Abstract
Background
Childhood sexual abuse (CSA) is a traumatic life event associated with an increased lifetime risk for psychopathology/morbidity. The long-term biological consequences of CSA-elicited stress on chromosomal stability in adults are unknown. The primary aim of this study was to determine if the rate of acquired chromosomal changes, measured using the cytokinesis-block micronucleus assay on stimulated peripheral blood lymphocytes, differs in adult female monozygotic twins discordant for CSA.
Methods
Monozygotic twin pairs discordant for CSA were identified from a larger population-based sample of female adult twins for whom the experience of CSA was assessed by self-report (51 individuals including a reference sample). Micronuclei (MN) contain chromatin from structurally normal or abnormal chromosomes that are excluded from the daughter nuclei during cell division and serve as a biomarker to assess acquired chromosomal instability.
Results
Female twins exposed to CSA exhibited a 1.63-fold average increase in their frequency of MN compared to their nonexposed genetically identical cotwins (Paired t-test, t 16 = 2.65, P = 0.017). No additional effects of familial factors were detected after controlling for the effect of CSA exposure. A significant interaction between CSA history and age was observed, suggesting that the biological effects of CSA on MN formation may be cumulative.
Conclusions
These data support a direct link between CSA exposure and MN formation measured in adults that is not attributable to genetic or environmental factors shared by siblings. Further research is warranted to understand the biological basis for the observed increase in acquired chromosomal findings in people exposed to CSA and to determine if acquired somatic chromosomal abnormalities/somatic clonal mosaicism might mediate the adult pathology associated with CSA.
Introduction
Childhood sexual abuse (CSA) not only compromises well-being in childhood but is also associated with a broad range of psychopathology and morbidity in adulthood [1], [2], [3], [4]. However, little is known about the biological mechanisms involved in mediating the long-term pathogenic effect of early-life trauma. One possible means for CSA to be biologically “embedded” in a manner that could lead to a latent pathologic consequence would be if it resulted in a change in the individual’s somatic cell DNA. Evidence shows childhood maltreatment predicts an increased risk of clinically relevant levels of inflammation in adulthood [5],[6], and inflammation-associated reactive oxygen/nitrogen species are known to cause DNA damage/chromosomal changes. Stress-related inflammation also leads to perturbations in the regulation/expression of several genes, including (but not limited to) nuclear factor-kappa B (NF-kβ), interleukin-1B, interleukin-6, and tumor necrosis factor-α [7], [8]. Additional evidence that early-life stress can lead to DNA-based alterations comes from reports of shortened telomeres in children exposed to adverse rearing settings [9], and in adults with a history of chronic or severe childhood illness [10] or childhood maltreatment [11], but the potential correlation between childhood maltreatment and telomere length is controversial [12].
Given that both telomere shortening and/or inflammation-related generation of oxygen and/or nitrogen species are phenomena that have been associated with an increased frequency of acquired chromosomal abnormalities [13], [14], [15], [16], [17], [18], [19], we hypothesize that an alternative or additional biological effect of stress could be the acquisition of somatic cell chromosomal instability. Moreover, since such damage can cause mutations and chromosomal abnormalities that disrupt cellular function and viability through aberrant gene expression and protein formation [20], [21], [22], the accumulation of chromosomal imbalances over time provides a plausible account by which CSA exposure could contribute to the development of later health and psychiatric symptoms observed in adults with a CSA history.
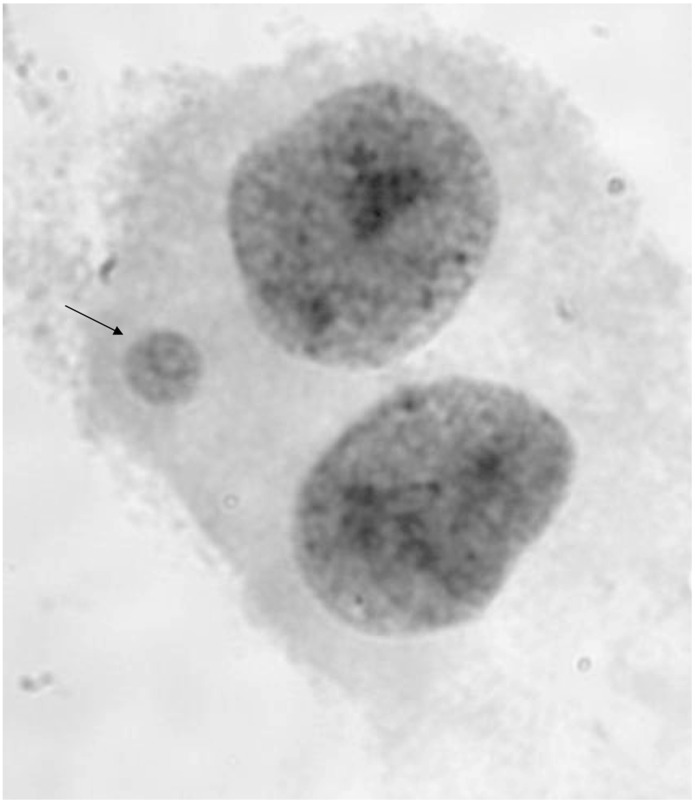
The cytokinesis-block micronucleus (CBMN) assay is an attractive biomarker for estimating chromosomal damage associated with environmental insults or exposures and is considered an acceptable alternative to data obtained from the assessment of metaphase chromosomal analyses [23], [24], since it is less labor intensive and less prone to producing artifacts than classical chromosomal studies [25]. Briefly, a micronucleus (MN) is a small chromatin-containing structure that can be visualized juxtaposed to the main daughter nuclei following the completion of mitosis (Figure 1). MN formation can occur spontaneously or in response to environmental exposures and can accumulate over several months or years [26]. MN form when whole chromosomes or chromosomal fragments fail to correctly migrate to spindle poles during mitosis [27], [28], [29]. The lagging chromosome(s) or fragment(s) are subsequently excluded from the daughter nuclei and are encased in their own nuclear envelope [27]. The exclusion of chromatin into a MN can result in alterations of cellular gene dosage, which, in turn, could result in abnormal gene expression and/or perturbations in cellular proliferation that could have a broad cascade of consequences on biological systems [30]. MN frequency is known to increase with age [23], [31], [32] and has been shown to be elevated in patients with several health conditions, including cancer [33], cardiovascular disease [34], [35], Alzheimer’s disease and Parkinson’s disease [36].
Figure 1. Giemsa stained micronucleus (MN) (arrow) and corresponding daughter binucleates.
By definition, a MN is no larger than one-third the size of the parental nuclei and appears adjacent to the binucleate.
MN frequency is influenced by both heritable genetic and environmental factors [32], [37]. However, the extent to which MN frequency is impacted by exposure to a traumatic event, such as CSA, is not known. One of the most robust approaches to determine the causal role of non-genetic influences on trait variation is to study monozygotic (MZ) twins who are discordant for exposure histories. Theoretically, because the DNA of MZ twins differs only for induced changes, they provide a unique opportunity to study the long-term biological impact of childhood traumatic events.
In the present study we tested the hypothesis that adult females who experienced CSA have a higher frequency of spontaneously occurring leukocyte MN than their identical twins who did not experience CSA. Although these twin pairs are quite rare, we elected to use a discordant identical twin study design since it allows one to control for known genetic influences on MN formation [32], [37] and provides an effective means for separating the causal effects of CSA from background familial risk factors known to associate with CSA.
Methods
Ethics Statement
Human subjects research was approved by the Virginia Commonwealth University IRB (#12407 and #179). Written informed consent was obtained from all research participants.
Sample and Assessment of Childhood Sexual Abuse
Female-female CSA discordant MZ twins were ascertained from the population based Virginia Adult Twin Study of Psychiatric and Substance Use Disorders [38] and the Mid-Atlantic Twin Registry (MATR) at Virginia Commonwealth University (VCU), with the details of ascertainment outlined elsewhere [39], [40], [41]. Briefly, the pairs derive from two related samples, born from 1934–1974, who were eligible to participate in the previous study [4] if both members responded to a mailed questionnaire (response rate was about 64%). Eighty-eight percent of the participants in the original study were first interviewed face to face in 1987–1989 (at which time their mean [SD] age was 30.1 [7.6] years [range, 17.0–55.0 years]). They were subsequently interviewed three additional times by telephone with at least a one year interval between follow-ups. The remaining 12% were first interviewed face-to-face in 1992–1994 and assessed a second time (with the same interview given to the rest of the sample during the fourth wave) by telephone in 1996–1997. During the second-wave interview, twins were queried about their willingness to respond to questions about CSA and their preferred method of assessment. Most preferred a mailed self-report questionnaire, which was employed using items developed by Martin et al. [42]. The initial item was:
“Before you were 16, did any adult, or any other person older than yourself, involve you in any unwanted incidents like (i) inviting or requesting you to do something sexual, (ii) kissing or hugging you in a sexual way, (iii) touching or fondling your private parts, (iv) showing their sex organs to you, (v) making you touch them in a sexual way or (vi) attempting or having sexual intercourse?”
Of the 1411 individuals completing this portion of the interview, there were 326 MZ twin pairs where both twins provided information about CSA exposure, of which 74 pairs were classified as discordant for CSA. For the present study, specimens were collected from 17 of these discordant MZ twin pairs (Table 1) in which one twin endorsed none of the CSA items and the other twin fell into one of three exclusive, hierarchical exposure categories: (1) non-genital (N = 3 pairs) [numbers (i), (ii) and (iv)], (2) genital (N = 8 pairs) [numbers (iii) and (v)] and (3) intercourse (N = 6 pairs) [number (vi)]. The mean age at the time of the first CSA incident was 10.7 years-old (s.d. = 3.9) and ranged from 5 to 16 years of age. These CSA discordant MZ twin pairs were invited to complete a health history questionnaire and submit blood samples (VCU IRB #12407). After providing their informed consent, blood samples were obtained by a health care provider of the participants’ choosing and shipped to our cytogenetics laboratory (overnight delivery carrier) at room temperature per standard procedures. A random sample of age-matched female MZ twins (7 pairs plus 3 individuals without a cotwin [N = 17]) was also obtained from the MATR to serve as an unselected reference sample (VCU IRB #179), with health history questionnaire completion and blood specimen processing occurring using the identical protocol. The mean current age of the discordant MZ twin pairs (Mean = 48.8, SD = 9.7) was not significantly different from that of the reference sample (Mean = 53.7, SD = 9.4) (t-test, t 29 = 1.47, P = 0.154).
Table 1. Rates and characteristics of the specific forms of childhood sexual abuse experienced by the affected individuals, their communication/support experience, and their perpetrator’s status (N = 17).
| Childhood sexual abuse | % | Perpetrator status | % |
| CSA type | Female | 0.06 | |
| Sexual invitation (i) * | 75.0 | Multiple individuals | 18.8 |
| Sexual kissing (ii) | 62.5 | Forced or threatened you | 47.1 |
| Fondling (iii) | 70.6 | Age of perpetrator(s) | |
| Exposing (iv) | 60.0 | <15 y | 15.0 |
| Sexual touching (v) | 26.7 | 15–18 y | 35.0 |
| Intercourse (vi) | 37.5 | 19–24 y | 20.0 |
| 25–49 y | 15.0 | ||
| After these incidents: | >50 y | 15.0 | |
| I told no one | 81.3 | ||
| I told someone and was believed and supported | 17.6 | Relationship with perpetrator | |
| Relative living at home | 17.6 | ||
| I told someone and was believed but not supported | 0.06 | Non-relative living at home | 0.0 |
| Relative not living at home | 6.0 | ||
| I told someone and was not believed, blame, or punished | 0.0 | Family friend or other important | 29.4 |
| adult not living at home | |||
| Telling someone put an end to the abuse | 100.0 | Acquaintance or neighbor | 41.2 |
| Stranger | 17.6 |
Type as listed in Methods sub-section, ‘Sample and Assessment of Childhood Sexual Abuse’. 70.6% of affected individuals experienced more than one CSA type. Participants were classified into three exclusive, hierarchical exposure categories: (1) non-genital (N = 3 pairs) [numbers (i), (ii) and (iv)], (2) genital (N = 8 pairs) [numbers (iii) and (v)] and (3) intercourse (N = 6 pairs) [number (vi)].
Assessment of Adult Psychopathology
A number of psychiatric and substance use disorders were assessed multiple times in the discordant twins using DSM-IIIR [43] or DSM-IV [44] criteria. Lifetime diagnosis of major depression, generalized anxiety disorder and alcohol and other drug dependence was assessed at the fourth interview by trained interviewers [35]. Lifetime panic disorder was assessed at earlier interviews only (waves 1 and 3). Further details of the diagnostic algorithms and diagnostic reliability can be found in Kendler et al. [38].
DNA Isolation and Zygosity Determination
Twin zygosity status was confirmed, using genomic DNA that was isolated from whole blood using the Puregene DNA Isolation Kit (Qiagen), based on the patterns of 13 highly polymorphic short tandem repeat sequences (AmpFlSTR® Profiler Plus® and Cofiler® kits, Applied Biosystems, Foster City, CA).
Cell Culture
To ensure that erythrocytes did not confound the recognition and scoring of MN, leukocytes were isolated using Histopaque-1077 (Sigma) and then established in culture according to standard procedures (RPMI 1640 media supplemented with 15% fetal calf serum and the mitogen phytohemagglutinin) [45]. Forty-four hours after initiation of the cultures, cytochalasin-B was added (3 µg/ml final concentration). Cells were harvested at 72 hours using standard techniques, including a 10-minute incubation in hypotonic solution (0.075 M KCl), and serial fixation (three times in 3∶1 methanol: acetic acid solution) [45]. Slides were made following standard procedures [46].
CBMN Assay
MN were visualized following Giemsa staining (4% Harleco Giemsa solution) and identified according to the criteria established by Fenech et al. [47] (Figure 1). The frequencies of MN observed in the cytochalasin-B blocked binucleated cells of the twins were calculated by averaging the values obtained from two replicate scores (1000 binucleates were evaluated from two independent areas of the slide for a total of 2000 binucleates per study participant). Given that differences in nuclear proliferation could impact observed MN frequencies, the nuclear division cytotoxicity index (NDCI) was calculated using Fenech’s adaptation of the protocol of Eastmond and Tucker [45], [48], as follows: NDCI = [Ap+Nec+M1+2(M2) +3(M3) +4(M4)]/N, where Ap = the number of apoptotic cells; Nec = the number of necrotic cells; M1; M2; M3; and M4 = the number of cells having 1, 2, 3, or 4 nuclei, respectively; and N = total number of cells scored (viable as well as non-viable). The cytogeneticists were blinded to twin pair membership and CSA exposure status.
Statistical Analysis
Differences in MN rates and NDCI values in the CSA exposed twins versus their nonexposed cotwins were assessed by a paired Student’s t-test. A general effect of CSA, not differentiated by severity of exposure, was examined in all tests since this was deemed appropriate based on the current literature [1], [3], [4]. A variance stabilizing square root transformation was applied to the MN frequency data for pairwise analyses, given that it was reasonable to assume the distribution of MN scores follow a Poisson distribution. The Wilcoxon signed rank test was also used as a nonparametric equivalent to the paired t-test since data transformations were not required and it provided an additional safeguard against biases sometimes encountered with modest sample sizes. Two-sided P-values were reported for all pairwise comparisons and exact P-values were calculated for non-parametric tests.
One could speculate that the nonexposed twin of pairs discordant for CSA could have elevated MN levels because of exposure to other shared adverse family factors not directly related to CSA or potentially from stress arising from knowledge that her cotwin was abused. To further test whether an effect of CSA on MN levels was restricted only to the abused twin a population sample of age-matched MZ female twins was incorporated in the analyses to serve as a reference group. Tests were performed using generalized mixed-effect models [49] with Poisson error distribution adjusting for covariance within families and the effect of age. Two fixed effect terms were included to specify the relevant contrasts among the different twin exposure classes. A CSA exposure term was coded positive for CSA exposed twins and negative for CSA nonexposed and reference sample twins. An additional term to indicate exposure to adverse familial environment was created where discordant pairs were coded as positive and reference sample twins as negative. Evidence for a CSA related family adversity effect beyond that of direct CSA exposure would be indicated by a significant coefficient for the second term while controlling for any influence of the first term. These models were also used for additional tests exploring differences in the rate of MN formation by age between CSA exposed and nonexposed twins. All analyses were performed using the R statistical programming language [50].
Results
Pair-wise Comparisons
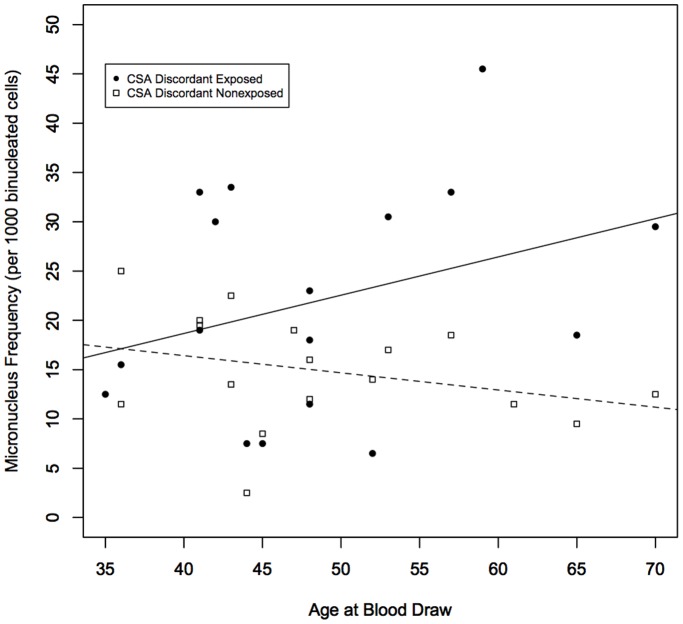
MZ twins exposed to CSA exhibited on average a 1.63-fold increase in the occurrence of MN compared to their nonexposed cotwin. The absolute MN frequency values were greater in the CSA exposed twins for 12 of the 17 discordant twin pairs (Figure 2A). Furthermore, the slope of the comparison line was near zero for 3 of the 5 pairs where their MN level was nominally higher in the CSA nonexposed twins. To determine if there might be differential levels of cellular proliferation/viability between the cotwins, their NDCI values were compared using a paired t-test, but showed no significant difference (t 16 = 0.66, P = 0.518) (Figure 2B). In contrast, a paired t-test comparison indicated a significantly higher frequency of MN formation in the CSA exposed twins (t 16 = 2.65, P = 0.017) and resulted in a significant Wilcoxon rank sum test (W = 124, P = 0.023). We then tested if these results were largely influenced by the discordant twin pair having the largest difference in MN level (the steepest slope in Figure 2A) by removal of this pair and repeating the analysis. This repeat assessment also showed a significant increase in MN frequency in the twins exposed to CSA (t 15 = 2.38, P = 0.031). CSA discordant cotwins did not differ on measured diet and lifestyle factors and no significant differences were found for rates of adult disease (Table 2). It should be clarified that the present sample was not designed to replicate the modest odds ratios reported by Kendler et al. between CSA exposure and the presence of adult psychiatric and substance disorders [4]. The goal of performing these latter tests was to examine whether the presence of adult health/behavioral conditions might confound the association between CSA status and MN formation.
Figure 2. Pairwise comparison of (A) MN frequencies (t 16 = 2.65, P = 0.017) and (B) nuclear division cytotoxicity index (t 16 = 0.66, P = 0.518) in CSA discordant MZ twin pairs (t 16 = 2.65, P = 0.017).

Table 2. Lifestyle characteristics and adult psychiatric and substance use disorders in CSA discordant MZ twin pairs.
| CSAExposed1 | CSA Nonexposed2 | Both Endorsed | Neither Endorsed | Odds Ratio3 | P * | |
| Medication Use4 | 1 | 3 | 7 | 5 | 0.3 | 0.63 |
| Green, Leafy VegetableIntake (5 days per week) | 1 | 6 | 4 | 5 | 0.2 | 0.13 |
| Smoking Status | ||||||
| Lifetime (>50 cigarettes) | 1 | 1 | 5 | 5 | 1.0 | 0.50 |
| Last 30 days (>15 days) | 2 | 0 | 0 | 10 | +∞ | 0.50 |
| Heart Disease | 1 | 1 | 0 | 13 | 1.0 | 1.0 |
| High Blood Pressure | 2 | 2 | 1 | 10 | 1.0 | 1.0 |
| Cancer Diagnosis | 0 | 2 | 0 | 13 | -∞ | 0.50 |
| Alcohol Dependence | 2 | 1 | 0 | 14 | 2.0 | 1.0 |
| Any Drug Abuse or Dependence | 2 | 0 | 0 | 15 | +∞ | 0.50 |
| Lifetime Depression | 5 | 2 | 5 | 5 | 2.5 | 0.45 |
| Lifetime Generalized Anxiety Disorder | 1 | 0 | 0 | 16 | +∞ | 1.0 |
| Panic Disorder | 2 | 0 | 0 | 12 | +∞ | 0.50 |
indicates pairs discordant for CSA and item where the exposed twin was positive for the item (n21).
indicates pairs discordant for CSA and item where the nonexposed twin was positive for the item (n12).
odds ratio for twin pairs doubly discordant for CSA and item (n21/n12).
prescription and non-prescription use for more than 1 year excluding birth control.
+/− ∞, value is positive/negative and infinite due to a null value in at least one category.
two-sided P value from exact binomial test.
Group Comparisons
The overall mean [SD] MN frequency in CSA exposed twins was 22.0 [11.3] compared to 14.9 [5.6] per 1000 cells in their nonexposed cotwins (Figure 3). The mean MN level in the unselected reference twins (14.2 [9.4]) was not significantly different from that of the CSA nonexposed set. While CSA exposure status was highly significant in this combined sample (P<0.001), there was no indication of an additional effect attributable to the familial environment (P = 0.406) based on results from generalized mixed-effect models. Removal of the most extreme value in the reference sample (greater than 3 standard deviations from the mean) resulted in a reduction of the mean MN level to12.3 and a more similar estimate of variability as the CSA nonexposed sample (SD = 5.4), but still yielded similar modeling results as the full sample.
Figure 3. MN frequencies for discordant MZ twin pairs and age-matched controls.
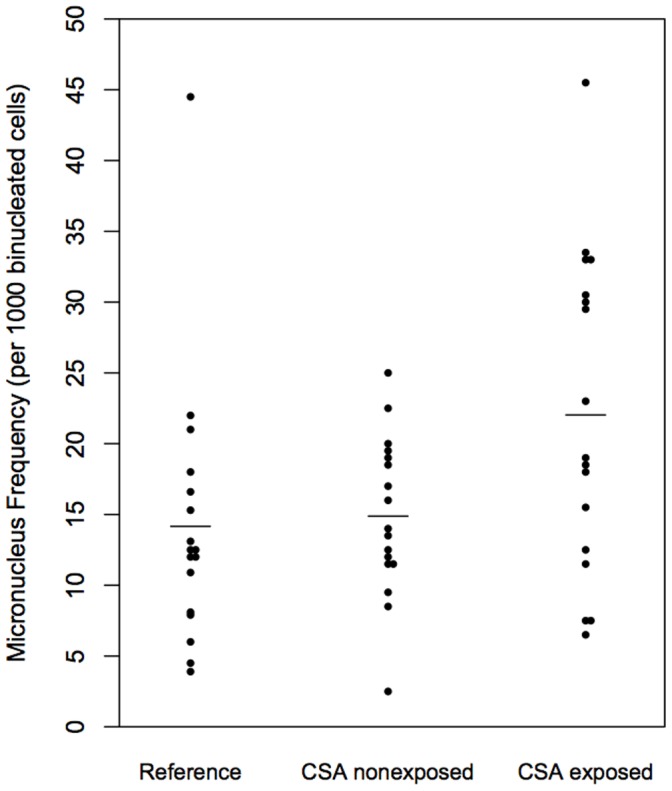
The mean level is indicated by a horizontal bar for controls (mean [SD] 14.2 [9.4]), CSA nonexposed (14.9 [5.6]) and CSA exposed (22.0 [11.3]) twins. CSA exposure status was significant in this combined sample (P<0.001), while there was no indication of an additional effect attributable to the familial environment (P = 0.406) based on results from generalized mixed-effect models.
Not surprisingly, the clearly established association of age with MN that has been shown by our lab and several previous investigators was not identified in this study, as estimated using mixed-effect models (P = 0.661), since the twins selected for study were from a narrow age range. However, the difference in MN frequencies between discordant cotwins showed a significant interaction with age, with the divergence increasing over time (coefficient [SE] = 0.030 [0.009], P = 0.0006) (Figure 4). Separate tests of age on each group indicated the interaction between age and CSA exposure was driven primarily by a significant increase of the level of MN in the CSA exposed group (coefficient [SE] = 0.017 [0.005], P = 0.001) rather than a decrease in MN level for the CSA nonexposed group (coefficient [SE] = −0.012 [0.007], P = 0.072). There was no increase in MN frequency with age over the limited age-matched range evaluated for the reference group (P = 0.361).
Figure 4. Relationship between MN frequency and age for CSA exposed and nonexposed twins.
A significant interaction effect was observed (coefficient [SE] = 0.030 [0.009], P = 0.0006) with the MN level in CSA exposed twins increasing with age while the MN level remained constant across the limited age range evaluated in CSA nonexposed twins.
Discussion
The present study yielded three main findings. Firstly, female twins exposed to CSA had an increased frequency of acquired somatic chromosomal changes (measured using MN), compared to their nonexposed, genetically identical (MZ) cotwins. Secondly, our analyses ruled out the hypothesis that the higher MN level was due to an indirect association through other shared familial risk factors. Thirdly, evidence was found for an increase in MN level with age in the CSA exposed twins that was not observed in their genetically identical nonexposed cotwins. This latter finding suggests that there may be a cumulative effect of CSA exposure on the frequency of acquired MN in lymphocytes.
CSA is Associated with MN Formation
The short- and long-term negative sequelae of CSA have been extensively documented. Kendler, et al. [51], who recently reported that the impact of environmental experiences, including traumatic event exposure, contributes substantially to stable and predictable inter-individual differences in symptoms of depression and anxiety that are observed by middle adult life. Although a number of possibilities exist, the biological mechanisms whereby childhood adversity “gets under the skin” to result in latent adult health/behavior consequences have not been well established [52], [53]. For example, there is ample support for the association of chronic psychological stress with persistent sensitizations of the hypothalamic-pituitary-adrenal axis and autonomic stress response [6], [53], [54]. The atypical development of stress reactivity could bring forth direct changes leading to acquired chromosomal abnormalities in lymphocytes through the induction of inflammatory factors known to influence MN formation, such as IL-6 [55]. Investigators have also shown that an increase in inflammatory activity can elicit the formation of reactive oxygen species, through the deleterious effects of chronically elevated glucocorticoid levels [55]. The resulting oxidative stress may lead to DNA or telomere damage/chromosomal aberrations, resulting in MN formation. Alternatively, epigenetic changes in genes associated with the cascade of biological responses to stress may lead to perturbations in mitotic apparatus formation, chromosomal alignment and/or DNA synthesis, which could subsequently lead to chromosomal malsegregation [55], [56], [57], [58], [59]. Evidence that methylation changes influence acquired chromosomal abnormality frequencies comes from studies of hypomethylated cells obtained following either: (1) in vitro induction (primarily using 5-azacytidine); or (2) as a result of mutation (cells from patients having immunonodeficiency, centromeric heterochromatin instability, and facial anomalies [ICF] syndrome, which is a condition in which the individuals have a mutation in the DNA methyltransferase 3b gene). The results of these investigation have shown increases in the rate of micronuclei associated with methylation alterations (particularly chromosomes 1, 9, and 16 in the samples from people having ICF), with observed delays in centromere separation being suggested as at least one means whereby the observed increase in somatic chromosomal abnormalities was acquired [59], [60], [61], [62], [63], [64].
The Accumulation of Damage Across the Lifespan
Interestingly, we observed a significant statistical interaction whereby only twins exposed to CSA displayed an increased MN level over the limited age range evaluated in this study. This association suggests that these individuals accumulated chromosomal changes over their lifespan, with this increase appearing to be in addition to normal, age-related or stochastic events. It is unlikely that this increase was limited to an effect of the normal aging process [21], [29], since it was not also seen in identical cotwins who were not exposed to CSA; nor was it seen in the negative control reference group. Extrapolating from data collected in our previous study of MN frequencies in healthy individuals [32], the “biological age” of the CSA exposed twins was inferred to be 9.9 years older, on average (95% CI, 2.8–17.1), than their CSA nonexposed cotwins. The accumulation of chromosomal instability acquired over the lifespan provides one plausible explanation for the non-specific adverse health effects of child maltreatment and suggests a framework for general susceptibility to a wide variety of adult illnesses and mental health outcomes. Alternatively, rather than chromosomal instability being causally related to the latent health problems observed in adults experiencing CSA, its presence could serve as an accessible and easily measured proxy for recognizing other biologically relevant changes that have occurred and could place the individual at an increased health risk.
Methodological Strengths and Limitations
In this study, the relationship between CSA and MN formation was tested using a powerful model; the discordant MZ twin design. Perhaps the most significant advantage of this approach is its ability to address issues related to direction of causality within cross-sectional data. Given that CSA has been shown to correlate with multiple family background risk factors, nearly all of which are shared by twins (such as interpersonal loss, family discord, and economic adversity), the discordant MZ twin design was effective for controlling for the effect of these influences. Without sufficient control in epidemiological samples, which necessitates the measurement of all confounding factors (some of which are unknown) the clustering of childhood adversities would likely serve to overestimate the association between CSA and MN formation. Similarly, the use of MZ discordant twins served as a control for factors related to MN formation that could be shared by twins, including but not limited to, inherited defects in genome maintenance [56], [57]. Another strength of this study design was our sampling of discordant twins who are currently in mid to late adulthood, thereby allowing an appreciable time for the accumulation of stress-related cellular damage to arise from an early life trauma (CSA occurred before 16 years of age). Aspects of this study that are novel include: (1) the conjecture that chromosomal instability could serve as a candidate system for the dysregulation of biological systems that could be “remembered” and accumulated through multiple cell divisions over time and; (2) the use of MN frequency as a potential biomarker for acquired biological changes that have accrued following CSA.
Although our discordant MZ twin study design provided a powerful test of our primary hypothesis, this investigation had methodological limitations. For instance, since we studied lymphocytes, the observation of an increased frequency of acquired chromosomal changes in twins experiencing CSA might have the greatest relevance to health problems associated with the cascade of biological effects mediated through the inflammatory system and may or may not be directly applicable to the acquisition of adult psychopathology. Indeed, this tissue sampling limitation may explain, at least in part, the lack of a clear relationship between MN frequency and the presence of adult psychopathology/morbidity for the limited number of conditions evaluated in our sample. However, it is of interest to note that chromosomal changes, primarily aneuploidy, are acquired in many tissues, normal brain and nerve cells, throughout development and that the brain and other somatic cells from individuals having a variety of health and/or psychiatric conditions show higher levels of acquired chromosomal abnormalities than controls [20], [65], [66], [67].
Summary
In summary, our study results showed increases in acquired chromosomal instability in female twins exposed to CSA, with the effect appearing to be cumulative with age, and independent of the family environment. Improvements in our understanding of this and other biological changes associated with CSA could lead to the development of biomarker panels for identifying individuals who are most at risk for acquiring health problems. In addition, these persisting biological alterations underscore the gravity of sexual abuse in children.
Funding Statement
This work was supported by grants from the National Institute on Aging [R01AG037986 (CJC, TPY)] and the National Institute of Environmental Health Sciences [R01 ES12074 (CJC)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nelson EC, Heath AC, Madden PA, Cooper ML, Dinwiddie SH, et al. (2002) Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Archives of General Psychiatry 59: 139–145. [DOI] [PubMed] [Google Scholar]
- 2. Bulik CM, Prescott CA, Kendler KS (2001) Features of childhood sexual abuse and the development of psychiatric and substance use disorders. Br J Psychiatry 179: 444–449. [DOI] [PubMed] [Google Scholar]
- 3. Dinwiddie S, Heath AC, Dunne MP, Bucholz KK, Madden PA, et al. (2000) Early sexual abuse and lifetime psychopathology: a co-twin-control study. Psychological Medicine 30: 41–52. [DOI] [PubMed] [Google Scholar]
- 4. Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, et al. (2000) Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry 57: 953–959. [DOI] [PubMed] [Google Scholar]
- 5. Danese A, Pariante CM, Caspi A, Taylor A, Poulton R (2007) Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America 104: 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heim C, Newport DJ, Miller AH, Nemeroff CB (2000) Long-term neuroendocrine effects of childhood maltreatment. JAMA : the journal of the American Medical Association 284: 2321. [PubMed] [Google Scholar]
- 7. Bartsch H, Nair J (2006) Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbeck’s archives of surgery/Deutsche Gesellschaft fur Chirurgie 391: 499–510. [DOI] [PubMed] [Google Scholar]
- 8. Miller GE, Chen E, Parker KJ (2011) Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin 137: 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, et al.. (2011) Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular Psychiatry. [DOI] [PMC free article] [PubMed]
- 10. Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, et al. (2010) Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS ONE 5: e10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, et al. (2010) Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biological Psychiatry 67: 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass D, Parts L, Knowles D, Aviv A, Spector TD (2010) No correlation between childhood maltreatment and telomere length. Biological Psychiatry 68: e21–22; author reply e23–24. [DOI] [PMC free article] [PubMed]
- 13. Harley CB (1991) Telomere loss: mitotic clock or genetic time bomb? Mutation Research 256: 271–282. [DOI] [PubMed] [Google Scholar]
- 14. Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, et al. (1992) Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. The EMBO journal 11: 1921–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Day JP, Limoli CL, Morgan WF (1998) Recombination involving interstitial telomere repeat-like sequences promotes chromosomal instability in Chinese hamster cells. Carcinogenesis 19: 259–265. [DOI] [PubMed] [Google Scholar]
- 16. Filatov L, Golubovskaya V, Hurt JC, Byrd LL, Phillips JM, et al. (1998) Chromosomal instability is correlated with telomere erosion and inactivation of G2 checkpoint function in human fibroblasts expressing human papillomavirus type 16 E6 oncoprotein. Oncogene 16: 1825–1838. [DOI] [PubMed] [Google Scholar]
- 17. Samper E, Goytisolo FA, Menissier-de Murcia J, Gonzalez-Suarez E, Cigudosa JC, et al. (2001) Normal telomere length and chromosomal end capping in poly(ADP-ribose) polymerase-deficient mice and primary cells despite increased chromosomal instability. The Journal of Cell Biology 154: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz JL, Jordan R, Liber H, Murnane JP, Evans HH (2001) TP53-dependent chromosome instability is associated with transient reductions in telomere length in immortal telomerase-positive cell lines. Genes, chromosomes & cancer 30: 236–244. [PubMed] [Google Scholar]
- 19. Leach NT, Rehder C, Jensen K, Holt S, Jackson-Cook C (2004) Human chromosomes with shorter telomeres and large heterochromatin regions have a higher frequency of acquired somatic cell aneuploidy. Mechanisms of ageing and development 125: 563–573. [DOI] [PubMed] [Google Scholar]
- 20. Iourov IY, Vorsanova SG, Yurov YB (2008) Chromosomal mosaicism goes global. Molecular Cytogenetics 1: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De S (2011) Somatic mosaicism in healthy human tissues. Trends in genetics : TIG 27: 217–223. [DOI] [PubMed] [Google Scholar]
- 22. Chen M, Huang JD, Deng HK, Dong S, Deng W, et al. (2011) Overexpression of eIF-5A2 in mice causes accelerated organismal aging by increasing chromosome instability. BMC Cancer 11: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonassi S, Fenech M, Lando C, Lin YP, Ceppi M, et al. (2001) HUman MicroNucleus project: international database comparison for results with the cytokinesis-block micronucleus assay in human lymphocytes: I. Effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environmental and molecular mutagenesis 37: 31–45. [PubMed] [Google Scholar]
- 24. Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M (2006) Chromosomal changes: induction, detection methods and applicability in human biomonitoring. Biochimie 88: 1515–1531. [DOI] [PubMed] [Google Scholar]
- 25. Battershill JM, Burnett K, Bull S (2008) Factors affecting the incidence of genotoxicity biomarkers in peripheral blood lymphocytes: impact on design of biomonitoring studies. Mutagenesis 23: 423–437. [DOI] [PubMed] [Google Scholar]
- 26. Kirsch-Volders M, Plas G, Elhajouji A, Lukamowicz M, Gonzalez L, et al. (2011) The in vitro MN assay in 2011: origin and fate, biological significance, protocols, high throughput methodologies and toxicological relevance. Archives of Toxicology 85: 873–899. [DOI] [PubMed] [Google Scholar]
- 27. Fenech M, Morley AA (1985) The effect of donor age on spontaneous and induced micronuclei. Mutation Research 148: 99–105. [DOI] [PubMed] [Google Scholar]
- 28. Lindberg HK, Wang X, Jarventaus H, Falck GC, Norppa H, et al. (2007) Origin of nuclear buds and micronuclei in normal and folate-deprived human lymphocytes. Mutation research 617: 33–45. [DOI] [PubMed] [Google Scholar]
- 29. Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, et al. (2011) Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 26: 125–132. [DOI] [PubMed] [Google Scholar]
- 30. Fenech M (2011) Micronuclei and their association with sperm abnormalities, infertility, pregnancy loss, pre-eclampsia and intra-uterine growth restriction in humans. Mutagenesis 26: 63–67. [DOI] [PubMed] [Google Scholar]
- 31. Miller B, Potter-Locher F, Seelbach A, Stopper H, Utesch D, et al. (1998) Evaluation of the in vitro micronucleus test as an alternative to the in vitro chromosomal aberration assay: position of the GUM Working Group on the in vitro micronucleus test. Gesellschaft fur Umwelt-Mutations-forschung. Mutation Research 410: 81–116. [DOI] [PubMed] [Google Scholar]
- 32. Jones KH, York TP, Juusola J, Ferreira-Gonzalez A, Maes HH, et al. (2011) Genetic and environmental influences on spontaneous micronuclei frequencies in children and adults: a twin study. Mutagenesis 26: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, et al. (2007) An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 28: 625–631. [DOI] [PubMed] [Google Scholar]
- 34. Murgia E, Maggini V, Barale R, Rossi AM (2007) Micronuclei, genetic polymorphisms and cardiovascular disease mortality in a nested case-control study in Italy. Mutation research 621: 113–118. [DOI] [PubMed] [Google Scholar]
- 35. Federici C, Botto N, Manfredi S, Rizza A, Del Fiandra M, et al. (2008) Relation of increased chromosomal damage to future adverse cardiac events in patients with known coronary artery disease. The American journal of cardiology 102: 1296–1300. [DOI] [PubMed] [Google Scholar]
- 36. Petrozzi L, Lucetti C, Scarpato R, Gambaccini G, Trippi F, et al. (2002) Cytogenetic alterations in lymphocytes of Alzheimer’s disease and Parkinson’s disease patients. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 23 Suppl 2S97–98. [DOI] [PubMed] [Google Scholar]
- 37. Surowy H, Rinckleb A, Luedeke M, Stuber M, Wecker A, et al. (2011) Heritability of baseline and induced micronucleus frequencies. Mutagenesis 26: 111–117. [DOI] [PubMed] [Google Scholar]
- 38.Kendler KS, Prescott CA (2006) Understanding the Causes of Psychiatric and Substance Use Disorders. New York: Guilford Press.
- 39. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ (1992) A population-based twin study of major depression in women. The impact of varying definitions of illness. Arch Gen Psychiatry 49: 257–266. [DOI] [PubMed] [Google Scholar]
- 40. Kendler KS, Prescott CA (1998) Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry 155: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 41. Kendler KS, Prescott CA (1999) A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry 56: 39–44. [DOI] [PubMed] [Google Scholar]
- 42. Martin J, Anderson J, Romans S, Mullen P, O’Shea M (1993) Asking about child sexual abuse: methodological implications of a two stage survey. Child Abuse and Neglect 17: 383–392. [DOI] [PubMed] [Google Scholar]
- 43.American Psychiatric Association (1987) Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition. Washington, DC: American Psychiatric Association.
- 44.American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association.
- 45. Fenech M (2000) The in vitro micronucleus technique. Mutation research 455: 81–95. [DOI] [PubMed] [Google Scholar]
- 46. Leach NT, Jackson-Cook C (2001) The application of spectral karyotyping (SKY) and fluorescent in situ hybridization (FISH) technology to determine the chromosomal content(s) of micronuclei. Mutation research 495: 11–19. [DOI] [PubMed] [Google Scholar]
- 47. Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, et al. (2003) HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutation research 534: 65–75. [DOI] [PubMed] [Google Scholar]
- 48. Eastmond DA, Tucker JD (1989) Identification of aneuploidy-inducing agents using cytokinesis-blocked human lymphocytes and an antikinetochore antibody. Environmental and Molecular Mutagenesis 13: 34–43. [DOI] [PubMed] [Google Scholar]
- 49.Faraway JJ (2006) Extending the Linear Model with R: generalized linear, mixed effects and nonparametric regression models. New York: Chapman & Hall/CRC.
- 50.R Development Core Team (2010) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 51.Kendler K, Eaves L, Loken E, Pedersen N, Middledop C, et al.. (2011) The impact of environmental experiences across the lifespan on symptoms of anxiety and depression. Psychological Science. [DOI] [PMC free article] [PubMed]
- 52. Scott J, Varghese D, McGrath J (2010) As the twig is bent, the tree inclines: adult mental health consequences of childhood adversity. Archives of general psychiatry 67: 111–112. [DOI] [PubMed] [Google Scholar]
- 53. McCrory E, De Brito SA, Viding E (2011) The impact of childhood maltreatment: a review of neurobiological and genetic factors. Frontiers in psychiatry/Frontiers Research Foundation 2: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miller GE, Chen E, Zhou ES (2007) If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin 133: 25–45. [DOI] [PubMed] [Google Scholar]
- 55. Yan B, Peng Y, Li CY (2009) Molecular analysis of genetic instability caused by chronic inflammation. Methods in molecular biology 512: 15–28. [DOI] [PubMed] [Google Scholar]
- 56.Samanta S, Dey P (2010) Micronucleus and its applications. Diagnostic cytopathology. [DOI] [PubMed]
- 57. van Leeuwen DM, Pedersen M, Knudsen LE, Bonassi S, Fenech M, et al. (2011) Transcriptomic network analysis of micronuclei-related genes: a case study. Mutagenesis 26: 27–32. [DOI] [PubMed] [Google Scholar]
- 58. Drazen JM, Yandava CN, Dube L, Szczerback N, Hippensteel R, et al. (1999) Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. NatGenet 22: 168–170. [DOI] [PubMed] [Google Scholar]
- 59. Herrera LA, Prada D, Andonegui MA, Duenas-Gonzalez A (2008) The epigenetic origin of aneuploidy. Current genomics 9: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fauth E, Scherthan H, Zankl H (1998) Frequencies of occurrence of all human chromosomes in micronuclei from normal and 5-azacytidine-treated lymphocytes as revealed by chromosome painting. Mutagenesis 13: 235–241. [DOI] [PubMed] [Google Scholar]
- 61. Hernandez R, Frady A, Zhang XY, Varela M, Ehrlich M (1997) Preferential induction of chromosome 1 multibranched figures and whole-arm deletions in a human pro-B cell line treated with 5-azacytidine or 5-azadeoxycytidine. Cytogenet Cell Genet 76: 196–201. [DOI] [PubMed] [Google Scholar]
- 62. Rodriguez MJ, Lopez MA, Garcia-Orad A, Vig BK (2001) Sequence of centromere separation: effect of 5-azacytidine-induced epigenetic alteration. Mutagenesis 16: 109–114. [DOI] [PubMed] [Google Scholar]
- 63. Stacey M, Bennett MS, Hulten M (1995) FISH analysis on spontaneously arising micronuclei in the ICF syndrome. J Med Genet 32: 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schmid M, Grunert D, Haaf T, Engel W (1983) A direct demonstration of somatically paired heterochromatin of human chromosomes. Cytogenet Cell Genet 36: 554–561. [DOI] [PubMed] [Google Scholar]
- 65. Faggioli F, Vijg J, Montagna C (2011) Chromosomal aneuploidy in the aging brain. Mechanisms of Ageing and Development 132: 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Iourov IY, Vorsanova SG, Yurov YB (2008) Molecular cytogenetics and cytogenomics of brain diseases. Current genomics 9: 452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kingsbury MA, Friedman B, McConnell MJ, Rehen SK, Yang AH, et al. (2005) Aneuploid neurons are functionally active and integrated into brain circuitry. Proceedings of the National Academy of Sciences of the United States of America 102: 6143–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]