Abstract
Precise positioning of the highly complex interdigitating podocyte foot processes is critical to form the normal glomerular filtration barrier, but the molecular programs driving this process are unknown. The protein atypical protein kinase C (aPKC)—a component of the Par complex, which localizes to tight junctions and interacts with slit diaphragm proteins—may play a role. Here, we found that the combined deletion of the aPKCλ/ι and aPKCζ isoforms in podocytes associated with incorrectly positioned centrosomes and Golgi apparatus and mislocalized molecules of the slit diaphragm. Furthermore, aPKC-deficient podocytes failed to form the normal network of foot processes, leading to defective glomerular maturation with incomplete capillary formation and mesangiolysis. Our results suggest that aPKC isoforms orchestrate the formation of the podocyte processes essential for normal glomerular development and kidney function. Defective aPKC signaling results in a dramatically simplified glomerular architecture, causing severe proteinuria and perinatal death.
During kidney development, podocytes extend primary processes and foot processes to surround glomerular capillaries.1 Podocyte and glomerular development can be classified into characteristic stages. During the comma-shaped body stage, undifferentiated podocytes are cuboidal epithelial cells with apical sited cell-cell contacts. During the s-shaped body stage, the cell-cell contacts move along the lateral membrane toward the basal sites of podocytes. Subsequently, at the capillary loop stage, podocytes stop cell division, start to express typical podocyte differentiation markers like the slit diaphragm proteins nephrin and Podocin, and extend primary processes and foot processes, which become connected by the slit diaphragm.1–6
There are only few cells known to undergo such dramatic phenotypic changes during development as podocytes. The signaling programs driving these unique complex changes in cell shape, function, and structure are incompletely understood. However, the asymmetric apical membrane expansion and the process formation suggest the involvement of apicobasal polarity complexes. Apicobasal polarity is established by three core polarity complexes, which subdivide the cellular membrane in an apical and a basolateral membrane compartment: the Crumbs complex, consisting of Crumbs, PATJ and PALS1, localizes to the apical membrane; the Par complex, consisting of Par3, Par6, and atypical protein kinase C (aPKC), localizes to tight junctions; and the Scribble complex, which contains Scribble, Lgl and Dlg, is located at adherens junctions and the basolateral membrane.7 We previously showed that the Par complex translocates together with the cell-cell contacts from apical to basal aspects of the podocyte.8,9 The Par complex interacts with the cytoplasmic tail of the slit diaphragm proteins nephrin and Neph1 and localizes to the insertion site of the slit diaphragm.8,10 Interaction of Par6 with the guanosine-5'-triphosphate (GTP)–bound form of the small GTPases Rac and Cdc42 results in a conformational change of Par6, which facilitates the activation of aPKC.11–14 The two isoforms of aPKC, aPKCλ/ι and aPKCζ, are very similar in structure, but are encoded by two different genes.15 Whereas constitutive aPKCλ/ι knockout results in embryonic lethality by embryonic day 9 (E9),16 aPKCζ knockout mice are viable.17 Recently, podocyte-specific conditional knockout mice of aPKCλ/ι (aPKCλ/ι PcKO) were analyzed. Although podocyte-specific deletion of aPKCλ/ι results in proteinuria, glomerulosclerosis, and death of knockout mice during adolescence,9,10 knockout podocytes still display regular foot processes connected by slit diaphragms at birth.10 These findings raise the possibility, that aPKCζ might at least partially compensate the loss of aPKCλ/ι during podocyte differentiation. To elucidate the full effect of the aPKC complex in development, we generated mice with a podocyte-specific knockout for both aPKC isoforms. This approach revealed that aPKC is a central regulator of podocyte process formation and glomerular maturation. Furthermore, we demonstrate that basal localization of the centrosome and the Golgi apparatus is a characteristic hallmark of podocyte cell polarity and that the loss of both aPKC isoforms causes an aberrant localization of these organelles.
Results
The Double aPKCλ/ι and aPKCζ Knockout in Podocytes Results in Severe Proteinuria and Perinatal Death
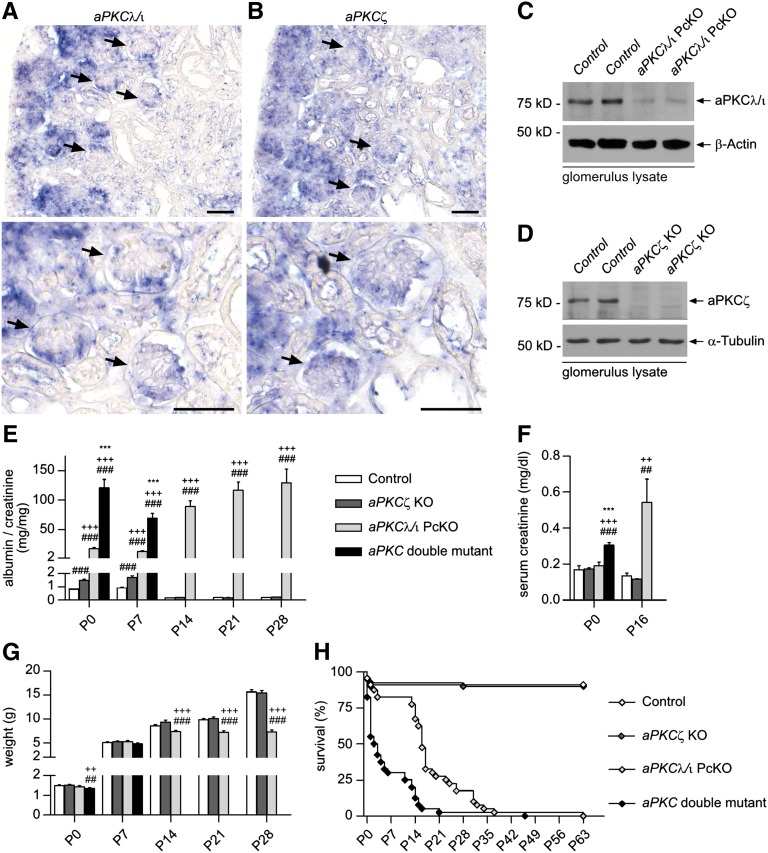
To visualize the expression of aPKCλ/ι and aPKCζ during glomerular development, we performed in situ hybridization analyses of kidneys of newborn mice. Both isoforms were highly expressed in podocytes of developing glomeruli (Figure 1, A and B). Next, aPKCζ−/−,17 aPKCλ/ιflox/flox,18 and NPHS2.Cre+ mice,19 which express Cre under control of a podocyte-specific promoter, were crossed to generate aPKC double mutant mice (NPHS2.Cre+;aPKCλ/ιflox/flox;aPKCζ−/−). Western blot analysis confirmed the loss of aPKCζ and significant reduction of aPKCλ/ι in glomerular lysates of knockout mice (Figure 1, C and D). At postnatal day (P) 0, aPKC double mutant mice already displayed massive proteinuria, whereas podocyte-specific aPKCλ/ι knockout resulted in significant lower albumin excretion, increasing to the level of double mutant mice around P14 (Figure 1E and Supplemental Figure 1). aPKCζ KO mice showed a mildly elevated albumin/creatinine ratio compared with control mice at P0 and P7. After completion of glomerulogenesis at or beyond P14, no difference was detected between control and aPKCζ KO mice. Unlike aPKCλ/ι PcKO mice, double mutant mice showed elevated serum creatinine already at birth (P0), indicating an impaired kidney function (Figure 1F), and a significant reduction in body weight compared with control and aPKCζ KO mice (Figure 1G). aPKCλ/ι PcKO mice exhibited developmental retardation from P14 onward, whereas aPKCζ KO mice showed no difference to control. Around 70% of double mutant mice died during the first week after birth with a median survival of 2.5 days (Figure 1H). The majority of aPKCλ/ι PcKO died between P14 and P21 with a median survival of 16 days, whereas aPKCζ KO mice showed no increased lethality compared with control mice.
Figure 1.
The double aPKCλ/ι and aPKCζ knockout in podocytes results in severe proteinuria and perinatal death. (A and B) In situ hybridization studies of kidneys of newborn mice reveal high expression of both aPKC isoforms, aPKCλ/ι and aPKCζ, in glomerular podocytes (arrows). (C and D) Western blot analyses confirm significant reduction of aPKCλ/ι and loss of aPKCζ in glomerulus lysates of the accordant knockout mice at P14. (E) Follow-up analysis of urinary albumin/creatinine ratio (n=7–48 each group). * P<0.05, ** P<0.01, *** P<0.001, aPKC double mutant compared with aPKCλ/ι PcKO; + P<0.05, ++ P<0.01, +++ P<0.001, aPKC double mutant or aPKCλ/ι PcKO respectively compared with aPKCζ KO; # P<0.05, ## P<0.01, ### P<0.001, aPKC double mutant, aPKCλ/ι PcKO or aPKCζ KO respectively compared with control. (F) Serum creatinine (n=7–12 each group). (G) Body weight (male mice, n=4–24 each group). (H) Survival (median survival aPKC double mutant = 2.5 days, aPKCλ/ι PcKO = 16 days. Log-rank (Mantel–Cox) test, P<0.001. Scale bars, 50 µm.
Severely Impaired Foot Process Network Formation in aPKC Double Mutant Mice
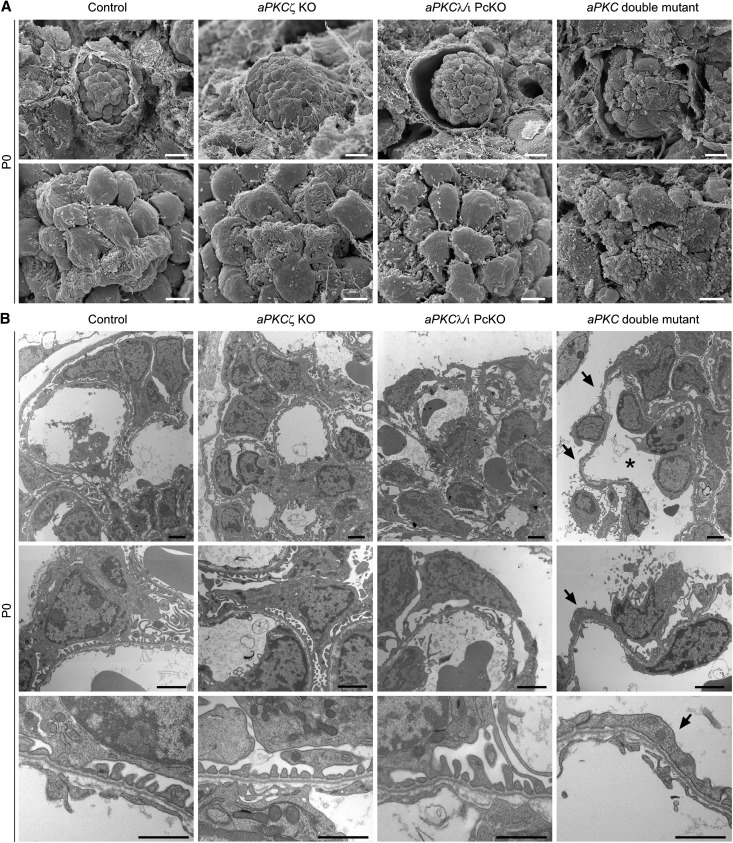
Histologic analysis of kidneys of newborn mice demonstrated tubular dilations with protein casts in aPKC double mutant mice (Supplemental Figure 2A). In scanning and transmission electron microscopy of kidneys of newborn mice, podocytes of control and aPKCζ KO mice displayed foot processes with no detectable abnormalities (Figure 2, A and B). aPKCλ/ι PcKO mice exhibited podocytes with partially broadened foot processes (Figure 2, A and B). Strikingly, podocytes of aPKC double mutant mice were covered with microvilli and blebs, but no foot processes could be detected (Figure 2A). In line with these findings, a distinct podocyte foot process network was not detected in aPKC double knockout by transmission electron microscopy (Figure 2B).
Figure 2.
Severely impaired foot process network formation in aPKC double mutant mice. (A) Whereas scanning electron micrographs of kidneys of control and aPKCζ KO mice show developing foot processes that enwrap the capillaries, podocyte processes of aPKCλ/ι PcKO mice display an irregular pattern. In aPKC double mutant mice, podocyte cell bodies are covered with microvilli and blebs. Podocyte processes cannot be detected. (B) Podocytes of newborn aPKCλ/ι PcKO mice exhibit partially broadened processes and long projections to the glomerular basement membrane. aPKC double knockout podocytes display no elaborated process network (arrows). Furthermore, capillary dilations are observed (asterisk). Scale bars, 10 µm in upper panel in A; 2.5 µm in lower panel in A; 2 µm in upper and middle panels in B; 1 µm in lower panel in B.
Glomerular Development Arrests at the Late Capillary Loop Stage in aPKC Double Mutant Mice
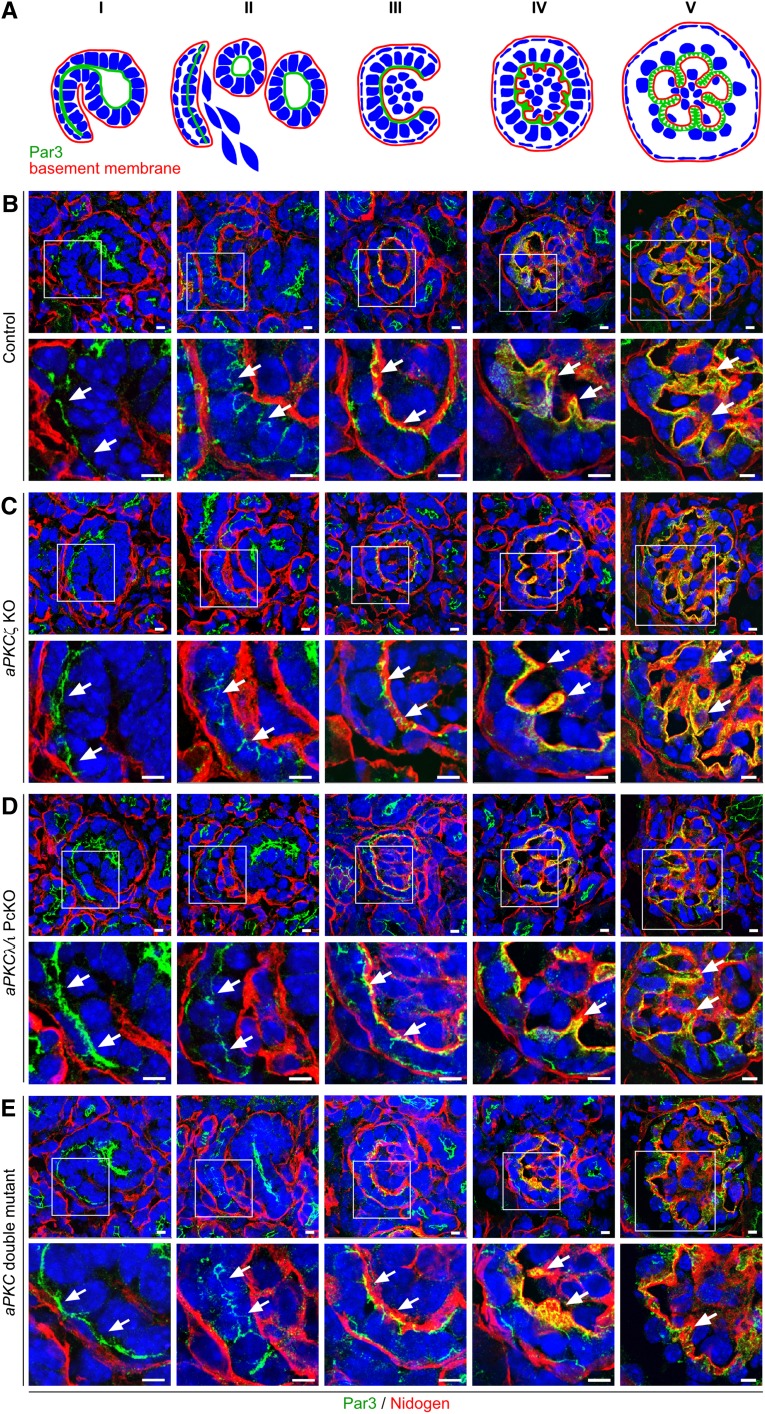
Because aPKC double mutants showed a disturbed podocyte process network development, individual maturation stages were further analyzed by confocal immunofluorescence microscopy using the junctional marker Par3. In the comma-shaped body stage, Par3 localizes at the apical sited cell-cell contacts of the undifferentiated podocytes. During the s-shaped body stage, Par3 translocates toward the basal membrane together with cell-cell contacts. Simultaneously, endothelial cells migrate into the cleft between immature podocytes and tubule cells. At the capillary loop stage, Par3 localizes at the basolateral sited cell-cell contacts. Subsequently, primary processes and foot processes develop, followed by the formation of slit diaphragms with Par3 localizing to the slit diaphragm insertions (see Figure 3A for schematic illustration).8,10 In early developmental stages, no differences were detectable between individual mutant mice and controls (Figure 3, B–E, I–III), which might be explained by the fact that Cre expression under control of the NPHS2 promoter occurs later during the capillary loop stage.19 Congruently, at the late capillary loop stage, process formation and glomerular infolding were severely impaired in aPKC double mutant mice. Whereas Par3-labeled podocyte processes extended into the glomerulus resulting in a partition of capillaries in control mice (Figure 3BV and Supplemental Figure 3), the infolding process was almost absent in aPKC double mutant mice. Only sporadic and unbranched Par3 positive protrusions were visible in these mice (Figure 3EV and Supplemental Figure 3). In contrast, glomerular development was unaffected in aPKCζ KO mice (Figure 3CV and Supplemental Figure 3), whereas Par3-positive stained extensions displayed less complex branching in aPKCλ/ι PcKO mice than in control mice (Figure 3DV and Supplemental Figure 3).
Figure 3.
Glomerular development arrests at late capillary loop stage in aPKC double mutant mice. Frozen kidney sections of newborn mice of the indicated genotypes (P0) were stained using antibodies against the polarity protein Par3 and the basement membrane marker nidogen and were subjected to confocal laser microscopy. Because glomerular development is asynchronous, kidneys of newborn mice display all glomerular developmental stages (from left to right) as follows: comma-shaped body (I), s-shaped body (II), early and late capillary loop stage (III and IV), and glomerulus (V). (A and B) Par3 is already expressed during the comma-shaped body stage (I) and localizes at the apical sited cell-cell junctions (arrows). During the s-shaped body stage (II), Par3 and the cell-cell junctions translocate to basal (arrows), where during capillary loop stage (IV) process development starts (arrows). In the following, glomerular surface infolding occurs (V), when Par3-positive stained protrusions enwrap the developing capillaries (arrows). (C) Whereas the glomerular development displays no obvious abnormality in aPKCζ KO mice, (D) Par3 positive protrusions display reduced branching in aPKCλ/ι PcKO mice. (E) The infolding process and enwrapping of the capillaries is almost completely absent in aPKC double mutant mice. Arrows mark localization of Par3. Scale bars, 5 µm.
aPKC Double Mutant Mice Exhibit a Disturbed Glomerular Maturation
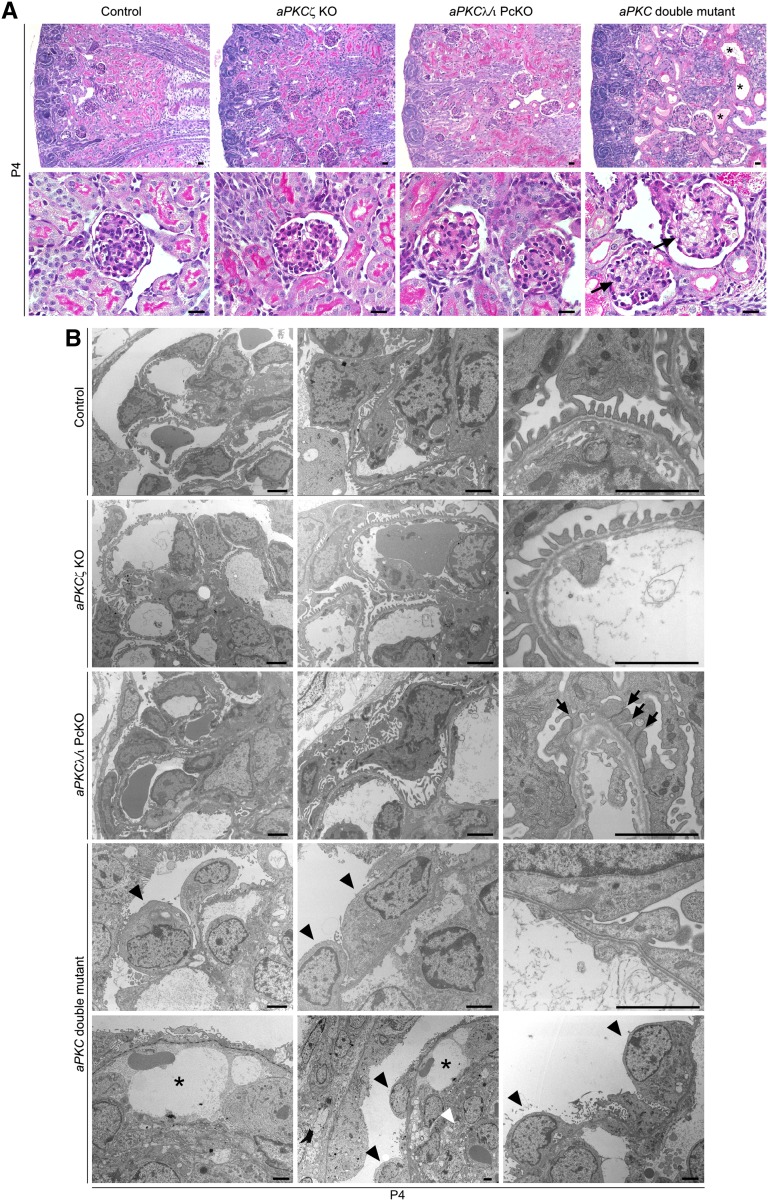
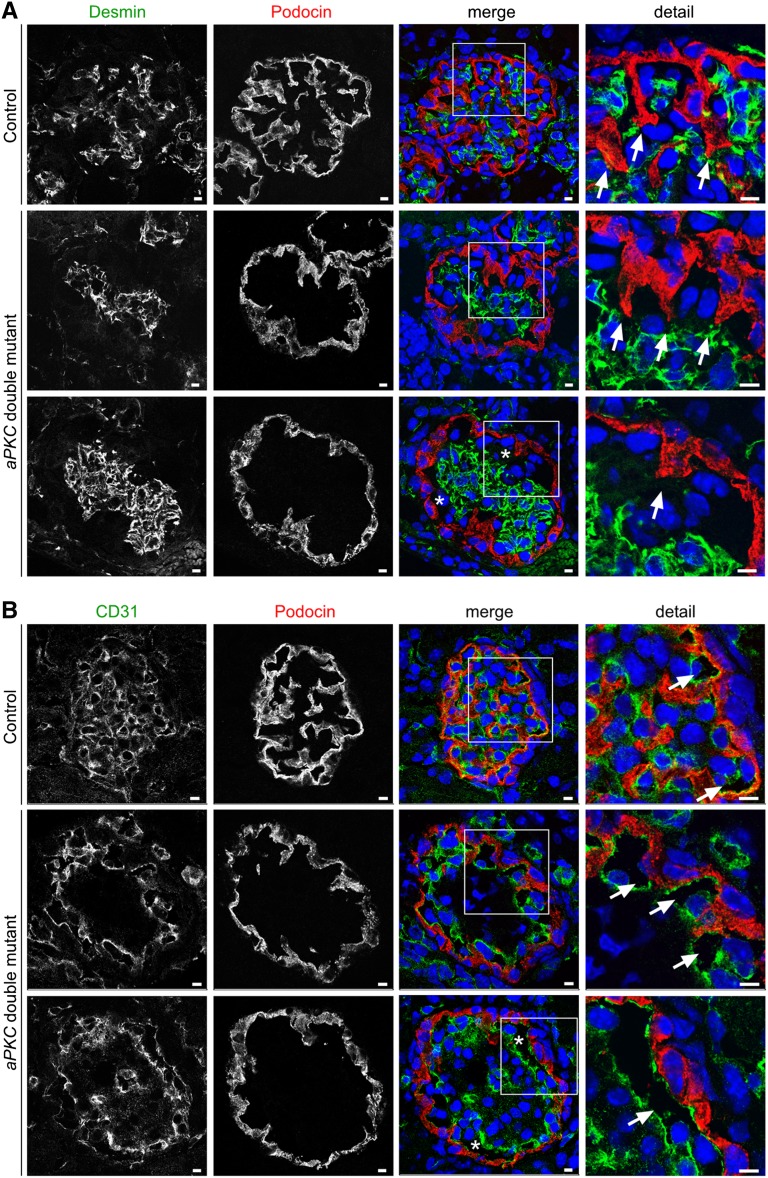
Glomerular maturation continues in mice during the first postnatal days with ongoing capillary formation and glomerular volume increase. To monitor this process, we analyzed glomeruli at P4 of surviving aPKC double mutant mice. Histologic analysis of kidneys at P4 revealed severe impairment in glomerular architecture of aPKC double mutant mice with capillary dilation, mesangiolysis, and mesangial hemorrhagia (Figure 4A). In transmission electron microscopy, aPKC double knockout podocytes displayed an undifferentiated morphologic appearance. Most podocytes lacked any processes and their cell bodies directly contacted the glomerular basement membrane (Figure 4B). The missing enwrapping of the glomerular capillaries by foot processes and basement membrane infolding was associated with capillary dilations and an impaired separation of the capillaries from the mesangium, accompanied with severe mesangiolysis. In contrast, control and aPKCζ knockout podocytes had regular spaced foot processes connected by slit diaphragms (Figure 4B), whereas broadened foot processes with narrowed filtration slits were detected in aPKCλ/ι PcKO mice (Figure 4B). Immunofluorescence staining of P4 kidney sections for the mesangial cell marker desmin, the endothelial marker CD31, and the podocyte marker podocin confirmed capillary dilation and the loss of podocyte projections in aPKC double mutant mice, ultimately resulting in the formation of capillary aneurysms (Figure 5, A and B) and in ballooned glomeruli, which almost completely lacked any capillary partition.
Figure 4.
aPKC double mutant mice display severely disturbed glomerular maturation. (A) Periodic acid–Schiff staining of P4 kidney sections of mice of the genotypes as indicated. Arrows mark mesangiolysis and mesangial hemorrhagia in aPKC double mutant mice. Asterisks mark tubular dilations with protein casts. (B) Ultrastructural analysis of P4 kidney sections reveals a regular foot process network in control and aPKCζ KO mice. In aPKCλ/ι PcKO mice, broadened foot processes with narrowed filtration slits (arrows) can be observed. In aPKC double mutant mice, podocytes (arrowheads) exhibit an undifferentiated appearance, sitting directly on the glomerular basement membrane. Asterisks indicate dilated capillaries, dotted line indicates the glomerular basement membrane, and white arrowhead indicates mesangiolysis. Scale bars, 20 µm in A; 2 µm in B.
Figure 5.
aPKC double mutant mice develop capillary aneurysms during glomerular maturation. (A) Frozen kidney sections of mice of the indicated genotypes at P4 are stained using antibodies against the mesangial marker desmin and the podocyte marker podocin and are subjected to confocal laser microscopy. Arrows indicate podocyte extensions to the mesangium, which regress during glomerular maturation in aPKC double mutant mice. (B) Frozen kidney sections of mice at P4 were stained using antibodies against the endothelial marker CD31 and the podocyte marker podocin. Arrows mark CD31-positive stained capillaries. In aPKC double mutant mice, massive aneurysms can be observed (asterisks). Scale bars, 5 µm.
Aberrant Expression of Slit Diaphragm Proteins and Incorrect Positioning of Centrosomes and Golgi Apparatus in aPKC Double Knockout Podocytes
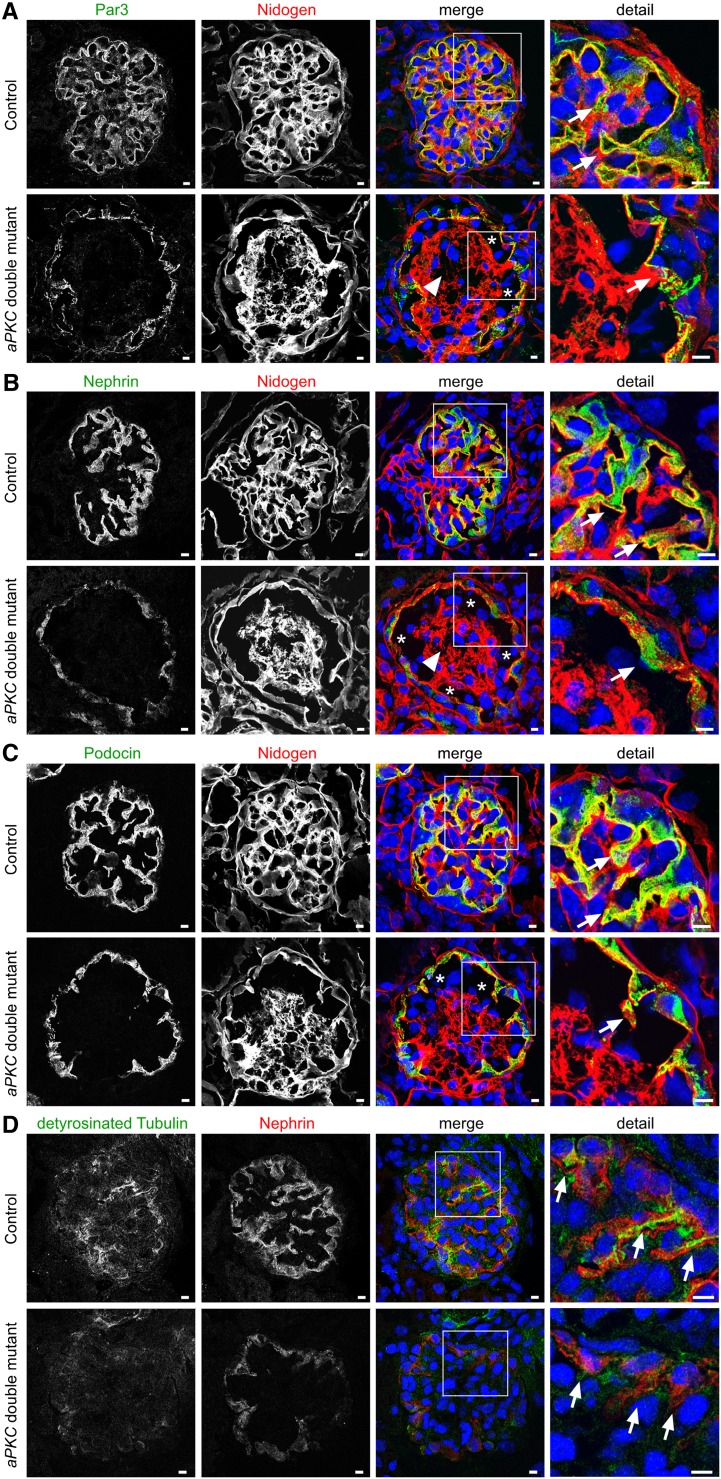
We next analyzed the expression pattern of Par3, the slit diaphragm proteins nephrin and podocin, and the primary process marker detyrosinated α-tubulin by confocal laser microscopy. Under physiologic conditions, slit diaphragm proteins distribute in a linear pattern along the capillary wall. In contrast, a granular and cytoplasmic distribution of Par3, nephrin, and podocin was detected in aPKC double knockout podocytes (Figure 6, A–C). In addition, nephrin staining seemed to be reduced (Figure 6B) and aPKC double knockout podocytes nearly completely lacked detyrosinated α-tubulin (Figure 6D), indicating a compromised microtubular network and primary process formation. To test whether growth hormones like vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which have a well documented role in glomerular maturation, are affected in aPKC double knockout mice, we performed in situ hybridization and immunofluorescence staining analysis for vascular endothelial growth factor A (VEGFA) and platelet-derived growth factor B (PDGFB) as well as their respective receptors. Supplemental Figure 4 indicates indeed a reduced glomerular expression of VEGFA and PDGFB during late glomerular maturation at P4 in aPKC double knockout mice, which might at least partially contribute to the observed aneurysm formation and mesangiolysis.
Figure 6.
Impaired expression of slit diaphragm proteins in aPKC double mutant mice. (A–C) Staining of frozen kidney sections at P4 for the slit diaphragm proteins Par3, nephrin, podocin, and the basement membrane marker nidogen. Arrows mark expression of the indicated proteins, asterisks mark aneurysms, and arrowheads label mesangiolysis. (D) Staining of frozen kidney sections at P4 for the podocyte primary process marker detyrosinated tubulin and the slit diaphragm protein nephrin. Arrows mark expression of detyrosinated tubulin. Scale bars, 5 µm.
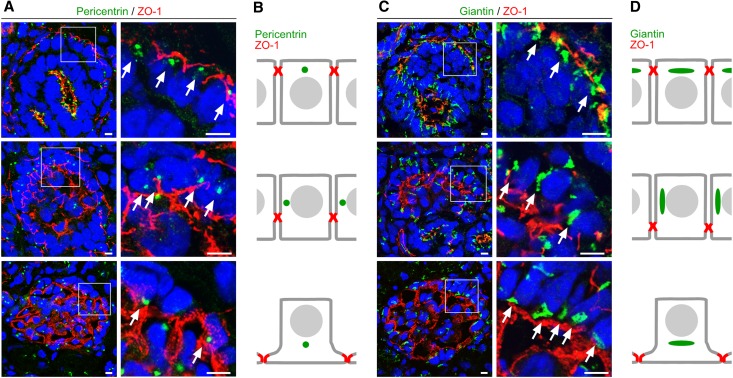
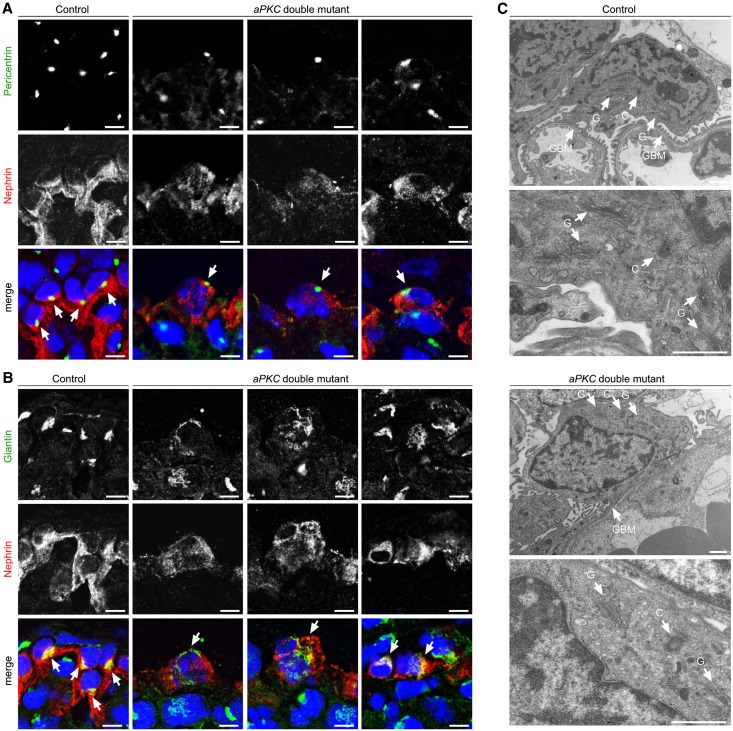
In neurons, localization of the centrosome and the Golgi apparatus precedes and determines the site of neuronal polarization and process outgrowth.20 In the comma-shaped body stage of the glomerulus, the centrosome and Golgi apparatus localize at the apical cell pole of podocytes (Figure 7, Supplemental Figures 5 and 6). Strikingly, unlike in tubular cells, where pericentrin (centrosome marker) and giantin (Golgi apparatus marker) signals remain in an apical position, in podocytes these markers translocate to the basal compartment during transition from the s-shaped body stage and the capillary loop stage to the glomerulus (Figure 7, Supplemental Figures 5 and 6). Although the centrosome and the Golgi apparatus localized to the basal side of the nucleus in podocytes of control mice, these subcellular structures were not restricted to this localization in aPKC double mutant mice (Figure 8, Supplemental Figures 7 and 8).
Figure 7.
Translocation of the centrosome and Golgi apparatus during podocyte differentiation. (A) Frozen kidney sections of newborn wild-type rat are stained using antibodies against the centrosome marker protein pericentrin (arrows) and the junction marker ZO-1 and are subjected to confocal laser microscopy (top, comma-shaped body stage; middle, late s-shaped body stage; bottom, immature glomerulus). (B) Schematic illustration of the pericentrin signal during podocyte differentiation. (C) Frozen kidney sections of newborn wild-type rat are stained using antibodies against the Golgi apparatus marker giantin (arrows) and ZO-1. Arrows mark translocation of the Golgi apparatus from apical to basal during podocyte differentiation (top, comma-shaped body stage; middle, late s-shaped body stage/early capillary loop stage; bottom, immature glomerulus). (D) Schematic illustration of the giantin signal during podocyte differentiation. Scale bars, 5 µm.
Figure 8.
Aberrant centrosome and Golgi apparatus localization in aPKC double knockout podocytes. (A and B) Frozen kidney sections of control and aPKC double mutant mice at P4 are stained for the podocyte marker nephrin, the centrosome marker pericentrin, or the Golgi apparatus marker giantin, respectively. Whereas the centrosome (arrows) and the Golgi apparatus (arrows) are restricted to the basal pole of the cytoplasm in control mice, aberrant localization (arrows) can be detected in aPKC double knockout podocytes. (C) Electron micrographs of P4 control and aPKC double knockout podocytes. C, centrosome; G, Golgi apparatus; GBM, glomerular basement membrane. Scale bars, 5 µm in A and B; 1 µm in C.
aPKC Is Required for Glomerular Maintenance
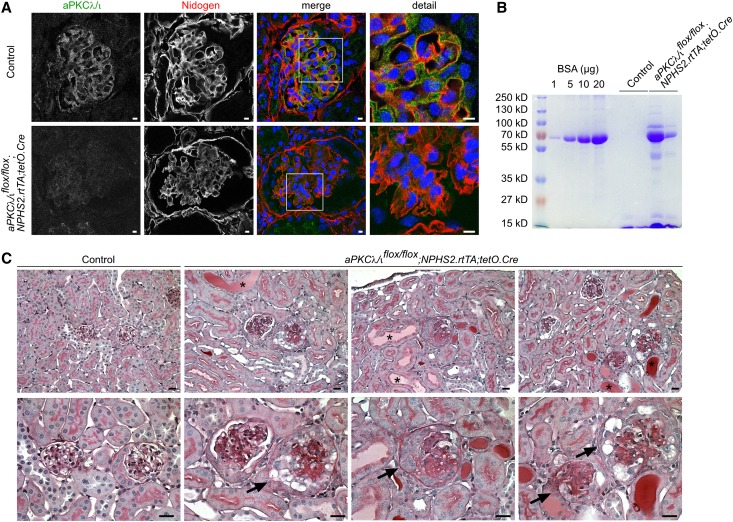
To investigate whether aPKC is required not only for development but also for maintenance of the glomerular filtration barrier, aPKCλ/ιflox/flox mice were crossed with NPHS2.rtTA;tetO.Cre mice to generate doxycycline-inducible podocyte-specific aPKCλ/ι knockout mice. Administration of doxycycline to the drinking water between 5 and 7 weeks of age resulted in loss of glomerular aPKCλ/ι expression, proteinuria, and glomerulosclerotic changes with synechia, crescent formation, and proteinaceous casts in dilated tubules (Figure 9).
Figure 9.
aPKC is required for glomerular maintenance. Time-dependant induction of aPKCλ/ι knockout in podocytes of aPKCλ/ιflox/flox;NPHS2.rtTA;tetO.Cre mice between 5 and 7 weeks of age results in the following: (A) loss of glomerular aPKCλ/ι expression, (B) proteinuria, and (C) glomerulosclerotic changes with synechia, crescent formation (arrows), and proteinaceous casts in dilated tubules (asterisks) 3 weeks after start of induction. Scale bars, 5 µm in A; 20 µm in C.
Discussion
Podocytes form the most outer part of the glomerular filtration barrier. To fully cover the expanding capillaries during glomerular development, podocytes have to undergo dramatic morphologic changes that are characterized by an apical membrane expansion, the formation of a complex structured interdigitating cell process network, and the lifting of the cell body into the Bowman’s space. Data on the molecular programs that orchestrate this conversion of a cuboidal podocyte precursor cell to the octopus-like differentiated podocyte remained elusive. Here, we identify the serine/threonine kinase aPKC as a fundamental regulator of podocyte development. Previously, we and others described that the podocyte-specific aPKCλ/ι knockout results in proteinuria and glomerulosclerosis between 2 and 4 weeks after birth (Supplemental Figures 2B and 9),9,10 whereas these mice initially showed fully established foot processes connected by slit diaphragms at birth.10 We speculated that the second aPKC isoform, aPKCζ, might partially compensate for the loss of aPKCλ/ι, resulting in the only mild developmental phenotype. To study the full effect of aPKC for podocyte differentiation and glomerular development, double mutant aPKC mice were generated.
Podocyte Process Formation Requires aPKC Function
We demonstrate that aPKC is required for podocyte differentiation and process network formation. The fundamental importance of aPKC for podocyte function is underlined by the fact that both isoforms are highly enriched in podocytes. Indeed, loss of aPKCλ/ι appears to be partially compensated by aPKCζ, suggesting that both isoforms can act synergistically. In agreement, aPKCζ knockout itself displays a slightly increased and transient albuminuria postnatally. In contrast, expression of only one aPKC isoform or different localization of the both isoforms have been reported in other tissues.21,22 Recently, it was demonstrated that aPKCζ and aPKCλ/ι are dispensable for certain cell types because the double knockout of aPKCζ and aPKCλ/ι in primitive and adult hematopoietic stem cells did not cause any obvious phenotype.23 In podocytes, aPKC seems not only to be important for podocyte maintenance and foot process assembly but also for the initial steps of process formation. To date, most published genetic and acquired injury models were reported to result in foot process effacement, but did not focus on the exact mechanism of podocyte process formation. For example, knockouts of other slit diaphragm proteins result in effaced, irregularly configured, or misguided foot processes, but not in such a severe failure of process formation. Mice lacking the slit diaphragm protein podocin reveal reduced number of foot processes with narrowed filtrations slits and lacking slit diaphragms.24 Knockout of nephrin results in irregular shaped foot processes without slit diaphragms.25 Neph1 deficiency causes misguidance and effacement of foot processes.26 The effect of aPKC for podocyte differentiation might be even underestimated in our conditional mouse model, because NPHS2.Cre-driven deletion of aPKCλ/ι does not occur before the capillary loop stage,19 which might be a reason for the unaffected initial differentiation steps in aPKC double mutant mice.
Podocytes exhibit astonishing similarities with neurons. Both cell types are postmitotic and develop long microtubule-based protrusions cobbled with short actin-based foot processes (podocytes) or dendritic spines (neurons). Interestingly, in primary cultured neurons, the Par complex localizes to the tip of an immature neurite to specify the axon and establishes neuronal polarity by the regulation of the neuronal microtubule machinery.27 Disrupting the function of the Par complex prevents neurites to differentiate into either axons or dendrites.28 At later steps of neuronal differentiation, the Par complex is required for the morphogenesis of the actin-based dendritic spines.29,30 It is conceivable to speculate that aPKC-mediated regulation of the microtubule and/or actin machinery in podocytes, like in neurons, contributes to cell process formation. Furthermore, the site of axon outgrowth in neurons is defined not only by the Par complex but also by the localization of the centrosome and the Golgi apparatus.20 Interestingly, we could discern that the centrosome and the Golgi apparatus translocate from the apical cell pole to the basal site of the nucleus during normal podocyte development. Correct basal centrosome positioning appears to fail in aPKC-deficient podocytes, indicating that aPKC might be required to instruct podocyte polarity via the positioning of the centrosome and the Golgi apparatus. It was consistently shown that in migrating astrocytes aPKC is needed for the centrosome and Golgi positioning toward the leading edge,31 which was controlled by Cdc42. Recently, a podocyte-specific Cdc42 knockout has been shown to cause severe nephrotic syndrome and postnatal lethality in mice, indicating that Cdc42 and aPKC could indeed act in the same pathway in podocytes.32
In summary, this study uncovers the fundamental role of aPKC for podocyte orientation, polarity, and process development.
Podocyte Process Formation and Polarity Establishment Contributes to Glomerular Maturation
Very little has been known on the contribution of podocytes to the development of the overall glomerular structure and capillary formation. This is partially due to the fact that, as mentioned above, most known genetic podocyte models still exhibit podocytes with an extending process network. Therefore, the aPKC double mutant mice offered a unique opportunity to study the influence on the glomerular development by podocytes that arrest at their development during the late capillary loop stage. In general, podocyte process formation starts at the capillary loop stage. Together with mesangial cells, extending processes and glomerular basement membrane appear to separate glomerular capillaries to form the immature glomerulus.
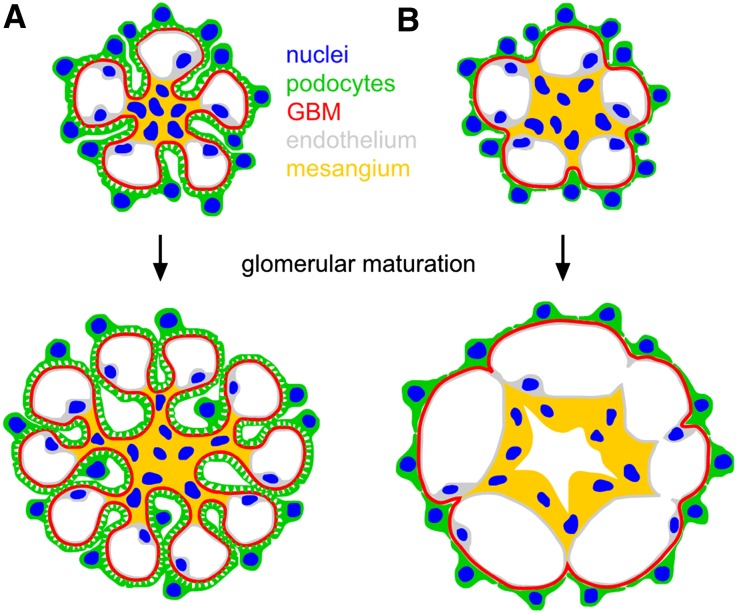
During perinatal glomerular maturation, arborization of surface infolding increases and further capillary lumina are formed, which are enwrapped by basement membranes and podocyte foot processes (Figure 10A). Because podocytes stop cell divisions at the capillary loop stage,1 the increase of glomerular surface area is associated with an increase of extensions of every single podocyte to cover the new shaped capillaries.
Figure 10.
The podocyte foot process network is required for glomerular maturation. (A) Under physiologic conditions, podocytes establish a foot process network during glomerular development and maturation. Surface infolding is formed by podocyte foot processes, glomerular basement membrane, and mesangial cells, separating the capillaries from each other. (B) In aPKC double mutant mice, failure to establish a process network results in an arrest of glomerular maturation.
Here we demonstrate that the failure of aPKC double knockout podocytes to extend their process network results in a dramatic phenotype with a reduced number of capillaries and massive capillary aneurysm formation of the remaining capillaries (Figure 10B). Subsequently, microruptures of the aneurysmatic capillaries seem to occur, leading to a spillover of erythrocytes and plasma components into the mesangium causing the extensive mesangiolysis seen in aPKC double mutant mice. These observations indicate that podocyte processes trigger the glomerular basement infolding and the shaping of the glomerular capillaries. Due to the polarization and differentiation defects, impaired secretion of soluble factors, such as VEGF,5 might contribute to the capillary phenotype (Supplemental Figure 4) because VEGFA expression is reduced in aPKC-deficient podocytes. However, complete absence of VEGFA in the glomerulus causes a different phenotype with rather small or missing capillary loops and diminished numbers of endothelial cells that always lack fenestrations.33 However, glomerular aneurysm formation and ballooning were described in a few other mouse models. For example, failure of mesangial cells to invade the glomerulus in mice lacking either PDGFB or PDGF receptor β results in massively enlarged glomeruli without a capillary tuft but a single capillary lumen.34 In addition, the genetic disruption of the connection between mesangial cells and the glomerular basement membrane leads to a lack of capillary convolution.35
Our model highlights the role of podocytes for glomerular development, indicating that the interplay of both the mesangial cells (from inside the glomerulus) and the podocyte process network (from the outer side) regulate the formation of the capillary tuft.
In summary, both aPKC isoforms are molecular guideposts to orchestrate fundamental podocyte polarity programs, podocyte development, and podocyte maintenance. In addition, our data illustrate that an extending foot process network is required for capillary formation and glomerular maturation.
Concise Methods
Mice
aPKCλ/ι-floxed mice (aPKCλ/ιflox/flox) and aPKCζ knockout mice (aPKCζ−/−) were previously described.17,18 NPHS2.Cre+ mice, which express Cre under control of a podocyte-specific promoter, were kindly provided by Lawrence Holzman (Renal, Electrolyte, and Hypertension Division, University of Pennsylvania School of Medicine, Philadelphia, PA).19 aPKCλ/ιflox/flox, aPKCζ−/− and NPHS2.Cre+ mice were crossed to generate aPKC double mutant mice (NPHS2.Cre+;aPKCλ/ιflox/flox;aPKCζ−/−), podocyte-specific aPKCλ/ι single knockout littermates (aPKCλ/ι PcKO) (NPHS2.Cre+;aPKCλ/ιflox/flox), and NPHS2.Cre negative aPKCζ knockout littermates (aPKCζ KO). NPHS2.Cre negative aPKCλ/ιflox/flox;aPKCζ+/+ littermates served as controls. NPHS2.rtTA;tetO.Cre mice were kindly provided by Susan Quaggin (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, University of Toronto, Toronto, Ontario, Canada).36 To generate doxycycline-inducible podocyte-specific aPKCλ/ι-knockout mice (aPKCλ/ιflox/flox;NPHS2.rtTA;tetO.Cre), aPKCλ/ι-floxed mice (aPKCλ/ιflox/flox) were crossed with NPHS2.rtTA;tetO.Cre mice; tetO.Cre negative littermates served as the control. For the induction of aPKCλ/ι deletion, mice received doxycycline hydrochloride (Sigma-Aldrich) via drinking water (2 mg/ml with 5% sucrose, protected from light) between 5 and 7 weeks of age. All animal studies were approved by the Committee on Research Animal Care, Regierungspräsidium Freiburg.
Urine and Serum Analyses
Urinary albumin and urinary creatinine were measured using a fluorometric albumin kit (Progen) and an enzymatic colorimetric creatinine kit (Labor+Technik) following the manufacturers’ instructions. Proteinuria was expressed as milligram of albumin per milligram of creatinine. For Coomassie gel analysis, BSA standard (1, 5, 10, and 20 μg) and 1 μl of urine of mice of the indicated genotypes were separated by 10% SDS-PAGE. The gel was stained by Coomassie blue.
Morphologic Analyses
Kidneys were fixed in 4% paraformaldehyde or glutaraldehyde, embedded in paraffin or Epon, and further processed for periodic acid–Schiff staining or electron microscopy, respectively. For scanning electron microscopy, glutaraldehyde fixed kidney samples were dehydrated by sequential incubation in 70% ethanol, 80% ethanol, 90% ethanol, and 100% ethanol, followed by incubation in 50:50 ethanol/Hexamethyldisilazane. After incubation in 100% Hexamethyldisilazane, the solvent was allowed to evaporate. Samples were coated with Gold (Zeiss Semco Nanolab7, Polaron Cool Sputter Coater E 5100, Balzer Cpd 020) and imaged using a Leo 1450 VP scanning electron microscope.
Immunofluorescence Staining of Kidney Sections
Kidneys were frozen in optimal cutting temperature compound and sectioned at 6 μm (Leica Kryostat). The sections were fixed with 4% paraformaldehyde, blocked in PBS containing 5% BSA, and incubated for 1 hour with primary antibodies as indicated. After washing the sections with PBS for several times, fluorophore-conjugated secondary antibodies (Invitrogen) were applied for 30 minutes. Images were taken using a Zeiss laser scan microscope equipped with a ×63 water immersion objective.
In Situ Hybridization
Whole mRNA extracts from P1 mouse kidney served as a template for RT-PCR and subsequent cloning of fragments of the coding sequence and 3′-untranslated region. The following primers were used: mPKCλ/ι.fp (5′-GTGTTACTGGTTGCCGAGGT-3′), mPKCλ/ι.rp (5′- GAGCTCAGGAAGAGCTGGAA-3′), mPKCζ.fp (5′-ACTAGCCAGTGCCTCCAAGA-3′), mPKCζ.rp (5′-GCAAATATGACCGGAGCCTA-3′), mVEGFA.fp (5′-GACTTGTGTTGGGAGGAGGA-3′), and mVEGFA.rp (5′-GGAAGGGAAGATGAGGAAGG-3′). PCR products were cloned into pBluescript II KS (-), linearized, and transcribed with T3 and T7 RNA polymerases (Promega) to generate sense and antisense digoxigenin-labeled probes (digoxigenin RNA labeling mix; Roche Applied Science). Kidneys were fixed overnight at 4°C in 4% paraformaldehyde, embedded in paraffin, and sectioned at 8 μm. For mRNA detection, slides were treated with proteinase K, refixed with 4% paraformaldehyde, acetylated by using acetic anhydride (0.25% acetic anhydride in 0.1 M triethanolamine; Sigma T-1377) and hybridized at 68°C in hybridization buffer (50% formamide, ×5 SSC, 50 g/ml yeast RNA, 1% SDS, 50 g/ml heparin, 0.1% probe). Stringency washes were performed with wash I (50% formamide, ×5 SSC pH 4.5, 1% SDS) and wash II (50% formamide, 2× SSC). For detection, slides were incubated with alkaline phosphatase-conjugated antidigoxigenin antibody (Roche Applied Science) 1:3000 at 4°C overnight followed by BM purple staining (Roche Applied Science). Digital photographs were captured on an Axioplan2 microscope (Zeiss).
Isolation and Characterization of Mouse Glomeruli
Glomeruli were isolated using Dynabead perfusion and were glass-glass homogenized in lysis buffer (containing 20 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate and 1% Triton X-100).37 After centrifugation (15,000×g, 15 minutes, 4°C) protein concentration was determined by Dc Protein-Assay (Bio-Rad). Equal amounts of protein were separated on SDS-page.
Antibodies
Antibodies were obtained from Millipore (anti-Par3 rabbit pAb, 07–330; anti-nidogen rat mAb, MAB1946; anti-WT1 mouse mAb, 05–753; anti-detyrosinated tubulin rabbit pAb, AB3201), Progen (anti-nephrin guinea pig pAb, GP-N2), Sigma (anti-α-tubulin mouse mAb, T6199; anti-β-Actin mouse mAb, A1978; anti-podocin rabbit pAb, P0372; anti-PDGFB rabbit pAb, HPA011972), Abcam (anti-WT1 rabbit pAb, ab15249; anti-giantin rabbit pAb, ab24586; anti-pericentrin rabbit pAb, ab4448), BD Biosciences (anti-CD31 rat mAb, 550274; anti-pericentrin mouse mAb, 611814), Invitrogen (anti-ZO-1 mouse mAb, 339100), Dako (anti-desmin mouse mAb, M0760), and Cell Signaling (anti-PKCζ rabbit mAb, 9368; anti-aPKCλ/ι rabbit mAb, 2998; anti-PDGFRβ rabbit mAb, 3169; anti-VEGFR2 rabbit mAb, 2479). Polyclonal antibody specific for aPKCλ/ι was raised in rabbit by injection of aPKCλ/ι amino acid 184–234 and was described previously.22 Nuclear staining reagent (To-Pro-3, T3605) and fluorophore-conjugated secondary antibodies were obtained from Invitrogen.
Statistical Analyses
Data were expressed as the mean ± SEM. Statistical comparisons were performed using two-tailed t test if not stated otherwise. Differences with P<0.05 were considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Charlotte Meyer and Ann-Kathrin Fritz (Renal Division, University Hospital Freiburg), and Evelyn Wätzig and Brigitte Plessow-Freudenberg (Institute of Pathology, University Hospital Freiburg) for excellent technical assistance. We are grateful to Claudia Gack (Biology I, Albert-Ludwigs-University, Freiburg) for her support with the scanning electron microscopy. We thank Nadja Herbach (Veterinary Pathology, Ludwig-Maximilians-University, Munich, Germany) for advice with the manuscript.
This study was supported by Else Kröner-Fresenius-Stiftung, Fritz-Thyssen-Stiftung, Deutsche Forschungsgemeinschaft, and the Excellence Initiative of the German Federal and State Governments (EXC 294) (all to T.B.H.), and Deutsche Nierenstiftung (to B.H.).
Footnotes
M.L., M.S., and T.B.H. contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012060582/-/DCSupplemental.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Reeves W, Caulfield JP, Farquhar MG: Differentiation of epithelial foot processes and filtration slits: Sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39: 90–100, 1978 [PubMed] [Google Scholar]
- 3.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kriz W: Ontogenetic development of the filtration barrier. Nephron, Exp Nephrol 106: e44–e50, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Vaughan MR, Quaggin SE: How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol 19: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Simons M, Hartleben B, Huber TB: Podocyte polarity signalling. Curr Opin Nephrol Hypertens 18: 324–330, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Pieczynski J, Margolis B: Protein complexes that control renal epithelial polarity. Am J Physiol Renal Physiol 300: F589–F601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartleben B, Schweizer H, Lübben P, Bartram MP, Möller CC, Herr R, Wei C, Neumann-Haefelin E, Schermer B, Zentgraf H, Kerjaschki D, Reiser J, Walz G, Benzing T, Huber TB: Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber TB, Hartleben B, Winkelmann K, Schneider L, Becker JU, Leitges M, Walz G, Haller H, Schiffer M: Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J Am Soc Nephrol 20: 798–806, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirose T, Satoh D, Kurihara H, Kusaka C, Hirose H, Akimoto K, Matsusaka T, Ichikawa I, Noda T, Ohno S: An essential role of the universal polarity protein, aPKClambda, on the maintenance of podocyte slit diaphragms. PLoS ONE 4: e4194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joberty G, Petersen C, Gao L, Macara IG: The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2: 531–539, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Qiu RG, Abo A, Steven Martin G: A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr Biol 10: 697–707, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T: A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2: 540–547, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, Ohno S: PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells 6: 721–731, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki A, Akimoto K, Ohno S: Protein kinase C lambda/iota (PKClambda/iota): A PKC isotype essential for the development of multicellular organisms. J Biochem 133: 9–16, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Soloff RS, Katayama C, Lin MY, Feramisco JR, Hedrick SM: Targeted deletion of protein kinase C lambda reveals a distribution of functions between the two atypical protein kinase C isoforms. J Immunol 173: 3250–3260, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Leitges M, Sanz L, Martin P, Duran A, Braun U, García JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J: Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell 8: 771–780, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M: Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117: 2289–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 20.de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG: Centrosome localization determines neuronal polarity. Nature 436: 704–708, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Oster H, Eichele G, Leitges M: Differential expression of atypical PKCs in the adult mouse brain. Brain Res Mol Brain Res 127: 79–88, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Helfrich I, Schmitz A, Zigrino P, Michels C, Haase I, le Bivic A, Leitges M, Niessen CM: Role of aPKC isoforms and their binding partners Par3 and Par6 in epidermal barrier formation. J Invest Dermatol 127: 782–791, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Sengupta A, Duran A, Ishikawa E, Florian MC, Dunn SK, Ficker AM, Leitges M, Geiger H, Diaz-Meco M, Moscat J, Cancelas JA: Atypical protein kinase C (aPKCzeta and aPKClambda) is dispensable for mammalian hematopoietic stem cell activity and blood formation. Proc Natl Acad Sci U S A 108: 9957–9962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roselli S, Heidet L, Sich M, Henger A, Kretzler M, Gubler MC, Antignac C: Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol 24: 550–560, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR: Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi SH, Jan LY, Jan YN: Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112: 63–75, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Insolera R, Chen S, Shi SH: Par proteins and neuronal polarity. Dev Neurobiol 71: 483–494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Macara IG: The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol 8: 227–237, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Macara IG: The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell 14: 216–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etienne-Manneville S, Hall A: Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 106: 489–498, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, Pawson T: Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol 23: 1149–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C: Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125: 3313–3322, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Kikkawa Y, Virtanen I, Miner JH: Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol 161: 187–196, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.