Abstract
The strain-specific capsular polysaccharide KR5 antigen of Sinorhizobium meliloti 41 is required both for invasion of the symbiotic nodule and for the adsorption of bacteriophage 16-3. In order to know more about the genes involved in these events, bacterial mutants carrying an altered phage receptor were identified by using host range phage mutants. A representative mutation was localized in the rkpM gene by complementation and DNA sequence analysis. A host range phage mutant isolated on these phage-resistant bacteria was used to identify the h gene, which is likely to encode the tail fiber protein of phage 16-3. The nucleotide sequences of the h gene as well as a host range mutant allele were also established. In both the bacterial and phage mutant alleles, a missense mutation was found, indicating a direct contact between the RkpM and H proteins in the course of phage adsorption. Some mutations could not be localized in these genes, suggesting that additional components are also important for bacteriophage receptor recognition.
The bacterial surface has great importance in recognition events necessary to develop pathogen or symbiotic interactions as well as for bacteriophage attachment. In rhizobia, symbiotic nitrogen-fixing partners of different leguminous plants, exopolysaccharides, capsular polysaccharides, and lipopolysaccharides may play an essential role in infection (14). Recently it was shown that Sinorhizobium meliloti 41 produces a strain-specific capsular polysaccharide, the KR5 antigen, which is related to the group II K antigens from Escherichia coli. It can provide the same function as exopolysaccharides during symbiotic nodule development. In the S. meliloti 41 exoB background, the KR5 antigen is essential for the invasion of host plants (21, 25, 26).
So far, three rkp gene clusters involved in biosynthesis of the KR5 antigen have been identified and shown to be important for the invasion of symbiotic hosts as well as for bacteriophage 16-3 infection. The rkp-1 region contains 10 genes (rkpA to rkpF) and appears to be involved in the production of a specific lipid carrier required for the biosynthesis of the KR5 antigen (12, 18, 21). The rkp-2 region harbors two genes (lpsL and rkpK), both required for lipopolysaccharide production; however, the second one (rkpK) encoding a UDP-glucose dehydrogenase, is also necessary for the synthesis of glucuronic acid, a precursor of the KR5 antigen (16). The rkp-3 region carries six strain-specific genes (rkpL to rkpQ) that are probably responsible for the synthesis of the pseudaminic acid component of the KR5 antigen. Additional genes have also been localized downstream from this cluster. Two of them (rkpZ and rkpY) affect capsular polysaccharide production, but three of them (rkpR and rkpTS) were identified only on the basis of their homology to genes involved in polysaccharide transport. Insertional mutations in these genes had no effect on the biosynthesis of KR5 antigen or on the development of symbiosis (17).
In most cases, biochemical and immunological analysis of rkp mutants demonstrated partial or complete loss of the KR5 antigen from the cell surface (16-18, 21). The mutants induced empty nodules on alfalfa and were unable to bind phage 16-3, suggesting that both the invasion process and phage attachment required the polysaccharide. Earlier studies indicated that the KR5 antigen serves as the receptor for the strain-specific bacteriophage 16-3 because the phage adsorption ability of purified bacterial envelope could be destroyed by β-glucosidase and β-glucuronidase but not by protease K treatment (25).
Bacteriophage 16-3, a temperate phage of S. meliloti 41, was studied intensively. Both genetic and physical maps of the phage chromosome have been published (9, 11, 19, 20), and many important loci were identified and analyzed in more detail, such as the attP site (9, 10), the main repressor c gene (6, 7), the site-specific recombination int and xis genes (29), and the immX region (5). A host range mutation was also identified (19), suggesting that isolation of bacterial mutants presenting an altered phage receptor is possible.
In order to know more about the biosynthesis of the KR5 antigen and about its role in the infection of host plants, we aimed to isolate and characterize special phage-resistant bacterial mutants of S. meliloti 41 that carry an altered phage receptor. These bacterial mutants were supposed either to produce an altered KR5 antigen or to present another surface structure that is used as a receptor by the host range phage mutants. In contrast to this hypothesis, in this work we show that the receptor of phage 16-3 is probably a protein complex involved in the biosynthesis of the KR5 antigen and that the bacterial RkpM protein and the H protein of phage 16-3 interact in the course of bacteriophage adsorption.
MATERIALS AND METHODS
Bacterial strains, bacteriophages, plasmids, and growth conditions.
Escherichia coli strains JM109 (32) and XL1-Blue (3) were used for cloning procedures. A derivative of S. meliloti 41, AK631, has been used as a background for genetic experiments for many years. It was shown recently that AK631 is an exoB mutant, unable to synthesize exopolysaccharides, but it is effective in symbiosis (23). In this study the original field isolate, RM41 (Exo+), was used for mutant isolation (see below). The strains isolated in this work are characterized in Table 1 and Fig. 1. Derivatives of strain AK631 carrying transposon Tn5 insertions in the rkp regions (Fig. 2) were isolated previously (16, 17, 21).
TABLE 1.
Complementation of phage receptor mutations in S. meliloti
| Strain tested | Cosmid introduced | Infection phenotypea
|
||
|---|---|---|---|---|
| 16-3 | 16-3 h5 | 16-3 h109 | ||
| 41 | None | S | S | S |
| GH4046 | None | R | S | R |
| GH4046 | pAT399 | S | S | NT |
| GH4046 | pAT399 orf140::Tn5(174) | S | S | NT |
| GH4046 | pAT399 rkpL::Tn5(187) | S | S | NT |
| GH4046 | pAT399 rkpM::Tn5(212) | R | S | NT |
| GH4046 | pAT399 rkpN::Tn5(107) | S | S | NT |
| GH4046 | pAT399 rkpO::Tn5(168) | S | S | NT |
| GH4046 | pAT399 rkpP::Tn5(105) | S | S | NT |
| GH4046 | pAT399 rkpQ::Tn5(124) | S | S | NT |
| GH4178 | None | R | S | R |
| GH4178 | pAT399 | S | S | NT |
| GH4178 | pAT399 rkpM::Tn5(212) | R | S | NT |
| GH4180 | None | R | S | S |
| GH4180 | pAT399 | R | S | S |
| GH4180 | pAT401 | S | S | S |
S, phage sensitive; R, phage resistant; NT, not tested.
FIG. 1.

Capsular polysaccharide (KPS) production of the phage receptor mutants. Phenol-water extracts from S. meliloti 41 and its phage 16-3-resistant derivatives (GH4046, GH4178, and GH4180) were separated on DOC-PAGE, and the gel was stained by the Alcian blue-silver method. HMW, high molecular weight; LMW, low molecular weight.
FIG. 2.
Physical-genetic map of the rkp-3 region of S. meliloti 41. The upper part presents the region covered by cosmid clones pAT399 and pAT401. The lower part shows the arrangement of rkp genes and Tn5 insertions applied in complementation experiments. The different shading reflects the phenotype of the insertions. Mutations in ORFs labeled in black result in severe failures in capsular polysaccharide production, phage 16-3 attachment, and symbiotic properties. Gray boxes represent genes that affect the molecular mass of the capsular polysaccharide produced. Mutants in this category are also phage 16-3 resistant and unable to invade host plants. Empty boxes represent ORFs where insertions result in no detectable changes. Restriction sites: E, EcoRI; B, BamHI; H, HindIII.
Bacteriophage 16-3 ΔNC (11) was kindly provided by L. Orosz and used as the background strain for mutant isolation. The host range mutants isolated in this study are characterized in Table 1.
For subcloning and sequencing plasmids, pBluescript SK(+) (Stratagene, La Jolla, Calif.) was applied. Cosmids pPP428, pAT330, pAT399, and pAT401 carrying the rkp regions and pAT399 derivatives carrying transposon Tn5 insertions (Fig. 2 and Table 1) were described previously (16, 17, 21).
Plasmids pPZS5 and pPZS10 are pSUP106 (22) derivatives containing the F+N and the N BamHI fragments (11) of the 16-3 h6 chromosome, respectively (Fig. 4).
FIG. 4.
Map of the h region of phage 16-3. The upper part shows the physical map of the phage with the host range (h) region. Solid bars represent the DNA clones used for identification of the h gene. Below the physical map of the h region, the positions of the h5 host range mutations and the changes in the DNA and protein sequences are presented. The empty circle represents an in-frame stop codon, while gray boxes show the relative positions of putative in-frame GTG, TTG, or CTG start codons preceded by ribosome binding site-like sequences. The first ATG codon is represented by the black box. The double open box shows the positions of the inverted repeats of the transcription termination signal-like sequence.
The media, antibiotic concentrations, and culture conditions for E. coli and S. meliloti strains and phage 16-3 sensitivity tests were described previously (24, 25).
Phage receptor and host range mutants.
Dilutions of an S. meliloti 41 wild-type strain RM41 culture at logarithmic phase were mixed with 108 phage 16-3 particles and plated in TA top agar. Phage-resistant colonies that appeared after 2 days were isolated and tested with a mixture of wild-type phage cultures grown from three independent 16-3 plaques. In this “host range screen,” 100 μl of overnight culture of the phage-resistant bacteria and 107 to 108 phage particles were mixed and plated. When any plaque (usually 10 to 200) appeared on a phage-resistant isolate, the bacterial and phage strains were considered receptor and host range mutants, respectively. Less than 1% of the isolated phage-resistant bacterial mutants proved to be receptor mutants. The phage adsorption test was carried out as described somewhere else (25).
Preparation and analysis of polysaccharides.
Extraction of surface polysaccharides by a modified hot phenol-water method and deoxycholate-polyacrylamide gel electrophoresis (DOC-PAGE) analysis of the samples were carried out as described previously (18, 26).
Genetic complementation experiments.
pLAFR1 cosmid clones carrying either wild-type or mutant rkp regions with different Tn5 insertions (Table 1) were introduced by triparental conjugation (15) into the receptor mutants. At least two independent tetracycline-resistant transconjugants were tested for phage 16-3 sensitivity and for production of capsular polysaccharide by DOC-PAGE.
DNA manipulations and sequence determination.
Standard procedures including DNA isolation, restriction enzyme digestion and ligation, agarose gel electrophoresis, and transformation of E. coli were performed by conventional methods (28) or as recommended by the suppliers. For sequencing the h region of phage 16-3, appropriate restriction fragments were subcloned into the vector pBluescript II SK(+) and their nucleotide sequences were determined in both directions with the BigDye terminator kit on an Applied Biosystems 373A sequencer (Perkin Elmer, Wellesley, Mass.).
Mutant alleles of the rkpM and h genes were amplified by PCRs with primers outside of the coding regions. DNA sequences were determined directly in both strands with the PCR primers and inside primers as well.
For amplification of the rkpM sequences primers Rm15 (GCATTAGGCCCGGGGAGAAGC) and Rm13 (CAGGCCGAAGGAACGGAACCTC) were applied. To determine the DNA sequence, four additional primers were used: Rm25 (CGAGCCAAGGTGGACTTCGTT), Rm23 (CAGGCCGGAATAGCTGTCAGG), Rm35 (CTGGCTCGGCGATATGATAAG), and Rm33 (AGCGAAATGCACGAGCACAAC).
The h region was amplified in two overlapping parts with primer pairs h51 and h31 (CAACTGCCGCGGAGATGG and GCGGGCTGCAAAATCCAGA) and h55 and h35 (CAGTTCGGCGCCATCCAC and CGGCATCTGGTTCGGGTAGA). Besides the above primers, h32 and h52 primers (CCGCCAGAAACGACTTCC and GCCATTGCCCGATGAGG) were used for sequence determination.
Computer-assisted sequence analysis.
PCGene software (designed by Amos Bairoch, Intelligenetics Corp., Mountain View, Calif.) and the GCG program package (8) were used for basic DNA sequence analysis and assembly. Amino acid homology searches were performed against the nonredundant database of the NCBI BLAST Network Service with the blastp program (1). Protein family and domain predictions were performed with different proteomics tools from the ExPASy Molecular Biology Server. Transmembrane domains were calculated with the TopPred2 program at the Stockholm University Server (www.sbc.su.se/≈erikw/toppred2/).
The codon preference program of the GCG software package was used to identify open reading frames (ORFs) showing codon usage similar to that of an average housekeeping S. meliloti gene as described previously (17).
Nucleotide sequence accession number.
The DNA sequence of the h region of phage 16-3 has been registered in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession number AJ567376. Coordinates in this publication are identical to those of the database record. The DNA sequence of the rkp-3 region was published earlier under accession number AJ245666.
RESULTS
Isolation of mutants affected in phage adsorption.
Many phage 16-3-resistant derivatives of S. meliloti 41 carrying the Tn5 insertion in one of the identified rkp genes were isolated and characterized previously (16, 17, 21, 25). Representatives of these mutants were not suitable to detect host range phage mutants, suggesting that either Tn5 insertion mutants are not applicable for this purpose or the gene(s) involved in the formation and modification of phage receptor has not been identified yet. In order to study the nature of the phage receptor, 146 spontaneous phage-resistant mutants were isolated and characterized further. Only one of these mutants, GH4046, was suitable to isolate host range phage mutants. When the wild-type phage population was titered on GH4046, host range mutant phages (representative 16-3 h5) appeared with a frequency of 10−5 to 10−6 suggesting that the phage receptor of this mutant had been modified but was still present on the bacterial surface.
Strain GH4046 was not able to adsorb phage 16-3 (99.9% of the phage particles remained in the supernatant) and produced no detectable capsular polysaccharide (KR5 antigen) according to DOC-PAGE analysis (Fig. 1). In contrast to the preliminary studies (25, 31), this result suggested that the phage receptor is not the KR5 antigen.
Receptor mutation affects the rkpM gene.
In order to decide which rkp gene is affected in strain GH4046, genetic complementation experiments were carried out by introducing the rkp-1, rkp-2, and rkp-3 gene regions separately on different cosmid clones (pPP428, pAT330, pAT399, and pAT401). Only pAT399 carrying the rkpLMNOPQ genes (Fig. 2) was able to complement the defect of GH4046, suggesting that the phage receptor mutation is located within this region (Table 1).
In a second series of complementation experiments, pAT399 cosmid derivatives carrying Tn5 insertions in one of the rkp genes were introduced and the phage sensitivity of the transconjugants was tested (Fig. 2). Only one cosmid derivative carrying an insertion in the rkpM gene was unable to restore the phage sensitivity of the mutant (Table 1), suggesting that the mutation affected the same gene, and therefore the mutant allele of GH4046 was designated rkpM4046.
In order to test whether another mutant allele of rkpM carrying a Tn5 insertion is also able to provide the “host range phenotype,” a derivative of S. meliloti that harbored the rkpM::Tn5(212) insertion was infected with the same 16-3 lysate that resulted in host range phage mutants on GH4046. In this experiment no phage plaques were detected, indicating that the rkpM4046 allele has a special feature not characteristic of the insertion mutants. It seemed probable that in contrast to Tn5 insertion mutants, the host range strain produced RkpM protein, although in a structurally altered form.
rkpM4046 allele harbors a missense mutation.
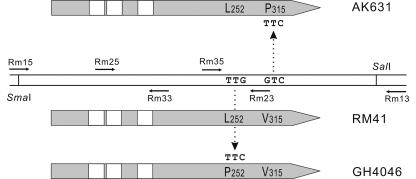
The nucleotide sequence of the rkpM4046 allele was established, and two missense mutations were detected when it was compared to the published sequence. Since the wild-type sequence was derived from strain AK631 and the rkpM4046 allele was isolated in the RM41 background, it was not possible to decide which mutations resulted in the receptor defect. Therefore, the DNA sequence of the rkpM gene from strain RM41 was also determined. In this case only one difference was found, suggesting that mutation in the TTG codon coding for Leu252 was responsible for phage resistance. In the rkpM4046 allele the corresponding codon is TTC, which determines Phe in the protein sequence (Fig. 3).
FIG. 3.
Missense mutations in the different rkpM alleles. Double horizontal bars in the middle represent the rkpM region of strain RM41. Horizontal arrows show the positions and directions of primers used for DNA sequence determination. Gray boxes represent the RkpM proteins encoded in the different alleles. White boxes represent the predicted transmembrane domains. Vertical arrows show the position and direction of the missense mutations. Change in rkpM4046 resulted in a host range mutation, while in rkpM631 they had no detectable effect.
The second mutation identified is probably a neutral one that is present in AK631 but not in RM41 and GH4046. In this case a GTC/TTC transversion resulted in the Val315Phe change in the RkpM sequence (Fig. 3).
Localization of a host range mutation on the physical map of phage 16-3.
The host range mutation h6 was mapped as tightly linked to the ts5 mutations on the genetic map of phage 16-3 (5, 19). On the physical map of the phage (11) the h6 mutation was localized by recombination marker rescue. In these experiments 16-3 ts2 phages were propagated on AK631 transconjugants harboring different cloned fragments of the 16-3 h6 DNA. When the h6 mutation was present, host range mutant phages appeared at least two orders of magnitude more frequently than in the control population (data not shown). With this method the h6 mutation was first localized between the BamHI (29) and the BamHI (36) restriction sites (on F+N BamHI fragments, pPZS5). In a second experiment it was localized between BamHI (34) and BamHI (36) restriction sites on the 1.16-kb N BamHI fragment (Fig. 4).
DNA sequence of the h region.
The DNA sequences of different subclones of the 6.18-kb EcoRI fragment from the pDH1 cosmid clone that carries a large portion of the phage genome (Fig. 4) were established. An ORF representing the putative h gene was localized on a 2,295-bp DNA fragment between an SphI and an StuI restriction site (Fig. 4).
On the 3′ end of the coding region, a transcription termination signal-like sequence was found with seven-base-long inverted repeats followed by a short poly(T) sequence, supporting that the identified ORF represents a functional gene (Fig. 4).
Prediction of the beginning of the coding sequence was uncertain. The first ATG, the most frequent translation start codon, was located more than 300 bp downstream of a 5′ translational stop codon (TGA) in the same frame. Between the 5′ stop codon and the first ATG, several other possible alternative start codons were found. Besides three in-frame GTG codons, several TTG and CTG codons may also initiate translation in this region. So far, two genes of phage 16-3 have been proved to use a non-ATG initiator codon: GTG for the int gene (29) and CTG for one of the immX coding sequences (5). In order to compare the putative product of the h gene with the protein databases, the first in-frame TTG codon at position bp 85 was used for translation, resulting in a 703-amino-acid protein (Fig. 4). A ribosome binding site-like sequence (GGAG) 8 bp upstream from this codon made the existence of this translational start probable.
A non-ATG translational start was also supported by the codon preference analysis, since codon usage characteristic for an average S. meliloti gene appeared around position 80 in the coding frame of the predicted h gene and was continued to the end of the coding sequence.
The putative H protein showed no significant homology to any other protein with known function in the databases. Only a putative protein encoded in Mesorhizobium loti shared 30% identity with it (E value = 5e-49).
h5 allele carries a missense mutation.
In order to prove that the ORF identified in the isolated region is involved in phage receptor recognition, the nucleotide sequence of a host range allele was also established. The original h6 allele was no longer available, and therefore the newly isolated h5 allele was analyzed. With PCR primer pairs, the DNA region was amplified from the 16-3 h5 mutant in two overlapping parts and their nucleotide sequences were determined on both strands directly with the help of h region-specific primers. The sequence data showed that the 16-3 h5 host range mutant carried one missense mutation on the 3′ region of the coding frame of the putative h gene. A wild-type GGC codon (from bp 1846 to 1848) encoding a Gly was altered by transition to GAC, which corresponds to Asp (Fig. 4).
Additional factors may take part in phage-receptor interactions.
We wanted to know whether additional amino acids of the identified proteins or other structural elements are involved in phage adsorption, and therefore a second series of mutant isolations was carried out by the method described above.
Two types of bacterial mutants showing different phenotypes in the phage test were identified. Interestingly, when the same wild-type phage population was titered on the new mutants, four times more host range phages were detected on some strains (representative GH4180) than on others (representative GH4178). This indicated that at least one type of host range phage mutant is not able to infect GH4178. Several phage mutants isolated on GH4180 were tested both on the mutant (GH4046, GH4178, and GH4180) and on the wild-type strain. Similar to 16-3 h5, some of them (representative 16-3 h105) were able to infect all of the bacteria tested, while a new category represented by mutant 16-3 h109 was not able to infect GH4046 and GH4178 (Table 1).
By complementation experiments, it was proved that GH4178 carries a mutant allele of rkpM similar to GH4046. What is more, the DNA sequence analysis of rkpM4178 showed that it has the same missense mutation as rkpM4046.
In contrast to the above mutants, complementation analysis of mutant GH4180 showed that it has no mutation either in the rkpM or in other rkp genes in the surrounding region. Instead of pAT399, cosmid clone pAT401 was able to restore the phage-sensitive phenotype of GH4180 (Table 1), suggesting that the defect in this mutant is located on the right side of the rkp-3 region (Fig. 2).
The nucleotide sequence of the h region of the newly isolated host range phage mutants was also determined in a way similar to that described above. It was proved that the h105 allele harbors the same missense mutation as the h5 allele. Interestingly, no mutation was detected in the region sequenced when DNA from phage 16-3 h109 was amplified, suggesting that in this case another locus is affected by the host range mutation.
DOC-PAGE analysis of the polysaccharides of the new bacterial mutants supported the genetic data. A capsular polysaccharide pattern characteristic of KR5 antigen was not detected in samples prepared from GH4178, while capsular polysaccharide prepared from GH4180 was almost similar to the wild-type capsular polysaccharide, suggesting that in this case a basic biosynthesis gene was not affected by the mutation (Fig. 1).
DISCUSSION
Analysis of phage-resistant bacterial mutations and host range bacteriophage mutations makes it possible to get information on the molecular nature of the phage receptor and on the interaction between the phage and host molecules involved in recognition. In addition, using bacterial point mutants, we aimed to get more information about the biosynthesis of the capsular polysaccharide, KR5 antigen, of S. meliloti 41.
Host range mutations affecting the S. meliloti 41 and phage 16-3 connection were isolated several years ago and the h6 mutation was mapped on the phage chromosome (19, 20). Unfortunately, the molecular nature of the mutations has not been investigated, and the mutant strains described are not available any more. Therefore, a new mutant isolation procedure was carried out, and both bacterial and host range phage mutants were examined.
In the first screen, many spontaneous phage 16-3-resistant mutants were isolated from S. meliloti 41, but only one of these mutants, GH4046, was able to interact with host range phage mutants that appeared in a wild-type phage lysate. This suggests that the phage receptor is present, although with an altered structure, on the surface of GH4046. Genetic complementation analysis revealed that the mutation is located in the rkpM gene. A surface polysaccharide, now described as KR5 antigen, was proposed previously to form the phage 16-3 receptor (25, 31). DOC-PAGE characterization of GH4046 did not support this idea because it produced no capsular polysaccharide at all. In contrast to GH4046, other mutants carrying transposon Tn5 insertions in the rkpM gene show no host range phenotype, suggesting that the rkpM4046 allele of GH4046 represents a special mutation resulting in minor changes in the protein structure. Indeed, a missense mutation that resulted in the Leu252Phe substitution has been identified as the only difference between the wild-type and the mutant allele.
The exact function of RkpM in the biosynthesis of KR5 antigen has not been investigated. On the basis of sequence homology, RkpM belongs to the DegT/EryC aminotransferase family (17). Members of this family take part in the biosynthesis of some antibiotics and polysaccharides. Three transmembrane domains in the RkpM sequence were predicted at positions 50 to 70, 73 to 93, and 123 to 143 that probably orient the C-terminal part of the protein to the periplasmic space. To interpret the phenotype of the rkpM4046 allele, it is proposed that the N-terminal region of the RkpM protein is inserted into the inner membrane, and the C-terminal region, affected by the missense mutation, is presented on the cell surface (Fig. 3).
Interestingly, mutations in almost all of the rkp genes result in a clear phage-resistant phenotype (no host range phage mutants could be isolated), indicating that any defect in the biosynthesis blocks the presentation of the phage receptor. Mutant allele rkpM4046 is a rare exception where a single missense mutation destroys the biosynthetic function of the RkpM protein (no capsular polysaccharide) but the assembly of the phage receptor is almost correct, which makes the isolation of host range phage mutants possible. According to our hypothesis, the C-terminal part of the RkpM protein takes part in the formation of the phage receptor, and the identified missense mutation blocks the recognition and attachment process.
Host range missense mutation of phage 16-3 identified in the h5 allele supports this idea. This mutation resulted in a Gly/Asp substitution in the C-terminal part of the putative H protein, which supported our prediction that the h gene encodes the tail fiber protein of phage 16-3. These results resemble that of the E. coli-lambda phage system, where missense mutations in the malB gene resulted in phage resistance but phage mutants harboring a missense mutation at the 3′ part of the J gene could infect the mutant bacteria as well as the wild-type strain (4, 30). The H protein of 16-3 showed no significant homology either to the J protein of lambda phage or to any other known protein in the databases.
The head and tail proteins of 16-3 were characterized earlier, and the molecular mass of one of the two tail proteins (detected as a weaker band on the SDS-PAGE gels) was 72 kDa (13). This is in good agreement with the 76-kDa (703 amino acids) predicted molecular mass of the H protein.
In order to identify additional amino acid residues that affect the receptor-phage interaction, a second mutant screen was carried out. Two types of mutation pairs were characterized. Interestingly, mutant GH4178 carried the same allele identified in GH4046, indicating that there is only one or a very few possible changes that result in bacterial mutants accessible to host range phage mutants. In contrast, more positions of the malB gene in E. coli were identified that resulted in the isolation of host range lambda mutations and changes in more positions of the J gene of lambda phage were reported to result in host range mutations (4, 30).
A new type of S. meliloti 41 mutant, GH4180, was also identified, where the mutation affected another rkp gene within the rkp-3 region, indicating that the receptor for 16-3 consists of more than one structural component. It is very probable that the mutation in GH4180 is in the rkpZ gene, since rkpZ mutants produce an almost-wild-type capsular polysaccharide (2, 17, 27) similar to that of GH4180, and there is no other gene in this region of the mutation which results in a similar phenotype. In addition, one may speculate that the wild-type recognition process also requires the KR5 antigen itself, and this was the reason the phage adsorption ability of the purified bacterial envelope could be destroyed by carbohydrate-specific treatments (25, 31).
With the help of GH4180, a second mutation outside of the h gene of phage 16-3 was also identified that influenced the attachment of phage particles. It is interesting that besides the h mutation, another locus, Ant, influencing the antigenic properties of the phage, was also mapped previously on the 16-3 chromosome. The Ant mutation was speculated to represent another possible type of host range mutation (19), and therefore h109 may represent an allele of the Ant gene. The locations and characteristic features of these new host range mutations will be investigated in the future.
Acknowledgments
We are grateful to L. Orosz and P. Papp for advice on the work and on the preparation of the manuscript. We appreciate the skillful technical assistance of M. Miklósvári and J. Keidl.
This work was supported by grant OTKA T038377.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Brzoska, P. M., and E. R. Signer. 1991. lpsZ, a lipopolysaccharide gene involved in symbiosis of Rhizobium meliloti. J. Bacteriol. 173:3235-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. Xl1-Blue: a high efficiency plasmid transforming RecA Es. BioTechniques 5:376-379. [Google Scholar]
- 4.Clement, J., E. Lepouce, C. Marchal, and M. Hofnung. 1983. Genetic study of a membrane protein: DNA sequence alterations due to 17 lamB point mutations affecting adsorption of phage lambda. EMBO J. 2:77-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csiszovszki, Z., Z. Buzas, S. Semsey, T. Ponyi, P. Papp, and L. Orosz. 2003. immX immunity region of Rhizobium phage 16-3: two overlapping cistrons of repressor function. J. Bacteriol. 185:4382-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallmann, G., F. Marincs, P. Papp, M. Gaszner, and L. Orosz. 1991. The isolated N-terminal DNA binding domain of the c repressor of bacteriophage 16-3 is functional in DNA binding in vivo and in vitro. Mol. Gen. Genet. 227:106-112 [DOI] [PubMed]
- 7.Dallmann, G., P. Papp, and L. Orosz. 1987. Related repressor specificity of unrelated phages. Nature 330:398-401. [Google Scholar]
- 8.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorgai, L., F. Olasz, M. Berenyi, G. Dallmann, A. Pay, and L. Orosz. 1981. Orientation of the genetic and physical map of Rhizobium meliloti temperate phage 16-3. Mol. Gen. Genet. 182:321-325. [Google Scholar]
- 10.Dorgai, L., I. Papp, P. Papp, M. Kalman, and L. Orosz. 1993. Nucleotide sequences of the sites involved in the integration of phage 16-3 of Rhizobium meliloti 41. Nucleic Acids Res. 21:1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorgai, L., G. Polner, E. Jonas, N. Garamszegi, Z. Ascher, A. Pay, G. Dallmann, and L. Orosz. 1983. The detailed physical map of the temperate phage 16-3 of Rhizobium meliloti 41. Mol. Gen. Genet. 191:430-433. [DOI] [PubMed] [Google Scholar]
- 12.Epple, G., K. M. G. M. vanDerDrift, J. E. Thomas-Oates, and O. Geiger. 1998. Characterization of a novel acyl carrier protein, RkpF, encoded by an operon involved in capsular polysaccharide biosynthesis in Sinorhizobium meliloti. J. Bacteriol. 180:4950-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdei, S., B. Dudas, L. Orosz, and E. Duda. 1982. Identification of structural proteins of Rhizobium meliloti temperate phage 16-3. J. Gen. Virol. 62:145-152. [DOI] [PubMed] [Google Scholar]
- 14.Fraysse, N., F. Couderc, and V. Poinsot. 2003. Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur. J. Biochem. 270:1365-1380. [DOI] [PubMed] [Google Scholar]
- 15.Friedman, A. M., S. R. Long, S. E. Brown, W. J. Buikema, and F. M. Ausubel. 1982. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289-296. [DOI] [PubMed] [Google Scholar]
- 16.Kereszt, A., E. Kiss, B. Reuhs, R. W. Carlson, A. Kondorosi, and P. Putnoky. 1998. Novel rkp gene clusters of Sinorhizobium meliloti involved in capsular polysaccharide production and the invasion of the symbiotic nodule: rkpK gene encodes for a UDP-glucose dehydrogenase. J. Bacteriol. 180:5426-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiss, E., A. Kereszt, F. Barta, S. Stephens, B. Reuhs, L., A. Kondorosi, and P. Putnoky. 2001. The rkp-3 gene region of Sinorhizobium meliloti Rm41 contains strain-specific genes that determine K antigen structure. Mol. Plant-Microbe Interact. 14:1395-1403. [DOI] [PubMed] [Google Scholar]
- 18.Kiss, E., B. Reuhs, J. Kim, A. Kereszt, G. Petrovics, P. Putnoky, I. Dusha, R. W. Carlson, and A. Kondorosi. 1997. The rkpGHI and -J genes are involved in capsular polysaccharide production by Rhizobium meliloti. J. Bacteriol. 179:2132-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orosz, L., and T. Sik. 1970. Genetic mapping of rhizobiophage 16-3. Acta Microbiol. Acad. Sci. Hung. 17:185-194. [PubMed] [Google Scholar]
- 20.Orosz, L., Z. Svab, A. Kondorosi, and T. Sik. 1973. Genetic studies on Rhizobio-phage 16-3. Genes and functions on the chromosome. Mol. Gen. Genet. 125:341-350. [PubMed] [Google Scholar]
- 21.Petrovics, G., P. Putnoky, B. Reuhs, J. Kim, T. A. Thorp, K. D. Noel, R. W. Carlson, and A. Kondorosi. 1993. The presence of a novel type of surface polysaccharide in Rhizobium meliloti requires a new fatty acid synthase-like gene cluster involved in symbiotic nodule development. Mol. Microbiol. 8:1083-1094. [DOI] [PubMed] [Google Scholar]
- 22.Priefer, U. B., R. Simon, and A. Puhler. 1985. Extension of the host range of Escherichia coli vectors by incorporation of RSF1010 replication and mobilization functions. J. Bacteriol. 163:324-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putnoky, P., E. Grosskopf, D. T. C. Ha, G. B. Kiss, and A. Kondorosi. 1988. Rhizobium fix genes mediate at least two communication steps in symbiotic nodule development. J. Cell Biol. 106:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putnoky, P., and A. Kondorosi. 1986. Two gene clusters of Rhizobium meliloti code for early essential nodulation functions and a third influences nodulation efficiency. J. Bacteriol. 167:881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putnoky, P., G. Petrovics, A. Kereszt, E. Grosskopf, D. T. C. Ha, Z. Banfalvi, and A. Kondorosi. 1990. Rhizobium meliloti lipopolysaccharide and exopolysaccharide can have the same function in the plant-bacterium interaction. J. Bacteriol. 172:5450-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuhs, B. L., R. W. Carlson, and J. S. Kim. 1993. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J. Bacteriol. 175:3570-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuhs, B. L., M. N. Williams, J. S. Kim, R. W. Carlson, and F. Cote. 1995. Suppression of the Fix− phenotype of Rhizobium meliloti exoB mutants by lpsZ is correlated to a modified expression of the K polysaccharide. J. Bacteriol. 177:4289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Semsey, S., I. Papp, Z. Buzas, A. Patthy, L. Orosz, and P. Papp. 1999. Identification of site-specific recombination genes int and xis of the Rhizobium temperate phage 16-3. J. Bacteriol. 181:4185-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werts, C., V. Michel, M. Hofnung, and A. Charbit. 1994. Adsorption of bacteriophage lambda on the LamB protein of Escherichia coli K-12: point mutations in gene J of lambda responsible for extended host range. J. Bacteriol. 176:941-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams, M. N., R. I. Hollingsworth, S. Klein, and E. R. Signer. 1990. The symbiotic defect of Rhizobium meliloti exopolysaccharide mutants is suppressed by lpsZ+, a gene involved in lipopolysaccharide biosynthesis. J. Bacteriol. 172:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanisch-Perron, C., J. Viera, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]