Abstract
The difficulty in accessing mammalian nephrons in vivo hinders the study of podocyte biology. The Drosophila nephrocyte shares remarkable similarities to the glomerular podocyte, but the lack of a functional readout for nephrocytes makes it challenging to study this model of the podocyte, which could potentially harness the power of Drosophila genetics. Here, we present a functional analysis of nephrocytes and establish an in vivo system to screen for renal genes. We found that nephrocytes efficiently take up secreted fluorescent protein, and therefore, we generated a transgenic line carrying secreted fluorescent protein and combined it with a nephrocyte-specific driver for targeted gene knockdown, allowing the identification of genes required for nephrocyte function. To validate this system, we examined the effects of knocking down sns and duf, the Drosophila homologs of nephrin and Neph1, respectively, in pericardial nephrocytes. Knockdown of sns or duf completely abolished the accumulation of the fluorescent protein in pericardial nephrocytes. Examining the ultrastructure revealed that the formation of the nephrocyte diaphragm and lacunar structure, which is essential for protein uptake, requires sns. Our preliminary genetic screen also identified Mec2, which encodes the homolog of mammalian Podocin. Taken together, these data suggest that the Drosophila pericardial nephrocyte is a useful in vivo model to help identify genes involved in podocyte biology and facilitate the discovery of renal disease genes.
The nephron is the basic functional and structural unit of the kidney, with the glomerulus controlling ultrafiltration and the renal tubule responsible for reabsorption and secretion. The glomerular podocyte forms the main filtration barrier by sending out interdigitating foot processes that are separated by ∼40-nm-wide slit pores created by the slit diaphragm.1 Proteins passing the filtration barrier are reabsorbed by the renal proximal tubule through receptor-mediated endocytosis.2 Genetic defects affecting podocyte ultrafiltration lead to proteinuria and kidney failure.3,4 Significant progress has been made to identify genes involved in kidney function and diseases in the last decade.5–9 However, the low accessibility of mammalian nephrons in vivo has hindered our understanding of the genetic network controlling podocyte function. The genetic intractability of the mammalian systems also makes it difficult to identify genes required for podocyte function.
The Drosophila excretory system is composed of pericardial nephrocytes, garland nephrocytes, and malpighian tubules.10 Because Drosophila has an open circulatory system, the fly heart can also be viewed as the sole blood vessel. Pericardial nephrocytes are strategically positioned around the three pairs of ostia (the inflow tracts of the fly heart). The hemolymph is filtered by the pericardial nephrocytes before it enters the heart tube and pumped out at the anterior open end of the aorta (Figure 1A).11 The Drosophila nephrocyte has a highly specialized filtration structure called the nephrocyte diaphragm, which shares remarkable similarities to the slit diaphragm structure of the mammalian glomerular podocytes.12,13 The nephrocyte foot processes created by extensive infolding of the plasma membrane generate ∼30-nm slit pores in Drosophila nephrocytes compared with the 30- to 50-nm slit pores created by podocyte foot processes in mammalian glomerulus. These nephrocyte diaphragms and the basement membrane covering the entire nephrocyte together form a size- and charge-selective filtration barrier to filter Drosophila hemolymph.
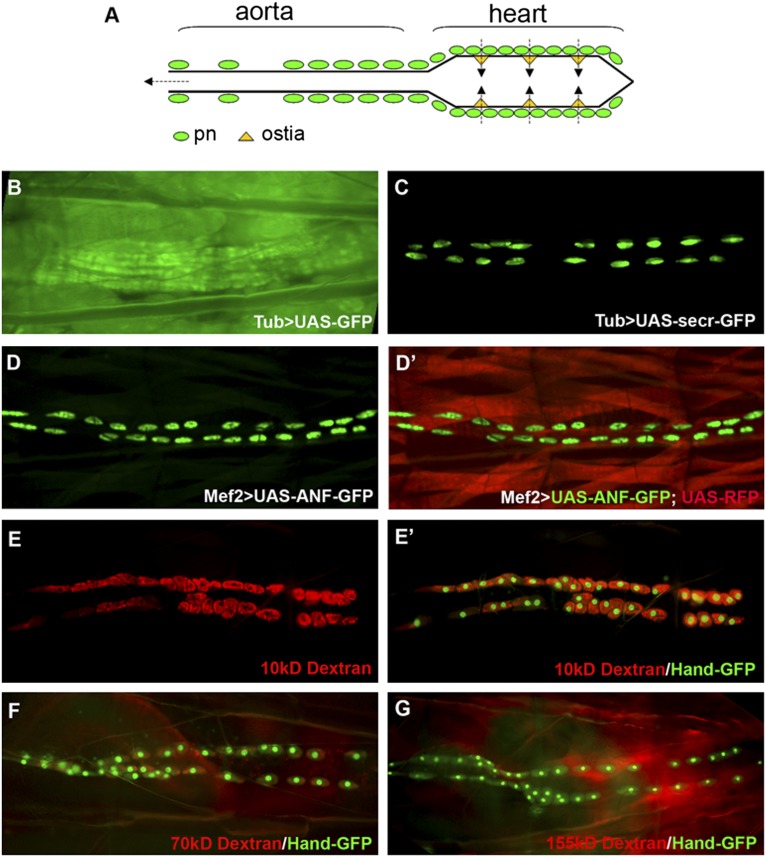
Figure 1.
Drosophila pericardial nephrocytes uptake secreted proteins. (A) Schematic drawing of Drosophila heart. pn, pericardial nephrocyte. (B) Larva with Tub-Gal4 and UAS-GFP showed ubiquitous expression of GFP in all tissues. (C) Larvae with Tub-Gal4 and UAS-secr-GFP showed GFP accumulation specifically in nephrocytes. (D and D′) Embryos with Mef2-Gal4 and UAS-ANF-GFP together with UAS-RFP showed specific accumulation of ANF-GFP in pericardial nephrocytes (green) and RFP in muscles (red). (E–G) Larva with Hand-GFP transgene (green) injected with (E and E′) 10-, (F) 70-, or (G) 155-kD fluorescence-labeled Dextran (red) showed size-dependent Dextran uptake, with (E and E′) high-efficient uptake of 10-kD Dextran, (F) less-efficient uptake of 70-kD Dextran, and (G) no uptake of 155-kD Dextran.
The remarkable similarities of the filtration structure shared by Drosophila nephrocytes and mammalian podocytes suggest that these two cell types are evolutionarily related, making Drosophila a potentially ideal model system to study podocyte biology in vivo. The powerful genetic tools and tremendous genetic resources of Drosophila make it an ideal model for large-scale genetic screen to identify and study novel genes that are involved in podocyte function and renal diseases.14,15 However, the lack of a reliable functional readout for nephrocytes made it difficult to utilize the power of Drosophila genetics. Here, we present a novel functional analysis for Drosophila nephrocytes and use it to develop a genetic screen system to identify genes required for nephrocyte function. We provide evidence to support the notion that this reporter system can be used as a direct readout for nephrocyte filtration function and that the Drosophila pericardial nephrocyte is an ideal model to facilitate the identification and study of podocyte-related renal disease genes.
In our attempt to analyze some secretion pathway mutants that affect Drosophila heart development, we observed that nephrocytes can uptake secreted green fluorescent protein (GFP) efficiently (Figure 1, B–D). The Gal4-UAS system is a versatile genetic tool in Drosophila. The system has two parts: the Gal4 gene, encoding the yeast transcription activator protein GAL4, and the UAS (Upstream Activation Sequence), where Gal4 specifically binds to activate gene transcription.16 When a fly carrying Tubulin (Tub)-Gal4 is crossed to a fly carrying UAS-GFP, the GFP reporter gene will be expressed in the pattern driven by the Tub-Gal4, which is ubiquitous in all tissues (Figure 1B). However, when we overexpressed GFP fused with the wingless secretion peptide (UAS-secr-GFP) with the same ubiquitous Tub-Gal4, the secreted GFP was only observed in the nephrocyte (Figure 1, C compared with B), suggesting that nephrocytes have the ability to collect secreted GFP from circulating hemolymph. To further verify this remarkable function of nephrocytes, we used a muscle-specific driver called Mef2-Gal4, which is not expressed in nephrocytes, to overexpress GFP fused with a different secretion peptide called rat atrial natriuretic factor (ANF). In the same larva, we overexpressed a nonsecreted red fluorescent protein (RFP) to show where the Mef2-Gal4 is activated. We found that the secreted ANF-GFP could only be found in nephrocytes (Figure 1D), whereas the nonsecreted RFP labels all the myocardial and somatic muscle cells in which the Mef2-Gal4 activates both UAS-RFP and UAS-ANF-GFP (Figure 1D′). We also tested six different Gal4 drivers to overexpress secreted GFP in various tissues and discovered that, regardless of the tissues in which the secreted GFP was originally expressed, it was always accumulated in nephrocytes (data not shown), providing a reliable and robust functional readout for nephrocytes.
We also verified that pericardial nephrocytes could uptake dyes of different sizes, but the efficiency is dependent on the size. When 10-kD fluorescent-labeled Dextran was injected into Drosophila larvae, this small-size dye was accumulated by pericardial nephrocyte very efficiently within a few hours (Figure 1E). Hand-GFP transgene, which is strongly expressed in cardioblasts and pericardial nephrocytes,17–19 was used to label pericardial nephrocytes (Figure 1E′). The efficiency of dye uptake decreased with larger size; 24 hours after injection, only a low level of 70-kD Dextran accumulation was observed in the pericardial nephrocytes (Figure 1F), and extra-large 155-kD Dextran was not found in pericardial nephrocytes (Figure 1G). These data suggested that one of the major functions of pericardial nephrocytes is to filter the hemolymph and uptake proteins and large molecules, with a size-dependent efficiency.
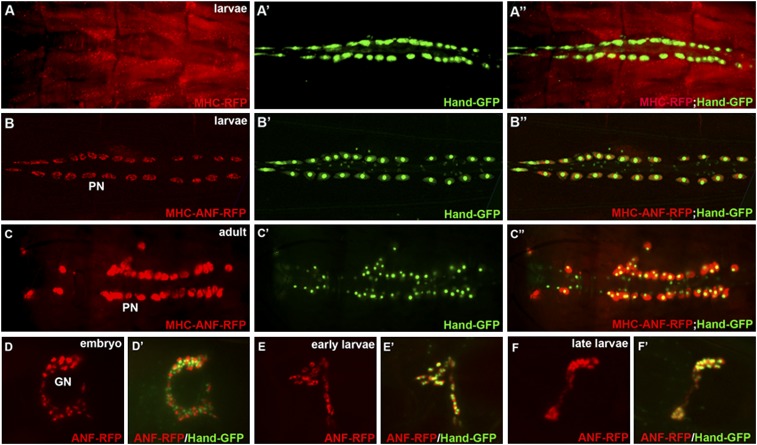
To develop a genetic screen for nephrocyte function, we needed to generate a single transgene to visualize fluorescent protein uptake in the nephrocyte. We used myosin heavy chain (MHC) enhancer to drive an RFP reporter gene fused with the ANF secretion peptide (MHC-ANF-RFP) or without the ANF (MHC-RFP) as a control. The control MHC-RFP transgene was specifically expressed in myocardial and somatic muscle cells (Figure 2, A–A″, red), whereas the secreted MHC-ANF-RFP driven by the same MHC enhancer was only found in pericardial nephrocytes and garland nephrocytes, the two different types of nephrocytes in Drosophila. (Figure 2, B–B″, red). The accumulation of ANF-RFP is remarkably strong in newly hatched adult flies (Figure 2, C–C″). We also observed ANF-RFP accumulation in garland nephrocytes at embryonic (Figure 2, D and D′) and larval stage (Figure 2, E–F′) but not in the adult stage, indicating that garland nephrocytes function like the mammalian embryonic kidney at early developing stages, and pericardial nephrocytes are the major filtration cell type in the adult stage of Drosophila.
Figure 2.
Generation and characterization of the MHC-ANF-RFP transgenic reporter as a nephrocyte functional readout. (A–A″) The control MHC-RFP reporter is expressed in the muscles, reflecting the pattern of the MHC enhancer. (B–B″) The MHC-ANF-RFP reporter can only be observed in nephrocytes. (C–C″) The MHC-ANF-RFP reporter can only be observed in pericardial nephrocytes in adult flies. (D–F) The MHC-ANF-RFP reporter can also be found in garland nephrocytes (GNs) in (E and E′) late embryonic stage and (E and E′) early and (F and F′) late larval stages. Hand-GFP (green) was used to label both pericardial and garland nephrocytes.
In Drosophila, a UAS-based transgenic RNA interference (RNAi) library has been established,20 with more than 30,000 RNAi transgenic lines covering the entire genome. Each UAS-RNAi transgenic line carries specific hairpin against a particular gene, allowing tissue-specific gene knockdown for every single gene in the Drosophila genome.20 To specifically knock down a particular gene in pericardial nephrocytes, we combined the functional readout transgene MHC-ANF-RFP and the pericardial cell marker Hand-GFP with the Dot-Gal4 driver21 (Figure 3A), which drives specific expression in pericardial nephrocytes (Figure 3B). By crossing the fly line carrying MHC-ANF-RFP, Hand-GFP, and Dot-Gal4 to thousands of UAS-RNAi lines, we are able to screen thousands of genes for their specific functional requirement in pericardial nephrocytes (Figure 3A). This genetic screen allows efficient scanning of the entire genome for genes required for pericardial nephrocyte function.
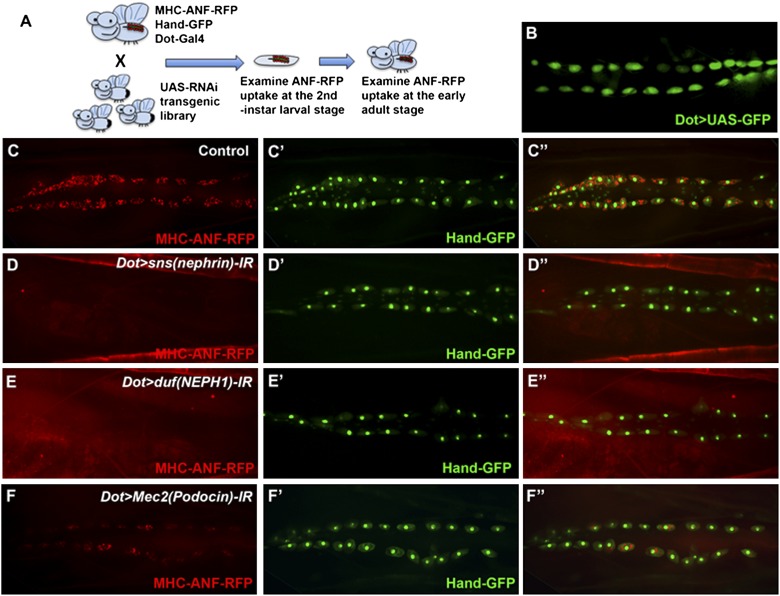
Figure 3.
Establishing and validating a genetic screen system for genes required for Drosophila nephrocyte function. (A) Schematic drawing of the genetic screen system for genes required for nephrocyte function. (B) Dot-Gal4, which drives pericardial nephrocyte-specific expression, was used to specifically knock down gene expression in pericardial nephrocytes. (C and C′) The fly line carrying MHC-ANF-RFP, Hand-GFP, and Dot-Gal4. The MHC-ANF-RFP reporter (red) is accumulated in pericardial nephrocytes overlapping with Hand-GFP (green). (D–F) Pericardial nephrocyte-specific RNAi knockdown of (D) sns, (E) duf, or (F) Mec2 completely blocked or dramatically reduced the MHC-ANF-RFP reporter uptake in pericardial nephrocytes. Hand-GFP (green) was used to label pericardial nephrocytes when ANF-RFP uptake is blocked or reduced.
Genes encoding slit diaphragm components have been shown to be highly conserved from Drosophila to humans and required for nephrocyte function.12,13 The molecular cores of the slit diaphragm structure are nephrin/Neph-1 in mammals, and their corresponding fly orthologs are Sticks and stones (Sns)/Dumbfounded (Duf). Genetic defects in nephrin are responsible for the human congenital nephrotic syndrome of the Finnish type, leading to heavy proteinuria and kidney failure.5,22 To validate that our in vivo functional analysis system can be used to identify essential renal genes, we first examined the ANF-RFP uptake in pericardial nephrocytes with sns or duf knockdown. We found that RNAi knockdown of sns or duf using Dot-Gal4 completely abolished ANF-RFP protein accumulation in pericardial nephrocytes (Figure 3, C–E″). The absence of ANF-RFP accumulation could result from defects in filtration at either the filtration barrier or the endocytic process at the lacunae structure. Transmission electron microscopy (TEM) analysis of the ultrastructure of pericardial nephrocytes in sns knockdown larvae revealed that nephrocyte diaphragms and lacunar channel structures were dramatically disrupted compared to control pericardial nephrocytes (Figure 4). These results validated our RNAi screening system and suggested that this nephrocyte functional reporter system can be used to identify genes required for nephrocyte function and structure.
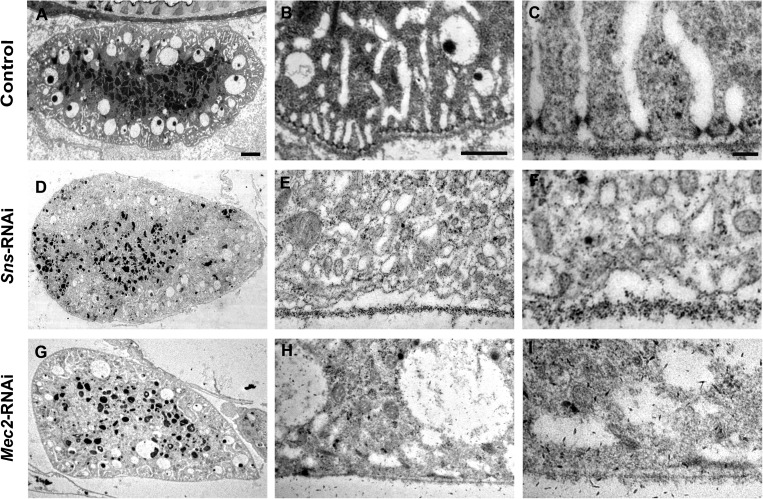
Figure 4.
Ultra-structure analysis of pericardial nephrocytes with sns or Mec2 RNAi. (A–C) Ultra-structure of pericardial nephrocytes from Dot-Gal4/MHC-ANF-RFP/Hand-GFP larvae as a wild-type control. (D–F) sns RNAi knock down led to (D) reduced numbers of endocytic vesicles, (E) shortened lacuna structures, and (F) abolished nephrocyte diaphragms. (G–I) Mec2 RNAi knock down in nephrocytes led to similar defects. Scale bars, 2 μm in A, D, and G; 500 nm in B, E, and H; 100 nm in C, F, and I.
We performed a preliminary screen of 1,000 genes in the Drosophila genome and identified over 70 genes required for nephrocyte function (Supplemental Table 1). All of these positive hits identified from the nephrocyte functional screen have highly conserved human homologs. Among them, Mec2 encodes the Drosophila homologs of Podocin, another essential slit diaphragm component that has been directly linked to renal disease.5,6,23,24 Pericardial nephrocyte-specific knockdown of Mec2 led to significant reduction of ANF-RFP uptake (Figure 3F). Although the overall morphology of pericardial nephrocytes appeared normal (Figure 3F′) from the Hand-GFP reporter when Mec2 was knocked down, the TEM analysis showed ultrastructure defects similar to the defects of Sns knockdown (Figure 4, D–F). Mec2 RNAi in nephrocytes also led to reduced numbers of vacuoles and lysosomes (Figure 4G), shortened lacuna channels (Figure 4H), and disrupted nephrocyte diaphragm (Figure 4I), suggesting that Mec2 functions together with Sns in maintaining nephrocyte diaphragm, the primary filtration structure in pericardial nephrocytes.
We also identified other Drosophila genes with human homologs that have been linked to renal diseases, including two novel Drosophila genes CG11592 and CG32702, which encode two renal disease genes Amnionless and Cubilin,25,26 respectively. Together with the slit diaphragm genes, these results strongly suggested that Drosophila nephrocytes can be modeled to study known nephrotic syndrome-associated genes and identify novel genes potentially involved in kidney diseases.
Many of the genes that we identified from the screen suggested the common roles of these genes and genetic pathways in the renal function of both Drosophila and mammals. For example, we identified the small GTPases Rac1 and Cdc42 from our screen. These small GTPases have been suggested to play essential roles in podocyte function and are involved in glomerular diseases.27–30 We also identified many genes involved in actin cytoskeleton (Supplemental Table 1) that are known to be essential for podocyte function as well.31 Additional genes identified from our screen, such as the endocytosis genes, signaling pathway genes, vesicle trafficking genes, and motor genes, suggested the involvement of new genetic pathways in renal function and podocyte biology. Detailed analysis of these genes will be performed using the powerful genetic tools provided in Drosophila. Because this list was generated from our initial round of genetic screen for about 1000 genes, we estimate that about 7% of the genes in the genome are required for nephrocyte function. The high efficiency of this screen makes it possible to scan the entire genome in the near future to identify all the genes involved in nephrocyte function in Drosophila. Such knowledge will greatly facilitate our understanding of the genetic network involved in renal function and renal disease.
Concise Methods
Fly Strains
Flies were reared on standard food at room temperature or 29°C for RNAi screening. The following strains were used in this study: w1118, Hand-GFP, Dot-Gal4, actin-Gal4, tubulin-Gal4, Dmef2-Gal4; a large collection of UAS-RNAi transgenic lines were acquired from the Bloomington Drosophila stock center and the Vienna RNAi center.
Generation of MHC-ANF-RFP and MHC-RFP Transgenic Lines
To generate MHC-RFP construct, a 2.5-kb MHC promoter region32 was amplified from w1118 genomic DNA and then inserted into pRed-H-Pelican vector. To generate the MHC-ANF-RFP construct, the full-length rat ANF cDNA was amplified from the genomic DNA of the UAS-ANF-GFP transgenic fly and inserted into pRed-H-Pelican in frame with RFP. The 2.5-kb MHC promoter region was then inserted before the basal promoter upstream of the ANF-RFP fusion protein. These constructs were introduced into flies by standard P element-mediated germ-line transformation.
In Vivo Nephrocyte Filtration Assay
AlexaFluo568-Dextran (10 kD, 0.05 mg/ml), TexasRed-Dextran (70 kD, 0.5 μg/ml; Molecular Probes), and TRIC-Dextran (155 kD, 5 mg/ml; Sigma) were injected into the Hand-GFP transgenic flies at the third-instar larval stage. Injected larvae were placed in grape juice agar plates with yeast paste and examined under fluorescent microscopes for Dextran uptake in pericardial nephrocytes.
EM
TEM was carried out using standard procedures. Briefly, third-instar larvae were injected with 2.5% glutaraldehyde and 4% paraformaldehyde fixation solution for initial fixation of 4 hours followed by cutting tips and overnight regular fixation. The larvae were then stained for 2 hours in 3% uranyl acetate and dehydrated through a series of concentration gradients of ethanol and propylene oxide. Samples were embedded in Epon resin and polymerized for 24 hours at 60°C. The samples were then ultrathin-sectioned (70 nm), mounted on copper grids (FCF-2010-Cu; Electron Microscopy Sciences), and stained with uranyl acetate and lead citrate. The sections were observed with a Philips CM100 electron microscope (FEI, Hillsboro, OR) at 60 kV.
RNAi-Based Nephrocyte Functional Screen Procedure
Virgins from the MHC-ANF-RFP, Hand-GFP, and Dot-Gal4 transgenic lines were crossed to males from over 1000 different UAS-RNAi transgenic lines at 25°C; 2 days after crossing, flies were transferred to small collection cages with grape juice agar plates to collect the embryos for 24 hours at 25°C. Collected embryos were aged for 48 hours at 29°C and then subjected to examination of the RFP accumulation in pericardial nephrocytes under fluorescent microscopes. The RFP uptakes were examined again in newly hatched adults from the original vials that were kept at 25°C.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the Bloomington stock center and the Vienna Drosophila RNAi center for Drosophila stocks. We thank the Microscopy and Image Analysis Laboratory at the University of Michigan for technical support and assistance in the transmission electron microscopy analysis. We thank Judith Connett for editing assistance.
Z.H. was supported by National Institutes of Health Grant R01HL090801 and American Heart Association Grant AHA-0630178N.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Drosophila Nephrocyte: Back on Stage,” on pages 161–163.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012080769/-/DCSupplemental.
References
- 1.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Christensen EI, Verroust PJ, Nielsen R: Receptor-mediated endocytosis in renal proximal tubule. Pflugers Arch 458: 1039–1048, 2009 [DOI] [PubMed] [Google Scholar]
- 3.D’Agati VD: The spectrum of focal segmental glomerulosclerosis: New insights. Curr Opin Nephrol Hypertens 17: 271–281, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt F: Genetic kidney diseases. Lancet 375: 1287–1295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR: Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denholm B, Skaer H: Bringing together components of the fly renal system. Curr Opin Genet Dev 19: 526–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choma MA, Suter MJ, Vakoc BJ, Bouma BE, Tearney GJ: Physiological homology between Drosophila melanogaster and vertebrate cardiovascular systems. Dis Model Mech 4: 411–420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, Ruiz-Gómez M, Skaer H, Denholm B: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM: Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136: 2335–2344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cagan RL: The Drosophila nephrocyte. Curr Opin Nephrol Hypertens 20: 409–415, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Simons M, Huber TB: Flying podocytes. Kidney Int 75: 455–457, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Brand AH, Perrimon N: Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Yi P, Han Z, Li X, Olson EN: The mevalonate pathway controls heart formation in Drosophila by isoprenylation of Ggamma1. Science 313: 1301–1303, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Han Z, Olson EN: Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 132: 3525–3536, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Han Z, Yi P, Li X, Olson EN: Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development 133: 1175–1182, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ: A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kimbrell DA, Hice C, Bolduc C, Kleinhesselink K, Beckingham K: The Dorothy enhancer has Tinman binding sites and drives hopscotch-induced tumor formation. Genesis 34: 23–28, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Tryggvason K: Unraveling the mechanisms of glomerular ultrafiltration: Nephrin, a key component of the slit diaphragm. J Am Soc Nephrol 10: 2440–2445, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Frishberg Y, Rinat C, Megged O, Shapira E, Feinstein S, Raas-Rothschild A: Mutations in NPHS2 encoding podocin are a prevalent cause of steroid-resistant nephrotic syndrome among Israeli-Arab children. J Am Soc Nephrol 13: 400–405, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS: Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest 112: 209–221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlstedt-Fröberg V, Pettersson T, Aminoff M, Dugué B, Gräsbeck R: Proteinuria in cubilin-deficient patients with selective vitamin B12 malabsorption. Pediatr Nephrol 18: 417–421, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Storm T, Emma F, Verroust PJ, Hertz JM, Nielsen R, Christensen EI: A patient with cubilin deficiency. N Engl J Med 364: 89–91, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, Pawson T: Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol 23: 1149–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, Antignac C, Pollak M, Kopp JB, Winn MP, Shaw AS: Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest 121: 4127–4137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attias O, Jiang R, Aoudjit L, Kawachi H, Takano T: Rac1 contributes to actin organization in glomerular podocytes. Nephron, Exp Nephrol 114: e93–e106, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T: Modification of mineralocorticoid receptor function by Rac1 GTPase: Implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Hess NK, Singer PA, Trinh K, Nikkhoy M, Bernstein SI: Transcriptional regulation of the Drosophila melanogaster muscle myosin heavy-chain gene. Gene Expr Patterns 7: 413–422, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.