Abstract
Frameshift mutations in a poly(G) track at the flhB gene of Pseudomonas putida DOT-T1E are responsible for the diminished swimming of this strain on semisolid medium, which contrasts with the high swimming ability of P. putida KT2440, which does not exhibit a poly(G) track at the flhB gene. We previously showed that a mutant lacking FlhB was more sensitive to solvents than the wild-type strain (Segura et al., J. Bacteriol., 183:4127-4133, 2001). In this study, we show that swimming ability correlates with solvent tolerance in P. putida DOT-T1E, so that growth conditions favoring a functional flhB gene (growth on semisolid medium) resulted in increased innate tolerance to a sudden toluene shock.
The flagellar biosynthetic pathway in gram-negative bacteria has been extensively studied (1, 14). It involves the sequential expression, localization, and assembly of subunits leading to a mature flagellum. Control of at least 50 genes, including those encoding the chemosensory apparatus, is required for flagellum formation (15). These motility genes are controlled at the transcriptional level in a hierarchical fashion, allowing bacteria to stringently control the production and assembly of flagellum subunits in response to environmental signals and to sense organelle structural intermediates (10). The flagellar export apparatus is involved mainly in flagellum assembly, but it has recently been reported that this system is parasitized to export proteins that are unrelated to flagellum assembly, i.e., export of a phospholipase to the periplasmic space or the outer medium in Yersinia enterocolitica (27) and export of lipases and hemolysin in Xenorhabdus nematophilus (9).
In Pseudomonas putida DOT-T1E and P. putida S12, flagellar genes have been shown to be involved in organic solvent tolerance, although the role of their proteins in solvent tolerance remains unclear (13, 24). Organic solvents with a logPow (logarithm of its partition coefficient in n-octanol and water) between 1.5 and 4.0 are extremely toxic for microorganisms and other living cells because they partition preferentially in the cytoplasmic membrane, disorganizing its structure and impairing vital functions. Several elements have been suggested to be involved in the response to these toxic chemicals. Some responses, such as increased rigidity of the cell membrane via the isomerization of cis-unsaturated fatty acids to the corresponding trans isomers, occur very rapidly (the reaction has been observed just 2 min after the exposure of the cell to organic solvents), whereas others, such as the increase in the content of phospholipids, can take place up to 30 min after addition of the solvent (12, 20). Although changes in phospholipid fatty acids are not essential for solvent tolerance, they probably represent a first response that allows the cells to gain time for de novo biosynthesis of other components involved in tolerance.
The efflux of the organic solvents by efflux pumps of the resistance, nodulation, and cell division (RND) family is probably the most important mechanism of solvent tolerance in gram-negative bacteria (22). In P. putida DOT-T1E, three efflux pumps are mainly involved in solvent tolerance (17, 21, 23). The TtgABC and TtgGHI pumps are expressed at a certain level in the absence of toluene. Expression of the TtgDEF and TtgGHI efflux pumps is increased when toluene is present in the culture medium (6, 17, 21, 23). Therefore, the TtgABC and TtgGHI efflux pumps are involved in the innate tolerance response, and TtgGHI is also involved in induced resistance. TtgDEF, on the other hand, seems to be involved only in induced tolerance.
Given that mutated FliP, FlhB, and other proteins involved in flagellum export were sensitive to solvents, we hypothesized that the flagellar export machinery could be involved in the possible translocation of toluene tolerance proteins (such as some components of the efflux pumps) to the periplasmic space (13, 24).
In the course of our investigation, reversible frameshift mutations in a short homopolymeric tract of guanine residues located at the 5′ region of the flhB gene were found in P. putida DOT-T1E. In this study, we show that phase variation in the P. putida DOT-T1E flhB gene influences its swimming ability and its tolerance to toluene shocks. Translational variation caused by frameshift mutations has been shown to be a widespread mechanism for adaptation to new environments.
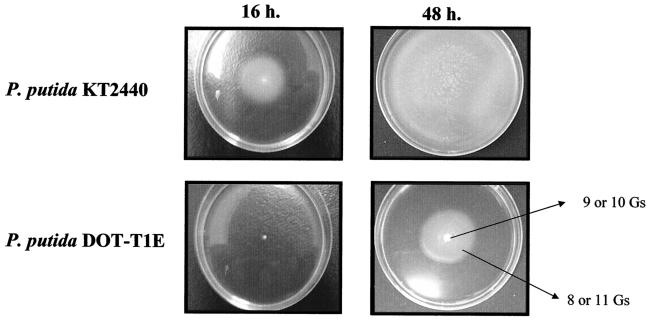
P. putida DOT-T1E presented retarded motility in soft-agar plates with respect to P. putida KT2440.
P. putida KT2440 (8) and P. putida DOT-T1E (19) cells were pregrown on Luria-Bertani (LB) liquid or solid medium and inoculated as a single spot in the center of a semisolid LB plate whose agar concentration was 0.3% (wt/vol). P. putida KT2440 showed a swimming halo of around 4 cm in diameter after 16 h, whereas DOT-T1E cells needed about 48 h to achieve a similar-sized swimming halo (Fig. 1). Given that the growth rate of both strains is similar in liquid LB medium, 60 ± 1 min for P. putida DOT-T1E versus 66 ± 2 min for P. putida KT2440, the above results suggested that P. putida DOT-T1E has to adapt to the new medium. If this were the case, the adapted cells would have an increased capability for swimming. When P. putida DOT-T1E cells pregrown on LB soft-agar plates for 48 h were used to inoculate in a single spot a new soft-agar plate, the 4-cm swarming halo was observed 16 h after the inoculation (data not shown). These results indicate that in LB liquid or solid medium P. putida DOT-T1E cells do not have the ability to swim and suggest that change(s) needs to occur in the population in response to the new environment. In fact, when we observed the cells from the liquid and soft-agar medium under the microscope, we detected two different cell morphologies in the liquid culture: large nonmotile cells, and smaller motile cells. Cultures from soft-agar plates contained only small motile cells.
FIG. 1.
Morphology of P. putida DOT-T1E and P. putida KT2440 colonies in soft-agar plates.
The flhB gene of P. putida DOT-T1E is translationally out of frame when the cells are grown on LB liquid or solid medium but not in soft-agar medium.
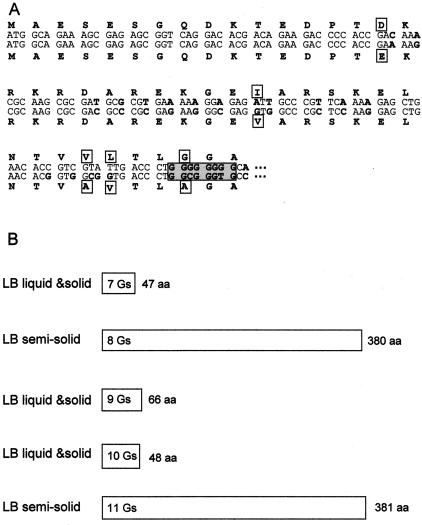
Some of the flagellar genes, fliLMNOPQR and flhBA, had previously been cloned and identified in our laboratory (24). In other bacteria, these genes are involved in the formation of the flagellar export apparatus (16). Sequence analysis of the P. putida DOT-T1E flagellar operon (GenBank accession number AF031418) revealed a run of eight G's at the 5′ end of the flhB gene (Fig. 2A). Phase variation due to a high frequency of reversible frameshift mutations in poly(A), poly(G), or poly(T) stretches have been described in several bacterial genes as response mechanisms to different environmental conditions (3, 4, 5, 11, 18, 25, 26, 28). To analyze whether the stretch of G's within the flhB gene was involved in the retarded swimming of P. putida DOT-T1E, PCR and appropriate primers (available upon request) were used to amplify a DNA fragment (2) within this flagellar region by using as a template chromosomal DNA from several independent DOT-T1E cultures grown on either liquid or solid LB medium as well as from cells pregrown on soft-agar plates for 48 h. When the inoculum came indistinctly from liquid or solid LB medium, three different sequences were found that differed only in the number of G's (10, 9, or 7). In all cases, the translated FlhB protein was truncated shortly after the run of G's (Fig. 2B). The flhB sequence obtained from cells growing on soft-agar plates contained a string of 8 or 11 G's, and the corresponding open reading frame extended over 380 amino acids in the first case and 381 amino acids in the latter, approximately the length of other FlhB proteins described for several microorganisms, i.e., Escherichia coli, Pseudomonas aeruginosa, Rhodobacter sp., and Campylobacter sp. (GenBank sequences NP416394, AAG04838, D32203, and D90830).
FIG. 2.
(A) Alignment of the 5′ ends of P. putida DOT-T1E (upper sequence) and P. putida KT2440 (lower sequence) flhB genes. The deduced protein sequences are also shown. Amino acid differences between the two strains are shown inside squares. Changes in nucleotides are in bold. The eight Gs in P. putida DOT-T1E and the corresponding sequence in P. putida KT2440 are shown in the shaded box. (B) Schematic representation of the sizes of five different polypeptides deduced from the sequences obtained. The length of each deduced protein is shown on the right. The number of Gs within the flhB gene is shown inside the protein representation. aa, amino acids.
Moreover, when we sequenced the DNA extracted from the cells of the inner part of the soft-agar colony, the flhB gene contained 9 or 10 G's, and its translation yielded a truncated FlhB peptide. When the DNA came from the cells from the border of the colony, the corresponding G stretch in the flhB gene contained 8 or 11 G's encoding a full-length protein. These results explain why when the cells used to inoculate the soft-agar plates came from the swimming halo they produced a swimming halo of 4 cm after only 16 h (as in P. putida KT2440).
A frameshift mutation in a poly(T) strip within the flhA gene was reported for Campylobacter coli. The authors of that study suggested that these frameshift mutations allowed the cell to save energy under circumstances where motility was not necessary (18), which is clearly the case for P. putida DOT-T1E growing on liquid medium in which the nutrients are homogenously distributed.
Since P. putida KT2440 did not exhibit the adaptive behavior described above for the DOT-T1E strain, we expected not to find the strip of G's within the flhB gene of this strain. Using an appropriate set of primers, we amplified and sequenced the flhB gene from P. putida KT2440 chromosomal DNA. The nucleotide sequence was 75% identical over the whole length of the genes from both strains. In the flhB sequence of P. putida KT2440, we found the 5′-GGCGGGTG-3′ sequence instead of the eight-G strip (Fig. 2A).
Swimming motility in P. putida DOT-T1E can be improved by introducing the P. putida KT2440 flhB gene in trans.
We cloned the P. putida KT2440 flhB gene in the pBRR1MCS5 vector to yield plasmid pED17, which was electroporated into P. putida DOT-T1E as previously described (7). P. putida DOT-T1E(pED17) exhibited swimming ability similar to that of P. putida KT2440; i.e., it produced a 4-cm halo in soft-agar plates when inoculated as a spot by using cells grown on liquid LB medium. In a similar way, we cloned the flhB gene that carries eight G's in pBRR1MCS5 (plasmid pANA98), and we introduced it by electroporation into the P. putida DOT-T1E strain. However, the recombinant cells were not able to form the 4-cm swimming halo after 16 h. When we sequenced the flhB gene of the plasmid extracted from these cells, nine G's were present in the gene, indicating that the frameshift variation also took place in the plasmid.
Implications of the frameshift mutations for solvent tolerance in P. putida DOT-T1E.
P. putida DOT-T1E is a toluene-tolerant microorganism, but tolerance is influenced by growth conditions. In fact, only 1 out of 104 cells survives a sudden toluene shock (19, 21). However, preinduction of the cells through their exposure to low toluene concentrations led to the survival of almost 100% of the cells after a sudden 0.3% (vol/vol) toluene shock. Innate and inducible tolerance to toluene in P. putida DOT-T1E is compromised by mutations in a series of toluene efflux pumps (17, 21, 23) of proteins involved in phospholipid turnover (Segura et al., unpublished data) and mutations in the flagellar export apparatus (24). Interestingly, it has been reported that the solvent-tolerant P. putida DOT-T1E strains with knockout mutations in either the fliP or flhB gene are more sensitive to toluene than the parental strain. An flhB::ΩKm mutant is unable to resist a sudden addition of 0.3% (vol/vol) toluene when cultures are not preinduced (24).
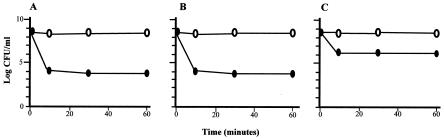
In light of the new findings described above, we hypothesized that only part of the P. putida DOT-T1E cells grown on liquid culture make the FlhB protein, and that most of the cells within the population have a truncated FlhB protein. If the FlhB protein is necessary for solvent tolerance (as shown previously), all the cells in the liquid culture that did not carry the in-frame protein should be toluene sensitive. This frameshift mutation could be a plausible explanation for the fact that only 1 in 10,000 cells of a wild-type liquid culture survived the toluene shock (Fig. 3A).
FIG. 3.
Survival rate of P. putida DOT-T1E pregrown in liquid LB medium (A), solid LB medium (B), and soft-agar medium (C). Cells were collected very carefully by using a loop, letting them stand for 10 min on LB, and then centrifuging the culture at 2,000 rpm (Desaga MC-2; Sarstedt-Gruppe) for 1 min to pellet the soft agar. Cells were suspended in LB until they reached a density of around 109 CFU per ml. The sample was divided into two halves; one was kept as a control (open symbols), and the other was treated with 0.3% (vol/vol) toluene (closed symbols). At the indicated time, the number of CFU/ml was determined.
As mentioned above, reiterative growth of P. putida DOT-T1E cells on soft agar led to a high swimming ability and to the synthesis of a functional FlhB protein. To test whether the presence of a complete FlhB protein improved toluene tolerance, we recovered P. putida DOT-T1E cells from soft-agar plates and LB solid plates and studied their survival rate after toluene shocks. We found that cells pregrown on soft-agar plates were more resistant (about 1 out of 102 cells survived) to sudden toluene shocks than those pregrown on LB solid plates (1 out of 104) or on liquid medium (Fig. 3). These results suggest that, in P. putida DOT-T1E, the presence of an intact FlhB protein helps the cell to overcome the toxic effect of toluene.
Based on the above results, we even expected differential survival of DOT-T1E growing on a soft-agar plate, depending on where the cells had been taken from, since cells from the inner part of the colonies showed 9 or 10 Gs and did not produce FlhB, whereas cells from the border of the swimming halo had 8 or 11 Gs and made a functional FlhB protein. Cells from different parts of a colony in a soft-agar plate were suspended in LB liquid medium, and the survival rate after the addition of 0.3% (vol/vol) toluene was determined. We found that cells from the border of the colony were relatively tolerant to toluene (survival rate was 1 out of 102 cells), whereas cells from the inner part of the colony were less resistant (survival rate was 1 out of 104).
In support of the finding that a functional FlhB protein increased solvent tolerance is the finding that P. putida DOT-T1E(pED17) exhibited behavior similar to that of P. putida DOT-T1E taken from the border of the colony.
In conclusion, the results presented in this study reveal a clear phenomenon of phase variation related to motility in P. putida DOT-T1E. This phenotype correlates with mutations in a G strip that either yielded or did not yield a functional FlhB protein. Early studies by Ramos et al. (21) revealed that P. putida DOT-T1E with innate solvent tolerance also showed traits related to phase variation; i.e., 1 out of 104 cells survived a sudden toluene shock. Our group and other groups had previously found that mutations in the flagellar export machinery led to solvent sensitivity in P. putida DOT-T1E (24) and S12 (13). Interestingly, we have now found in P. putida DOT-T1E a phase variation associated with flagellum biosynthesis linked to the flhB gene, which correlates with the innate solvent tolerance of this strain. Although our results should not be interpreted as meaning that the FlhB protein is sufficient per se for solvent resistance, they support the finding that the presence of intact flagellar machinery is critical for the immediate and innate solvent-tolerant response in this strain. While it is clear that the lack of FlhB directly influences the swimming ability of DOT-T1E, we cannot ascertain whether the lack of FlhB is directly responsible for solvent sensitivity or whether it is simply a side effect.
Acknowledgments
This study was supported by a grant from the European Commission (QLK3-CT-2001-00435) and the Comisión Interministerial de Ciencia y Tecnología (BIO-2003-00515).
We thank Inés Abril for secretarial assistance and Carmen Lorente for checking the English in the manuscript.
REFERENCES
- 1.Aizawa, S. 1996. Flagellar assembly in Salmonella typhimurium. Mol. Microbiol. 19:1-5. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. F. Kingstom, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1991. Current protocols in molecular biology. Greene Publishing Associates, New York, N.Y.
- 3.Carroll, P. A., K. T. Tashima, M. B. Rogers, V. J. DiRita, and S. B. Calderwood. 1997. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol. Microbiol. 25:1099-1111. [DOI] [PubMed] [Google Scholar]
- 4.Chen, C. J., C. Elkins, and P. F. Sparling. 1998. Phase variation of hemoglobin utilization in Neisseria gonorrhoeae. Infect. Immun. 66:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danaher, R. J., J. C. Levin, D. Arking, C. L. Burch, R. Sandlin, and D. C. Stein. 1995. Genetic basis of Neisseria gonorrhoeae lipooligosaccharide antigenic variation. J. Bacteriol. 177:7275-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duque, E., A. Segura, G. Mosqueda, and J. L. Ramos. 2001. Global and cognate regulators control the expression of the organic solvent efflux pumps TtgABC and TtgDEF of Pseudomonas putida. Mol. Microbiol. 39:1100-1106. [DOI] [PubMed] [Google Scholar]
- 7.Enderle, P. J., and M. A. Farwell. 1998. Electroporation of freshly plated Escherichia coli and Pseudomonas aeruginosa cells. BioTechniques 25:954-958. [DOI] [PubMed] [Google Scholar]
- 8.Franklin, F. C. H., M. Bagdasarian, M. M. Bagdasarian, and K. N. Timmis. 1981. Molecular and functional analysis of the TOL plasmid PWW0 from Pseudomonas putida and cloning of the genes for the entire regulated aromatic ring-cleavage pathway. Proc. Natl. Acad. Sci. USA 78:7458-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Givaudan, A., and A. Lanois. 2000. flhDC, the flagellar master operon of Xenorhabdus nematophilus: requirement for motility, lipolysis, extracellular hemolysis, and full virulence in insects. J. Bacteriol. 182:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikebe, T., S. Iyoda, and K. Kutsukake. 1999. Structure and expression of the fliA operon of Salmonella typhimurium. Microbiology 145:1389-1396. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson, A. B., G. Nyberg, and S. Normark. 1991. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 10:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junker, F., and J. L. Ramos. 1999. Involvement of the cis/trans isomerase Cti in solvent resistance of Pseudomonas putida DOT-T1E. J. Bacteriol. 181:5693-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieboom, J., R. Bruinenberg, I. Keizer-Gunnink, and J. A. M. de Bont. 2001. Transposon mutations in the flagella biosynthetic pathway of the solvent-tolerant Pseudomonas putida S12 result in a decreased expression of solvent efflux genes. FEMS Microbiol. Lett. 198:117-122. [DOI] [PubMed] [Google Scholar]
- 14.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 15.Macnab, R. M. 2000. Microbiology: action at a distance—bacterial flagellar assembly. Science 290:2086-2087. [DOI] [PubMed] [Google Scholar]
- 16.Minamino, T., and R. M. Macnab. 1999. Components of the Salmonella flagellar export apparatus and classification of export substrates. J. Bacteriol. 181:1388-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosqueda, G., and J. L. Ramos. 2000. A set of genes encoding a second toluene efflux system in Pseudomonas putida DOT-T1E is linked to the tod genes for toluene metabolism. J. Bacteriol. 181:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park, S. F., D. Purdy, and S. Leach. 2000. Localized reversible frameshift mutation in the flhA gene confers phase variability to flagellin gene expression in Campylobacter coli. J. Bacteriol. 182:207-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos, J. L., E. Duque, M. J. Huertas, and A. Haidour. 1995. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J. Bacteriol. 177:3911-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos, J. L., E. Duque, J. J. Rodriguez-Herva, P. Godoy, A. Haidour, F. Reyes, and A. Fernandez-Barrero. 1997. Mechanisms of solvent tolerance in bacteria. J. Biol. Chem. 272:3887-3890. [DOI] [PubMed] [Google Scholar]
- 21.Ramos, J. L., E. Duque, P. Godoy, and A. Segura. 1998. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J. Bacteriol. 180:3323-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 23.Rojas, A., E. Duque, G. Mosqueda, G. Golden, A. Hurtado, J. L. Ramos, and A. Segura. 2001. Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J. Bacteriol. 183:3967-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segura, A., E. Duque, A. Hurtado, and J. L. Ramos. 2001. Mutations in genes involved in the flagellar export apparatus of the solvent-tolerant Pseudomonas putida DOT-T1E strain impair motility and lead to hypersensitivity to toluene shocks. J. Bacteriol. 183:4127-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theiss, P., and K. S. Wise. 1997. Localized frameshift mutation generates selective, high-frequency phase variation of a surface lipoprotein encoded by a Mycoplasma ABC transporter operon. J. Bacteriol. 179:4013-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassenaar T. M., J. A., Wagenaar, A. Rigter, C. Fearnley, D. G. Newell, and B. Duim. 2002. Homonucleotide stretches in chromosomal DNA of Campylobacter jejuni display high frequency polymorphisms as detected by direct PCR analysis. FEMS Microbiol. Lett. 18:77-85. [DOI] [PubMed] [Google Scholar]
- 27.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang, Q., and K. S. Wise. 1997. Localized reversible frameshift mutation in an adhesin gene confers a phase-variable adherence phenotype in mycoplasma. Mol. Microbiol. 125:859-869. [DOI] [PubMed] [Google Scholar]