tubes for the same period. Both cultures were maintained in chondrogenic, serum free medium supplemented with combined IGF-I and TGF-β1. Half of the culture medium was replaced with fresh medium every 5 days.
Morphological analyses
After harvesting, CNCs were washed with phosphate-buffered saline (PBS), and observed macroscopically with stereomicroscope.
For histological and immunohistological analyses, CNC were washed in PBS, fixed in 4% phosphate-buffered paraformaldehyde at 4°C for 30 minutes, dehydrated through a graded series of ethanol, infiltrated with Histo-Clear, embedded in paraffin, and sectioned at a thickness of 8 μm. For histological analysis, sections were then deparaffinized in Histo-Clear, rehydrated using a graded series of ethanol, and stained with hematoxylin and eosin (H&E) or alcian blue (pH 1.0). Immunohistochemical analysis was used to detect collagen types II and IX, aggrecan, and cartilage proteoglycan link protein. To detect collagens types II and IX, sections were pre-digested with 300 U/mL of hyaluronidase at 37°C for 15 minutes before incubation in 15 μg/mL of antibodies to collagen type II or IX (Development Studies Hybridoma Bank, Iowa City, IA). For the detection of aggrecan and link protein, sections were pre-digested for 15 minutes at 37°C in 1.5 U/mL of chondroitinase, and then incubated with either 10 μg/mL of aggrecan antibody (Development Studies Hybridoma Bank, Iowa City, IA) at 37°C for 1 hour or with 6 μg/mL of link protein antibody (Development Studies Hybridoma Bank, Iowa City, IA) at 4°C overnight. Broad Spectrum alkaline phosphatase-conjugated secondary antibodies (Zymed Lab, San Francisco, CA) were used for detection and developed using BCIP-NBT. Tissue sections without treatment with primary antibodies served as controls.
Quantitative and conventional RT-PCR analyses
Total RNA was isolated from CNC samples with 800 μL Trizol reagent. After addition of 160 μl chloroform to the homogenized samples, RNA was precipitated using 400 μL of isopropanol. RNA pellets were dissolved in 20 μL of RNase- and DNase-free water and RNA yields were estimated based on A260. First strand cDNA was reverse transcribed from 3 μg of total RNA using the SuperScript First-Strand Synthesis System kit (Invitrogen, Carlsbad, CA) and amplified for real-time PCR, with detection using iQ SYBR Green supermix in the Bio-Rad iCycler iQ system19. Gene-specific oligonucleotide primers were used for collagen type II, aggrecan, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control for mRNA loading was used20.
For conventional RT-PCR, first strand cDNA was processed for PCR using gene-specific oligonucleotide primers for collagen types II, IX, and X, cartilage oligomeric matrix protein (COMP), aggrecan, and GAPDH. Thirty cycles were used to amplify all gene sequences, and the PCR products were electrophoretically analyzed and visualized after ethidium bromide staining.
[3H]-Proline and [35S]-sulfate incorporation assays
To estimate collagen biosynthesis rate, fresh medium with a final concentration of 10 μCi/mL [3H]-proline and 5 μCi/mL [35S]-sulfate (Amersham Bioscience, Buckinghamshire, UK) was added to the cultures 24 hours prior to harvest. The harvested CNC cultures were thoroughly washed with PBS containing 1 mM proline and 0.8 mM sodium sulfate, and digested with 300 μg/mL papain in 20 mM sodium phosphate, pH 6.8, 5 mM EDTA, 2 mM dithiothreitol (DTT) at 60°C for 18 hours. Aliquots were analyzed for radioactivity by liquid scintillation counting, and the results normalized to the amount of DNA determined using the RediPlate 96 PicoGreen dsDNA assay (Invitrogen, Carlsbad, CA).
Sulfated glycosaminoglycan (sGAG) assay
sGAGs were extracted from CNC samples following a method modified from a previous report21. Briefly, samples were harvested, washed with PBS, and digested with 300 μg/mL papain in 20 mM sodium phosphate, pH 6.8, 5 mM EDTA, 2 mM DTT at 60°C for 18 hours. The extract was cleared by centrifugation and analyzed for sGAG and DNA. For sGAG, the sample was reacted with the Blyscan dye reagent composed of 1, 9-dimethylmethylene blue (DMMB) for 30 minutes, and the unbound dye solution was removed by centrifugation. Bound dye was released from the insoluble sGAG-dye complex, and quantified spectrophotometrically on the basis of A656. The total amount of sGAG was calculated from a standard curve using chondroitin 4-sulfate as a standard. DNA analysis was carried out using the RediPlate 96 PicoGreen dsDNA Assay and the result was used to normalize the sGAG amount.
Mechanical testing
The compressive mechanical property of CNCs harvested from the static and dynamic cultures was measured using a custom-made mechanical tester22. CNCs were rinsed and 8 mm-diameter test specimens were obtained by coring. Each specimen was trimmed to ensure a flat surface and the thickness was measured with a digital micrometer. Stress relaxation test in unconfined compression was carried out as follows. A pre-conditioning load of 0.02 N was first applied until equilibrium was achieved. Stress relaxation was carried out at a constant deformation of 1 μm/s until 10% strain was achieved, after which specimens were allowed to relax to equilibrium. The equilibrium compressive Young's modulus was calculated as the ratio of the equilibrium stress to the strain.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical analysis was performed with Student's t-test and significance was determined at p < 0.05 or p < 0.01.
Results
Morphology of CNC
Efficient cell seeding into the nanofibrous scaffold was achieved using the procedure described here (Fig. 1A). Specifically, as shown in Fig. 1B-D, H&E histology revealed that at 1 day after cell seeding, well dispersed cells were seen distributed among the nanofibers and also found throughout the entire 3 mm thick CNC construct. While the centrifugation-mediated method efficiently produced high-density cell loading, higher magnification revealed populations of distinct, individual cells surrounded by the nanofibers. This finding suggested that the seeding process used here effectively allowed cell penetration into the scaffold matrix, unlike the limited and uneven cell distribution observed in the nanofibrous scaffold by gravity cell seeding.
After 42 days, cultured CNC constructs developed into a shiny, cartilage-like tissue (Fig. 1E,F). The new tissue produced in CNC appeared to encapsulate and coalesce chondrocytes and nanofibers into a fused piece of cartilage. The CNC-derived cartilage construct exhibited favorable tissue integrity and was capable of retaining its shape after physical deformation, such as bending and compression, suggesting the presence of an elastic, biological tissue. In addition, the shape and size of CNC based cartilage were controlled by the shape of culture containers and the amount of nanofibers, respectively. In this study, the circular cartilage was shaped by the 50-mL conical tubes used during the culture. CNCs exhibited an irregular surface and edge, probably due to uneven tissue growth rates within the construct.
Effects of growth factors
Cartilage ECM gene expression in CNC cultures upon treatment with different growth factors was analyzed quantitatively using real time RT-PCR. After 42 days of culture, the mRNA levels of collagen type II (Fig. 2A) and aggrecan (Fig. 2B) in the TGF-β1 and IGF-I/TGF-β1 treated cultures were significantly higher (p < 0.01) than those in IGF-I and control cultures. IGF-I cultures expressed a similar level of collagen type II and a lower level of aggrecan, compared to those in the control group. TGF-β1 and IGF-I/TGF-β1 treated cultures showed comparable mRNA levels of collagen type II and aggrecan. These results suggested that TGF-β1, compared to IGF-I, dominantly regulated collagen type II and aggrecan expression in CNC culture.
Figure 2.
(A and B) Cartilage-specific gene expression in CNC cultures stimulated with different growth factors for 42 days analyzed by quantitative RT-PCR. (A) Collagen type II; (B) aggrecan. TGF-β1 and IGF-I/TGF-β1 treated cultures showed a significantly higher level of both collagen type II and aggrecan mRNA expression compared to IGF-I and control cultures. mRNA values of each gene were normalized to those of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (C, D, and E) Collagen and sGAG synthesis in CNC cultures stimulated with different growth factors for 42 days analyzed by [3H]-proline incorporation, [35S]-sulfate incorporation, and 1, 9-dimethylmethylene blue (DMMB) assay. (C) [3H]-Proline incorporation. IGF-I/TGF-β1 treated cultures exhibited the highest rate of proline incorporation, i.e., collagen production. (D) [35S]-Sulfate incorporation. IGF-I/TGF-β1 treated cultures had highest rate of sGAG production. (E) sGAG content. Both TGF-β1 and IGF-I/TGF-β1 treated cultures showed the highest sGAG content. All data were normalized to DNA content. All values were mean ± SD. *p < 0.05, **p < 0.01, compared to control without growth factor supplementation, n = 3 in A and B, n = 4 in C, D, and E.
Biochemical analyses of ECM components were also used to compare the effects of growth factors in CNC cultures. At Day 42, both TGF-β1 and IGF-I/TGF-β1 treated cultures showed higher level (p < 0.05) of collagen biosynthesis, estimated on the basis of [3H]-proline incorporation, than IGF-I treated culture, which was similar to the control group (Fig. 2C). The biosynthesis and accumulation of sGAG were estimated on the basis of [35S]-sulfate incorporation and using the DMMB assay, respectively. sGAG synthesis in IGF-I/TGF-β1 culture was significantly higher (p < 0.01) than other cultures (Fig. 2D), whereas in TGF-β1 culture, sGAG synthesis was comparable to that in IGF-I culture. On the other hand, accumulation of sGAG in both TGF-β1 and IGF-I/TGF-β1 cultures was significantly higher (p < 0.01) than that in both IGF-I and control culture (Fig. 2E). The results of the gene and biochemical analyses thus clearly showed that TGF-β1 and IGF-I/TGF-β1 treatments up-regulated collagen and sGAG production in CNC cultures.
Effects of culture conditions
CNCs harvested from both static and dynamic cultures appeared as circular, tissue-like constructs, 1.3 cm in diameter and more than 0.5 cm in thickness (Fig. 3A). Macroscopically, after 42 days of culture, the engineered constructs showed neo-tissue that filled the pores and embedded the nanofibers. The CNCs from the dynamic cultures in RWV bioreactors were generally slightly larger than static cultures kept in the 50-mL conical tubes for the entire culture period. Moreover, dynamically grown cartilage showed a glossy, smooth surface and edge, whereas statically grown cartilage had a rough surface and edge, suggesting that the dynamic environment in the bioreactor effectively smoothed the surface of the construct. In addition, by culture day 42, the weight of CNCs in the dynamic culture increased 12-fold, significantly more (p < 0.01) than the 10-fold increase of those in the static culture (Fig. 3B).
Figure 3.

(A) Macroscopic morphology of CNC based engineered cartilage constructs grown in dynamic or static culture for 42 days. (Right) Dynamically grown constructs maintained in RWV bioreactor showing a smooth, glossy surface and a larger size than statically grown cartilage (Left) with a more bumpy surface and rough edge. Scale in 1 mm divisions. (B) Weight increase of CNC based engineered cartilage cultured in different conditions after 42 days. A 12-fold increase is observed in dynamically grown cartilage, which is statistically higher than that of statically grown cartilage (~ 10 folds). All values are mean ± SD. **p < 0.01, n = 6.
Expression of cartilage ECM genes in the different culture environments was analyzed using RT-PCR. At Day 42, both static and dynamic cultures expressed the cartilage-specific ECM markers, collagen types II, IX, and X, aggrecan, and COMP (Fig. 4A), suggesting that chondrocytes in the CNCs maintained their phenotype. Specifically, the mRNA levels of collage type IX and aggrecan in the dynamic culture were higher than those in the static culture, indicating that the bioreactor culture environment enhanced the expression of cartilage-specific genes. It is noteworthy that both static and dynamic cultures expressed a detectable, low level of collagen type X mRNA, suggesting that a small number of chondrocytes might have undergone hypertrophy.
Figure 4.
(A) Comparison of cartilage-specific gene expression in Day 42 static and dynamic CNC cultures analyzed by RT-PCR. Both cultures were supplemented with TGF-β1 and IGF-I. Total RNA was analyzed for collagen types II (Col II), IX (Col IX), and X (Col X), cartilage oligomeric matrix protein (COMP), and aggrecan (AGN), with GAPDH as a control. Collagen types IX and X and aggrecan gene expressions are up-regulated in dynamic CNC cultures, whereas expression of other genes are similar in both culture conditions. S, static culture; D, dynamic culture. (B-G) Histological comparison of CNC based engineered cartilage grown in static and dynamic cultures after 42 days. (B and C) H&E staining; (D-G) alcian blue staining. Spindle or elongated cells are found in static cultures (B), whereas more cells with round or oval, chondrocytic morphology are seen in dynamic cultures (C). Less intense alcian blue staining, indicating a lower content of proteoglycan, is seen in static cultures (D) than dynamic cultures (E). Low magnification view of alcian blue stained CNCs showed less proteoglycan accumulation at the periphery of statically grown CNCs (F) than in similar regions of dynamically grown CNCs (G). (H-O) Immunolocalization of cartilage-specific matrix proteins in static and dynamic CNCs cultured for 42 days. Both types of CNCs showed positive staining for collagen types II (H and I) and IX (J and K), aggrecan (AGN) (L and M), and cartilage proteoglycan link protein (LP) (N and O), but more intense staining was shown in dynamically grown CNCs. Bar = 25 μm (B-E, and H-O) or 3 mm (F, G).
At Day 42, H&E staining demonstrated that cells in static cultures were variable in shape and size; both spindle-shaped and oval cells, large and small, were seen (Fig. 4B). In comparison, in dynamic cultures, relatively fewer spindle-shaped cells were present, and the majority of the cells observed were oval or round (Fig. 4C). It is noteworthy that, compared to static cultures, dynamically grown CNCs contained a higher percentage (60% versus 25%) of cells embedded in lacunae. This phenotype resembled that of chondrocytes in native, hyaline cartilage, suggesting that the enhanced nutrition/waste exchange facilitated by the rotatory bioreactor influenced chondrogenic differentiation. Positive alcian blue staining showed that cells in both static (Fig. 4D) and dynamic (Fig. 4E) cultures were surrounded by sulfated proteoglycan rich ECM, typical of cartilage. Positive staining was generally seen throughout the CNC, with static cultures exhibiting more peripheral staining (Fig. 4F), while dynamic cultures (Fig. 4G) had more intense alcian blue staining overall. As Figure 1B shows that the seeding method described here resulted in even distribution of cells in the construct, the non-uniform sGAG staining found here was unlikely to be the result of uneven cell dispersion. Alcian blue staining was present throughout the entire construct, but appeared more intense in certain regions, suggesting that the production of sGAG could be a function of cell location within the construct, i.e. cells in the cortical region produced more sulfated proteoglycan than those in the core. This difference was probably related to a difference in the efficiency of nutrient/waste exchange. More efficient nutrient/waste exchange occurred in the region close to the surface of the construct, and correlated with higher sGAG production.
Immunohistochemistry demonstrated the presence of the cartilage ECM markers, collagen types II and IX, aggrecan, and cartilage proteoglycan link protein, in both dynamic and static cultures at Day 42. Dynamic cultures (Fig. 4I,K,M,O) showed stronger and more wide-spread staining for these markers compared to static cultures (Fig. 4H,J,L,N). Specifically, staining for collagen type II and aggrecan in static cultures was primarily around the cells, whereas in dynamic cultures, the staining was more prolific, with collagen type II positive signal distributed throughout the CNC and aggrecan staining over half of the specimen. Consistent with the gene expression data, the histological results suggested that cells in dynamic cultures produced more cartilage ECM macromolecules than those in static cultures.
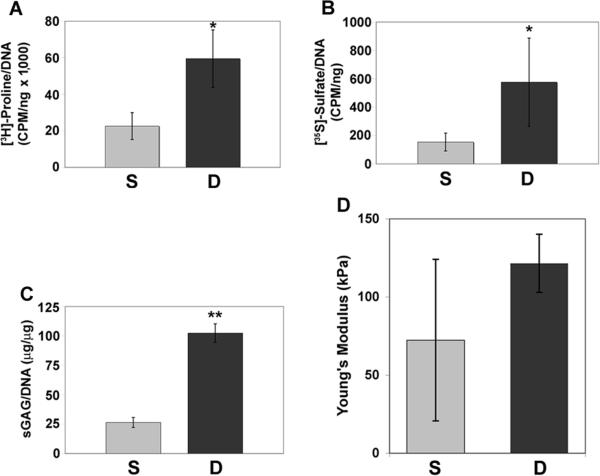
Biochemical analyses further confirmed these findings. The dynamic culture showed significantly enhanced rate of collagen synthesis, representing 3-fold increase compared to the static culture (Fig. 5A). The dynamic culture was also consistently higher (p < 0.05) than the static culture in both synthesis rate and accumulation of sGAG (Fig. 5B,C). These results suggested that the dynamic culture condition favored the production of collagen and sGAG in the chondrogenic CNC.
Figure 5.
Comparison of collagen and sGAG synthesis in CNCs cultured for 42 days in static and dynamic culture analyzed by (A) [3H]-Proline incorporation, (B) [35S]-sulfate incorporation, and (C) DMMB assay. Dynamically grown CNCs showed a higher rate of (A) collagen synthesis ([3H]-proline incorporation) and (B) sGAG production as well as (C) a higher sGAG content. (D) Equilibrium compressive Young's modulus of static and dynamic culture of CNCs on culture day 42. All data are normalized to DNA content. S, static culture; D, dynamic culture. All values are mean ± SD. *p < 0.05, **p < 0.01, n = 3.
Mechanical property is a critical criterion to assess the quality of engineered cartilage. Dynamically cultured CNCs showed equilibrium compressive Young's modulus of 122 ±19 kPa, compared to static culture (72 ± 52 kPa) (Fig. 5D). Despite the lack of statistical significance, a positive trend is indicated upon the use of the RWV bioreactor, suggesting that dynamic culture likely provided mechanically derived stimuli to enhance the cartilaginous quality of the CNCs.
Discussion
A significant and practical challenge in using nanofibers as a scaffold for tissue engineering is that the small pore size between fibers could impede cells from entering the scaffold during seeding or migrating between pores during culture. Because ultra fine nanofibers pack more tightly than larger fibers, the interfiber pores are substantially smaller. Our previous study showed that, while there is a wide range of pore sizes in a nanofibrous scaffold, a large percentage of pores were less than 25 μm23. These pore sizes are at the same scale as a cell and may deter free passage of cells. When introduced onto a nanofibrous scaffold using conventional, gravity cell seeding approaches, the initially seeded cells often clog the pores and prevent subsequent cells from penetrating the scaffold, resulting in uneven cell distribution. To circumvent this technical problem, gentle aspiration of cells into the nanofibrous scaffold by imbibing with filter paper yielded improved, but still less than satisfactory, results. We report here a significantly improved approach to improve cell seeding in nanofibrous scaffolds: chondrocytes were mixed with nanofibers and the mixture packed by gentle centrifugation to create cell-dispersed nanofibrous composites. This effective cell seeding method resulted in CNCs that contain phenotypically similar cells evenly distributed throughout the entire composite, overcoming the pore-size related obstacle.
The application of centrifugation to tightly pack cells and nanofibers to form CNCs is similar to the production of high density cell pellets, commonly used to study chondrogenesis of mesenchymal stem cells2 and redifferentiation of dedifferentiated chondrocytes24. The process simulates high density cellular condensation observed during early cartilage formation in the developing embryonic limb bud. Cell condensation, enhanced by centrifugation, should promote cell-cell interactions that are necessary for chondrogenesis or chondrocyte redifferentiation25. In this study, because of the presence of nanofibers within the scaffold, cells in the CNCs attain not only cell-cell but also cell-matrix biomaterial interactions. We have previously reported that the interaction between chondrocytes and nanofibers favorably supports the maintenance of chondrocytic phenotype8. This effect is likely to be related to the unique, three-dimensional structure of the nanofibers, which mimics native ECM, and may stimulate chondrocyte activity by regulating their cytoskeleton or shape20. Geometric cues via cell-nanofiber interactions are likely to play a critical role for effective cartilage regeneration in this study. In addition, the centrifugation process tightly packs chondrocytes in the CNCs, promoting a spherical morphology, which also enhances chondrocytic phenotype26.
The use of CNCs to tissue-engineer cartilage readily permits the adjustment of the size and the shape of the construct by varying the amount of fibers used and the shape of the culture container. We are currently investigating the optimal ratio between cell number and fiber quantity for efficacious cartilage formation. A key concern for a large, engineered tissue is that cells in the central core may undergo premature cell death due to inefficient nutrition/waste diffusion and gas exchange. Similar size limitation applies to cell pellets in vitro27,28. Interestingly, in CNC based engineered cartilage, the nanofibrous matrix appears to serve as a filler, creating space to allow nutrients and wastes to diffuse more effectively. Therefore, larger cartilage constructs can be produced using this approach.
Bioreactors are culture devices in which the medium dynamically circulates to promote cell and tissue growth via enhancing nutrient transport and mechanical stimulation29. Engineered cartilage, when cultured in bioreactors, has demonstrated a significant improvement of cartilaginous tissue generation, compared to that in static cultures such as flasks or Petri dishes16. Our findings reported here clearly demonstrate that bioreactor culture of the CNCs results in a more smoothened surface, increases tissue weight, and enhances chondrocytic phenotype. These beneficial effects have also been observed in other engineered cartilage using different scaffolds and bioreactors30. The most obvious enhancement seen here is that the bioreactor-grown CNC cartilage constructs exhibit a glossy, smooth tissue surface as well as a more rigid structure compared to those cultured statically. It is known that a tissue-engineered construct cultured in an RWV bioreactor encounter low shear stress when the rotation speed of the chamber is set to balance the weight of the construct and the hydrodynamic force15. Since this manipulation generates a randomized gravity or simulated microgravity environment inside the chamber, the construct essentially undergoes continuous free-fall with maximal enhancement of medium diffusion into the construct. During the 42 days of culture, while the CNCs increased in weight, the speed of rotation was gradually increased from 15 rpm to 30 rpm to keep the construct floating. Interestingly, in our bioreactor system, the CNCs were not completely stationary and immobile, but instead moved up and down and occasionally underwent rotation. It is likely that these movements generated mechanical stimuli, including shear stress and hydrodynamic influences, that result in smoothing of the CNCs and enhancement of matrix production. The extent of mechanical stimulation is highly dependent on the geometric shape of the construct. Uni-geometric constructs, such as spheres or cubes, are suspended more stably in the medium, compared to disc-shaped constructs, such as the CNCs used here.
Growth factors have been shown to be critical in the induction and maintenance of chondrocyte phenotype and active in the regulation of cell proliferation and differentiation. In cartilage tissue engineering, members of TGF-β superfamily and IGF are the most potent signaling proteins regulating chondrocytic activities11. This study investigates the effects of TGF-β1, IGF-I, and their combination in CNC cultures. The results showed that TGF-β1 alone has positive effects on chondrocytic gene expression and cartilaginous ECM production, whereas IGF-I alone has no significant effects. Combined administration of multiple growth factors has been suggested as a means to optimize cartilage growth for tissue engineering31. It is noteworthy that the effects of multiple growth factors are not simply additive, but may be synergistic or counteractive. For instance, IGF-I, together with bFGF and TGF-β2, has been shown to increase cartilage-specific ECM expression and enhance the histological features of engineered cartilage32. Conversely, Kaplan et al. have shown that the combination of IGF-I and TGF-β did not improve histological features and mechanical performance of engineered cartilage33. Interestingly, our results also showed that the combination of TGF-β1 and IGF-I treatment does not synergically promote chondrocytic gene expression and matrix production. That the effects of TGF-β1 treatment alone are comparable to those of combined growth factor treatment suggests that IGF-I has a less significant effect on the cartilage phenotype. Our observation is thus different from the previously reported positive effect of IGF-I on the production of cartilaginous matrix34. Possible explanations may include the lower IGF-I dose (50 ng/mL versus 100 ng/mL) used here and/or a difference in terms of cell types used and the timing of growth factor treatment35.
In conclusion, this study clearly indicates the feasibility of cell-based cartilage tissue engineering using chondrocytes seeded efficiently and uniformly in a biodegradable nanofibrous scaffold and cultured in a dynamic environment maintained with a rotary bioreactor. Further studies will begin to explore the applicability of the engineered cartilage construct in articular cartilage repair in vivo.
Acknowledgements
The authors thank Dr. Robert Mauck for providing assistance in mechanical testing. This research was supported by Intramural Research Program of NIAMS, NIH (Z01 AR 41131).
References
- 1.DeLise AM, Stringa E, Woodward WA, Mello MA, Tuan RS. Embryonic limb mesenchyme micromass culture as an in vitro model for chondrogenesis and cartilage maturation. Methods Mol Biol. 2000;137:359. doi: 10.1385/1-59259-066-7:359. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Ma T, Kniss DA, Lasky LC, Yang ST. Effects of filtration seeding on cell density, spatial distribution, and proliferation in nonwoven fibrous matrices. Biotechnol Prog. 2001;17:935. doi: 10.1021/bp0100878. [DOI] [PubMed] [Google Scholar]
- 4.Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. J Biomed Mater Res. 2000;51:376. doi: 10.1002/1097-4636(20000905)51:3<376::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Kim BS, Putnam AJ, Kulik TJ, Mooney DJ. Optimizing seeding and culture methods to engineer smooth muscle tissue on biodegradable polymer matrices. Biotechnol Bioeng. 1998;57:46. [PubMed] [Google Scholar]
- 6.Freed LE, Hollander AP, Martin I, Barry JR, Langer R, Vunjak-Novakovic G. Chondrogenesis in a cell-polymer-bioreactor system. Exp Cell Res. 1998;240:58. doi: 10.1006/excr.1998.4010. [DOI] [PubMed] [Google Scholar]
- 7.Solchaga LA, Tognana E, Penick K, Baskaran H, Goldberg VM, Caplan AI, Welter JF. A rapid seeding technique for the assembly of large cell/scaffold composite constructs. Tissue Eng. 2006;12:1851. doi: 10.1089/ten.2006.12.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 9.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Darling EM, Athanasiou KA. Articular cartilage bioreactors and bioprocesses. Tissue Eng. 2003;9:9. doi: 10.1089/107632703762687492. [DOI] [PubMed] [Google Scholar]
- 11.Kuo CK, Li WJ, Mauck RL, Tuan RS. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 12.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 13.Hu JC, Athanasiou KA. Low-density cultures of bovine chondrocytes: effects of scaffold material and culture system. Biomaterials. 2005;26:2001. doi: 10.1016/j.biomaterials.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Unsworth BR, Lelkes PI. Growing tissues in microgravity. Nat Med. 1998;4:901. doi: 10.1038/nm0898-901. [DOI] [PubMed] [Google Scholar]
- 15.Lappa M. Organic tissues in rotating bioreactors: fluid-mechanical aspects, dynamic growth models, and morphological evolution. Biotechnol Bioeng. 2003;84:518. doi: 10.1002/bit.10821. [DOI] [PubMed] [Google Scholar]
- 16.Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE, Vunjak-Novakovic G. Modulation of the mechanical properties of tissue engineered cartilage. Biorheology. 2000;37:141. [PubMed] [Google Scholar]
- 17.Li WJ, Cooper JA, Jr., Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12:1775. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 21.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 22.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 23.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 24.Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker HJ, Scheid A, Shakibaei M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308:371. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 25.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, McCaffery JM, Spencer RG, Francomano CA. Hyaline cartilage engineered by chondrocytes in pellet culture: histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J Anat. 2004;205:229. doi: 10.1111/j.0021-8782.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muraglia A, Corsi A, Riminucci M, Mastrogiacomo M, Cancedda R, Bianco P, Quarto R. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci. 2003;116:2949. doi: 10.1242/jcs.00527. [DOI] [PubMed] [Google Scholar]
- 28.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Vunjak-Novakovic G, Obradovic B, Martin I, Freed LE. Bioreactor studies of native and tissue engineered cartilage. Biorheology. 2002;39:259. [PubMed] [Google Scholar]
- 31.Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Chua KH, Aminuddin BS, Fuzina NH, Ruszymah BH. Interaction between insulin-like growth factor-1 with other growth factors in serum depleted culture medium for human cartilage engineering. Med J Malaysia. 2004;59(Suppl B):7. [PubMed] [Google Scholar]
- 33.Kaplan BA, Gorman CR, Gupta AK, Taylor SR, Iezzoni JC, Park SS. Effects of transforming growth factor Beta and insulin like growth factor 1 on the biomechanical and histologic properties of tissue-engineered cartilage. Arch Facial Plast Surg. 2003;5:96. doi: 10.1001/archfaci.5.1.96. [DOI] [PubMed] [Google Scholar]
- 34.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Bursac PM, Vunjak-Novakovic G, Freed LE. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem Biophys Res Commun. 2001;286:909. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 35.van Osch GJ, Mandl EW, Marijnissen WJ, van der Veen SW, Verwoerd-Verhoef HL, Verhaar JA. Growth factors in cartilage tissue engineering. Biorheology. 2002;39:215. [PubMed] [Google Scholar]