Abstract
The insect nephrocyte and the mammalian glomerular podocyte are similar with regard to filtration, but it remains unclear whether there is an organ or cell type in flies that reabsorbs proteins. Here, we show that the Drosophila nephrocyte has molecular, structural, and functional similarities to the renal proximal tubule cell. We screened for genes required for nephrocyte function and identified two Drosophila genes encoding orthologs of mammalian cubilin and amnionless (AMN), two major receptors for protein reabsorption in the proximal tubule. In Drosophila, expression of dCubilin and dAMN is specific to nephrocytes, where they function as co-receptors for protein uptake. Targeted expression of human AMN in Drosophila nephrocytes was sufficient to rescue defective protein uptake induced by dAMN knockdown, suggesting evolutionary conservation of Cubilin/AMN co-receptors function from flies to humans. Furthermore, we found that Cubilin/AMN-mediated protein reabsorption is required for the maintenance of nephrocyte ultrastructure and fly survival under conditions of toxic stress. In conclusion, the insect nephrocyte combines filtration with protein reabsorption, using evolutionarily conserved genes and subcellular structures, suggesting that it can serve as a simplified model for both podocytes and the renal proximal tubule.
The nephron is the basic functional and structural unit of the kidney, with the glomerulus controlling ultrafiltration and the renal tubule supporting reabsorption and secretion. The glomerular podocyte forms the main filtration barrier by sending out interdigitating foot processes that are separated by ∼40-nm-wide slit pores created by the slit diaphragm.1 Proteins passing the filtration barriers are reabsorbed by the renal proximal tubule through receptor-mediated endocytosis.2 Genetic defects affecting either podocyte ultrafiltration or renal proximal tubule reabsorption lead to proteinuria and kidney failure.1–3 Significant progress has been made to identify genes involved in kidney function and diseases in the last decade.4 However, our understanding of the genetic network required for kidney function has been hindered by the relative intractability of characteristics of mammalian nephron structure and function in vivo. The emergence of simpler and more genetically tractable models for the renal system, such as Drosophila5,6 and zebrafish,7,8 provide new insights and opportunities to facilitate our understanding of renal biology.
The Drosophila excretory system is composed of nephrocytes and malpighian tubules.5,9 There are two different types of nephrocytes in Drosophila, the pericardial nephrocytes that flank the heart tube and the garland nephrocytes that are near the proventriculus. Both pericardial and garland nephrocytes have highly specialized filtration structures called nephrocyte diaphragms, which share remarkable similarities to the slit diaphragm structure of the mammalian glomerular podocytes.5,10 Because Drosophila has an open circulatory system, the fly heart functions as both a pump and a major blood vessel. Pericardial nephrocytes are strategically positioned around the three pairs of ostia (the inflow tracts of fly heart); therefore, the hemolymph gets filtered by the pericardial nephrocytes before it enters the heart tube and is pumped out at the anterior end of the heart.
The remarkable similarities of the filtration structure shared by Drosophila nephrocytes and mammalian podocytes suggest that these two cell types are evolutionarily related. However, nephrocytes are stand-alone cells that are spacially separated from the malpighian tubules, whereas the glomerulus and the renal tubule are integral parts of the nephron. How the structure and function of the nephrocyte are related to the structure and function of the mammalian nephron remains unclear. It is also unknown whether insects possess protein reabsorption machinery similar to mammals.
Our previous study showed that Drosophila nephrocytes can uptake secreted fluorescent proteins from hemolymph, which can be used as a reliable functional readout for nephrocytes in vivo. Based on this functional readout, we designed a genetic screen system for genes required for nephrocyte function (F. Zhang et al., submitted data). Here, we present the identification of two novel Drosophila genes that are essential for nephrocyte functions. These two genes encode Drosophila homologs of mammalian Cubilin and Amnionless (AMN), two major receptors for protein reabsorption in renal proximal tubules.
In mammals, Cubilin was first identified as the intrinsic factor–vitamin B12 receptor11 and then as an albumin binding protein that is important for renal proximal tubule protein reabsorption.12 Cubilin is also involved in the renal clearance of hemoglobin.13 AMN was identified to form a functional receptor complex with cubilin14 and is required for endocytic function of Cubilin in renal proximal tubules.15 AMN interacts with the epidermal growth factor (EGF) domains of cubilin and is responsible for membrane attachment and export of the complex from the endoplasmic reticulum.16,17 Conditional knockout of Cubilin in mouse renal proximal tubules led to dramatically reduced protein uptake by proximal tubules and proteinuria.18 Proteinuria was also observed in patients with Gräsbeck–Imerslund syndrome, which is caused by genetic mutations of Cubilin,19 and a patient disease mutation was mapped to a single amino acid change in Cubilin.20
In this study, we found that the Drosophila Cubilin and AMN are specifically expressed in nephrocytes and function as co-receptors for protein uptake, similar to their roles in the mammalian proximal tubules. Furthermore, we showed that targeted expression of human AMN in Drosophila nephrocytes is sufficient to rescue the protein uptake defect caused by Drosophila AMN (dAMN) RNA interference (RNAi) knockdown, suggesting that function of these protein reabsorption receptors is evolutionarily conserved from flies to humans. Using transmission electron microscopy (TEM), we showed that Drosophila Cubilin (dCubilin) and dAMN are also essential for the maintenance of nephrocyte ultrastructure. We also showed that one of the major physiologic roles of protein reabsorption in Drosophila is to remove toxins from the hemolymph and that lack of protein reabsorption affects fly survival under toxic conditions. Our discoveries suggest that the insect nephrocyte combines filtration with protein reabsorption using evolutionarily conserved genes and subcellular structures and that it can serve as a simplified model for both podocytes and renal proximal tubules.
Results
Identification of Two Novel Drosophila Genes (CG32702 and CG11592) Essential for the Function of Pericardial Nephrocytes
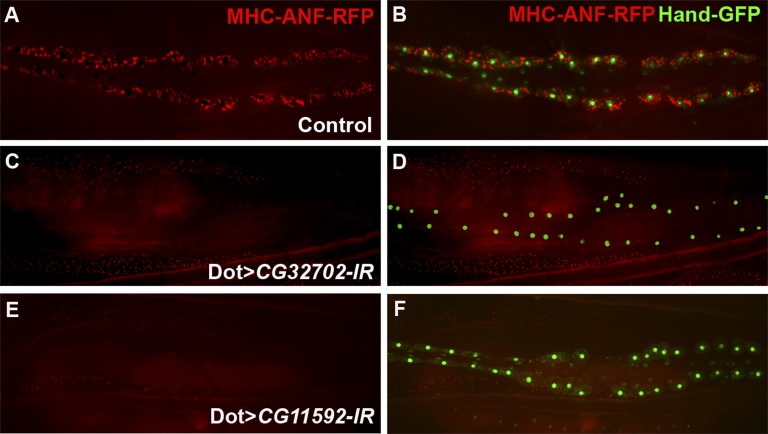
In our previous studies, we developed a tissue-specific RNAi screen to identify genes required for nephrocyte function in vivo (F. Zhang et al., submitted data). The transgenic fly line myosin heavy chain (MHC) -atrium natriuretic factor (ANF) -red fluorescent protein (RFP) expresses RFPs fused with rat ANF in the muscles driven by the MHC promoter. The secreted ANF-RFP fusion protein can be efficiently internalized into pericardial nephrocytes, which are labeled by Hand-green fluorescent protein (GFP)21 in nucleus (Figure 1, A and B). By combining the functional readout (MHC-ANF-RFP) of nephrocytes with a pericardial nephrocyte-specific driver Dot-Gal4, we can screen the upstream activation sequence (UAS) -RNAi library22 for genes that are required for nephrocyte function efficiently. From our initial phase of unbiased RNAi genetic screen, we identified about 70 genes required for nephrocyte function in vivo from ∼1000 RNAi lines targeting different genes in the Drosophila genome (F. Zhang et al., submitted data). Among them, pericardial nephrocyte-specific RNAi knockdown of two novel Drosophila genes, CG32702 and CG11592, completely blocked ANF-RFP uptake in pericardial nephrocytes (Figure 1, C–F) without affecting the overall morphology and survival of the nephrocytes (based on the intact nucleus in adult flies that are labeled by Hand-GFP).
Figure 1.
Two novel Drosophila genes, CG32702 and CG11592, are required for nephrocyte function. (A and B) Secreted ANF-RFP (red) is accumulated in pericardial nephrocytes labeled with Hand-GFP (green). (C–F) Pericardial nephrocyte-specific RNAi knockdown of (C and D) CG32702 or (E and F) CG11592 completely blocked the accumulation of ANF-RFP (red), but the survival of pericardial nephrocytes was not affected based on Hand-GFP (green) expression in the nephrocytes.
CG32702 and CG11592 Encode Drosophila Orthologs of Cubilin and Amn
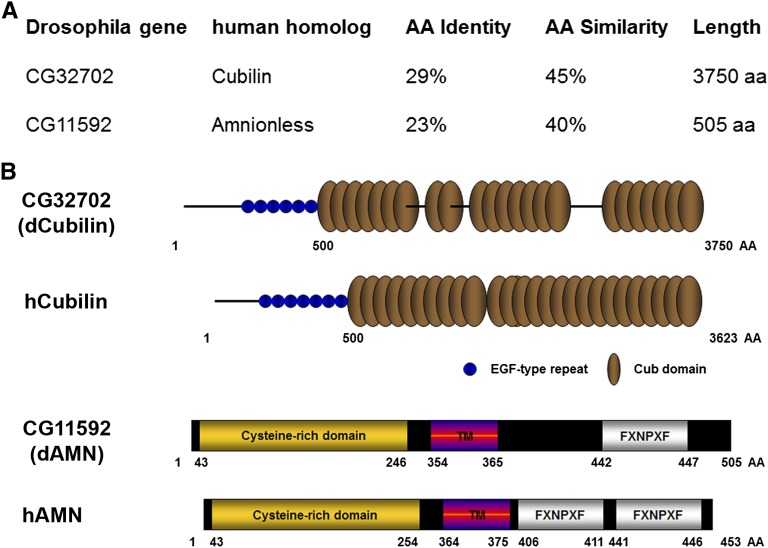
Protein amino acid sequence analysis showed that CG32702 and CG11592 encode fly orthologs of human Cubilin and AMN, respectively (Figure 2A). CG11592 encodes a 505-amino acid polypeptide and shares 40% similarity with human AMN. CG32702 encodes a 3750-amino acid polypeptide and shares 45% similarity with human Cubilin protein. dCubilin (CG32702) contains 6 EGF-type repeats and 26 Complement C1r/C1s, Uegf and Bone morphogenic protein-1 (CUB) domains compared with human Cubilin, which has 7 EGF-type repeats and 27 CUB domains (Figure 2B). dAMN (CG11592), like its human ortholog AMN,16 contains an N-terminal cysteine-rich domain, a transmembrane domain, and a C-terminal FXNPXF internalization signal domain (Figure 2B).
Figure 2.
CG32702 and CG11592 encode Drosophila orthologs of human Cubilin and Amn, respectively. (A) Amino acid sequence analysis of Drosophila CG32702 and CG11592 showing their identity and similarity to human Cubilin and AMN, respectively. (B) Protein domain structure analysis of Drosophila CG32702 and CG11592. TM, transmembrane domain.
dCubilin and dAMN Are Specifically Expressed in Nephrocytes
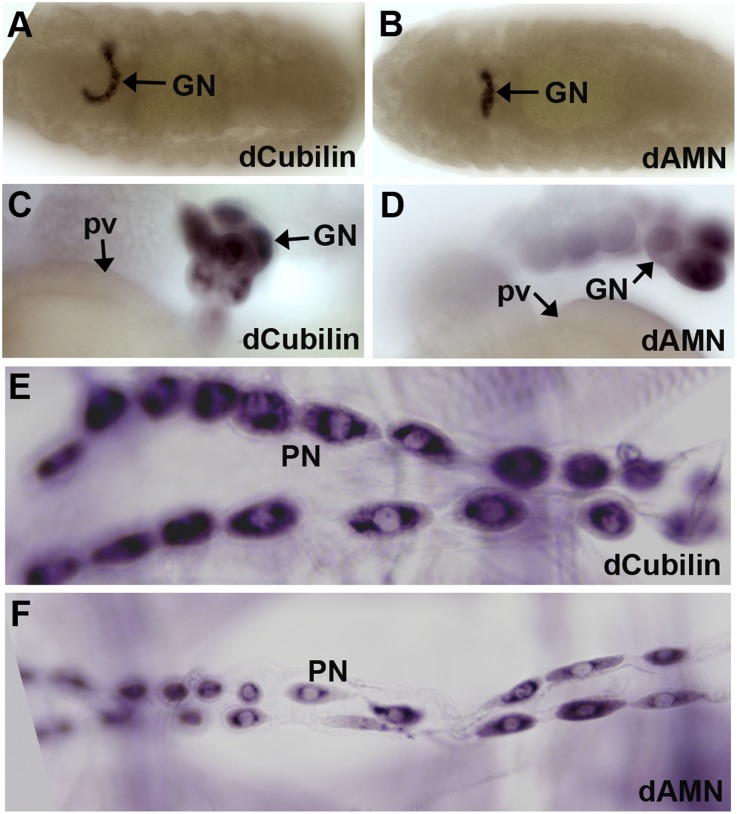
To address whether the gene products of dCubilin and dAMN are required specifically in nephrocytes, we examined the expression pattern of dCubilin and dAMN. In situ hybridization showed that these two genes are specifically expressed in Drosophila nephrocytes (Figure 3). At embryonic stage, dCubilin and dAMN are only expressed in the garland nephrocytes (Figure 3, A and B). At larval stage, dCubilin and dAMN are highly expressed in both garland and pericardial nephrocytes (Figure 3, C–F). Garland nephrocytes start to uptake ANF-RFP at the embryonic stage, whereas the pericardial nephrocytes start to uptake ANF-RFP from the larval stage (F. Zhang et al., submitted data). The expression of dCubilin and dAMN seems to reflect the functional maturation of these two types of nephrocytes. Because Cubilin and AMN are highly expressed in mammalian renal proximal tubules, it is reasonable to speculate that Drosophila nephrocytes and the mammalian renal proximal tubules are evolutionarily related.
Figure 3.
Drosophila Cubilin and AMN are specifically expressed in nephrocytes. (A and B) In situ hybridization showed that dCubilin and dAMN are specifically expressed in garland nephrocytes (GNs) at embryonic stage. (C–F) dCubilin and dAMN are specifically expressed in (C and D) GNs and (E and F) pericardial nephrocytes (PNs) at larval stage. pv, proventriculus.
Drosophila Cubilin and AMN Function Together as a Receptor Complex for Protein Uptake in Nephrocytes
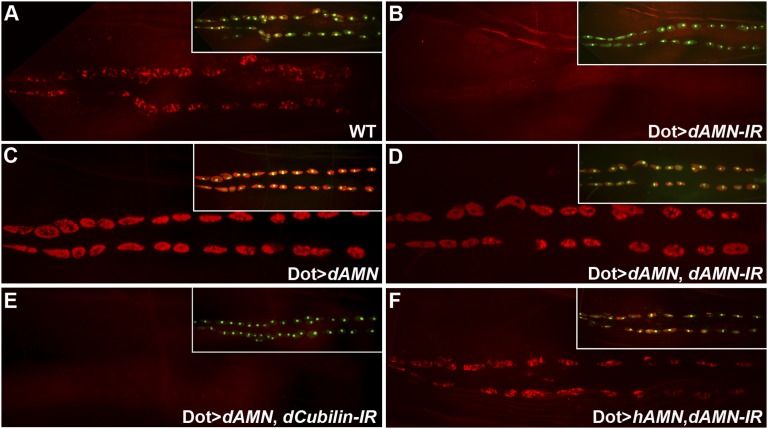
Cubilin and AMN are two major receptors for receptor-mediated protein reabsorption in the renal proximal tubules. Cubilin binds to ligands directly through its CUB repeat domains. AMN does not bind to ligands directly but is required for the apical membrane localization of Cubilin.17,23 To test whether dCubilin and dAMN act in a similar way as in human renal proximal tubule, we examined the genetic interaction between dCubilin and dAMN. Targeted overexpression of dAMN in nephrocytes led to increased ANF-RFP uptake (Figure 4, A–C). Because dAMN should not bind ligands directly based on its domain structure and the studies of mammalian AMN, it is likely that the increased amount of dAMN in nephrocytes leads to more efficient accumulation of dCubilin on the nephrocyte membrane, which leads to increased protein uptake. Supporting this assumption, we found that targeted overexpression of dAMN in nephrocytes was sufficient to rescue the defect of ANF-RFP uptake in dAMN RNAi knockdown larvae (Figure 4D), but it could not rescue the protein uptake defect in dCubilin RNAi knockdown larvae (Figure 4E), suggesting that dAMN and dCubilin, like their mammalian homologs, function together as a receptor complex.
Figure 4.
Drosophila Cubilin and AMN function together as a receptor complex for protein uptake in nephrocytes. (A) Pericardial nephrocytes of wild-type (WT) larvae efficiently uptake ANF-RFP. (B) Pericardial nephrocyte-specific RNAi knockdown of dAMN completely blocked ANF-RFP uptake. (C) Overexpression of dAMN in pericardial nephrocytes increased ANF-RFP uptake. (D and E) Overexpression of dAMN in pericardial nephrocytes (D) could rescue the blocked ANF-RFP uptake caused by dAMN RNAi knockdown but (E) could not rescue defect caused by dCubilin RNAi knockdown. (F) Overexpression of human AMN in pericardial nephrocytes rescued the ANF-RFP uptake defect caused by dAMN RNAi knockdown. Insets show combined Hand-GFP (green) and ANF-RFP (red) for the same larva as depicted in each panel.
Human AMN Can Functionally Substitute Drosophila dAMN in Pericardial Nephrocytes
Because Drosophila dAMN shares remarkable homology with human AMN (hAMN), we tested whether hAMN could functionally substitute dAMN in Drosophila nephrocytes. Using the same Dot-Gal4 that drives pericardial nephrocyte-specific expression, we overexpressed hAMN cDNA in pericardial nephrocytes with dAMN RNAi knockdown. The blocked ANF-RFP uptake caused by Drosophila dAMN RNAi knockdown was rescued by hAMN (Figure 4F), suggesting that the Cubilin/AMN receptor complex is evolutionarily conserved from flies to humans and that Drosophila nephrocytes and vertebrate renal proximal tubules use the same receptor complex for protein reabsorption.
Cubilin/AMN-Mediated Protein Reabsorption in Nephrocytes Is Required for Toxin Removal
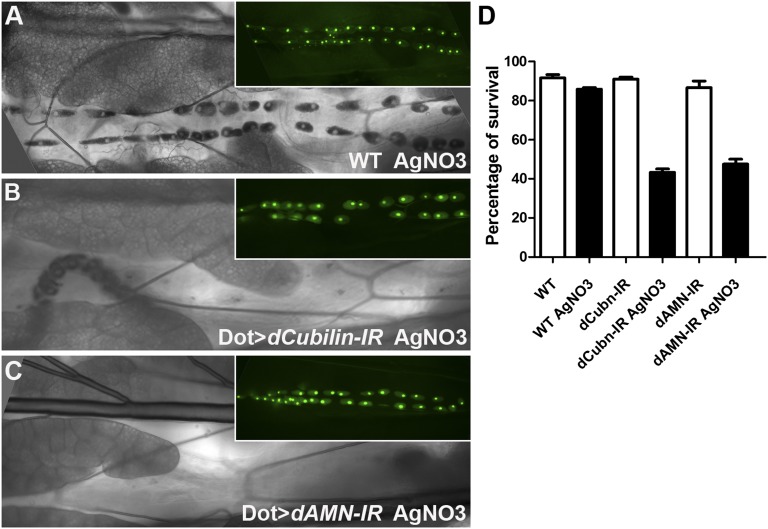
Nephrocyte-specific knockdown of dCubilin or dAMN does not cause lethality during any developmental stages, and adult flies appear normal. Because nephrocytes can remove toxins, such as silver nitrate (AgNO3), from the hemolymph,5 we tested whether the toxin-removal function of nephrocytes is dependent on Cubilin/AMN-mediated protein reabsorption by feeding larvae silver nitrate. We found that silver nitrate accumulates significantly in the Drosophila pericardial nephrocytes (Figure 5A), and the nephrocyte-specific RNAi knockdown of dCubilin or dAMN completely blocked silver nitrate uptake (Figure 5, B and C), indicating that Cubilin/AMN-mediated protein uptake is essential for the toxin removal function of nephrocytes.
Figure 5.
Cubilin/AMN-mediated protein uptake in nephrocytes is required for toxin resistance. (A) Silver nitrate (AgNO3) is efficiently collected by pericardial nephrocytes in wild-type (WT) Drosophila larvae. (B and C) Pericardial nephrocyte-specific RNAi knockdown of (B) dCubilin or (C) dAMN completely blocked silver nitrate uptake. Insets in A–C show Hand-GFP (green) for the same larva as depicted in each panel. (D) Toxin treatment did not affect wild-type fly survivability but significantly reduced the survivability of flies with pericardial nephrocyte-specific knockdown of dCubilin or dAMN.
We also observed that, although knockdown of dCubilin or dAMN did not cause significant changes in viability under normal conditions (Figure 5D), the viability of these flies was reduced dramatically under toxin stress conditions (Figure 5D). These data suggest that dCubilin and dAMN are required to remove toxins from hemolymph, which is essential for animal survival in hostile environments.
Cubilin/AMN-Mediated Protein Reabsorption Is Required for Maintaining Nephrocyte Subcellular Structures
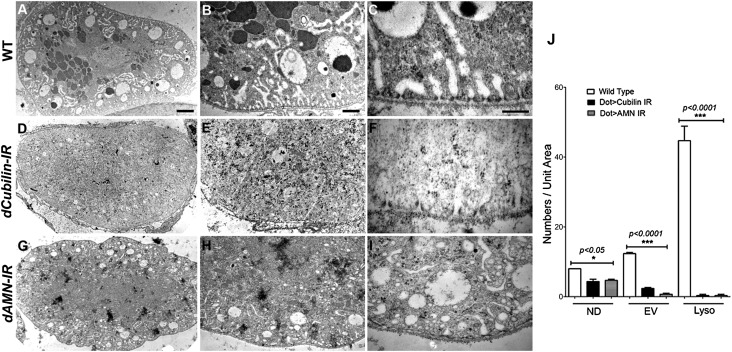
To investigate the roles of dCubilin and dAMN in nephrocyte function in greater details, we examined the ultrastructure of pericardial nephrocytes from dCubilin or dAMN RNAi knockdown larvae. In wild-type pericardial nephrocytes, three distinct layers can be found: the filtration layer containing the basement membrane and the nephrocyte diaphragms, the lacunar channel network mixed with clear endocytic vacuoles, and a layer containing lots of electron-dense lysosomes (Figure 6, A–C). In dCubilin or dAMN RNAi knockdown pericardial nephrocytes, the numbers of endocytic vesicles and vacuoles were dramatically reduced, whereas electron-dense lysosomes were nearly absent (Figure 6, D, E, G, and H). Nephrocyte diaphragms were present, but their numbers were reduced (Figure 6, F and I, red arrowheads). Lacunar structures were also dramatically shortened (Figure 6, F and I, red asterisks). These phenotypic changes were quantified and summarized (Figure 6J). The results suggest that blocking the Cubilin/AMN-mediated protein reabsorption abolished a large part of endocytic trafficking in nephrocytes, leading to dramatic reduction of endocytic vesicles, vacuoles, and lysosomes and affecting the maintenance of the lacunar structures that rely on dynamic endocytic cycling.
Figure 6.
Cubilin/AMN-mediated protein uptake is essential for maintaining subcellular structures of Drosophila nephrocytes. (A–C) The wild-type (WT) Drosophila nephrocytes show distinct layers of (A and B) lacuna network and endocytic vacuoles and lysosomes. (C) Nephrocyte diaphragms and lacunae densely populate the nephrocyte surface. (D–F) Pericardial nephrocytes with dCubilin RNAi knockdown have (D and E) fewer endocytic vesicles and vacuoles and almost no electron-dense lysosomes. (F) The number of nephrocyte diaphragms is reduced, and the lacunae become much shorter. (G–I) dAMN RNAi knockdown also reduced the number of (G and H) endocytic vacuoles and electron-dense lysosomes, with (I) a reduced number of nephrocyte diaphragms and shorter lacunae. There is no dramatic change of basement membrane. Scale bars, 4 μm in A, D, and G; 1 μm in B, E, and H; 200 nm in C, F, and I. (J) Quantification of the ultrastructure changes in nephrocytes with dCubilin or dAMN RNAi. ND, nephrocyte diaphragm; EV, endocytic vacuole; Lyso, lysosomes.
Discussion
In this study, we show that the Drosophila nephrocyte shares remarkable molecular, structural, and functional similarities with the mammalian renal proximal tubule. Molecularly, the Drosophila nephrocyte uses Cubilin and AMN, the two major receptors for protein reabsorption in the renal proximal tubule, to uptake proteins from hemolymph. Structurally, the lacuna structure of nephrocytes resembles the brush border structure in renal proximal tubules for generating enormous membrane surface for reabsorption. Functionally, both the nephrocyte and the renal proximal tubule have high endocytic activity, with large numbers of endosomes, lysosomes, and endocytic vacuoles. Given the remarkable similarity between Drosophila nephrocytes and mammalian podocytes, our findings suggest that the Drosophila nephrocyte combines the molecular, structural, and functional features of both the podocyte and the renal proximal tubule cell.
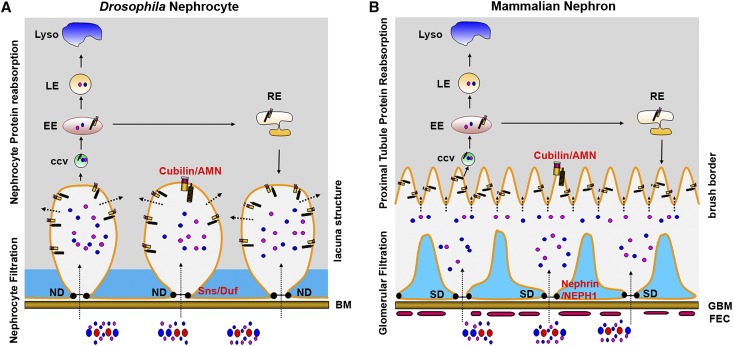
To better compare the similarities between Drosophila neophytes and the mammalian nephron, we made models to show the essential molecules and subcellular structures in these two different renal systems for filtration and protein reabsorption (Figure 7). Both the mammalian blood and Drosophila hemolymph contain proteins and particles with different sizes. In mammals, these proteins and particles are filtered through the three-layer filtration system, and they enter the space between the glomerulus and the renal proximal tubule. In Drosophila, these proteins and particles are filtered through the nephrocyte basement membrane and the nephrocyte diaphragm, and then, they enter the space of the lacuna network. The lacuna network, similar to the brush border structure of the proximal tubule cells, generates a large membrane surface for Cubilin/AMN-mediated protein uptake. After ligand proteins bind to the Cubilin/AMN receptor, the receptor/ligand complex enters the cell through clatherin-mediated endocytosis (F. Zhang et al., unpublished data). The endocytosed receptor/ligand complex is sorted in early endosomes to be processed to either late endosomes and then lysosomes for protein degradation or recycling endosomes for membrane protein recycling. Therefore, the Drosophila nephrocyte performs the filtration and protein reabsorption function using conserved genes and subcellular structures that are similar to those genes and structure used by the mammalian glomerular podocyte and renal proximal tubules (Figure 7).
Figure 7.
The Drosophila nephrocyte combines podocyte filtration with renal proximal tubule protein reabsorption using conserved molecules and similar subcellular structures. Essential molecules and subcellular structures involved in filtration and protein reabsorption in (A) Drosophila nephrocytes and (B) the mammalian glomerulus and renal proximal tubules are shown. Ultrafiltration is indicated by long dotted arrows, and protein reabsorption is shown by short dotted arrows. BM, basement membrane; ND, nephrocyte diaphragm; CCV, clatherin-coated vesicles; EE, early endosome; LE, late endosome; RE, recycling endosome; FEC, fenestrated endothelium cell; GBM, glomerular basement membrane; SD, slit diaphragm.
Our study is the first to show that insects have protein reabsorption ability through their nephrocytes. Although renal proximal tubule protein reabsorption is undoubtedly essential for mammals, it is not expected that animals with the open circulatory system, such as flies, have similar protein reabsorption ability using conserved mechanisms. Using the toxin resistance assay, we showed that protein reabsorption in insects is important, at least for detoxification. It is likely that there are other important roles for protein reabsorption, such as keeping the amino acid balance or removal of unwanted proteins. Our studies also suggest that the molecular basis of toxin removal is the binding of toxins to proteins in the Drosophila hemolymph followed by the Cubilin/AMN-mediated protein uptake in nephrocytes. The large number of lysosomes and vacuoles in nephrocytes facilitates the process and store of toxins to protect other cell types.
Our finding brings new insight for the comparison between invertebrate and vertebrate excretory systems. The nephrocyte was previously considered to perform the filtration function similar to the vertebrate glomerulus, and the Malpighian tubules were believed to execute the reabsorption function similar to the renal tubules. Our finding suggests that the insect nephrocyte functions equivalent to the vertebrate glomerulus and the renal proximal tubule for both filtration and protein reabsorption and that the Malpighian tubules are similar to the loop of Henle and the renal distal tubule, which are mainly used for water and salt reabsorption. Interestingly, a recent study showed that a mouse kidney-derived stem cell can differentiate into either podocytes or renal proximal tubule cells,24 suggesting that podocytes and renal proximal tubule cells are closely related. Our genetic screen system makes it possible to efficiently screen every gene in the fly genome for a comprehensive understanding of the genetic control of filtration and protein reabsorption, the two basic functions of the renal system. The fact that human AMN could functionally substitute the Drosophila AMN in our rescue experiment showed the remarkable evolutionary conservation of the genes involved in renal functions from flies to humans. Therefore, the knowledge gained from Drosophila will bring new insights and opportunities to study the biology of podocytes and proximal tubule cells, the two essential cell types involved in proteinuria, the hallmark of kidney disease.
Concise Methods
Fly Strains
Flies were reared on standard food at 25°C. All UAS-Gal4 crosses were performed at 29°C. Dot-Gal4 was obtained from the Bloomington Drosophila stock center. Transgenic RNAi lines were obtained from the Bloomington Drosophila stock center and the Vienna Drosophila RNAi Center. Generation of MHC-ANF-RFP was described in detail elsewhere (Zhang et al., submitted data). Hand-GFP was used to label nephrocytes at all developmental stages.
RNAi-Based Nephrocyte Functional Screen Procedure
Details of the screen procedures are described in a companion paper (F. Zhang et al., submitted data). Briefly, 10 virgins of pMHC-ANF-RFP, Hand-GFP; Dot-Gal4 flies were crossed with 5 males of UAS-RNAi transgenic line in vials at 25°C; 2 days after crossing, flies were transferred to small collection cages with grape juice agar plates for 24 hours at 25°C. Collected embryos were aged for 48 hours at 29°C, and then, they were subjected to examination of the RFP signal in pericardial nephrocytes under confocal microscope. RFP uptakes were examined again in newly hatched adult progenies from the original vials.
In Situ Hybridization
Whole-mount in situ hybridization to embryos and third-instar nephrocytes was carried out using standard procedures. Briefly, fixed embryos or dissected third-instar larvae were incubated with probes in hybridization buffer at 55°C for 24 hours. The samples were then incubated with anti-Digoxigenin antibody for 2 hours followed by staining.
Generation of the UAS-dAMN and UAS-hAMN Transgenic Lines
To generate UAS-dAMN construct, 1.5-kb full-length cDNA of dAMN (CG11592) was amplified from first-strand cDNA and then inserted into pUAST vector. To generate UAS-hAMN construct, a 1.5-kb hAMN fragment was released from pCDNA3-hAMN (Stephan M .Tanner, Ohio State University) and then inserted into pUAST construct. These constructs were introduced into flies by standard P element-mediated germ-line transformation.
Confocal Imaging and TEM
Fluorescence microscopy was carried out using a Zeiss LSM 510-META Laser Scanning Confocal Microscope. TEM was carried out using standard procedures. Briefly, third-instar wandering larvae of the indicated genotypes were fixed with Sorensen phosphate buffer containing 4% paraformaldehyde and 2.5% glutaraldehyde. The processed samples were analyzed by Philips CM100 TEM.
Quantification of Ultrastructure in TEM Images
Quantification of TEM images was done by counting the number of nephrocyte diaphragms and lacuna channels in a 2500 × 1500-nm rectangular area along the plasma membrane with ×46,000 magnification TEM images. Numbers of lysosomes were counted from ×2000 magnification TEM images containing the section of entire cells. Images from five different nephrocytes of the same genotype were counted, and the results are expressed as mean ± SD. Statistical analyses were performed using GraphPad Prism software. The results are expressed as mean ± SD. Statistical significance was defined as P<0.05.
Toxin Stress Assay
Flies with the appropriate genotype were allowed to lay eggs on standard apple juice plates for 24 hours. After aging for 24 hours, freshly emerging first-instar larvae from the collecting plates were transferred to agar-only plates supplemented with regular yeast paste or yeast paste containing AgNO3 (2.0 g yeast in 3.5 ml 0.005% AgNO3 solution) and allowed to develop at 25°C until adulthood. The percentage of eclosing adults was scored.
Disclosures
None.
Acknowledgments
We thank the Bloomington stock center and the Vienna Drosophila RNAi Center for Drosophila stocks. We thank the Microscopy and Image Analysis Laboratory at the University of Michigan for their technical support in transmission electron microscopy. We want to specially thank Dotty Sorenson and Sasha Meshinchi for their assistance in electron microscopy. We thank Dr. Stephan M. Tanner for sharing human amnionless cDNA. We thank Judith Connett for editing assistance.
Z.H. was supported by National Institutes of Health Grant R01HL090801 and American Heart Association Grant AHA-0630178N.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Drosophila Nephrocyte: Back on Stage,” on pages 161–163.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Christensen EI, Verroust PJ, Nielsen R: Receptor-mediated endocytosis in renal proximal tubule. Pflugers Arch 458: 1039–1048, 2009 [DOI] [PubMed] [Google Scholar]
- 3.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt F: Genetic kidney diseases. Lancet 375: 1287–1295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, Ruiz-Gómez M, Skaer H, Denholm B: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cagan RL: The Drosophila nephrocyte. Curr Opin Nephrol Hypertens 20: 409–415, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Ebarasi L, Oddsson A, Hultenby K, Betsholtz C, Tryggvason K: Zebrafish: A model system for the study of vertebrate renal development, function, and pathophysiology. Curr Opin Nephrol Hypertens 20: 416–424, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Drummond IA: Kidney development and disease in the zebrafish. J Am Soc Nephrol 16: 299–304, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Denholm B, Skaer H: Bringing together components of the fly renal system. Curr Opin Genet Dev 19: 526–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM: Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136: 2335–2344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozyraki R, Fyfe J, Kristiansen M, Gerdes C, Jacobsen C, Cui S, Christensen EI, Aminoff M, de la Chapelle A, Krahe R, Verroust PJ, Moestrup SK: The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat Med 5: 656–661, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow TE, Moestrup SK, Christensen EI: Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest 105: 1353–1361, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, Moestrup SK, Christensen EI: Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol 13: 423–430, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Fyfe JC, Madsen M, Højrup P, Christensen EI, Tanner SM, de la Chapelle A, He Q, Moestrup SK: The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 103: 1573–1579, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Strope S, Rivi R, Metzger T, Manova K, Lacy E: Mouse amnionless, which is required for primitive streak assembly, mediates cell-surface localization and endocytic function of cubilin on visceral endoderm and kidney proximal tubules. Development 131: 4787–4795, 2004 [DOI] [PubMed] [Google Scholar]
- 16.He Q, Madsen M, Kilkenney A, Gregory B, Christensen EI, Vorum H, Højrup P, Schäffer AA, Kirkness EF, Tanner SM, de la Chapelle A, Giger U, Moestrup SK, Fyfe JC: Amnionless function is required for cubilin brush-border expression and intrinsic factor-cobalamin (vitamin B12) absorption in vivo. Blood 106: 1447–1453, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coudroy G, Gburek J, Kozyraki R, Madsen M, Trugnan G, Moestrup SK, Verroust PJ, Maurice M: Contribution of cubilin and amnionless to processing and membrane targeting of cubilin-amnionless complex. J Am Soc Nephrol 16: 2330–2337, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R: Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahlstedt-Fröberg V, Pettersson T, Aminoff M, Dugué B, Gräsbeck R: Proteinuria in cubilin-deficient patients with selective vitamin B12 malabsorption. Pediatr Nephrol 18: 417–421, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Storm T, Emma F, Verroust PJ, Hertz JM, Nielsen R, Christensen EI: A patient with cubilin deficiency. N Engl J Med 364: 89–91, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Han Z, Olson EN: Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development 132: 3525–3536, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ: A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ahuja R, Yammani R, Bauer JA, Kalra S, Seetharam S, Seetharam B: Interactions of cubilin with megalin and the product of the amnionless gene (AMN): Effect on its stability. Biochem J 410: 301–308, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Fuente Mora C, Ranghini E, Bruno S, Bussolati B, Camussi G, Wilm B, Edgar D, Kenny SE, Murray P: Differentiation of podocyte and proximal tubule-like cells from a mouse kidney-derived stem cell line. Stem Cells Dev 21: 296–307, 2012 [DOI] [PubMed] [Google Scholar]