Abstract
Background
Vibrio vulnificus is an opportunistic human pathogen that is widely distributed in estuarine environments and is capable of causing necrotizing fasciitis and sepsis. In Japan, based on epidemiological research, the incidences of V. vulnificus were concentrated in Kyusyu, mainly in coastal areas of the Ariake Sea. To examine the virulence potential, various genotyping methods have recently been developed. This study aimed to investigate the distribution of virulence markers among V. vulnificus isolates of clinical and environmental origin in three coastal areas with different infection incidences and to determine whether these isolates have the siderophore encoding gene viuB.
Methodology/Principal Findings
We examined the distribution of genotypes of the 16S ribosomal ribonucleic acid (rRNA) gene, vvhA, vcg, and capsular polysaccharide (CPS), and the presence of viuB in 156 isolates collected from patients and environmental samples in Japan. The environmental samples were collected from three coastal areas: the Ariake Sea, Ise & Mikawa Bay, and Karatsu Bay. The results showed disparity in the ratios of genotypes depending on the sample origins. V. vulnificus isolates obtained from patients were classified into the clinical type for all genotypes. In the environmental isolates, the ratios of the clinical type for genotypes of the 16S rRNA gene, vvhA, and vcg were in the order of the Ariake Sea>Ise & Mikawa Bay>Karatsu Bay. Meanwhile, CPS analysis showed no significant difference. Most isolates possessed viuB.
Conclusions
Many V. vulnificus belonging to the clinical type existed in the Ariake Sea. Three coastal areas with different infection incidences showed distinct ratios of genotypes. This may indicate that the distribution of clinical isolates correlates with the incidence of V. vulnificus infection.
Introduction
Vibrio vulnificus is a gram-negative halophilic bacterium, which inhabits warm coastal and estuarine waters worldwide. This microorganism infects humans by the consumption of contaminated seafood or contact of a wound with seawater and causes necrotizing fasciitis and sepsis. The mortality rate is high (>50%), and the latency period is short (within 24 to 48 h) [1], [2]. Patients with underlying disease such as liver dysfunction, alcoholic cirrhosis, or hemochromatosis are particularly susceptible to this life-threatening infection [1].
In addition to the United States, where V. vulnificus infections occur frequently, V. vulnificus infections have been reported in Japan, South Korea, Taiwan, Australia, Israel, and India [3]–[10]. In recent years, infections have been reported in high-latitude areas of the Baltic Sea coast such as Denmark and Sweden, indicating the spread of the organism [11], [12]. In Japan, a recent epidemiological survey showed that 185 cases of V. vulnificus infections occurred from 1975 to 2005 [13]. In particular, there were many clinical reports on the western part of Japan. Northern Kyushu (Saga, Nagasaki, Fukuoka, and Kumamoto prefectures), which encloses the Ariake Sea, the inland sea with large rivers, accounted for approximately 40% of all the reported cases (77 cases), whereas Aichi Prefecture, which is located roughly in the center of Japan and encloses the Ise & Mikawa Bay, accounted for about 10% (19 cases). In contrast, there was no report of infection in Karatsu Bay, the northern area of Saga Prefecture in northern Kyushu that encloses the open sea, during our investigation. There have been many reports about environmental factors that influence V. vulnificus, e.g., temperature and salinity of seawater in various areas [10], [14]–[16]. The optimal growth environment of V. vulnificus is a seawater temperature of ≥20°C and salinity of 15–20‰ [17]. However, a direct correlation of the incidence of V. vulnificus infections and other environmental factors (such as pH, dissolved oxygen, turbidity, and chlorophyll a) has not been proven.
V. vulnificus is a heterogeneous bacterial species that exhibits variation in strain virulence. In terms of virulence, it has been classified based on phenotypical and serological characteristics [18]–[20]. Recently, because of the need for rapid differentiation of strains with human virulence potential, genotyping systems based on deoxyribonucleic acid (DNA) polymorphisms at some loci have been developed. Nilsson et al. [21] showed that polymorphic variants generally included two genotypes such as the 16S ribosomal ribonucleic acid (rRNA) gene types A and B that significantly correlated with the non-clinical (environmental) and clinical isolates, respectively. Senoh et al. [22] found that the hemolysin gene (vvhA) could differentiate between two genotypes, termed type 1 and type 2, correlating with clinical and non-clinical types, respectively. Similarly, random amplification of polymorphic DNA typing by Rosche et al. [23] showed the E-type and C-type sequence variants at the virulence-correlated gene (vcg) locus, correlating with the non-clinical and clinical isolates, respectively. Capsular polysaccharide (CPS) is required for virulence, and many reports of lethality in animal models are clearly related to CPS expression [24]–[26]. Chatzidaki-Livanis et al. [27] observed heterogeneity within the CPS operon sequences at the same locus in different strains and referred to these as allele 1 and allele 2. These alleles diverged from sequences encoding hypothetical proteins (HPs). HP1 and HP2 alleles correlated with clinical and non-clinical types, respectively. In addition, multiplex polymerase chain reaction (PCR) studies by Panicker et al. [28] showed that clinical isolates were more likely to be positive for the siderophore-encoding gene viuB than environmental isolates. Regarding the prevalence of viuB, Bogard et al. [29] found that serum survivability of the viuB-positive isolate was greater than that of the viuB-lacking ones. Bacterial genotyping methods focusing on differences between clinical and non-clinical types have been performed for individual or two to four combinations of genes; however, genotypic analysis of a combination of five genes participating in V. vulnificus infection has not been reported. In addition, genotypic analysis of V. vulnificus in areas with different infection incidences based on epidemiological investigation has rarely been performed.
In the present study, we examined the distribution of genotypes of the 16S rRNA gene, vvhA, vcg, CPS, and the presence of viuB among V. vulnificus isolates of clinical (16 isolates) and environmental (140 isolates) origin. Furthermore, to determine the genotypes of V. vulnificus from areas with different infection incidences, we characterized the isolates collected from the Ariake Sea, Ise & Mikawa Bay, and Karatsu Bay.
Results
Genotypic Analysis of V. vulnificus
Tables S1, S2 summarize the genetic profiles of all 156 isolates. The 16 clinical isolates are shown in Table S1, and 140 environmental isolates are shown in Table S2. Genotypes of V. vulnificus were designated on the basis of 16S rRNA, vvhA, vcg, and CPS allele types, and the presence or absence of viuB. Genotypes of 16S rRNA, vvhA, and vcg types could be divided into two groups. Genotype of CPS could be divided into two groups; however, approximately 30% of the isolates could not be categorized into either group because PCR amplicon was not detected.
Genotypic Characterization of Isolates Collected from Patients and the Ariake Sea
The distribution of genotypes and the presence of viuB in V. vulnificus from the clinical and Ariake Sea isolates are shown in Table 1. Based on 16S rRNA genetic analysis, 15 among the 16 (93.8%) V. vulnificus isolates from patients (clinical isolates) belonged to type B. Similarly, in the clinical isolates, both the C-type and allele 1 were classified as the clinical type in terms of the vcg type and CPS allele, and the proportion was high (93.8%, 15/16 isolates). All the isolates possessed viuB. These results were in agreement with previous reports on the clinical type [21], [23], [29]. The prevalence rate of vvhA, which is classified into type 1 (clinical type), was 68.8% (11/16 isolates). Most of the Ariake Sea isolates were classified as type B, type 1, and C-type, which are clinical types in genotype of the 16S rRNA gene, vvhA type, and vcg type, respectively, and their prevalence rates were 90.5% (105/116 isolates), 85.3% (99/116 isolates), and 91.4% (106/116 isolates), respectively. All the isolates possessed viuB. In contrast, the prevalence rate of allele 1, which is the clinical type by CPS analysis, was 50.9%. Thus, the clinical type (the 16S rRNA gene type B, vvhA type 1, and vcg C-type) was more frequent in genotypes of the Ariake Sea isolates, except by CPS analysis, and its ratios between clinical and non-clinical types showed no statistically significant difference between the Ariake Sea and clinical isolates in all the loci (Table 1). When we subcategorized the isolates from the Ariake Sea as having seafood and non-seafood sources and compared the genotypes, there were no significant differences among both the sources.
Table 1. Distribution of genotypes and viuB proportion of V. vulnificus according to clinical and Ariake Sea isolates.
| % with genotype | % with profile | viuBproportion(%) | |||||||||||||||
| rRNA gene | vvhA | vcg | CPS | Profile | |||||||||||||
| B | A | type 1 | type 2 | NAa | C-type | E-type | C/E | allele 1 | allele 2 | NAa | 1 | 2 | 4 | Untypeable | no profile | ||
| Clinical (n = 16) | 93.8 | 6.3 | 68.8 | 31.3 | 0.0 | 93.8 | 6.3 | 0.0 | 93.8 | 6.3 | 0.0 | 68.8 | 6.3 | 25.0 | 0.0 | 0.0 | 100.0 |
| Ariake Sea (n = 116) | 90.5 | 0.9 | 85.3 | 13.8 | 0.9 | 91.4 | 6.9 | 1.7 | 50.9 | 19.0 | 30.2 | 81.9 | 5.2 | 6.9 | 2.6 | 3.4 | 100.0 |
| Seafood (n = 14) | 85.7 | 14.3 | 85.7 | 14.3 | 0.0 | 85.7 | 14.3 | 0.0 | 64.3 | 28.6 | 7.1 | 71.4 | 7.1 | 7.1 | 14.3 | 0.0 | 100.0 |
| Non-seafood (n = 102) | 91.2 | 8.8 | 85.3 | 13.7 | 1.0 | 92.2 | 5.9 | 2.0 | 49.0 | 17.6 | 33.3 | 83.3 | 4.9 | 6.9 | 1.0 | 3.9 | 100.0 |
NA, not amplified.
rRNA, ribosomal ribonucleic acid; CPS, capsular polysaccharide.
Genotypic Characterization of Three Areas with Different V. vulnificus Incidences
The distribution of genotypes and viuB prevalence among the environmental V. vulnificus isolates from three areas (the Ariake Sea, Ise & Mikawa Bay, and Karatsu Bay; shown in Fig. 1) are shown in Table 2. In the Ariake Sea, Ise & Mikawa Bay, and Karatsu Bay isolates, the prevalence rate of 16S rRNA gene type B (clinical type) was 90.5%, 63.6%, and 38.5%, respectively; that of vvhA type 1 (clinical type) was 85.3%, 72.7%, and 38.5%, respectively; and that of vcg C-type (clinical type) was 91.4%, 72.7%, and 30.8%, respectively. Two isolates (A-56 and A-68) collected from the Ariake Sea possessed both vcg C and vcg E. The prevalence rate of viuB in the Ariake Sea and Ise & Mikawa Bay isolates was 100%, whereas that in the Karatsu Bay isolates was 84.6%. Two viuB-negative isolates (K-5 and K-10) that originated from Karatsu Bay were the non-clinical type in genotypes of 16S rRNA gene, vvhA, and vcg. In contrast, CPS loci analysis showed no statistically significant difference among areas. The prevalence rate of CPS allele 1 accounted for approximately 50% and 30% of isolates that could not be classified in any area. Except for CPS, geographical disparity was observed in the ratios of genotypes, and clinical types were observed in the order of the Ariake Sea>Ise & Mikawa Bay>Karatsu Bay isolates. Significant differences were found in all loci in the distributions of genotypes in these three areas (P<0.01). These results suggested that the prevalence ratios of clinical- and non-clinical-type V. vulnificus differed between the three areas in Japan.
Figure 1. Map of sampling points.
The prefectures indicated by dots are areas where V. vulnificus infections occurred. 1, Saga; 2, Nagasaki; 3, Fukuoka; 4, Kumamoto; and 5, Aichi prefectures.
Table 2. Distribution of genotypes and viuB proportion of V. vulnificus according to environmental isolate origins.
| % with genotype | % with profile | viuBproportion(%) | |||||||||||||||
| rRNA gene | vvhA | vcg | CPS | Profile | |||||||||||||
| B | A | type 1 | type 2 | NAa | C-type | E-type | C/E | allele 1 | allele 2 | NAa | 1 | 2 | 4 | Untypeable | no profile | ||
| Ariake Sea (n = 116) | 90.5 | 0.9 | 85.3 | 13.8 | 0.9 | 91.4 | 6.9 | 1.7 | 50.9 | 19.0 | 30.2 | 81.9 | 5.2 | 6.9 | 2.6 | 3.4 | 100.0 |
| Seafood (n = 14) | 85.7 | 14.3 | 85.7 | 14.3 | 0.0 | 85.7 | 14.3 | 0.0 | 64.3 | 28.6 | 7.1 | 71.4 | 7.1 | 7.1 | 14.3 | 0.0 | 100.0 |
| Non-seafood (n = 102) | 91.2 | 8.8 | 85.3 | 13.7 | 1.0 | 92.2 | 5.9 | 2.0 | 49.0 | 17.6 | 33.3 | 83.3 | 4.9 | 6.9 | 1.0 | 3.9 | 100.0 |
| Ise & Mikawa Bay | 63.6 | 36.4 | 72.7 | 27.3 | 0.0 | 72.7 | 27.3 | 0.0 | 63.6 | 9.1 | 27.3 | 63.6 | 27.3 | 0.0 | 9.1 | 0.0 | 100.0 |
| Non-seafood (n = 11) | |||||||||||||||||
| Karatsu Bay (n = 13) | 38.5 | 61.5 | 38.5 | 61.5 | 0.0 | 30.8 | 69.2 | 0.0 | 53.8 | 15.4 | 30.8 | 30.8 | 61.5 | 0.0 | 7.7 | 0.0 | 84.6 |
| Seafood (n = 4) | 75.0 | 25.0 | 75.0 | 25.0 | 0.0 | 50.0 | 50.0 | 0.0 | 50.0 | 50.0 | 0.0 | 50.0 | 25.0 | 0.0 | 25.0 | 0.0 | 100.0 |
| Non-seafood (n = 9) | 22.2 | 77.8 | 22.2 | 77.8 | 0.0 | 22.2 | 77.8 | 0.0 | 55.6 | 0.0 | 44.4 | 22.2 | 77.8 | 0.0 | 0.0 | 0.0 | 77.8 |
NA, not amplified.
rRNA, ribosomal ribonucleic acid; CPS, capsular polysaccharide.
Genotypic Profiles According to Areas
Combinations of the three main genotypic profiles, as described Sanjuán et al. [30], were examined according to the region of isolation. Profile 1 (clinical type) consisted of genotypes of 16S rRNA gene type B, vvhA type 1, and vcg C-type, whereas profile 2 (non-clinical type) consisted of genotypes of 16S rRNA gene type A, vvhA type 2, and vcg E-type. The combination of 16S rRNA gene type B, vvhA type 2, and vcg C-type observed in this study was designated as profile 4 (Profile 3 has already been defined with a different gene profile, i.e., the combination of 16S rRNA gene type AB, vvhA type 1, and vcg E-type, by Sanjuán et al. [30]). Other combinations were assigned untypeable. The prevalence rate of profiles for the clinical and environmental isolates is shown in Fig. 2. Among the clinical isolates, the prevalence rate of profile 1 was 69%. In the Ariake Sea, Ise & Mikawa Bay, and Karatsu Bay isolates, the percentages of profile 1 were 82%, 64%, and 32%, respectively. In contrast, the prevalence rate of the isolates classified as profile 2 was 6%, 5%, 27%, and 65%, respectively. Profile 4 was only observed in the clinical (25%) and Ariake Sea (6%) isolates. These results also showed that the prevalence rate of clinical-type V. vulnificus inhabiting the Ariake Sea was higher than that of the clinical isolates. Among the isolates from the three areas, the genotypic profile of the Karatsu Bay isolates was significantly differed from that of the clinical ones (P<0.05). When we subcategorized the environmental isolates from the Ariake Sea and Karatsu Bay as having seafood and non-seafood sources and compared the genotypes and profiles (Table 2), there was no statistically significant difference among both the sources. This suggests that regardless of the different sources such as seafood and non-seafood sources, their genotypes and profiles were present at the same rate.
Figure 2. Ratio of genotypic profile according to isolate origins.
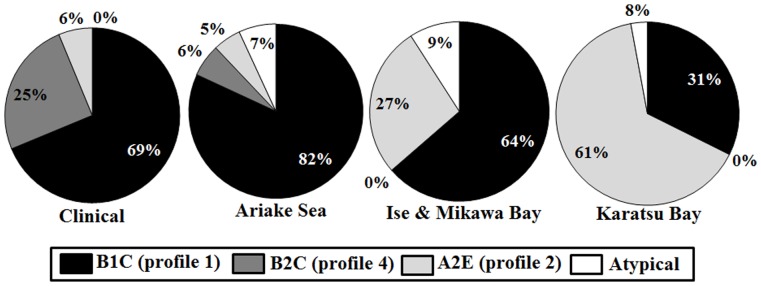
The ratio of profile of clinical and environmental isolates is shown. The three main genotypic profiles were combined. Profile 1 (clinical type) consisted of 16S rRNA gene type B, vvhA type 1, and vcg C-type; profile 2 (non-clinical type) consisted of type A, type 2, and E-type; and profile 4 consisted of type B, type 2 and C-type. Other combination types were set to untypeable.
Discussion
Although it is clear that V. vulnificus inhabits coastal marine waters worldwide, the number of people infected with V. vulnificus is less compared with the microbial load. The use of genotyping analysis has been prompted to determine the virulence potential between the clinical-type and non-clinical-type V. vulnificus isolates [18]–[20], [31].
We first investigated 16 clinical isolates reported in areas surrounding the Ariake Sea using five biomarkers: the 16S rRNA gene, vvhA, vcg, CPS, and viuB. Many V. vulnificus isolates obtained from patients were classified into the clinical type for all genotypes. These distributions were mostly in agreement with the typing results reported previously [21]–[23], [30], suggesting that clinical isolates have essentially the same genotypes in any region.
Interestingly, when genotypes were investigated in terms of V. vulnificus isolated from three areas (the Ariake Sea, Ise & Mikawa Bay, and Karatsu Bay) the prevalence rates of clinical and non-clinical types differed in each area (Table 2). The prevalence rate of clinical-type V. vulnificus isolated from the Ariake Sea was overwhelmingly high (approximately 90%) and of that isolated from Ise & Mikawa Bay was comparatively high (approximately 70%). Meanwhile, most (approximately 65%) of the isolates from Karatsu Bay belonged to the non-clinical type. Kim and Jeong [32] reported that in seawater, oysters, and sediment of the Hadong area on the southern coast of South Korea, 35% of isolates were classified as A type (non-clinical type) and 65% as B type (clinical type) by 16S rRNA gene analysis. Warner and Oliver [16] reported that in seawater samples from Alligator Bay on the eastern coast of North Carolina, 46.9% of isolates belonged to the non-clinical type (vcg E-type) and 53.1% belonged to the clinical type (vcg C-type), whereas in Cedar Key, FL, 37.3% of isolates belonged to the non-clinical type and 62.7% belonged to the clinical type. Compared with these results, the number of V. vulnificus isolates belonging to the clinical type was much higher in the Ariake Sea. Although the number of V. vulnificus isolates obtained from Ise & Mikawa Bay and Karatsu Bay was limited, the distribution of three genotypes (16S rRNA gene, vvhA, and vcg) was in agreement with each area. Therefore, we concluded that genotypes of V. vulnificus differed by region in Japan.
Based on the 16S rRNA gene, vvhA, and vcg, we classified the isolates into three profiles (Fig. 2). In the clinical isolates, profile 3 (16S rRNA gene type B, vvhA type 2, and vcg C-type) was observed (25%). In the environmental isolates, profile 4 was observed only in the Ariake Sea (6%) isolates and not in the Ise & Mikawa Bay and Karatsu Bay ones. To our knowledge, there are few reports about profile 4. It can be said that profile 4 was the feature of the clinical isolates reported in areas surrounding the Ariake Sea near Saga Prefecture and the isolates collected from the Ariake Sea in Japan.
On investigating the epidemiological and clinical characteristics of V. vulnificus infection reported in Japan, we found that approximately 40% of cases occurred in four prefectures around the Ariake Sea and approximately 10% were reported in Aichi Prefecture, which encloses Ise & Mikawa Bay [14]. In our study, V. vulnificus was isolated from the Ariake Sea and Ise & Mikawa Bay, which are semi-closed seas with relatively low salinity and warm seawater temperatures. In contrast, there was no report of V. vulnificus infection in the northern area of Saga Prefecture, which encloses Karatsu Bay, during our investigation. Karatsu Bay faces the Sea of Japan, and neither salinity nor seawater temperature seem to be affected by changes in the weather. Therefore, we supposed that the Ariake Sea and Ise & Mikawa Bay were more suitable for V. vulnificus than Karatsu Bay. Yahiro et al. reported [33] that more V. vulnificus isolates were detected around the inland sea area with many rivers (where V. vulnificus infections were reported) than in the open sea area in Kumamoto Prefecture. Thus, inhabitation of a large number of V. vulnificus may be responsible for the large number of infections reported. In addition to this report, we found that statistically more clinical-type V. vulnificus existed in the epidemic areas of infection such as the Ariake Sea and Ise & Mikawa Bay, whereas more non-clinical-type V. vulnificus existed in the non-epidemic area of infection such as the Karatsu Bay as described above. Therefore, our results suggest that genotype distributions of V. vulnificus in the region are related to infection incidence.
In CPS analysis, a difference was not seen in the distribution of two alleles among three areas; 50%–60% of the isolates belonged to the clinical type (allele 1) and 10%–20% belonged to the non-clinical type (allele 2). Interestingly, in approximately 30% of the isolates, both alleles 1 and 2 could not be identified by PCR in all three areas. Han et al. reported that approximately 20% of the isolates could not be amplified by the same method [34]. Chatzidaki-Livanis et al. reported that one environmental strain could not be divided either allele 1 or 2 in 33 clinical and 35 environmental strains [27]. HP segments including CPS alleles were inserted between wza and wzb in V. vulnificus [27]. Although we tried to confirm an intact HP gene using PCR primers (forward primer: wza region, reverse primer: wzb region), most isolates could not be amplified (data not shown); therefore, it was proposed that these isolates may not contain the HP gene. Considering that all the isolates collected from patients could be distributed into either allele 1 or allele 2, the existence of this gene may be associated with human infection.
In the present study, we investigated genotypes of V. vulnificus isolated from three areas: the Ariake Sea, Ise & Mikawa Bay, and Karatsu Bay. We found that there was regional difference in genotypes of V. vulnificus that was relevant to infection rate in each area. In addition, we found that significantly larger numbers of clinical-type V. vulnificus existed in the epidemic areas of infection such as the Ariake Sea and Ise & Mikawa Bay, suggesting that the genotype distribution of V. vulnificus is related to infection incidence. To date, there are no unique virulent biomarkers because all clinical and environmental isolates are equally virulent in animal and cell culture pathogenesis models [35]. Continuous sampling and multiple analyses may lead to discovery of an effective new virulence biomarker for V. vulnificus.
Materials and Methods
Bacterial Strains and Culture Preparation
A total of 156 Vibrio vulnificus isolates, including 16 clinical and 140 environmental isolates (116 isolated from the Ariake Sea, 11 from Ise & Mikawa Bay, and 13 from Karatsu Bay), were used in this study (Fig. 1). The clinical isolates were collected from patients with V. vulnificus infection at hospitals in Saga Prefecture and 19 other emergency hospitals in the region where we have established an information network surrounding the Ariake Sea near Saga Prefecture for V. vulnificus infection during the period of 1984–2010. Permission to examine medical records for identifying cases of V. vulnificus infection was granted from each institution. Patients were suffering from liver dysfunction (n = 15), and multiple endocrine neoplasia (n = 1), and the mortality rate was 75%. The source of infection was the ingestion of raw fish/shellfish and garnish contacted with raw fish (n = 14) or unknown (n = 2).
Microbial identification of V. vulnificus was determined in agar plate cultures and confirmed by biochemical methods from clinical specimens, including blood, blister fluids, and necrotizing tissue samples, at each hospital. The environmental isolates were collected from seawater, mud, oysters, and fish collected from the Ariake Sea and Karatsu Bay in Saga Prefecture and Ise & Mikawa Bay in Aichi Prefecture from April to September in 2001, 2002, and 2007–2011. In particular, a number of samples were collected during the summer months of July–September. No specific permits were required for sampling. The sampling location is not privately-owned or protected in any way, and the field studies did not involve endangered or protected species. The V. vulnificus isolates from the Ariake Sea were collected near Saga Prefecture. Oysters were collected, their external surfaces were washed and opened, and the entire oyster tissue was ground with seawater. Seawater (10 ml) was centrifuged, and 9 ml was then discarded. Aliquot (1 ml) was used as sea water sample. Mud was added directly. All the samples were aerobically grown at 30°C or 37°C in modified Zobell broth containing 0.5% peptone and 0.1% yeast extract in seawater (24.3 PSU). The cultivate solution was then plated onto the ES Vibrio agar plate (Eiken Chemical Co., Ltd., Tokyo, Japan) and incubated at 30°C for 15–24 h. Perspective V. vulnificus colonies on plates were picked onto a new plate. All the isolates were aerobically grown at 30°C or 37°C in modified Zobell broth. Genomic DNA was isolated from each culture using a DNA isolation kit (QIAamp® DNA Mini Kit; Qiagen, Hilden, Germany) according to the manufacturer’s instructions and used as template in PCR assays.
Genotyping
The V1–V3 region of the bacterial 16S rRNA gene was amplified from genomic DNA isolated from each strain using PCR with a pair of eubacterial universal primers: 8UA (5′-AGAGTTTGATCCTGGCTCAG-3′) and 519B (5′-ATTACCGCSGCTGCTG-3′) [36], [37]. PCR was performed as described by Nilsson et al. [21]. Genotypes were determined depending on sequence homology to V. vulnificus ATCC27562T representative of type A (GenBank accession number X76333) or V. vulnificus C7184 representative of type B (GenBank accession number X76334) as reported previously [21]. An 813-bp segment of the hemolysin gene (vvhA) was targeted for PCR amplification using two primer sets of vvhA-1F (5′-AGATTAAGTGTGTGTTGCACAAGCGGTG-3′) and vvhA-1R (5′-ACCGAAAACAGCGCTGAAGGAAGAACGGTA-3′) pair, and vvhA-2F (5′-AAATTAAGTGCGTGCTACACACAAGTGGTG-3′) and vvhA-2R (5′-A CTGAGAAGAGTGCTGAAGGGATTACCGTA-3′) pair [22]. vvhA amplified with vvhA-1F and vvhA-1R was designated as type 1 and that amplified with vvhA-2F and vvhA-2R was designated as type 2. PCR was performed with Ex Taq polymerase (Takara Bio, Shiga, Japan) as described previously [22]. Genotyping of vcg was performed using PCR according to Rosche et al. [23]. The primers P1 (5′-AGCTGCCGATAGCGATCT-3′) and P3 (5′-CGCTTAGGATGATCGGTG -3′) were used for identification of the C-type isolates. The primers P2 (5′-CTCAATTGACAATGATCT-3′) and P3 were used for identification of the E-type isolates. Genomic DNA from each isolate was subjected to a separate PCR reaction with each of the two primer sets. PCR was performed as described by Rosche et al. [23]. To detect specific CPS alleles, the V. vulnificus isolates were examined using PCR according to Han et al. [34]. Two primer pairs were used: primers HP1F (5′-TTTGGGTTTGAAAGGCTTG-3′) and HP1R (5′-GTGCCTTTGCGAAT TTTGAT-3′) were used to detect HP1 in V. vulnificus MO6-24/O (CPS allele 1) and primers HP2F (5′-TTCCATCAAACATCGCAGAA-3′) and HP2R (5′-CTTTTGTCCGGCTTCTATGC-3′) were used to detect HP2 in V. vulnificus YJ016/O (CPS allele 2) at the same locus. PCR was performed under the conditions described by Han et al. [34]. A 342-bp product was detected in a PCR reaction with the HP1 primer set, and a 152-bp product was detected with the HP2 primer set. PCR detection of viuB was performed using a primer set reported by Jones et al. [38]: forward primer (5′-GGTTGGGCACTAAAGGCAGAT-3′) and reverse primer (5′-TCGCTTTCTCCGGGGCGG-3′). PCR was performed with Ex Taq polymerase (Takara Bio) as described by Jones et al. [38].
Statistical Analysis
Statistical analyses were performed using the chi-squared test or Fisher’s exact test. Statistical significance was determined using a P-value of 0.05. All analyses were performed using Microsoft Excel (Excel Toukei 2010; Social Survey Research Information, Tokyo, Japan).
Supporting Information
Clinical isolates in this study along with their respective origins and properties.
(DOC)
Environmental isolates used in this study along with their respective sources and properties.
(DOC)
Acknowledgments
We thank the Clinical Laboratory of Saga Medical School Hospital for assistance in sample collection. We also thank collaborators within the hospitals of the Vibrio vulnificus network. We would like to thank Enago (www.enago.jp) for the English language review.
Funding Statement
This study was supported by the Ariake Sea Research Project, Saga University, Japan (http://www.ariake.civil.saga-u.ac.jp/index_e.html). The funder had no role in study design,data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
References
- 1. Matsumoto K, Ohshige K, Fujita N, Tomita Y, Mitsumizo S, et al. (2010) Clinical features of Vibrio vulnificus infections in the coastal areas of the Ariake Sea, Japan. J Infect Chemother 16: 272–279. [DOI] [PubMed] [Google Scholar]
- 2. Blake PA, Merson MH, Weaver RE, Hollis DG, Heublein PC (1979) Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N Engl J Med 300: 1–5. [DOI] [PubMed] [Google Scholar]
- 3. Hlady WG, Klontz KC (1996) The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis 173: 1176–1183. [DOI] [PubMed] [Google Scholar]
- 4. Inoue Y, Ono T, Matsui T, Miyasaka J, Kinoshita Y, et al. (2008) Epidemiological survey of Vibrio vulnificus infection in Japan between 1999 and 2003. J Dermatol 35: 129–139. [DOI] [PubMed] [Google Scholar]
- 5. Park SD, Shon HS, John NJ (1991) Vibrio vulnificus septicemia in Korea: clinical and epidemiologic findings in seventy patients. J Am Acad Dermatol 24: 397–403. [DOI] [PubMed] [Google Scholar]
- 6. Hsueh PR, Lin CY, Tang HJ, Lee HC, Liu JW, et al. (2004) Vibrio vulnificus in Taiwan. Emerg Infect Dis 10: 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maxwell EL, Mayall BC, Pearson SR, Stanley PA (1991) A case of Vibrio vulnificus septicaemia acquired in Victoria. Med J Aust 154: 214–215. [DOI] [PubMed] [Google Scholar]
- 8. Bisharat N, Raz R (1996) Vibrio infection in Israel due to changes in fish marketing. Lancet 348: 1585–1586. [DOI] [PubMed] [Google Scholar]
- 9. Bisharat N, Agmon V, Finkelstein R, Raz R, Ben-Dror G, et al. (1999) Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Lancet 354: 1421–1424. [DOI] [PubMed] [Google Scholar]
- 10. Strom MS, Paranjpye RN (2000) Epidemiology and pathogenesis of Vibrio vulnificus . Microbes Infect 2: 177–188. [DOI] [PubMed] [Google Scholar]
- 11. Dalsgaard A, Frimodt-Møller N, Bruun B, Høi L, Larsen JL (1996) Clinical manifestations and molecular epidemiology of Vibrio vulnificus infections in Denmark. Eur J Clin Microbiol Infect Dis 15: 227–232. [DOI] [PubMed] [Google Scholar]
- 12. Melhus A, Holmdahl T, Tjernberg I (1995) First documented case of bacteremia with Vibrio vulnificus in Sweden. Scand J Infect Dis 27: 81–82. [DOI] [PubMed] [Google Scholar]
- 13. Oishi H, Ura Y, Mitsumizo S, Nakashima M (2006) A collective review of Vibrio vulnificus infection in Japan (in Japanase). Kansenshogaku Zasshi 80: 680–689. [DOI] [PubMed] [Google Scholar]
- 14. Randa MA, Polz MF, Lim E (2004) Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl Environ Microbiol 70: 5469–5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warner E, Oliver JD (2008) Population structures of two genotypes of Vibrio vulnificus in oysters (Crassostrea virginica) and seawater. Appl Environ Microbiol 74: 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inoue Y, Miyasaka J, Ono T, Ihn H (2007) The growth of Vibrio vulnificus and the habitat of infected patients in Kumamoto. BioSci Trends 1: 134–139. [PubMed] [Google Scholar]
- 17. Mahmud ZH, Neogi SB, Kassu A, Huong BTM, Jahid IK, et al. (2008) Occurrence, seasonality and genetic diversity of Vibrio vulnificus in coastal seaweeds and water along the Kii Channel, Japan. FEMS Microbiol Ecol 64: 209–218. [DOI] [PubMed] [Google Scholar]
- 18. Biosca EG, Amaro C, Larsen JL, Pedersen K (1997) Phenotypic and genotypic characterization of Vibrio vulnificus: proposal for the substitution of the subspecific taxon biotype for serovar. Appl Environ Microbiol 63: 1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colodner R, Raz R, Meir I, Lazarovich T, Lerner L, et al. (2004) Identification of the emerging pathogen Vibrio vulnificus biotype 3 by commercially available phenotypic methods. J Clin Microbiol 42: 4137–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tison DL, Nishibuchi M, Greenwood JD, Seidler RJ (1982) Vibrio vulnificus biogroup 2: new biogroup pathogenic for eels. Appl Environ Microbiol 44: 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nilsson WB, Paranjype RN, DePaola A, Strom MS (2003) Sequence polymorphism of the 16S rRNA gene of Vibrio vulnificus is a possible indicator of strain virulence. J Clin Microbiol 41: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Senoh M, Miyoshi S, Okamoto K, Fouz B, Amaro C, et al. (2005) The cytotoxin-hemolysin genes of human and eel pathogenic Vibrio vulnificus strains: comparison of nucleotide sequences and application to the genetic grouping. Microbiol Immunol 49: 513–519. [DOI] [PubMed] [Google Scholar]
- 23. Rosche TM, Yano Y, Oliver JD (2005) A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical or environmental isolation. Microbiol Immunol 49: 381–389. [DOI] [PubMed] [Google Scholar]
- 24. Simpson LM, White VK, Zane SF, Oliver JD (1987) Correlation between virulence and colony morphology in Vibrio vulnificus . Infect Immun 55: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wright AC, Powell JL, Kaper JB, Morris JG Jr (2001) Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus . Infect Immun 69: 6893–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Powell JL, Wright AC, Wasserman SS, Hone DM, Morris JG Jr (1997) Release of tumor necrosis factor alpha in response to Vibrio vulnificus capsular polysaccharide in vivo and in vitro models. Infest Immun 65: 3713–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chatzidaki-Livanis M, Jones MK, Wright AC (2006) Genetic variation in the Vibrio vulnificus group 1 capsular polysaccharide operon. J Bacteriol 188: 1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panicker G, Vickery MC, Bej AK (2004) Multiplex PCR detection of clinical and environmental strains of Vibrio vulnificus in shellfish. Can J Microbiol 50: 911–922. [DOI] [PubMed] [Google Scholar]
- 29. Bogard RW, Oliver JD (2007) Role of iron in human serum resistance of the clinical and environmental Vibrio vulnificus genotypes. Appl Environ Microbiol 73: 7501–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanjuán E, Fouz B, Oliver JD, Amaro C (2009) Evaluation of genotypic and phenotypic methods to distinguish clinical from environmental Vibrio vulnificus strains. Appl Environ Microbiol 75: 1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aznar R, Ludwig R, Amann RI, Schleifer KH (1994) Sequence determination of rRNA genes of pathogenic Vibrio species and whole-cell identification of Vibrio vulnificus with rRNA-targeted oligonucleotide probes. Int J Syst Bacteriol 44: 330–337. [DOI] [PubMed] [Google Scholar]
- 32. Kim MS, Jeong HD (2001) Development of 16S rRNA targeted PCR methods for the detection and differentiation of Vibrio vulnificus in marine environments. Aquaculture 193: 199–211. [Google Scholar]
- 33. Yahiro S, Matsumoto K, Miyasaka J, Harada S (2008) Relation between Vibrio vulnificus infection and environmental factor (in Japanese). Kumamoto Prefectural Institute of Public Health and Environmental Science Report 38: 27–32. [Google Scholar]
- 34. Han F, Pu S, Hou A, Ge B (2009) Characterization of clinical and environmental types of Vibrio vulnificus isolates from Louisiana oysters. Foodborne Pathog Dis 6: 1251–1258. [DOI] [PubMed] [Google Scholar]
- 35. DePaola A, Nordstrom JL, Dalsgaard A, Forslund A, Oliver J, et al. (2003) Analysis of Vibrio vulnificus from market oysters and septicemia cases for virulence markers. Appl Environ Microbiol 69: 4006–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Acinas SG, Antón J, Rodríguez-Valera F (1999) Diversity of free-living and attached bacteria in offshore Western Mediterranean waters as depicted by analysis of genes encoding 16S rRNA. Appl Environ Microbiol 65: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bertilsson S, Cavanaugh CM, Polz MF (2002) Sequencing-independent method to generate oligonucleotide probes targeting a variable region in bacterial 16S rRNA by PCR with detachable primers. Appl Environ Microbiol 68: 6077–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones MK, Warner E, Oliver JD (2008) Survival of and in situ gene expression by Vibrio vulnificus at varying salinities in estuarine environments. Appl Environ Microbiol 74: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical isolates in this study along with their respective origins and properties.
(DOC)
Environmental isolates used in this study along with their respective sources and properties.
(DOC)