Abstract
The mammalian collecting duct comprises principal and intercalated cells, which maintain sodium/water and acid/base balance, respectively, but the epigenetic contributors to the differentiation of these cell types remain unknown. Here, we investigated whether the histone H3 K79 methyltransferase Dot1l, which is highly expressed in principal cells, participates in this process. Taking advantage of the distribution of aquaporin 2 (Aqp2), which localizes to principal cells of the collecting duct, we developed mice lacking Dot1l in Aqp2-expressing cells (Dot1lAC) and found that these mice had approximately 20% fewer principal cells and 13%–16% more intercalated cells than control mice. This deletion of Dot1l in principal cells abolished histone H3 K79 methylation in these cells, but unexpectedly, most intercalated cells also had undetectable di-methyl K79, suggesting that Aqp2+ cells give rise to intercalated cells. These Aqp2+ cell-derived intercalated cells were present in both developing and mature kidneys. Furthermore, compared with control mice, Dot1lAC mice had 40% higher urine volume and 18% lower urine osmolarity with relatively normal electrolyte and acid-base homeostasis. In conclusion, these data suggest that Dot1l deletion facilitates the differentiation of some α- and β-intercalated cells from Aqp2-expressing progenitor cells or mature principal cells.
The renal collecting duct in the mammalian kidney plays a critical role in the regulation of extracellular volume, osmolarity, and pH. It contains at least three structurally and functionally distinct cell types: principal cells (PCs), α-intercalated cells (α-ICs), and β- intercalated cells (β-ICs).1 PCs regulate Na+ and water homeostasis. α-ICs and β-ICs are involved in acid and bicarbonate secretion, respectively.2,3 Aqp2 is a well accepted PC marker, whereas carbonic anhydrase type II (CAII) is expressed in all ICs.4,5 The proton-pumping V-ATPase is a complex, multisubunit enzyme consisting of at least 13 subunits, including A, B1, and B2. It is highly expressed in the plasma membranes of some epithelial cells, including ICs in the kidney.6 The kidney variant of the band 3 Cl−/HCO3− exchanger (AE1) and the sodium-independent chloride/iodide transporter Pendrin are present in α-IC and β-IC, respectively.7
β-ICs in primary culture behave as stem cells and give rise to α-ICs, PCs, and PC/IC “hybrids,” based on functional characteristics and expression of IC and PC markers. Unlike β-IC, sorted PCs maintain their PC phenotype as terminally differentiated cells.8 Metabolic acidosis reduced β-ICs and increased α-ICs without changing the total IC population, indicating β-IC conversion to α-IC.9 Mice lacking the extracellular matrix protein hensin/DMBT1 in ICs contained no typical α-ICs.10 Inactivation of the forkhead transcription factor Foxi1 resulted in a single cell type (Aqp2+ CAII+) that lacks other IC markers (Pendrin, AE1, AE4, and V-ATPase B1) in the cortex and medulla, raising the possibility that PCs and ICs are differentiated from epithelial precursor cells characterized by Foxi1− Aqp2+ CAII+. 11 Both hesin/DMBT1 and Foxi1 mutant mice develop distal renal tubular acidosis. In CAII-deficient mice, ICs (evaluated by AE1 and H+-ATPase expression) were reduced and replaced by PCs.4 However, whether ICs are derived from PCs or Aqp2+ progenitor cells has not been demonstrated.
Disruptor of telomeric silencing (Dot1) was first cloned in yeast as a gene affecting telomeric silencing.12 Dot1 and its mammalian homologues (Dot1l) encode a methyltransferase specific for histone H3 K79.13–15 Dot1l regulates transcription, development, erythropoiesis, differentiation, proliferation, and leukemogenesis.16–18 Global deletion of Dot1l causes embryonic lethality.19
We previously cloned mouse Dot1l, observed high mRNA expression in mouse kidney and strong H3 dimethyl K79 (m2K79) staining in PC and IC, and identified five alternative splicing variants (Dot1a-e).15,20 Our work also revealed that Dot1a regulates mRNA expression and activity of the epithelial Na+ channel in mouse cortical collecting duct M1 and mouse inner medulla collecting duct line 3 (IMCD3) cells.20–22 However, the function of Dot1l in vivo in mouse kidney has not been directly addressed.
In this study, we used our Aqp2:Cre and newly developed Dot1lf/f mice to generate connecting tube/collecting duct (CNT/CD)–specific Dot1l-deficient (Dot1lf/f Aqp2:Cre or Dot1lAC) mice and then determined whether Dot1l deletion affects PC/IC differentiation. To our knowledge, this study provides for the first time direct in vivo evidence that ICs as defined by standard markers can be derived from Aqp2-expressing cells. Derived ICs are observed in both developing and adult kidneys. Dot1l deletion facilitates this process, leading to an increase in IC/PC ratio and urine volume. This study highlights the plasticity of renal tubular epithelia, the origin of most ICs from Aqp2+ cells, and the significance of Dot1l inactivation in renal physiology and pathology.
Results
Generation of CNT/CD-specific Dot1l Conditional Knockout Mice
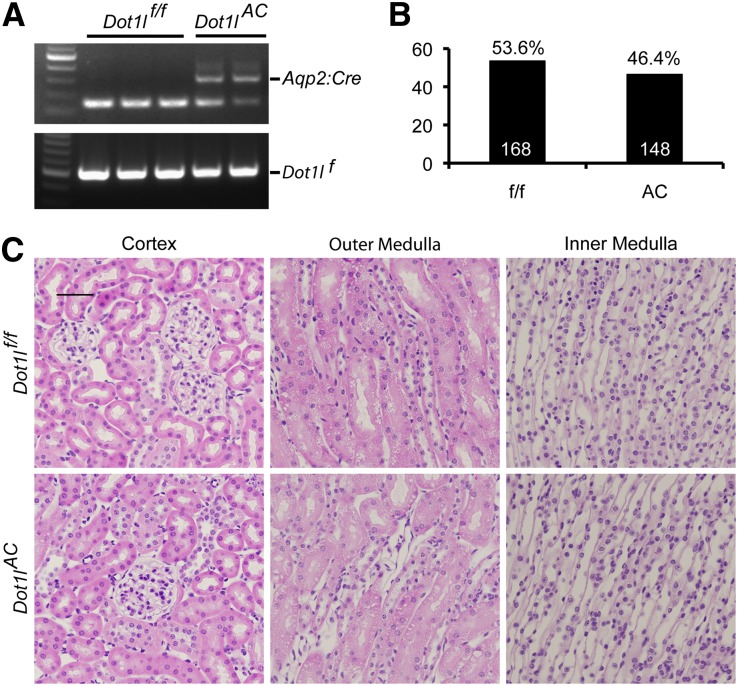
We generated a Dot1l conditional knockout line using the LoxP-Cre system (Dot1lf/f), which removed most Dot1l function, including the methyltransferase activity upon Cre-mediated recombination.16 This line was crossed with Aqp2:Cre mice,23 which express Cre under the control of regulatory elements of the mouse Aqp2 gene (Figure 1A). The resulting Dot1lf/f Aqp2:Cre mice were termed Dot1lAC, which were normal appearing and fertile. We found the expected 1:1 Mendelian distribution in the offspring of Dot1lAC backcrossed with Dot1lf/f (Figure 1B). Histologic analysis revealed no abnormalities in the cortex, outer medulla (OM), and inner medulla (IM) of both Dot1lf/f and Dot1lAC mice (Figure 1C).
Figure 1.
Generation and characterization of Dot1lAC mice. (A) Representative agarose gel images showing PCR-based genotyping of genomic DNA. PCR was conducted with primers to amplify a 150-bp endogenous Aqp2 and 300-bp Aqp2:Cre transgene in the same reaction or with primers to amplify a 510-bp floxed Dot1l allele (Dot1lf). (B) Summary of Dot1lf/f (ff) and Dot1lAC (AC) littermates from the crosses between the two genotypes. (C) Representative images of hematoxylin and eosin staining showing normal kidney histologic features in both Dot1lf/f and Dot1lAC mice.
Dot1l Is Solely Responsible for All Methylation Events at Histone H3 K79
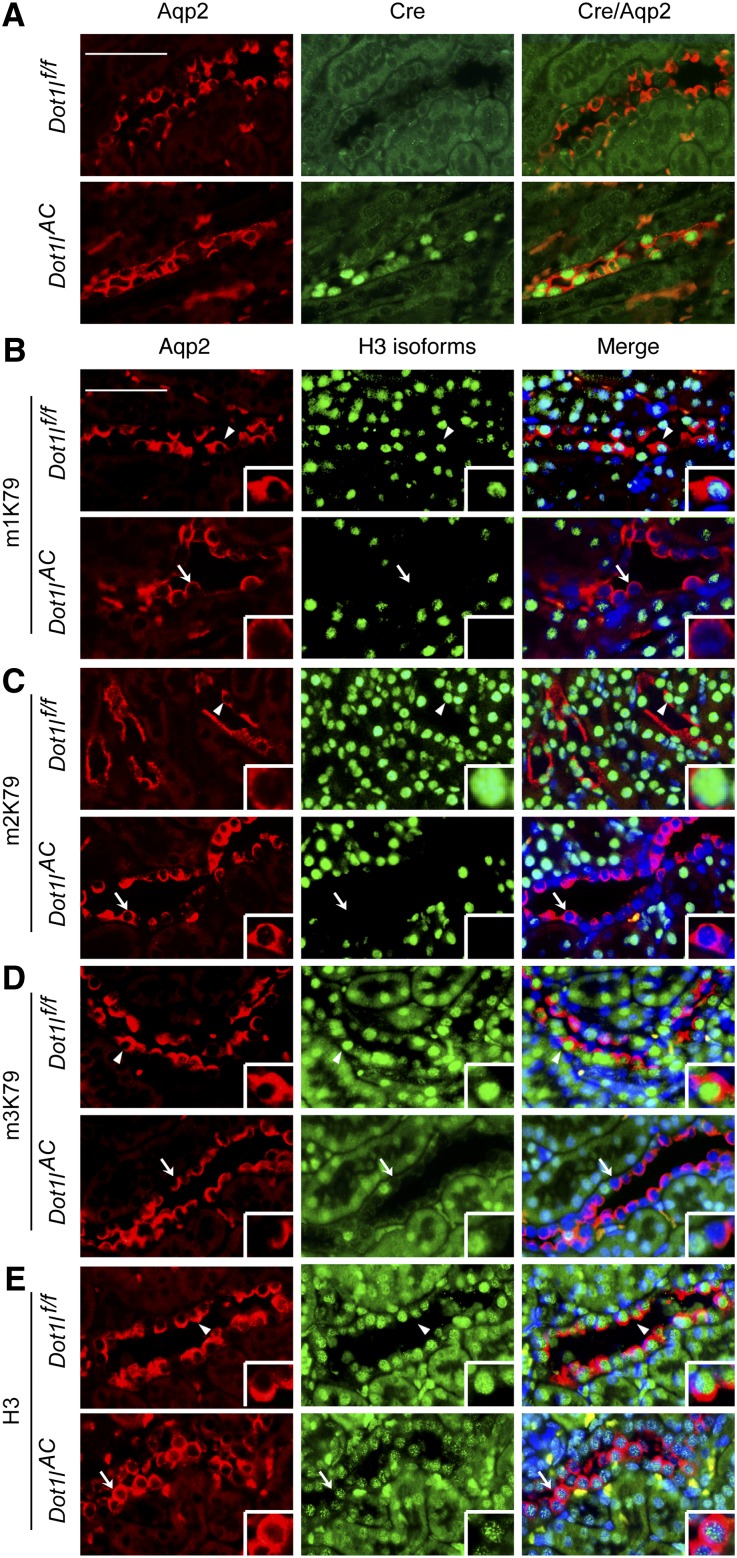
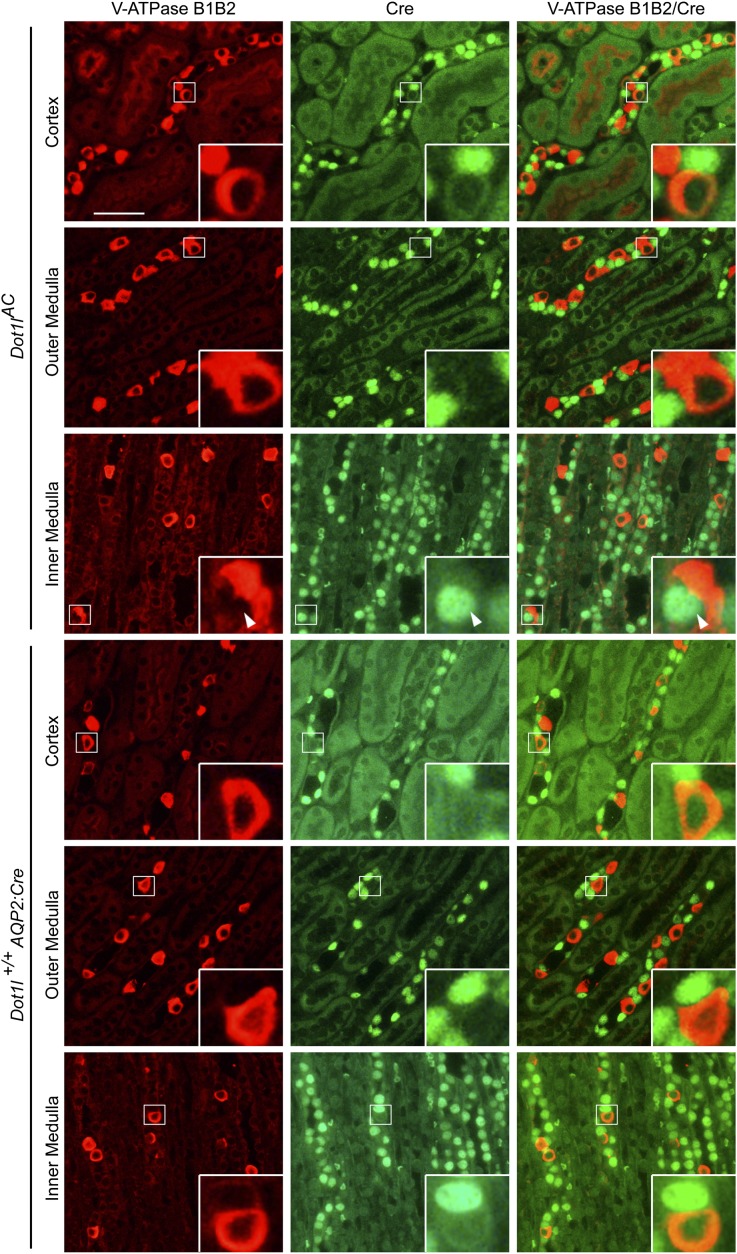
To determine whether Cre is expressed in PCs, we performed double immunofluorescence (IF) using chicken anti-Aqp224–26 and rabbit anti-Cre antibodies. As expected, Cre was detected in Aqp2+ PC in Dot1lAC mice and was absent in other (i.e., Aqp2−) cells in both groups (Figure 2A).
Figure 2.
Dot1l is disrupted and solely responsible for all methylation events at histone H3 K79. (A) Representative IF images showing Cre expression in Aqp2-expressing cells in Dot1lAC, but not in control kidneys. (B–E) Representative IF images showing Dot1l deletion abolished H3 mono-, di-, and tri-methyl K79 (m1K79, m2K79, and m3K79) without affecting total H3 in Aqp2-expressing cells in Dot1lAC but not in control kidneys. Aqp2 was stained in red. All other markers were in green. Nuclei were stained with DAPI (blue). Arrowhead and arrows: Cells with intact or abolished H3 K79 methylation, respectively, are amplified in the inserts. Scale bar: 50 μM and 12.5 μM for insert.
Similar IF with antibodies specific for H3 mono-, di-, and trimethyl K79 (m1, m2, and m3K79, respectively) detected these modifications in the PC of Dot1lf/f, but not in most PCs in Dot1lAC mice (Figure 2, B–D). The undetectable level of H3 K79 methylation did not result from a lack of DNA, as evidenced by 4′,6-diamidino-2-phenylindole staining, or in a general effect on total H3. Dot1l loss had little effect on total histone H3 (Figure 2E) and H3 dimethylation at either K9 or K36 (Supplemental Figure S1). These results collectively demonstrate that Dot1l is solely responsible for all known methylation events at H3 K79, consistent with previous in vitro studies,16,19 and that it is efficiently and specifically disrupted in most PC.
Dot1lAC versus Dot1lf/f Mice Have Significantly Fewer PCs and More ICs
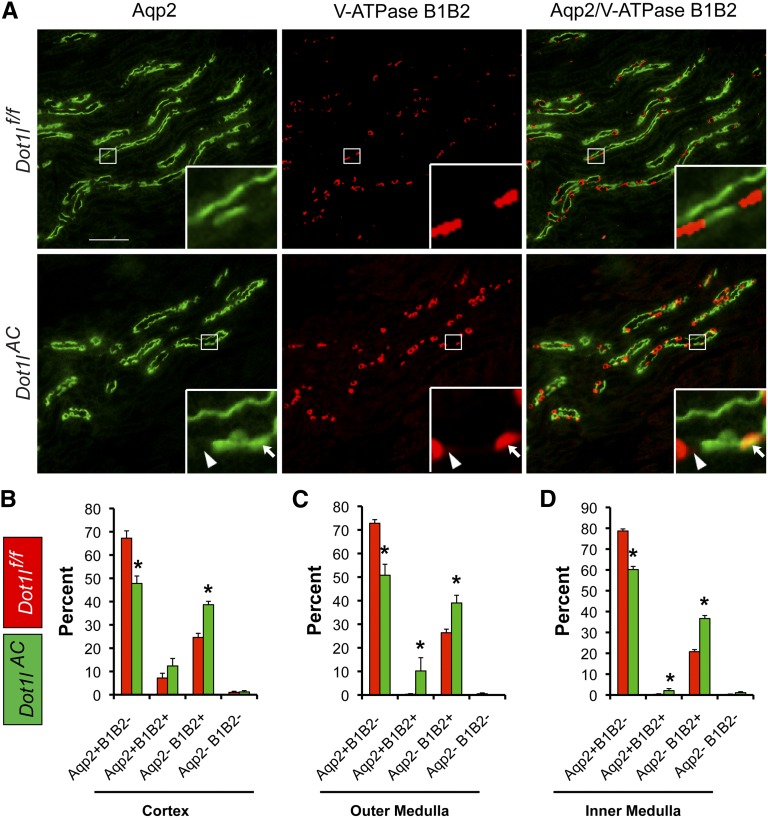
To determine whether Dot1l deletion affects abundance of PCs versus ICs, we conducted double IF with the anti-Aqp2 antibody to label the PCs and a rabbit antibody specifically recognizing V-ATPase B1 and B2 subunits (referred as B1B2 hereafter) to identify the ICs. Representative IF images from OM of Dot1lf/f and Dot1lAC mice are shown in Figure 3A.
Figure 3.
Dot1lAC mice have fewer PCs and more ICs than control mice. (A) Representative IF images showing expression of PC marker Aqp2 (green) and IC markers V-ATPase subunits B1 and B2 (red) in the OM of adult Dot1lf/f and Dot1lAC mice. Boxed areas were amplified in the insert. Arrowhead and arrows: Aqp2−B1B2− and Aqp2+B1B2+ cells, respectively. Scale bar: 100 μM and 17.5 μM for insert. (B–D). CNT/CD cells in the cortex (B), OM (C), and IM (D) of Dot1lf/f and Dot1lAC mice were categorized into four groups as indicated, based on the expression of the two markers. The relative abundance of each cell group was graphed.
We focused on the CNT/CD tubules identified by at least one Aqp2+ cell. All other tubules lacking an Aqp2+ cell were excluded from analyses. On the basis of labeling of these two antibodies, we categorized the cells in the CNT/CD into four types: Aqp2+B1B2−, Aqp2+B1B2+, Aqp2−B1B2+, and Aqp2−B1B2−, all of which were observed in the two genotypes. To estimate their relative abundance, we counted more than 200 CNT/CD tubules with approximately 2000 cells in the cortex, OM and IM from two to three mice per genotype, and results are summarized (Figure 3, B–D). Dot1lAC versus Dot1lf/f mice significantly decreased PCs (Aqp2+B1B2−) by about 20% throughout the kidney, which may contribute to polyuria (see below), and increased ICs (Aqp2−B1B2+) by 13%–16% and double-positive cells by about 3%–11%. Aqp2−B1B2− cells were observed occasionally (approximately 1%) in the CNT/CD in each group. Both double-positive and double-negative cells may represent intermediate states in the derivation of ICs from Aqp2+ cells (see below).
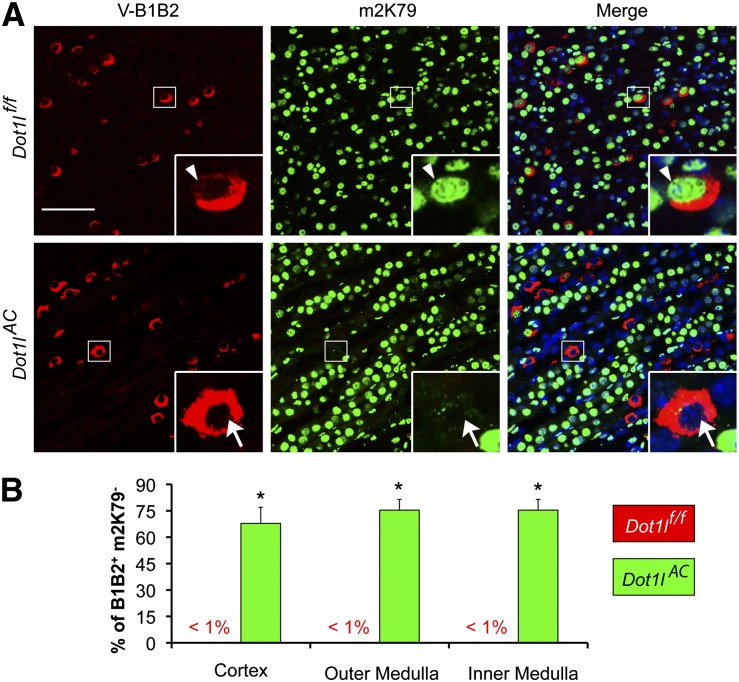
Like PCs, Most ICs in Dot1lAC Mice Have No Detectable m2K79
To determine whether some ICs originate from Aqp2+ cells, double IF was performed with the anti-m2K79 to mark the Cre-mediated Dot1l deletion and thus the “initial” identity of the Aqp2+ cells, along with the anti–V-ATPase B1B2 antibody to identify the ICs. We focused on CNT/CD, recognized by both m2K79+ and m2K79− cells in the tubules. The lack of m2K79 staining is most likely due to Aqp2-promoter–driven, Cre-mediated Dot1l deletion. In Dot1lf/f mice, m2K79 was discernible in nearly all of the B1B2+ cells throughout the kidney (Figure 4A). However, there were two types of ICs in Dot1lAC mice. The first type resembled those in Dot1lf/f mice and is the “canonical” IC (B1B2+ m2K79+). The rest of the B1B2+ cells (68%–74% of the cells) had near background or no detectable m2K79 staining (B1B2+ m2K79−) (Figure 4B). Thus, most of ICs in the CNT/CD are likely derived from cells that originally express Aqp2.
Figure 4.
Most of ICs have no detectable m2K79 in Dot1lAC mice. (A) Representative IF images showing IC markers V-ATPase subunits B1 and B2 (red) and histone H3 di-methyl K79 (m2K79, green) in the IM of adult Dot1lf/f and Dot1lAC mice. Boxed areas were amplified in the insert. Arrowhead and arrows: Cells with intact or abolished m2K79, respectively. Scale bar: 50 μM and 12.5 μM for insert. (B) The percentages of B1B2+m2K79− in the total of B1B2+ cells in the cortex, OM, and IM were estimated and graphed. Error bar represents SEM. *P<0.05 versus Dot1lf/f.
Loss of m2K79 in the ICs of Dot1lAC Mice Is Not a Result of Promiscuous Cre Expression
Because a 7-kb Atp6v1b1 promoter directed Cre expression not only in all ICs but also in half of the PCs in the connecting segment,27 it is possible that the Aqp2:Cre transgene activated Cre in the original IC, leading to generation of B1B2+m2K79− cells. To check for this possibility, kidney sections from Dot1lAC and Dot1l+/+ Aqp2:Cre were analyzed for B1B2 and Cre coexpression. Although B1B2+Cre+ cells were occasionally (<1%) observed in Dot1lAC mice, none were found in the cortex, OM and IM in Dot1l+/+Aqp2:Cre mice (Figure 5). These results not only demonstrate the specificity and capability of Aqp2:Cre transgene in trans-activating Cre in PC but also indicate that most of the B1B2+ m2K79− cells originate from Aqp2-expressing cells.
Figure 5.
Cre is not promiscuously expressed in ICs. Representative IF images showing lack of coexpression of the IC markers V-ATPase subunits B1 and B2 (red) with Cre (green) throughout the whole kidneys of adult mice as indicated. Boxed areas were amplified in the insert. Arrowhead: A potential B1B2+Cre+ cell occasionally observed in Dot1lAC. Scale bar: 50 μM and 12.5 μM for insert.
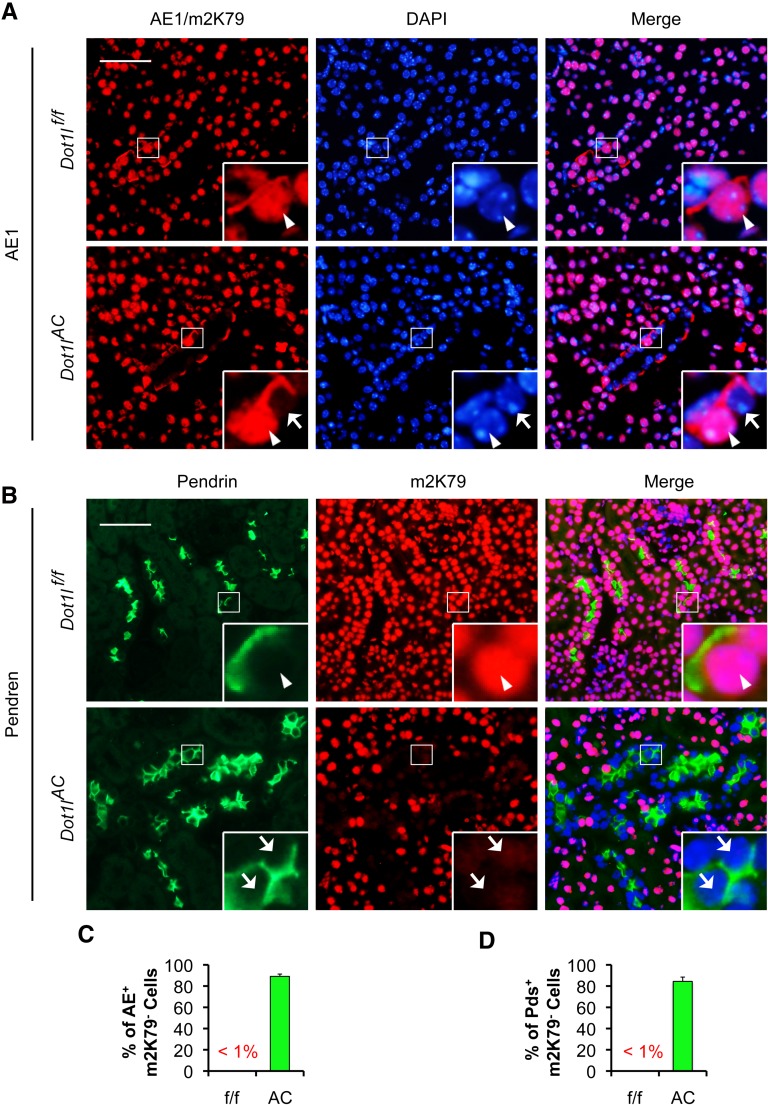
Most α-ICs and β-ICs Have No Detectable m2K79
In addition to B1B2, ICs express other markers, including V-ATPase A,28 CAII,5 AE1,29 and pendrin.30 AE1 is expressed in the basolateral plasma membrane of α-IC.29 Pendrin is found in the apical membrane of the β-IC and non-A, non-B ICs in the cortical CD.30 To determine whether the derived ICs express these IC markers, coexpression of m2K79 with these IC markers was assessed. An antibody specific for V-ATPase B1 was included to independently verify B1 expression. Most cells expressed these markers in the Dot1lf/f mice that also expressed m2K79 (Figures 6, A and B, and Supplemental Figure S2, A–C). However, the majority of the cells stained with these IC markers in the Dot1lAC mice displayed barely detectable m2K79. The percentages of m2K79− cells were 89%, 84%, 87%, 87%, and 90% in all of AE+, Pendrin+, V-ATPase A+, V-ATPase B1+, and CAII+ cells, respectively (Figures 6, C and D, and Supplemental Figure S2, D–F). It should be stressed that both of the AE1 and m2K79 antibodies were raised in rabbit. Because AE1 and m2K79 reside in the basolateral membrane and nuclei, respectively, we were still able to score their expression with the same fluorescent color (Figure 6A). In brief, ICs including α-IC and β-IC probably originate from Aqp2+ cells and express all six IC markers examined.
Figure 6.
Most α-ICs and β-ICs have no detectable m2K79 in Dot1lAC mice. (A and B) Representative IF images showing coexpression of m2K79 with AE1 (A) or Pendrin (B), which are markers of α-IC and β-IC, respectively, in Dot1lf/f mice, and undetectable m2K79 in most α-ICs and β-ICs in Dot1lAC mice. AE1 and m2K79 reside in the basolateral membrane and nuclei, respectively, allowing determination of their expression even though they were detected by two rabbit antibodies and visualized by the same color (red). Boxed areas were amplified in the insert. Arrowhead and arrows: Cells with intact or abolished m2K79, respectively. Scale bar: 50 μM and 12.5 μM for insert. (C and D). Percentages of X+m2K79− in the total of X+ cells, where X indicates each of the IC markers as indicated, were calculated and graphed. Error bars represent SEM.
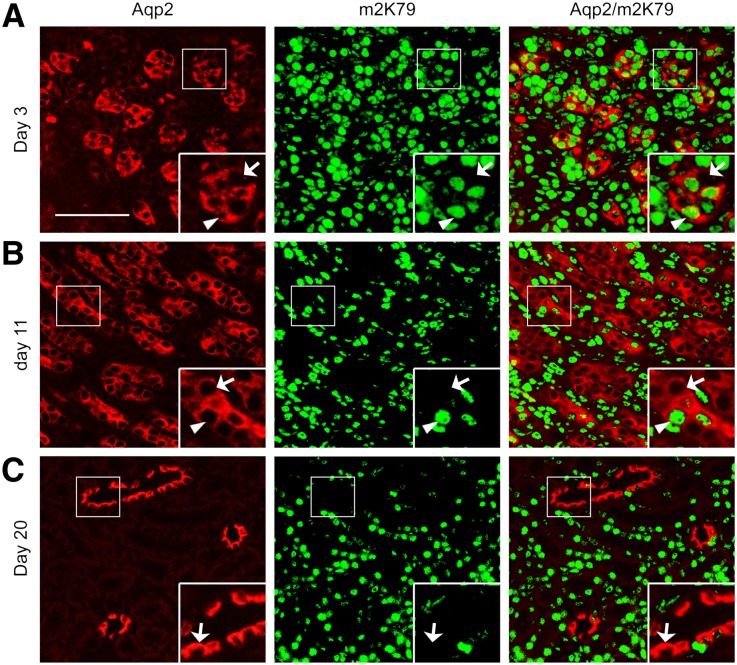
Derivation of ICs from Aqp2+ Cells Occurs in the Developing Kidneys
To determine when in development the derivation of ICs from Aqp2+ cells occurs, we first evaluated Aqp2 and m2K79 co-staining in kidneys from 3-, 11-, and 20-day old pups. For Dot1lf/f mice, the vast majority of Aqp2+ cells were positive for m2K79 in all three stages (Supplemental Figure S3). For Dot1lAC mice, some of Aqp2+ cells at day 3 and most of Aqp2+ cells at the other two stages lacked detectable m2K79 (Figure 7, A–C). Aqp2:Cre thus directs Cre-mediated Dot1l removal in the majority of Aqp2+ cells by day 11 and 20, but detection is still possible at day 3.
Figure 7.
Cre-dependent abolishment of m2K79 occurs in the Aqp2-expressing cells of the developing kidneys. Representative IF images showing undetectable m2K79 in some of Aqp2+ cells in 3-day-old Dot1lAC kidneys and in most of Aqp2+ cells in 11- and 20-day-old Dot1lAC kidneys. Arrowhead and arrows: Cells with intact or abolished H3 K79 methylation, respectively, are amplified in the inserts. Scale bar: 100 μM and 50 μM for insert. For developing kidneys of Dot1lf/f mice, see Supplemental Figure S3.
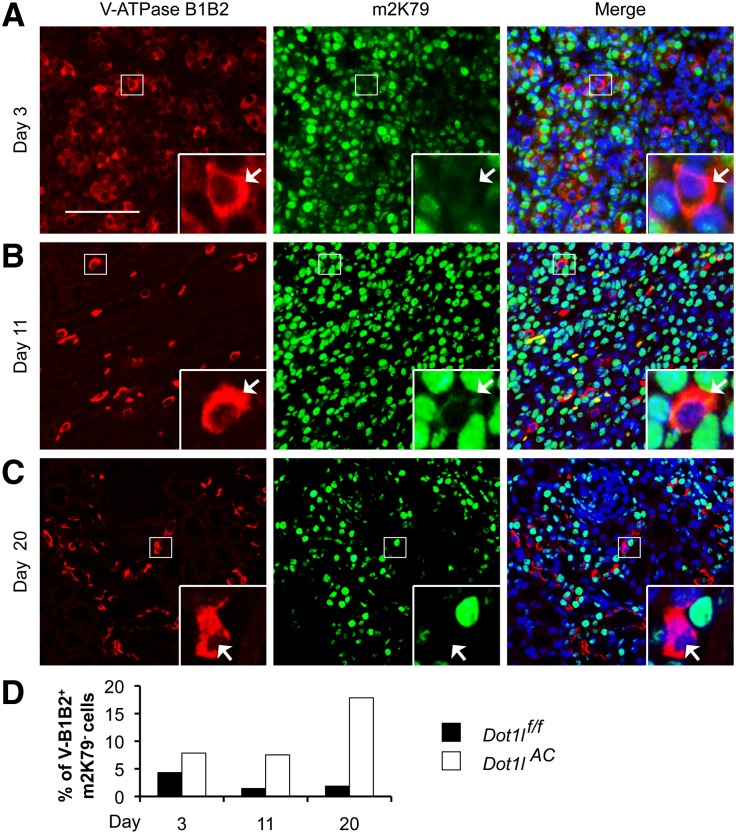
For each of the three stages, we examined 325–1024 B1B2+ cells/genotype and found that 3%, 1.5%, and 1.8% of them in Dot1lf/f mice were m2K79−. These cells were increased to 10.4%, 7.5%, and 17.8% in corresponding Dot1lAC littermates (Figure 8 and Supplemental Figure S4). Because m2K79 levels fluctuate throughout the cell cycle, reaching the lowest point in G2,13 undetectable m2K79 in some B1B2+m2K79− and Aqp2+m2K79− cells may stem from cell cycle–dependent regulation rather than from Dot1l deletion, causing their occasional presence in Dot1lf/f mice and overestimation of the percentages of the derived ICs. Nevertheless, Dot1lAC versus Dot1lf/f had higher percentages of B1B2+m2K79− in all of the three stages examined (Figure 8D), suggesting that the derivation of IC from Aqp2+ cells occurs as the cells develop.
Figure 8.
Derivation of ICs from Aqp2-expressing cells occurs in the developing kidneys. (A–C) Representative IF images showing undetectable m2K79 in some of B1B2+ cells in 3-, 11-, and 20-day-old Dot1lAC mice. Arrows: Cells with abolished m2K79 are amplified in the inserts. Scale bar: 100 μM and 25 μM for insert. For developing kidneys of Dot1lf/f mice, see Supplemental Figure S4. (D). Percentages of B1B2+m2K79− cells in total of B1B2+ cells were estimated and graphed.
Dot1lAC Mice Displayed Polyuria without Severely Impaired Electrolyte and Acid-Base Balance
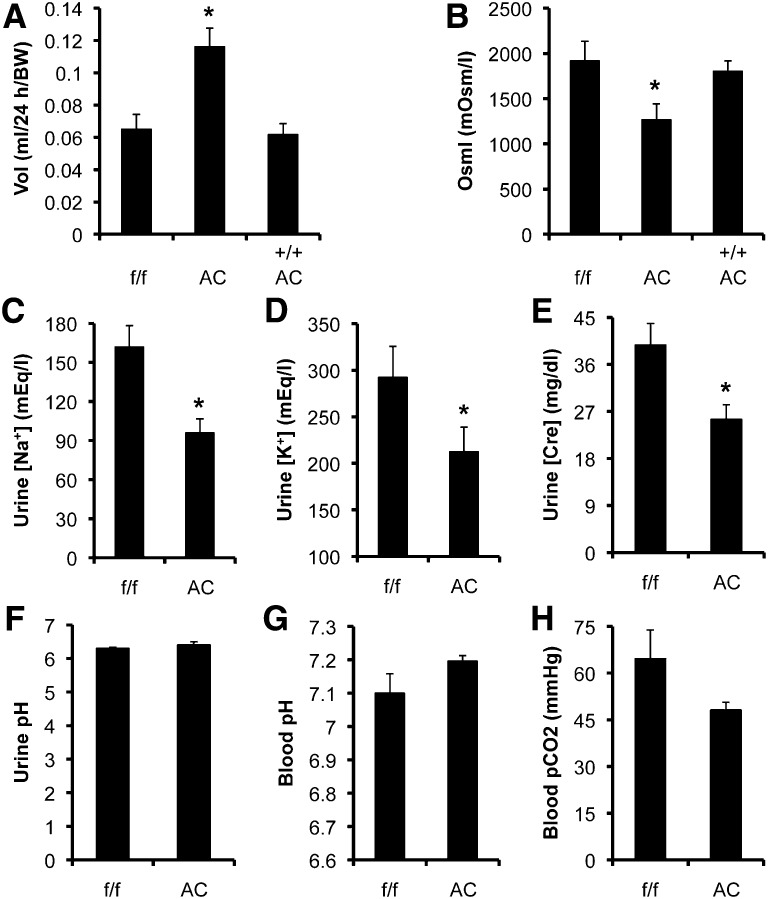
To determine the consequence of Dot1l deletion in renal physiology, we performed metabolic analysis. Twenty-four-hour urine analysis of Dot1lAC, Dot1lf/f, and Dot1l+/+ Aqp2:Cre mice (n=6–8 mice/group) revealed relatively hypoosmotic polyuria in Dot1lAC mice, compared with the other two control groups. Dot1lAC versus Dot1lf/f mice had elevated urine volume by 83±29% and decreased urine osmolarity by 34±9.1%. However, neither the urine volume nor the urine osmolarity of Dot1l+/+ Aqp2:Cre mice were significantly different from Dot1lf/f mice (Figure 9, A and B), suggesting that the relatively hypoosmotic polyuria of Dot1lAC mice primarily results from deletion of Dot1l and that the presence of the Aqp2:Cre transgene itself alone has little effect on urine production and concentration.
Figure 9.
Dot1lAC mice have impaired urine concentration ability without severely impaired electrolyte and acid-base balance. (A–F) Twenty-four-hour urine analyses of Dot1lf/f (f/f), Dot1lAC (AC), and Dot1l+/+ Aqp2Cre (++ AC) mice (n=8 mice per group) were conducted to measure the parameters as indicated. (G and H). Blood analyses (n=6 mice/group) for the parameters as indicated. For additional measurements, see Supplemental Figures S5 and -S6. *P<0.05 versus Dot1lf/f.
Accordingly, Dot1l+/+ Aqp2:Cre mice were excluded from analysis of other urinary measures. Dot1lAC versus Dot1lf/f mice had significantly lower urinary [Na+], [K+], and [creatinine] with similar urine pH (Figure 9, C–F). Similarly, excretion of Na+ and K+; [Na+]/[creatinine]; [K+]/[creatinine]; body weight; and diastolic, systolic, and mean BP did not significantly differ between the two genotypes (Supplemental Figure S5, A–F).
Blood analyses (n=6 mice/group) were conducted to measure 12 parameters ([Na+], [K+], [Cl−], total carbon dioxide content [TCO2], [pCO2], [HCO3−], anion gap, base excess in extracellular fluid, pH, [BUN], [hematocrit], and [hemoglobin]). Dot1lAC mice had mildly reduced pCO2 and increased pH (Figure 9, G and H), with no differences in the other 10 measures between the two groups (Supplemental Figure S6, A–J). Taken together, the urine and blood measurements suggest that Dot1lAC mice have polyuria without severe impairment in maintaining normal electrolyte and acid-base balance. Hypokalemia, which causes polyuria, is unlikely to be a contributory factor for the polyuria in these mice.
Discussion
In this study, we (1) describe the first kidney-specific Dot1ll conditional knockout mouse line; (2) identify relatively hypoosmotic polyuria with apparently normal electrolyte and acid-base homeostasis as the major phenotype of these mutant mice; (3) reveal that the polyuria may be attributable to a significant reduction in the number of PC; and (4) provide strong in vivo evidence that ICs, including α-IC and β-IC, are derived from Aqp2+ cells;
Given that Aqp2 is a widely accepted PC marker, our current study suggests that ICs are derived from PCs and that this process is facilitated by Dot1l deletion. Once derived ICs acquire IC identity after Cre-mediated removal of Dot1l and thus abolishment of m2K79, they lose the Aqp2+ phenotype including the original ability to sustain Aqp2 and Cre expression driven by the Aqp2:Cre transgene and eventually render Aqp2 and Cre undetectable. However, because derivation of IC from Aqp2+ cells begins as early as day 3 after birth, it remains unknown whether Aqp2+ cells acquire complete function of PC before giving rise to ICs. In addition, adult kidneys may contain Aqp2-expressing “stem” cells that differentiate into ICs. Nevertheless, our findings support that PCs and ICs differentiate from Foxi1− Aqp2+ CAII+ epithelial precursor cells11 and PCs are not terminally differentiated as observed in vitro.8 The difference between the latter and our study may stem from the use of two totally different model systems. The canonical IC in adult Dot1lAC mice accounted for only 10%–33% of the total IC population. Such underrepresentation of canonical ICs in adults may result from depletion of ICs in developing kidneys.31 Because derived ICs were <18% (Figure 8D), most of the presumably deleted ICs are the canonical ICs.
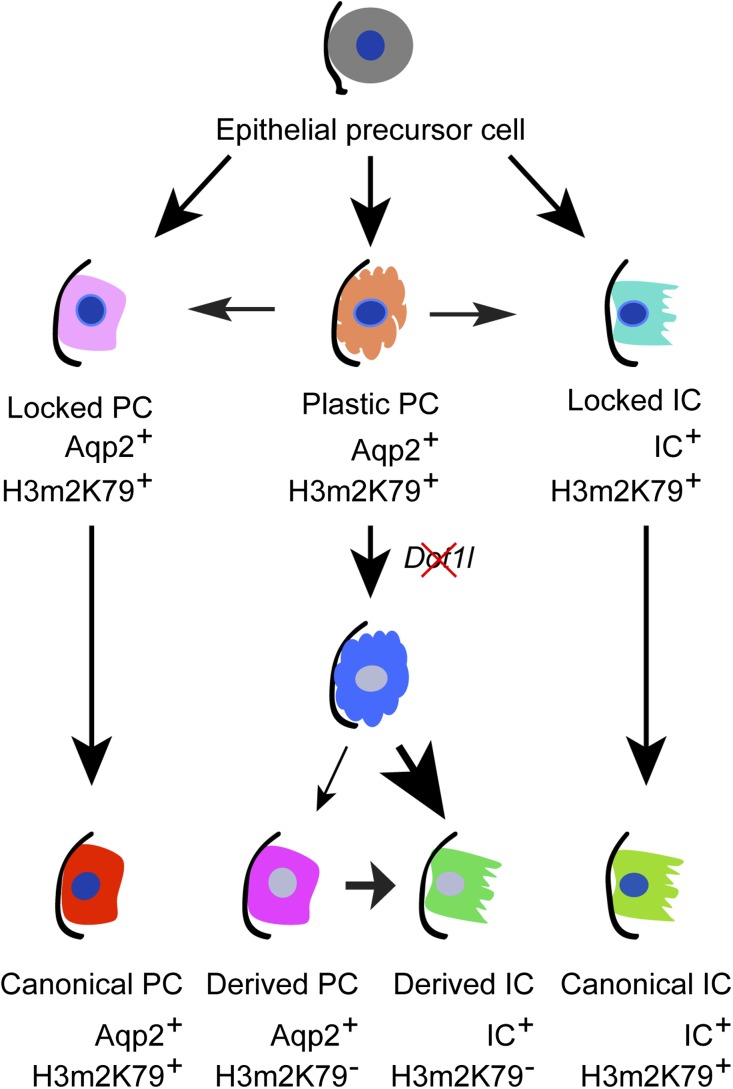
The detailed molecular mechanism governing derivation of ICs from Aqp2+ cells is unclear. Because IF with the anti-H3m2K79 clearly demonstrated high expression of Cre at postnatal day 3 (Figure 8 and Supplemental Figure S3), it is very unlikely that variation in Cre expression is the sole factor contributing to the developmental difference in H3m2K79 expression. It is also possible that developmental programs vary in the “same” Aqp2+ cell population as they do in differentiated Th1 and Th2-helper cells, and that these programs can be irreversibly “locked” as they are in some GATA3-expressing Th2 cells, while other GATA3 expressing cells remain plastic.32,33 Accordingly, we propose a hypothetic model (Figure 10). Aqp2+ progenitor cells differentiate into “locked” PCs, “locked” ICs, and “plastic” PCs in a Dot1l-independent manner. The first two types eventually differentiate into the canonical PCs and ICs characterized by the coexpression of H3m2K79 with PCs and ICs markers, respectively. The plastic PCs are also Aqp2+ and may switch to locked PCs or ICs. Dot1l deletion enhances differentiation of the plastic PCs into ICs, leading to generation of derived ICs (IC+ H3m2K79−) and an increase in IC population. In this regard, Dot1a may repress IC markers in the locked and plastic PC, but not in locked ICs. In other words, like H3m3K27,32,33 Dot1a-mediated H3m2K79 may mark repression in a gene- and lineage-specific manner. It can be speculated that Dot1a represses the expression of IC markers and sequesters an unknown repressor to permit expression of PC markers, including Aqp2. As a result, the cells exhibit the PC phenotype. Deletion of Dot1l abolishes Dot1a-mediated repression of the IC markers in the plastic PC and releases the unknown repressor to silence PC markers, facilitating the derivation. Future studies are required to test this idea in the plastic PCs.
Figure 10.
A hypothetical differentiation model for principal and intercalated cells. Epithelial precursor cells differentiate into irreversibly “locked” PCs and ICs as well as “plastic” PCs. Although the locked PCs and ICs eventually mature into the canonical PCs and ICs, respectively, the plastic PCs can give rise to PCs or ICs. Inactivation of Dot1l favors derivation of ICs from the plastic PCs. The phenotype of each cell type in terms of Aqp2, IC markers, and H3m2K79 is also shown.
Because of its activity in the majority of renal Aqp2+ cells, Aqp2:Cre transgene is very useful for generation of CNT/CD-specific knockout mice to study the function and physiologic consequence of the target genes in the developing and adult kidneys. Although three other Aqp2 promoters have been used to drive Cre for inactivation of endothelin-1 and peroxisome proliferator-activated receptor γ in PC,34–36 it remains unknown whether they are also active in the developmental stages.
Concise Methods
Reagents
The primary antibodies used are chicken anti–Aqp2 LC54 (a gift from James B. Wade, University of Maryland School, Baltimore, MD); chicken anti–V-ATPase A and anti–V-ATPase B1;37 rabbit anti-H3 (Millipore); anti-H3 mono-, di-, and tri-methyl K79 (abcom); anti-Cre (Sigma); anti-AE1 (Alpha Diagnostic); and four antibodies (rabbit anti–V-ATPase B1 and B2, goat anti-AQP2, mouse anti–V-ATPase B1 and B2, and mouse anti-CAII) from Santa Cruz. The secondary antibodies are Dylight 594-AffiniPure goat antichicken IgG (Jackson ImmunoResearch Laboratorie), Alexa Fluor 488-conjugated goat antirabbit IgG and Alexa 594-conjugated goat antimouse IgG (Invitrogen).
Generation, Genotyping, and Characterization of Dot1lAC Mice
Dot1lf/f and AQP2Cre mice have been previously described.16,23 Offspring from the cross between Dot1lf/f and Aqp2:Cre was backcrossed with Dot1lf/f to generate double transgenic animals (Dot1lf/f Aqp2:Cre), which is termed Dot1lAC. Genotyping was performed as described.16 Dot1lAC mice were constantly backcrossed with Dot1lf/f mice for at least 10 generations to get and maintain the highly pure C57BL6 background. Littermates from the cross between these Dot1lf/f and Dot1lAC mice were used for all of the current studies. Mice age 2–5 months were used unless otherwise indicated.
IF Studies
IF was conducted as we reported before,25,38 with the following modifications. Adobe Photoshop CS4 was used to examine the IF images and to score the staining of each primary antibody. To estimate the relative abundance of PCs and ICs and to calculate the percentages of derived ICs, we examined two to four mice per genotype per stage in the related double IF experiments. Except 3-day-old pups, 5–10 fields were taken from the whole kidney in each mouse. Cell counting was restricted to tubules consisting of at least one PC. In the experiments involving Aqp2 staining, these tubules were identified by the presence of at least one Aqp2+ cells. In the experiments without Aqp2 labeling, these tubules were located by the coexistence of cells with some positive and others negative for IC marker labeling or for m2K79 staining. For the experiments evaluating coexpression of V-ATPase B1B2 and m2K79 in the 3-day-old kidneys, all of the cells strongly stained with the anti–V-ATPase B1B2 were included in counting except those without a clear DAPI-stained nucleus. This exception was also applied to all other experiments involving evaluation of m2K79 status. The percentage of derived ICs was defined as the number of X+m2K79− cells divided by the sum of the number of X+m2K79+ cells and the number of X+m2K79− cells, where X indicates an IC marker.
We could not determine Dot1a protein expression on a cell-by-cell basis. The Dot1a antibodies tested did not recognize mouse Dot1a in IHC and IF experiments. This is a limitation in the field. Dot1a protein was not examined by IF or IHC in the Dot1l-deficient embryos,19 in the heart of the cardiac-specific Dot1l knockout mice39 or in the peripheral blood nucleated cells of Dot1lf/f Vav-Cre mice.40 Instead, IF and/or IB with the anti-H3m2K79 assessed inactivation of Dot1l function and thus disrupted Dot1l expression.19,39,40
Metabolic Balance Studies
After acclimation for 3 days to Tecniplast metabolic cages (Exton, PA) with free access to water and the normal Na+ (0.4%) pellet diet (the regular laboratory chow), BP measurement with CODA tail-cuff BP system (Kent Scientific, Torrington, CT) and 24-hour urine collecting were carried out as we recently reported.41
All animal studies were performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Texas Health Science Center at Houston Animal Welfare Committee.
Blood and Urine Measurements
Urinary [Na+], [K+], and [creatinine] were measured with an analyzer (Roche Cobas Integra 400 plus) in the Clinical Pathology Laboratory, Department of Veterinary Medicine and Surgery, University of Texas MD Anderson Cancer Center as described.41 Urine osmolarity was determined by vapor pressure (Wescor Vapro Vapor Pressure Osmometer 5520, Scimetrics, Houston, TX).41 Blood measures were assessed using VetScan i-STAT 1 (ABAXIS) according to the manufacturer’s instruction.
Statistical Analyses
Quantitative data are presented as mean ± SEM. A t test was used with the statistical significance set at P<0.05.
Supplementary Material
Acknowledgments
We thank Dr. James Wade for sharing the chicken Aqp2 antibody. We thank Dr. Hua A. Jenny Lu and Dr. Dennis Brown for kindly providing the chicken antibodies against V-ATPase A and B1 subunits and constructive comments in the preparation of the manuscript.
This work was supported by the following grants: National Institutes of Health Grants R01 DK080236 (to W.Z.Z.), American Society of Nephrology Carl W. Gottschalk Research Scholar grant (to W.Z.Z.), American Heart Association grant (0865271F to W.Z.Z.), and the National Natural Science Foundation of China grant 81070552 (to Q.L.Z.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Salt and Pepper Distribution of Cell Types in the Collecting Duct,” on pages 163–165.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012080866/-/DCSupplemental.
References
- 1.Bagnis C, Marshansky V, Breton S, Brown D: Remodeling the cellular profile of collecting ducts by chronic carbonic anhydrase inhibition. Am J Physiol Renal Physiol 280: F437–F448, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Al-Awqati Q: Plasticity in epithelial polarity of renal intercalated cells: Targeting of the H(+)-ATPase and band 3. Am J Physiol 270: C1571–C1580, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Verlander JW, Madsen KM, Tisher CC: Structural and functional features of proton and bicarbonate transport in the rat collecting duct. Semin Nephrol 11: 465–477, 1991 [PubMed] [Google Scholar]
- 4.Breton S, Alper SL, Gluck SL, Sly WS, Barker JE, Brown D: Depletion of intercalated cells from collecting ducts of carbonic anhydrase II-deficient (CAR2 null) mice. Am J Physiol 269: F761–F774, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Brown D, Kumpulainen T, Roth J, Orci L: Immunohistochemical localization of carbonic anhydrase in postnatal and adult rat kidney. Am J Physiol 245: F110–F118, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Brown D, Paunescu TG, Breton S, Marshansky V: Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol 212: 1762–1772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall SM: Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens 14: 480–484, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Fejes-Tóth G, Náray-Fejes-Tóth A: Differentiation of renal beta-intercalated cells to alpha-intercalated and principal cells in culture. Proc Natl Acad Sci U S A 89: 5487–5491, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Barasch J, Al-Awqati Q: Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Eladari D, Leviel F, Tew BY, Miró-Julià C, Cheema F, Miller L, Nelson R, Paunescu TG, McKee M, Brown D, Al-Awqati Q: Deletion of hensin/DMBT1 blocks conversion of beta- to alpha-intercalated cells and induces distal renal tubular acidosis. Proc Natl Acad Sci U S A 107: 21872–21877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergström G G, Enerbäck S: Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest 113: 1560–1570, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer MS, Kahana A, Wolf AJ, Meisinger LL, Peterson SE, Goggin C, Mahowald M, Gottschling DE: Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics 150: 613–632, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, Struhl K, Zhang Y: Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol 12: 1052–1058, 2002 [DOI] [PubMed] [Google Scholar]
- 14.van Leeuwen F, Gafken PR, Gottschling DE: Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109: 745–756, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Hayashizaki Y, Kone BC: Structure and regulation of the mDot1 gene, a mouse histone H3 methyltransferase. Biochem J 377: 641–651, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang MJ, Wu H, Achille NJ, Reisenauer MR, Chou CW, Zeleznik-Le NJ, Hemenway CS, Zhang W: Histone H3 lysine 79 methyltransferase Dot1 is required for immortalization by MLL oncogenes. Cancer Res 70: 10234–10242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo SY, Granowicz EM, Maillard I, Thomas D, Hess JL: Requirement for Dot1l in murine postnatal hematopoiesis and leukemogenesis by MLL translocation. Blood 117: 4759–4768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y: hDOT1L links histone methylation to leukemogenesis. Cell 121: 167–178, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Jones B, Su H, Bhat A, Lei H, Bajko J, Hevi S, Baltus GA, Kadam S, Zhai H, Valdez R, Gonzalo S, Zhang Y, Li E, Chen T: The histone H3K79 methyltransferase Dot1L is essential for mammalian development and heterochromatin structure. PLoS Genet 4: e1000190, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Xia X, Jalal DI, Kuncewicz T, Xu W, Lesage GD, Kone BC: Aldosterone-sensitive repression of ENaCalpha transcription by a histone H3 lysine-79 methyltransferase. Am J Physiol Cell Physiol 290: C936–C946, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisenauer MR, Anderson M, Huang L, Zhang Z, Zhou Q, Kone BC, Morris AP, Lesage GD, Dryer SE, Zhang W: AF17 competes with AF9 for binding to Dot1a to up-regulate transcription of epithelial Na+ channel alpha. J Biol Chem 284: 35659–35669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Chen L, Zhou Q, Zhang W: AF17 facilitates Dot1a nuclear export and upregulates ENaC-mediated Na+ transport in renal collecting duct cells. PLoS ONE 6: e27429, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronzaud C, Loffing J, Bleich M, Gretz N, Gröne HJ, Schütz G, Berger S: Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Coleman RA, Wu DC, Liu J, Wade JB: Expression of aquaporins in the renal connecting tubule. Am J Physiol Renal Physiol 279: F874–F883, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Reisenauer MR, Wang SW, Xia Y, Zhang W: Dot1a contains three nuclear localization signals and regulates the epithelial Na+ channel (ENaC) at multiple levels. Am J Physiol Renal Physiol 299: F63–F76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC: Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem 281: 18059–18068, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, Nelson RD: The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HA, Breton S, Brown D: Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Sabolić I, Brown D, Gluck SL, Alper SL: Regulation of AE1 anion exchanger and H(+)-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int 51: 125–137, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE: Pendrin: An apical Cl-/OH-/HCO3- exchanger in the kidney cortex. Am J Physiol Renal Physiol 280: F356–F364, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Cha JH, Tisher CC, Madsen KM: Role of apoptotic and nonapoptotic cell death in removal of intercalated cells from developing rat kidney. Am J Physiol 270: F575–F592, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Yagi R, Zhu J, Paul WE: An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol 23: 415–420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, Schones DE, Peng W, Sun HW, Paul WE, O’Shea JJ, Zhao K: Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30: 155–167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE: Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, Kohan DE: Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol 288: F912–F920, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T: Collecting duct-specific deletion of peroxisome proliferator-activated receptor gamma blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci U S A 102: 9406–9411, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Păunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D: cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Xia X, Zou L, Xu X, LeSage GD, Kone BC: In vivo expression profile of a H+-K+-ATPase alpha2-subunit promoter-reporter transgene. Am J Physiol Renal Physiol 286: F1171–F1177, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Nguyen AT, Xiao B, Neppl RL, Kallin EM, Li J, Chen T, Wang DZ, Xiao X, Zhang Y: DOT1L regulates dystrophin expression and is critical for cardiac function. Genes Dev 25: 263–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, Feng Z, Punt N, Daigle A, Bullinger L, Pollock RM, Richon VM, Kung AL, Armstrong SA: MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell 20: 66–78, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Wu H, Pochynyuk OM, Reisenauer MR, Zhang Z, Huang L, Zaika OL, Mamenko M, Zhang W, Zhou Q, Liu M, Xia Y, Zhang W: Af17 deficiency increases sodium excretion and decreases blood pressure. J Am Soc Nephrol 22: 1076–1086, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.