Abstract
Type 2 diabetes associates with increased risk of mortality, but how kidney disease contributes to this mortality risk among individuals with type 2 diabetes is not completely understood. Here, we examined 10-year cumulative mortality by diabetes and kidney disease status for 15,046 participants in the Third National Health and Nutrition Examination Survey (NHANES III) by linking baseline data from NHANES III with the National Death Index. Kidney disease, defined as urinary albumin/creatinine ratio ≥30 mg/g and/or estimated GFR ≤60 ml/min per 1.73 m2, was present in 9.4% and 42.3% of individuals without and with type 2 diabetes, respectively. Among people without diabetes or kidney disease (reference group), 10-year cumulative all-cause mortality was 7.7% (95% confidence interval [95% CI], 7.0%–8.3%), standardized to population age, sex, and race. Among individuals with diabetes but without kidney disease, standardized mortality was 11.5% (95% CI, 7.9%–15.2%), representing an absolute risk difference with the reference group of 3.9% (95% CI, 0.1%–7.7%), adjusted for demographics, and 3.4% (95% CI, −0.3% to 7.0%) when further adjusted for smoking, BP, and cholesterol. Among individuals with both diabetes and kidney disease, standardized mortality was 31.1% (95% CI, 24.7%–37.5%), representing an absolute risk difference with the reference group of 23.4% (95% CI, 17.0%–29.9%), adjusted for demographics, and 23.4% (95% CI, 17.2%–29.6%) when further adjusted. We observed similar patterns for cardiovascular and noncardiovascular mortality. In conclusion, those with kidney disease predominantly account for the increased mortality observed in type 2 diabetes.
In 2012, there were an estimated 346 million individuals with diabetes worldwide.1 This number is expected to rise to >430 million by 2030.2 Diabetes is associated with substantially increased risk of mortality, particularly due to cardiovascular disease.3
Kidney disease, defined by increased urine albumin excretion and/or impaired GFR, is also strongly associated with increased risk of all-cause and cardiovascular mortality, both among persons with diabetes4,5 and in the general population.6–10 The critical effect of kidney disease on mortality in type 1 diabetes was emphasized in two recent reports.11,12 Each study demonstrated that excess mortality was confined to the subgroup with kidney disease.
The degree to which kidney disease captures risk of adverse health outcomes in type 2 diabetes has not been determined. The findings from type 1 diabetes may not extrapolate to type 2 diabetes because the latter is frequently associated with other comorbidities that affect mortality. This question has crucial public health implications because type 2 diabetes predominates among the 26 million US adults with diabetes13,14 and identifying predictors of excess mortality in type 2 diabetes is essential in order to optimally target risk-reduction strategies. The primary objective of this study was to quantify and compare the excess risk of all-cause and cause-specific mortality among individuals with type 2 diabetes in presence or absence of kidney disease.
Results
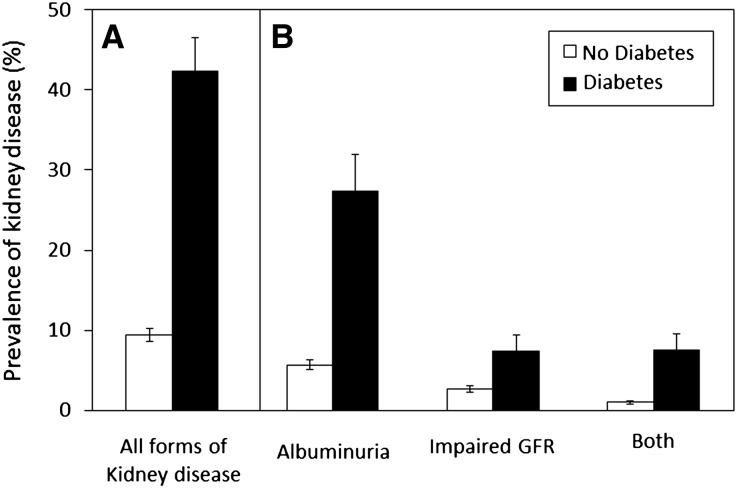
Of 15,762 individuals aged ≥20 years in the Third National Health and Nutrition Examination Survey (NHANES III), 15,046 have follow-up mortality data to 2006 and were included in this study (95.5%). Of these, 9.5% had type 2 diabetes (Table 1, Supplemental Table 1). Among persons with diabetes, 42.3% had kidney disease, as defined by albuminuria, impaired GFR or both (Figure 1). In comparison, 9.4% of people without diabetes had kidney disease. Participants with diabetes, kidney disease, or both were older and had higher mean systolic BP and higher mean concentrations of non-HDL cholesterol (Table 1).
Table 1.
Baseline characteristics of participants
| Variable | No Diabetes | Diabetes | ||||||
|---|---|---|---|---|---|---|---|---|
| No Kidney Disease | Kidney Disease | No Kidney Disease | Kidney Disease | |||||
| n | Weighted Proportion or Mean (SEM) | n | Weighted Proportion or Mean (SEM) | n | Weighted Proportion or Mean (SEM) | n | Weighted Proportion or Mean (SEM) | |
| 11,742a | 90.6(0.4) | 1874 | 9.4 (0.4) | 772 | 57.7 (2.1) | 658 | 42.3 (2.1) | |
| Age (yr) | 42 (15.0) | 57 (20) | 56 (13.4) | 62 (14.0) | ||||
| Female | 6203 | 51.5 (0.5) | 1026 | 59.9 (2.5) | 365 | 41.2 (2.6) | 370 | 57.4 (3.5) |
| Race | ||||||||
| White, not Hispanic | 5081 | 77 (1.3) | 664 | 72.7 (1.9) | 280 | 66.4 (3.2) | 198 | 69.1 (3.1) |
| Black, not Hispanic | 3191 | 10 (0.6) | 419 | 14.4 (1.2) | 278 | 17.8 (1.7) | 174 | 16.9 (2.1) |
| Mexican American | 3401 | 5.3 (0.4) | 300 | 4.5 (0.5) | 259 | 7.2 (0.9) | 191 | 6.8 (0.8) |
| Current smoker | 3262 | 29.9 (0.9) | 386 | 23.8 (1.9) | 155 | 20 (2.1) | 107 | 20.6 (3.2) |
| Body mass index (kg/m2) | 26.2 (5.4) | 26.9 (5.8) | 30.5 (6.7) | 31.1 (6.5) | ||||
| eGFR (ml/min per 1.73 m2)b | 102.1 (17.7) | 78.5 (28.5) | 92.9 (17.8) | 75.6 (26.7) | ||||
| Albumin/creatinine ratio (mg/g) | 7 (5.2) | 128 (369.5) | 10 (7.4) | 317 (873.0) | ||||
| Lipids | ||||||||
| Non-HDL cholesterol (mg/dl) | 150 (42.8) | 164.8 (47.2) | 170.4 (45.6) | 186.4 (52.9) | ||||
| Lipid-lowering agents (%)c | 169 | 1.6 (0.2) | 72 | 4.8 (1) | 40 | 9.3 (1.7) | 34 | 8.4 (1.8) |
| BP (mmHg) | ||||||||
| Systolic | 120 (15.4) | 135 (23.1) | 132 (16.8) | 140 (20.8) | ||||
| Diastolic | 74 (9.66) | 77 (11.97) | 76 (9.78) | 77 (10.56) | ||||
| Antihypertensive agents (%)c | 1442 | 10.3 (0.5) | 818 | 38.5 (1.6) | 265 | 34.7 (3) | 397 | 59.2 (3.9) |
| HbA1C (%) | 7.9 (1.7) | 8.3 (2.1) | ||||||
| Glucose-lowering agents (%)c | ||||||||
| None | 396 | 47.9 (2.8) | 253 | 38.7 (3) | ||||
| Oral agents only | 266 | 34.1 (2.4) | 226 | 37 (2.9) | ||||
| Insulin ± oral agents | 110 | 18 (2.8) | 179 | 24.3 (2.8) | ||||
| Duration of diabetes (yr)d | ||||||||
| Previously undiagnosed | 313 | 37.1 (2.4) | 173 | 30.9 (3.1) | ||||
| 0–4.9 | 180 | 27.4 (3) | 134 | 26.4 (2.9) | ||||
| 5–9.9 | 99 | 16.8 (2.9) | 86 | 13.6 (2.2) | ||||
| 10–19.9 | 112 | 13.4 (1.8) | 140 | 17.7 (2.6) | ||||
| ≥20 | 51 | 5.3 (1.1) | 108 | 11.4 (1.8) | ||||
Proportions reflect row percentages for each subgroup in the top row and means or columns percentages in all other rows. Means are listed with SEMs in parentheses. Proportions and means are weighed to NHANES sampling distributions. To convert GFR in ml/min to ml/s, multiply by 0.01667. To convert non-HDL cholesterol in mg/dl to mmol/L, multiply by 0.0259. The P values for comparison among the four groups were significant for all variables (0.002 for sex, 0.004 for body mass index, and <0.001 for all other variables).
The sample sizes for continuous variables (age, body mass index, eGFR, albumin/creatinine ratio, non-HDL cholesterol, BP and HbA1C) are listed in the first row.
GFR is calculated using the CKD-EPI equation.
Percentage of individuals using the listed medications.
Duration of diabetes was self-reported.
Figure 1.
Prevalence (A) and manifestations (B) of kidney disease in diabetic and nondiabetic subpopulations of the United States. Prevalence values are estimated percentages of total US population, calculated using NHANES sample weighing. Error bars indicate 95% CIs. Closed bars (▪) and open bars (□) represent prevalence in individuals with and without diabetes, respectively.
Ten-year cumulative all-cause mortality, standardized to average age, sex, and race in the whole population, was 19.1% (95% confidence interval [95% CI], 15.5–22.7) among people with diabetes compared with 8.6% (95% CI, 7.9–9.3) among people without diabetes. Standardized 10-year cumulative cardiovascular mortality was 11.2% (95% CI, 8.7–13.7) among people with diabetes compared with 4.0% (95% CI, 3.7–4.4) among people without diabetes. Standardized 10-year cumulative noncardiovascular mortality followed a similar pattern (Table 2).
Table 2.
Ten-year standardized all-cause and cardiovascular mortality by diabetes status
| Standardized Cumulative Incidence, % (95% CI) | |
|---|---|
| All-cause mortality | |
| No diabetes | 8.6 (7.9–9.3) |
| Diabetes | 19.1 (15.5–22.7) |
| Cardiovascular mortality | |
| No diabetes | 4.0 (3.7–4.4) |
| Diabetes | 11.2 (8.7–13.7) |
| Noncardiovascular mortality | |
| No diabetes | 6.3 (5.7–6.9) |
| Diabetes | 13.1 (9.8–16.4) |
Absolute differences in mortality risk were estimated using linear regression and were adjusted for age, sex, and race. Standardized 10-year all-cause cumulative incidences were estimated from the model for the mean levels of the covariates in the study population.
In the reference group consisting of people with no diabetes or kidney disease, 10-year cumulative all-cause mortality, standardized to population age, sex and race, was 7.7% (95% CI, 7.0–8.3). Among people with diabetes and no kidney disease, the standardized mortality was 11.5% (95% CI, 7.9–15.2), hence an absolute risk difference compared with the reference group of 3.9% (95% CI, 0.1–7.7) when adjusted for age, sex and race and 3.4% (95% CI, −0.3 to 7.0) after additional adjustment for smoking, BP, and cholesterol. Among individuals with both diabetes and kidney disease, standardized mortality was 31.1% (95% CI, 24.7–37.5), with an absolute risk difference of 23.4% (95% CI, 17.0–29.9) after adjustment for age, sex, and race and 23.4% (95% CI, 17.2–29.6) after additional adjustment for smoking, BP, and cholesterol (Table 3). The same patterns were observed for both cardiovascular and noncardiovascular mortality (Table 3). We observed an interaction between diabetes and kidney disease on the additive scale in which the presence of both was associated with a significantly greater increase in mortality than the sum of increase in mortality with each risk factor alone (P<0.01). This interaction was not present when evaluated on the multiplicative scale (P=0.86).
Table 3.
Ten-year standardized all-cause and cardiovascular mortality by diabetes and kidney disease status
| Number of Events | Standardized Cumulative Incidence, % (95% CI) | Adjusted Difference in Cumulative Incidence, % (95% CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| All-cause mortality | ||||
| None | 1027 | 7.7 (7.0–8.3) | 0 (Reference) | 0 |
| Diabetes | 168 | 11.5 (7.9–15.2) | 3.9 (0.1–7.7) | 3.4 (−0.3 to 7.0) |
| Kidney disease | 750 | 17.2 (14.6–19.7) | 9.5 (7.0–12.0) | 9.0 (6.6–11.4) |
| Diabetes and kidney disease | 332 | 31.1 (24.7–37.5) | 23.4 (17.0–29.9) | 23.4 (17.2–29.6) |
| Cardiovascular mortality | ||||
| None | 347 | 3.4 (3.1–3.7) | 0 | 0 |
| Diabetes | 68 | 6.7 (4.2–9.1) | 3.3 (0.7–5.8) | 3.0 (0.3–5.6) |
| Kidney disease | 343 | 9.9 (7.9–11.9) | 6.5 (4.5–8.5) | 6.1 (4.0–8.1) |
| Diabetes and kidney disease | 155 | 19.6 (14.7–24.4) | 16.1 (11.2–21.0) | 16.0 (11.1–20.9) |
| Noncardiovascular mortality | ||||
| None | 663 | 5.7 (5.2–6.3) | 0 | 0 |
| Diabetes | 97 | 7.2 (3.9–10.5) | 1.5 (−2.0 to 4.9) | 1.1 (−2.1 to 4.2) |
| Kidney disease | 404 | 11.7 (9.5–13.9) | 6.0 (3.9–8.1) | 6.0 (4.0–7.9) |
| Diabetes and kidney disease | 174 | 23.2 (16.5–29.9) | 17.5 (10.6–24.3) | 18.1 (11.4–24.8) |
Absolute differences in mortality risk were estimated using linear regression and were adjusted for age, sex, and race (model 1) or additionally adjusted for smoking, BP, and cholesterol (model 2). Standardized 10-year all-cause cumulative incidences were estimated from model 1 for the mean levels of the covariates in the study population. In the two columns to the right, adjusted cumulative incidence of mortality in each of the three other subgroups is compared with that of the no-diabetes, no-kidney disease subgroup. Cause of death was unknown for 1.1% of study participants.
We undertook additional analyses to confirm the robustness of the observed associations between kidney disease and mortality. We observed similar results in individuals who were and were not taking renin-angiotensin system (RAS) inhibitors (Supplemental Table 2), although small numbers of events were observed in some strata. We also evaluated the results after excluding 75 participants with estimated GFR (eGFR) <30 ml/min per 1.73 m2 (0.5% of the study population), and the results were not substantially different. Risk differences comparing participants with and without kidney disease were similar among participants with undiagnosed and diagnosed diabetes (Supplemental Figure 1). In addition, among individuals with type 2 diabetes, additional adjustment for diabetes-related variables (hemoglobin A1c [HbA1C], diabetes duration, and use of glucose-lowering medications) did not significantly alter the absolute difference in mortality comparing those with and without kidney disease (from 18.9% [95% CI, 12.6–25.2] to 17.9% [95% CI, 11.5–24.3]).
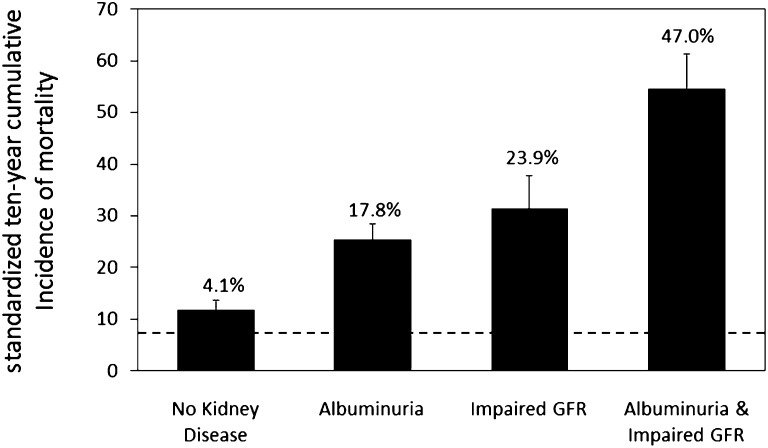
Albuminuria and impaired GFR were each independently associated with increased risks of all-cause mortality in presence or absence of diabetes (Figure 2, Supplemental Table 3). An interaction was detected between albuminuria and GFR among participants without diabetes on the additive (P<0.001) but not the multiplicative scale (P=0.31). This interaction was not statistically significant on either an additive or multiplicative scale in the smaller group with diabetes (P=0.23 and P=0.50, respectively). Associations of impaired GFR with increased mortality risk were observed across the range of albuminuria (Supplemental Figure 2).
Figure 2.
Ten-year mortality in type 2 diabetes by kidney disease manifestation. Absolute differences in mortality risk were estimated using linear regression and were adjusted for age, sex, and race. Standardized 10-year all-cause cumulative incidences were estimated for the mean levels of the covariates in the study population. The dashed line indicates mortality in persons without diabetes or kidney disease (the reference group). The numbers above bars indicate excess mortality above the reference group. Error bars indicate 95% CIs.
Discussion
Type 2 diabetes was associated with substantially increased risk of all-cause and cardiovascular mortality in the US population, as expected. However, this excess risk was concentrated in the subgroup of people with diabetes and kidney disease, manifest as albuminuria, impaired GFR, or both. Kidney disease was common among people with diabetes and associated with substantially increased mortality risk. Absent kidney disease, diabetes was not associated with a large increase in risk of mortality.
The robust association of kidney disease with mortality in persons with or without diabetes has been observed in previous studies.4–10,15 This study extends this work by examining how kidney disease influences the association of diabetes with mortality. Risk differences were evaluated on the absolute scale, which is particularly relevant to clinical care and public health because it reflects the marginal risk to individuals and populations, taking into account the baseline risk among unexposed members of the population.16–18 Using this approach, we observed an additive interaction between diabetes and kidney disease such that the coexistence of kidney disease and diabetes was associated with a considerably larger excess mortality than the sum of excess risks associated with either risk factor alone. Such an interaction was not observed when evaluated on the relative scale, which has been applied in prior studies.7,19 Our results highlight people with both type 2 diabetes and kidney disease as a population at particularly high risk of adverse health outcomes.
Mortality rates in the absence of kidney disease have been studied in type 1 diabetes11,12,20,21 and a small cohort of individuals with type 2 diabetes.22 In contrast to these studies, which used indirect comparison to population standards, this study compared mortality in individuals with diabetes and no kidney disease directly with mortality in a group of individuals with no diabetes or kidney disease from the same population, using identical measures for assessment and adjusting for major confounders. Surprisingly, in absence of kidney disease, type 2 diabetes was not associated with a large increase in mortality risk. These results suggest that persons with diabetes are heterogeneous with respect to their risk of all-cause and cardiovascular mortality, and that kidney disease powerfully identifies a subset of people with increased health risk.
As observed in prior studies, albuminuria and impaired GFR were independent risk factors for death.4,5,7,9,10,23 The observation that impaired GFR alone is associated with a high mortality risk among persons with diabetes is important because this manifestation of diabetic kidney disease in particular is increasing over time.14 In addition, there was an interaction between albuminuria and impaired GFR such that their combination was associated with a greater difference in mortality than the sum of their individual effects. This interaction was statistically significant only among the large number of participants without diabetes but did not reach statistical significance in the smaller group of participants with diabetes or when risk was evaluated on the relative scale, as in prior studies.10,24
The major limitation of our study is its observational nature. As such, it is not possible to determine whether kidney disease is causally related to excess mortality risk. Kidney disease may be a noncausal marker of cumulative vascular damage. For example, microalbuminuria is highly correlated with general endothelial dysfunction and may reflect widespread vascular damage beyond kidney injury.25 Alternatively, kidney disease may contribute directly to mortality by promoting cardiovascular risk factors such as hypertension, insulin resistance, oxidative stress, endothelial dysfunction, and inflammation. Either way, the presence of kidney disease robustly identifies a diabetes subpopulation at high risk of death.
Additional limitations of this study include the evaluation of kidney function and albuminuria status at a single point in time, lack of follow-up regarding new diabetes diagnoses or changes in kidney function, potential misclassification in determination of cause of death based on International Classification of Diseases codes, and the relatively low prevalence of RAS inhibitor use. Of these, single-point evaluation of kidney function and albuminuria status and lack of follow-up information would likely reduce observed differences in mortality due to nondifferential misclassification. Use of RAS antagonists has become more common since NHANES III,14 but subgroup analyses demonstrating similar associations of diabetes and kidney disease with mortality with or without use of these medications suggest that results are applicable to current medical care. It should be noted that our data do not address the effect of RAS antagonists on mortality, rather their effect on the association between kidney disease and mortality. Study strengths include wide external validity of the data, large sample size, and number of events, uniform assessment of kidney function and diabetes, comparison with population-internal controls, and evaluation of associations and interactions on the clinically relevant additive scale.
This study supports a renewed focus on the prevention and treatment of kidney disease in diabetes. Most importantly, the subgroup with kidney disease appears to carry most of the excess all-cause and cardiovascular mortality risk of type 2 diabetes. Therefore, we suggest that people with both diabetes and kidney disease be targeted for therapeutic interventions designed to reduce cardiovascular disease and mortality.
Concise Methods
Study Population
The NHANES is a population-based program of studies conducted by the National Center for Health Statistics of the US Centers for Disease Control and Prevention. NHANES combines data from interviews, physical examinations, and laboratory assays on collected blood and urine specimens to assess the health and nutritional status of civilian, noninstitutionalized children and adults in the United States. Elderly and ethnic minorities (non-Hispanic blacks and Mexican Americans) are oversampled to enable a more detailed assessment of these groups. NHANES III was conducted between 1988 and 1994 and provides nationally representative data for 33,994 individuals aged ≥2 months. This study uses data from NHANES III participants aged ≥20 years, who participated in a health examination and had available data on medications used, serum creatinine, and urine albumin and creatinine concentrations. Of these, we included only participants who had follow-up mortality data through 2006 (15,046 of 15,762 of NHANES III participants, 95.5%). All NHANES protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics and all participants signed written informed consent forms.
Diabetes Definition
Diabetes was defined as use of glucose-lowering medicines and/or HbA1C ≥6.5%, as per recent updates to diagnostic criteria for type 2 diabetes.14,26,27 HbA1C was used to detect diabetes in NHANES participants without necessitating fasting glucose measurements. HbA1C was measured using high-pressure liquid chromatography and standardized to the Diabetes Control and Complications Trials laboratory.28 We excluded participants with probable type 1 diabetes, defined as diabetes diagnosis before age 30 years, first insulin use within 2 years of diagnosis, and current insulin use. Diabetes duration was self-reported by questionnaire.29
Kidney Disease Definition
Kidney disease was defined as albuminuria, impaired GFR, or both. Albuminuria was defined as a urine albumin/creatinine ratio ≥30 mg/g.26,30 In NHANES, urine albumin and creatinine concentrations were measured in one random urine sample. Urine albumin was measured using a solid-phase fluorescent immunoassay, with intra-assay and inter-assay coefficients of variation <8%.31 Serum creatinine was measured using the kinetic Jaffe rate method.28 The NHANES III serum creatinine values were calibrated for use in the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation28 and the eGFR was calculated from the calibrated serum creatinine using the CKD-EPI equation.32 Impaired GFR was defined as a GFR ≤60 ml/min per 1.73 m2.
Other Clinical Characteristics
Age, sex, and race/ethnicity were obtained from questionnaire responses.29 Medicine use during the month before the NHANES physical examination was ascertained by in-person interviews. Body mass index was calculated as weight in kilograms divided by height in meters squared. BP was determined by the average of ≥3 consecutive measurements, separated by 30 seconds and after 5 minutes of rest. Total and HDL cholesterol were measured using a series of enzymatic reactions whose final product was measured using a calorimetric assay.33 Non-HDL cholesterol was evaluated because LDL cholesterol was not available in 56% of individuals (e.g., due to nonfasting status or triglyceride levels >400 mg/dl).28
Outcomes
The main outcomes were death from any cause and death from cardiovascular causes within 10 years of survey participation. These are binary outcomes that allow estimation of risk differences in the US population. Mortality data through December 31, 2006 were obtained by linkage to the National Death Index records using probabilistic matching,34 which was 98.5% complete. National Death Index records provide information on the date and underlying cause of death. Causes of death and definition of cardiovascular International Classification of Diseases codes are described in the Supplemental Material. There was no censoring for 10-year all-cause mortality. However, cause of death was unknown for 1.1% of the study population. Therefore, data on cause-specific mortality were available for 98.9% of this population.
Statistical Analyses
Raw cumulative incidences were estimated by taking the mean of the event indicators. Ten-year cumulative mortality was standardized to the age, sex, and race/ethnicity distribution of the study population. Standardized cumulative incidences were estimated by entering the average covariate values of the study population into the regression model with each possible value of diabetes and kidney disease in order to estimate the 10-year risk at the four possible levels of kidney disease and diabetes for a population with the covariates of the average US adults. Absolute differences in mortality risk were estimated using linear regression. Valid inference was obtained using a Taylor series linearization with adjustment for the sampling weights.35 Linear regression models were adjusted for categorical age (in 10-year categories), sex, and race/ethnicity; additionally adjusted for systolic and diastolic BP (continuous), use of BP medications (dichotomous), non-HDL cholesterol (continuous), and use of lipid-lowering medications (dichotomous); and, among persons with diabetes only, additionally adjusted for duration of diabetes (categories), use of glucose-lowering medications (categories), and HbA1C (continuous). Estimated measures of association were similar to those obtained from a time-to-event analysis using Cox proportional hazards regression.
All statistical analyses were performed using the Survey package36 in the R statistical software.37 The algorithms implement the same theory as the SUDAAN software.38 The analyses incorporated recommended sampling weights provided by the National Center for Health Statistics.29 These weights account for the differential probability of selection and nonresponse and allow for estimation and inference in the civilian, noninstitutionalized US population.35
Disclosures
None.
Supplementary Material
Acknowledgments
This manuscript was made possible by support from Grants 5K23DK089017-02, 1RO1DK087726, and 1R01DK088762 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and Grants 5R01HL070938-06 and 1R01HL096875 from the National Heart, Lung, and Blood Institute (NHLBI).
The funding organizations had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data or preparation, review, and approval of the manuscript. The findings and interpretations presented in this manuscript are solely the responsibility of the authors and do not necessarily represent the official viewpoints of the NIDDK, NHLBI, or the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070718/-/DCSupplemental.
References
- 1.World Health Organization: Diabetes Fact Sheet, 2012. Available at: http://www.who.int/mediacentre/factsheets/fs312/en/index.html Accessed September 12, 2012
- 2.Shaw JE, Sicree RA, Zimmet PZ: Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4–14, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Emerging Risk Factors Collaboration : Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364: 829–841, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Boer IH, Katz R, Cao JJ, Fried LF, Kestenbaum B, Mukamal K, Rifkin DE, Sarnak MJ, Shlipak MG, Siscovick DS: Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care 32: 1833–1838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J, ADVANCE Collaborative Group : Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szczech LA, Best PJ, Crowley E, Brooks MM, Berger PB, Bittner V, Gersh BJ, Jones R, Califf RM, Ting HH, Whitlow PJ, Detre KM, Holmes D, Bypass Angioplasty Revascularization Investigation (BARI) Investigators : Outcomes of patients with chronic renal insufficiency in the bypass angioplasty revascularization investigation. Circulation 105: 2253–2258, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J: Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 167: 1226–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bello AK, Hemmelgarn B, Lloyd A, James MT, Manns BJ, Klarenbach S, Tonelli M, Alberta Kidney Disease Network : Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol 6: 1418–1426, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M, Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groop PH, Thomas MC, Moran JL, Wadèn J, Thorn LM, Mäkinen VP, Rosengård-Bärlund M, Saraheimo M, Hietala K, Heikkilä O, Forsblom C, FinnDiane Study Group : The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58: 1651–1658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orchard TJ, Secrest AM, Miller RG, Costacou T: In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: A report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 53: 2312–2319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Centers for Disease Control and Prevention: 2011 National Diabetes Fact Sheet, 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed August 12, 2011
- 14.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J: Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305: 2532–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA: Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc 1: 8–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman KJ, Greenland S, Walker AM: Concepts of interaction. Am J Epidemiol 112: 467–470, 1980 [DOI] [PubMed] [Google Scholar]
- 17.Blot WJ, Day NE: Synergism and interaction: Are they equivalent? Am J Epidemiol 110: 99–100, 1979 [DOI] [PubMed] [Google Scholar]
- 18.Saracci R: Interaction and synergism. Am J Epidemiol 112: 465–466, 1980 [DOI] [PubMed] [Google Scholar]
- 19.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium: Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borch-Johnsen K, Andersen PK, Deckert T: The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 28: 590–596, 1985 [DOI] [PubMed] [Google Scholar]
- 21.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH: Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 313: 779–784, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutgers HL, Gerrits EG, Sluiter WJ, Ubink-Veltmaat LJ, Landman GW, Links TP, Gans RO, Smit AJ, Bilo HJ: Life expectancy in a large cohort of type 2 diabetes patients treated in primary care (ZODIAC-10). PLoS ONE 4: e6817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A: Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32: 219–226, 1989 [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association : Standards of medical care in diabetes—2011. Diabetes Care 34[Suppl 1]: S11–S61, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Expert Committee : International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32: 1327–1334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Centers for Disease Control and Prevention, National Center for Health Statistics: National Health and Nutrition Examination Survey III (1988–1994): Laboratory File Documentation, 2006. Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/nhanes/nhanes3/1A/lab-acc.pdf Accessed August 12, 2011
- 29.US Centers for Disease Control and Prevention, National Center for Health Statistics: National Health and Nutrition Examination Survey III (1988–1994): Household Adult Data File Documentation, 1996. Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/nhanes/nhanes3/1A/ADULT-acc.pdf Accessed August 12, 2011
- 30.KDOQI : KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 49[Suppl 2]: S12–S154, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Chavers BM, Simonson J, Michael AF: A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int 25: 576–578, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachorik P, Kwiterovich P: The measurement of plasma cholesterol, low density lipoprotein- and high density lipoprotein cholesterol. In: Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual, edited by Hommes F, New York, Wiley-Liss Inc., 1991, pp 425–429 [Google Scholar]
- 34.US Centers for Disease Control and Prevention, National Center for Health Statistics: National Health and Nutrition Examination Survey III (1988–1994): Linked mortality file. 2009. (Accessed August 12, 2011, at http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm)
- 35.National Center for Health Statistics: Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988–1994), Hyattsville, MD, US Centers for Disease Control and Prevention, 1996 [Google Scholar]
- 36.CRAN Package Repository: Survey: analysis of complex survey samples, 2011. Available at: http://CRAN.R-project.org/package=survey Accessed June 2011
- 37.R Development Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria, R Foundation for Statistical Computing, 2008
- 38.Lumley T: Complex Surveys: A Guide to Analysis Using R, Hoboken, NJ, Wiley, 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.