Abstract
Resistance to biotrophic pathogens is largely dependent on the hormone salicylic acid (SA) while jasmonic acid (JA) regulates resistance against necrotrophs. JA negatively regulates SA and is, in itself, negatively regulated by SA. A key component of the JA signal transduction pathway is its receptor, the COI1 gene. Mutations in this gene can affect all the JA phenotypes, whereas mutations in other genes, either in JA signal transduction or in JA biosynthesis, lack this general effect. To identify components of the part of the resistance against biotrophs independent of SA, a mutagenised population of NahG plants (severely depleted of SA) was screened for suppression of susceptibility. The screen resulted in the identification of intragenic and extragenic suppressors, and the results presented here correspond to the characterization of one extragenic suppressor, coi1-40. coi1-40 is quite different from previously described coi1 alleles, and it represents a strategy for enhancing resistance to biotrophs with low levels of SA, likely suppressing NahG by increasing the perception to the remaining SA. The phenotypes of coi1-40 lead us to speculate about a modular function for COI1, since we have recovered a mutation in COI1 which has a number of JA-related phenotypes reduced while others are equal to or above wild type levels.
Introduction
The ability of plants to prevent pathogen colonization relies on a complex network of genes and phytohormones. Salicylic acid (SA) is a well known hormone essential for activating plant basal defence responses, particularly against biotrophic pathogens (reviewed by [1]). An imbalance in basal levels of SA can dramatically alter plant resistance. For instance, Arabidopsis thaliana (Arabidopsis) plants with high levels of SA are more resistant to pathogens such as the bacteria Pseudomonas syringae pv. tomato isolate DC3000 (Pto) [2], while plants with lower SA levels are less resistant to Pto and other pathogens [3]. Furthermore, it has been shown that transgenic plants expressing the salicylate hydroxylase gene from Pseudomonas putida (NahG) can rapidly degrade SA [3] and are therefore more susceptible to biotrophic pathogens [4] such as Pto [5]. Once activated, SA resistance triggers a number of defence or pathogenesis-related genes including PR1. This gene is widely used as a marker for biotic stress and is required for various types of resistance, including Systemic Acquired Resistance (SAR). SAR acts to protect systemic leaves following earlier localized pathogen inoculation [6]. Considering the array of resistance responses to biotrophs, there is evidence for part of the resistance response being independent of SA [7].
A phytohormone with an intricate relationship with SA is jasmonic acid (JA). JA is required for a wide range of plant functions, from pollen maturation to activating defence responses against necrotrophic pathogens (reviewed by [8]). It also plays a minor role in activating defence responses against biotrophs, since exogenous application of jasmonates, i.e. methyl esther jasmonate (MeJA), can trigger defence against Pto, a response that appears dependent on activation of NPR1 [9]. JA is also important for inducing systemic induced susceptibility (SIS, [10]), which, in contrast to SAR, induces susceptibility in systemic leaves. As with SA, there are several genes that play a significant role in JA signal transduction, but only its receptor, COI1, is absolutely required for inducing all related phenotypes. SA and JA have been shown to negatively regulate each other, although there are examples of synergistic effects (reviewed in [11]).
This study aims to investigate resistance responses that are independent of SA. Although there are no viable biosynthetic mutants that are completely deficient of SA [12], NahG plants are severely depleted in SA [3]. Using a mutagenised NahG population and screening for suppressors of NahG susceptibility, we aimed to identify and characterize parts of the resistance response that would normally be masked by the abundance of SA. The results presented here show that part of the SA-independent defence response is dependent on JA perception. In addition, we show that an allele of COI1 displays a number of JA-related phenotypes reduced while others are equal to or above wild type levels.
Results
Design and implementation of a NahG suppressor screen
In a previous screen for loss of resistance to Pto, one of us (P.T.) recovered a promising Arabidopsis mutant, lra5 [13]. Further characterization showed that lra5 was in fact a stray NahG plant that contaminated the screen (data not shown). The NahGCW line was found to have some islands in the genome from the accession Ws-0 (data not shown), hence the name. Then, it was backcrossed five times with the accession Col-0. The transgene was inserted between the genes At2g46970 and At2g46980, and there were no differences in susceptibility to Pto with other Col-0 or Ws-0 NahG lines (data not shown).
We took advantage of the detailed characterization of this line to elucidate how the SA-dependent and independent branches of the resistance response interact, by using NahGCW to screen for mutations that suppress the susceptibility to Pto. Before embarking on the NahGCW suppressor screen, the conditions were optimized using several Arabidopsis mutants with an enhanced resistance against Pto [1]. We generated double mutants between NahGCW and cpr1 [14], cpr5 [15], dnd1 [16], and lsd1 [17]. With these plants, we fine-tuned a medium throughput screening protocol that would detect suppressors of NahG susceptibility. Figure S1 shows the proof of concept after optimizing the inoculations. With two inoculations of Pto, wild-type plants of the ecotypes Col-0, Ws-0 and Laer-0 can overcome the pathogen and grow almost unaffected, while NahGCW plants died or were severely affected. The enhanced disease resistance mutants in combination with NahGCW produced a small but detectable suppression of NahG, but in the case of cpr5 NahGCW, there is a strong suppression of the susceptibility (Figure S1, also described by [15]).
From 60 independent M2 families, 89 candidates were recovered and 40 selected for further characterization. These 40 putative mutants were crossed with Col-0, and their F2 progeny were inoculated with Pto. We identified 12 intragenic and 28 extragenic suppressors. Three of the intragenic suppressors were selected and characterized further to confirm that they were allelic and less susceptible to Pto than the parental line NahG (Figure S2A and S2B, respectively).
An extragenic suppressor, coi1-40, was selected for further characterization. In our conditions coi1-40 was not different to wild type in all the gross morphological phenotype (data not shown). This mutant was shown to contain a single nuclear mutation, which was recessive and effectively suppressed the susceptibility of NahG. coi1-40 was mapped to Chromosome II, between the markers C2-12916335 and BIO2-18012804 (an interval of 5.1 Mb).
Response to pathogens of coi1-40
Since NahG plants accumulate a very low but discernable level of SA [18], it was important to ascertain if the suppression of susceptibility in coi1-40 plants was related to alterations in SA levels or other mechanisms. The steady state levels of SA in coi1-40 were similar to Col-0, and coi1-40 NahGCW accumulated similar levels compared to NahGCW (Figure S3).
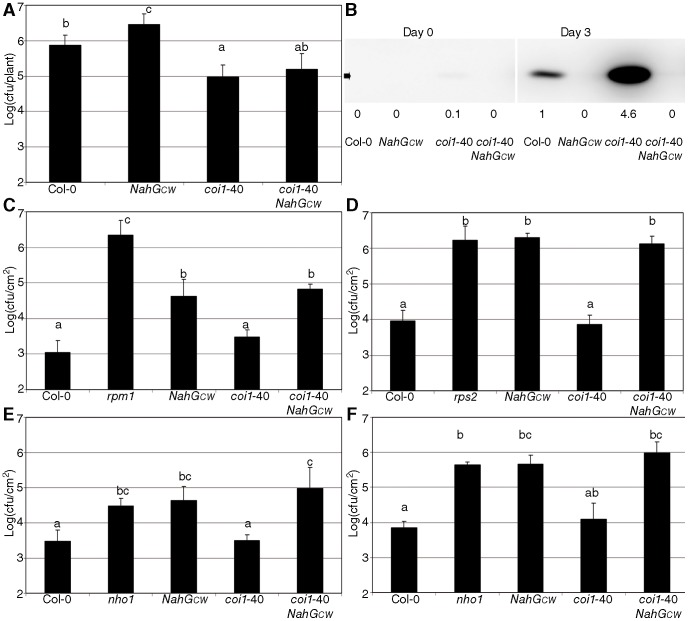
Identification of the suppressor mutants was based on visual inspection of disease symptoms. It is possible, however, that the reduced macroscopic disease symptoms did not reflect reduced pathogen growth. Therefore, more accurate measurements of Pto growth were performed. These measurements confirmed that coi1-40 is able to suppress the growth of Pto in a NahG background (Figure 1A). In fact, the single mutant was more resistant than Col-0, even in the NahG background, demonstrating that coi1-40 has a heightened basal resistance. The status of resistance can also be evaluated by the levels of the protein PR1 [19]. Upon Pto inoculation PR1 was strongly induced in coi1-40 compared to the Col-0 control, however, in coi1-40 NahGCW no induction was evident (Figure 1B). The same membranes were probed with anti-RuBisCO as an internal control (Figure 1B).
Figure 1. Characterization of resistance to biotrophs in coi1-40.
(A) Growth of Pto in the suppressor. Plants of the indicated genotypes were spray-inoculated with Pseudomonas syringae pv. tomato isolate DC3000 (Pto) at an OD600 of 0.1 when they were 18 days old. (B) PR1 Western blot of the indicated genotypes at day zero and three days post inoculation with Pto, inoculated as described in (A). The arrow indicates the position of PR1 (14 kDa). The same membrane was probed with anti-RuBisCO, as a loading and transferring control. The signal produced by anti-PR1 was quantified and normalized against the control of anti-RuBisCO. The data is shown in arbitrary units, where the amount in Col-0 inoculated with Pto is equal to one. (C) Growth of Pto(avrRpm1) in the suppressor. rpm1 is included as a control. (D) Growth of Pto(avrRpt2) in the suppressor. rps2 is added as a control. (E) Growth of Pseudomonas syringae pv. phaseolicola isolate NPS3121 in the suppressor. (F) Growth of Pseudomonas syringae pv. tabaci in the suppressor. In both (E) and (F) nho1 is used as a control. In the panels (C) to (F), 28 day-old plants were inoculated by hand infiltration with bacterial suspension at an OD600 of 2×10E-4, since it is the best way to characterize these resistances. The data represent the average and the standard deviation of three measurements, and in all the figures, the experiments were repeated three times with similar results. The letters above the bars indicate different homogeneous groups with statistically significant differences (Fisher's LSD Test, P<0.05).
NahG is not only susceptible to compatible pathogens like Pto, but also to some incompatible and non-host pathogens [20]. Figure 1C–D displays the behaviour of coi1-40 when inoculated with Pto(avrRpm1) [21] or Pto(avrRpt2) [22]. The presence of the avrRpm1 or avrRpt2 effectors converts Pto into an incompatible pathogen in the presence of the resistance genes RPM1 [23] and RPS2 [24], respectively, and coi1-40 did not suppress the susceptibility to either effector (Figure 1C and D). Analogous results were obtained when the genotypes were inoculated with the non-host pathogens Pseudomonas phaseolicola (Pph) and Pseudomonas tabaci (Ptab) (Figure 1E–F).
As mentioned previously, pathogen resistance can be activated independently of SA signalling. To assess the resistance of coi1-40 against necrotrophic pathogens, which is dependent on JA signalling [25], the mutants were inoculated with Plectosphaerella cucumerina (Figure 2). coi1-40 showed a marked increase in susceptibility to P. cucumerina one week after inoculation. Figure 2B shows the leaves of an experiment when the sampling is done two weeks after inoculation; note that coi1-40 NahGCW was slightly less susceptible than coi1-40 alone. ocp3, a mutant more resistant to P. cucumerina, is included as a control [26]. OCP3 is a homeodomain transcription factor, and a mutation in this gene renders plants more resistant to necrotrophic pathogens without affecting the resistance to biotrophs [26].
Figure 2. Resistance against necrotrophs in coi1-40.
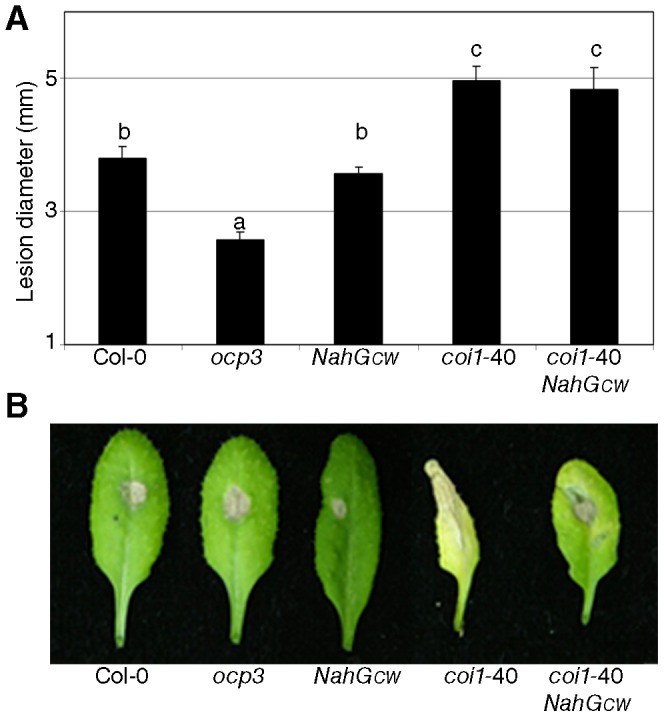
coi1-40 and its controls were inoculated with Plectosphaerella cucumerina by depositing a 6 µL drop of 5×10E6 spores/mL on a leaf, in 28-day-old plants. (A) Diameter of the lesion one week after inoculation. ocp3 is included as a control. The data represent the average and the standard error of 40 leaves. (B) Images of representative leaves two weeks after inoculation. The genotypes are shown in the same order as in (A).
JA defence-related phenotypes of coi1-40
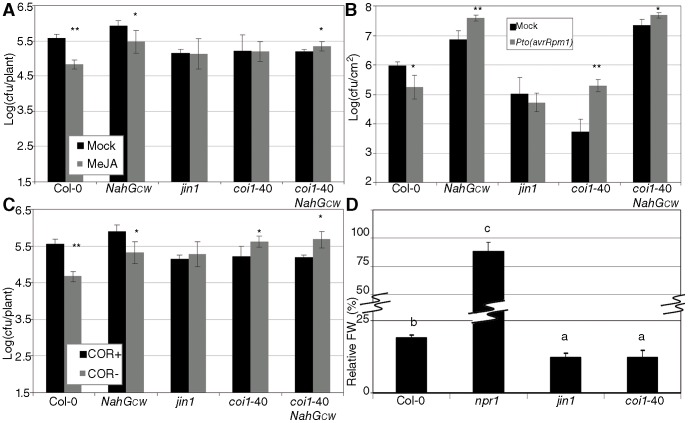
Given the enhanced susceptibility of coi1-40 to P. cucumerina and that resistance against necrotrophs is dependent on JA, we reasoned that it would be interesting to test a series of JA related phenotypes in coi1-40. Figure 3A shows the resistance induced by MeJA when Pto is inoculated one day later [27]. Both Col-0 and NahGCW respond to MeJA with a small but reproducible reduction in the levels of Pto, compared to the negative control jin1 (Jasmonate Insensitive 1, [28]). In these conditions, no resistance response was evident for coi1-40 and coi1-40 NahGCW in response to MeJA. SAR has been shown to be dependent on JA, [29] and in accordance wild-type Col-0 plants displayed SAR. However, NahGCW and jin1 (Figure 3B), coi1-40 and coi1-40 NahGCW had no SAR and Pto grew better in SAR conditions, especially for coi1-40 plants. This phenotype has been called SIS [10], and it is a systemic effect of coronatine. Some isolates like Pto are able to produce this chemical, a molecular mimic of the iso-leucine conjugate of jasmonic acid (JA-Ile, [30]), that functions as a virulence factor [31]. We inoculated coi1-40 and coi1-40 NahGCW and their controls with a Pto strain that lacks coronatine (Pto(cfa−), [31]), and a wild-type Pto strain (Figure 3C). Pto(cfa−) had a reduced growth in wild-type plants, compared to Pto, while in coi1-40 and coi1-40 NahGCW there were no detectable differences, or the differences were opposite to those on wild-type plants (Figure 3C).
Figure 3. Pathogen resistance phenotypes of coi1-40 related to JA.
coi1-40 and its controls were tested for: (A) Methyl jasmonate (MeJA) induced resistance. 17-day-old-plants were treated with either 100 µM MeJA (with 0.1% DMSO and 0.02% Silwet L-77) or a mock solution. One day later, Pto was inoculated and its growth measured as in Figure 1A. (B) Systemic Acquired Resistance. Three leaves of 28-day-old plants were hand infiltrated with either Pto(avrRpm1) or a mock solution. Two days later, Pto was inoculated and its growth in systemic leaves measured as described (see Methods). (C) Coronatine as a virulence factor. Bacteria with coronatine (Pto, COR+) or without coronatine (Pto(cfa−), COR−) were inoculated and their growth measured as in Figure 1A. (D) Negative crosstalk with SA. Plants of the indicated genotypes were treated four times with either 350 µM benzothiadiazole (BTH) or a mock solution, and their weight was recorded when 21 days old. The ratio between BTH treated and mock treated is shown as a percentage, with npr1 included as control. jin1 is included in all the panels as a control of no response to JA. Asterisks indicate statistically significant differences from the mock treatment (P<0.05 one asterisks, P<0.01 two) using the Student's t-test (two-tails).
One of the hallmarks of JA signalling in defence is the negative crosstalk with SA signal transduction [32]. Therefore, we measured the perception of benzothiadiazole (BTH, an analogue of SA, [33]) to check the status of crosstalk in coi1-40 plants. Wild-type, jin1 and coi1-40 plants had a considerable reduction in fresh weight, when compared to npr1 and wild type plants (Figure 3D). The increase in sensitivity to BTH for these mutants is consistent with less negative crosstalk from JA to SA signalling pathways.
JA growth-related phenotypes of coi1-40
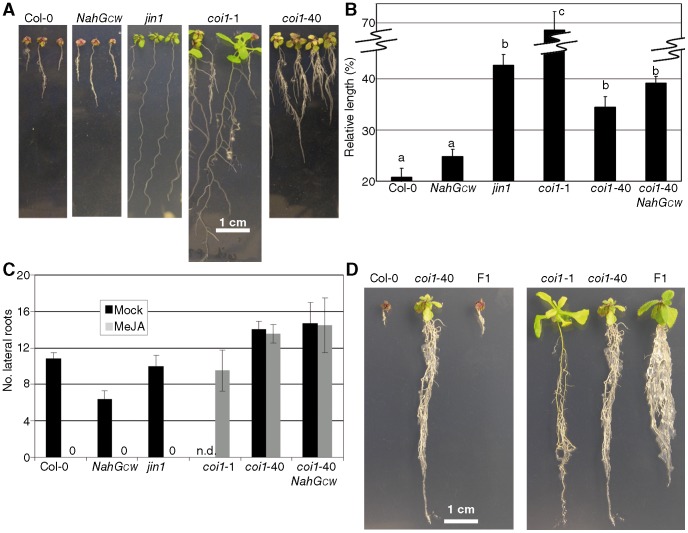
To determine whether perception of JA in coi1-40 was affected during development, we studied the phenotypes of coi1-40 alongside coi1-1. The receptor of JA, COI1 (Coronatine Insensitive 1, [34]), is an F-box protein and it is a key regulator of JA signalling, therefore null alleles are insensitive to JA. The plants were grown in vitro in the presence and absence of MeJA. In the absence of MeJA, all plants were comparable, while in the presence of MeJA, several phenotypes were evident (Figure 4A). Firstly, the aerial region of coi1-40 was larger than the wild-type control, although not as large as coi1-1. Secondly, the relative length of the primary root, in coi1-40 was intermediate between Col-0 and coi1-1 (Figure 4B). Thirdly, there was a profusion of secondary root growth (branching) in coi1-40.
Figure 4. Response of coi1-40 to JA in vitro.
coi1-40 and its controls were tested for: (A) Phenotype in plates. The indicated genotypes were grown in plates with Johnson's Media [67] supplemented with 50 µM MeJA. The pictures were taken 20 days after germination with the same settings and in the same experiment. In plates without MeJA the plants were the same size (data not shown). (B) Length of primary root. The plants were grown as described in (A), with and without 50 µM MeJA. At 10 days old, the lengths of the roots were measured in both conditions, and their ratio (MeJA treated divided by mock treated) expressed as a percentage. (C) Lateral roots. The plants were grown as described in (A), with and without 50 µM MeJA. At 14 days old, the number of lateral roots longer than 0.2 mm was counted in both conditions with the help of a magnifying glass. Note that in some genotypes like Col-0, the root does not grow in MeJA and therefore it is not possible to count lateral roots (marked as “0” in the figure). Since coi1-1 is not fertile, the number of lateral roots without MeJA was not counted (marked as not determined -n.d.- in the figure). (D) Phenotype of F1s between coi1-40 and Col-0 and between coi1-40 and coi1-1. The plants were grown as described in A, with 50 µM MeJA. The pictures were taken when the plants were 20 days old.
JA has been reported to induce root branching [35], therefore, to quantify this phenotype, we counted the number of lateral roots in 14 day-old seedlings grown in medium in the presence or absence of 50 µM MeJA. We found that coi1-40 had more lateral roots than coi1-1 when grown in the presence of MeJA, or Col-0 in mock conditions (Figure 4C). We could not sample coi1-1 in mock treatment at 10–14 days old, due to its lack of phenotype at this stage in a segregating population. Since JA-induced secondary branching increases the length of the root system we used total root dry weight as a measure of the size of the entire root system. This analysis revealed that the relative weight of coi1-40 roots was similar to coi1-1 (Figure S4).
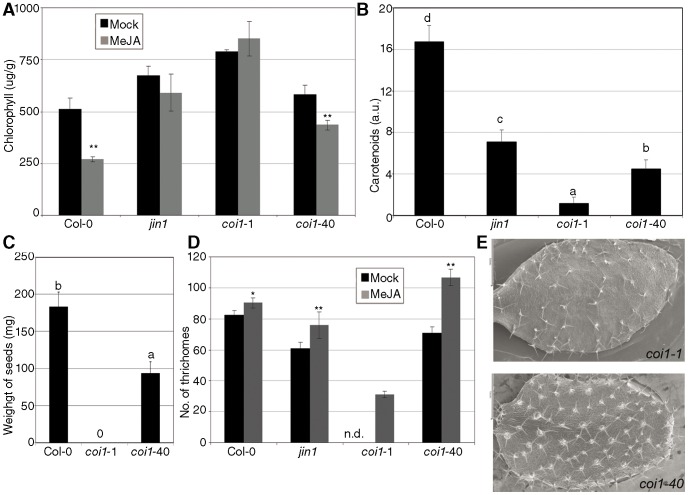
Senescence in leaves is accelerated by JA when incubated in the dark [36]. Figure 5A shows that, in wild-type plants, MeJA promoted a loss of chlorophyll. jin1 and coi1-1 did not show this effect, while coi1-40 was intermediate between coi1-1 and Col-0.
Figure 5. Allele specific phenotypes of coi1-40.
coi1-40 and its controls were tested for: (A) Senescence induced by JA. The indicated genotypes were grown in soil, and mature leaves from six-week-old plants were cut and floated on water with or without 100 µM MeJA. The amount of chlorophyll (in µg/g fresh weight) was measured after four days of darkness, with three groups of leaves of c. 1 g each. Previous to the sampling, coi1-1 plants were selected by PCR markers from a segregating population. (B) Carotenoids. 14-day-old seedlings, grown in 50 µM MeJA plates, were incubated in acetic methanol during 18 hours and the absorption of the extracts was measured. (C) Fertility. The total average seed set of eight plants grown in long day conditions. (D) Trichomes. Plants were grown in media with or without 10 µM MeJA, and when the fifth true leaf emerged, the number of trichomes was counted with the help of a magnifying glass. Since coi1-1 is not fertile, the number of trichomes without MeJA cannot be counted at this stage. (E) Detail of the distribution of trichomes in both coi1 alleles. Plants growing in MeJA plates, as indicated in (D) were visualized with a scanning electron microscope. The length of the bar (left of the picture) is 1 mm.
The synthesis and accumulation of carotenoids is activated by many abiotic and biotic signals, including JA. In addition, this biosynthesis is modulated by COI1 [37]. By growing plants in MeJA-containing media and measuring the levels of carotenoids (Figure 5B), we found that coi1-40 was intermediate between coi1-1 and Col-0. One of the main characteristics of coi1-1 is its lack of fertility. For this phenotype, coi1-40 was also found to be intermediate between wild-type and coi1-1 (Figure 5C).
The number of trichomes is another developmental phenotype partially dependent on JA, [38]. We found that the number of trichomes for coi1-40 was enhanced in the presence of MeJA (Figure 5D), and that the morphology was strikingly different to wild-type plants (Figure 5E, S6).
coi1-40 is an allele of coi1
coi1-40 and coi1-1 displayed quite different JA-dependent phenotypes, such as coi1-40 being fertile. However, both mutants shared certain phenotypes such as dry weight of the root system in MeJA, and coi1-40 mapped to an interval that contains COI1 (At2g39940). In order to check for complementation, the F1 plants from a cross between coi1-1 and coi1-40 or Col-0 and coi1-40 were obtained and grown in MeJA-containing media (Figure 4D). The F1 from the coi1-1 by coi1-40 cross showed identical phenotypes to coi1-40, when root length, number of lateral roots, and number of trichomes were assessed. This suggests that coi1-40 is an allele of COI1 (Figure S7) or that both mutations are in different genes that interact genetically and could give rise to non-allelic, non-complementation. However, in the F2 all plants were resistant to MeJA, and 3 in every 4 were similar to coi1-40, while 1 in every 4 was similar to coi1-1 (data not shown). Therefore, both mutations are allelic, and coi1-40 has allele-specific phenotypes. The F1 plants from the cross between Col-0 and coi1-40 displayed a wild type phenotype when grown in the presence of MeJA (Figure 4D) and the F2 segregated 3∶1 (Col-0 to coi1-40), indicating that coi1-40 is a recessive mutation. Sequencing of COI1 in coi1-40 revealed a single canonical EMS mutation in the gene, changing residue 22 from glutamic acid to lysine, indicating again that coi1-40 is allelic to coi1-1 (Figure S5).
The lateral root, length of the main root, and trichome phenotypes were dominant in coi1-40 with respect to coi1-1, but recessive with respect to wild type COI1 (Figure 4D and Figure S7). Once the mutation was identified, 60 F2 from coi1-1×coi1-40 and 60 F2 from Col-0 × coi1-40 crosses were analyzed with a molecular marker and the lateral root phenotype was found to cosegregate with the coi1-40 marker in both populations (data not shown). The trichome phenotype was also visually observed as cosegregating with the molecular marker in 70 F2s from the cross coi1-1×coi1-40, due to the low level of trichomes in coi1-1 (Figure 5D and E).
JA-induced expression of COI1-dependent genes in coi1-40
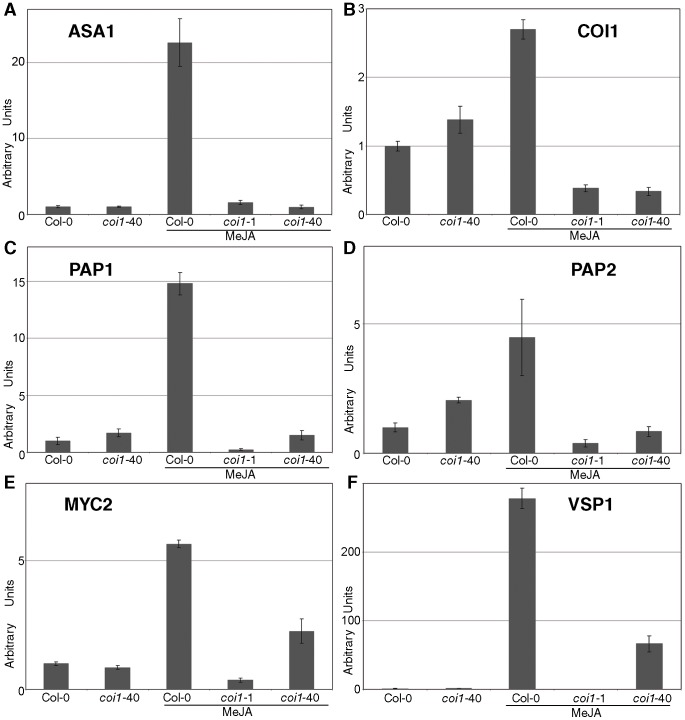
The differences in some of the JA-dependent phenotypes shown by Col-0 and the coi1-1 and coi1-40 mutants should mirror a molecular footprint. Therefore, we analyzed the effect of MeJA on the expression of six important genes involved in JA-related phenotypes (Figure 6). The ASA1 gene (Anthranilate Synthase α1, [35]) may modulate auxin biosynthesis in response to JA thus regulating lateral root formation. We analyzed the expression of this gene to address whether the lateral root phenotype of coi1-40 was dependent of this node (Figure 6A). No significant differences were observed; hence the phenotype of coi1-40 might be independent of this auxin pathway. The expression of COI1 was also examined and found to be identical in the two coi1 alleles (Figure 6B). PAP1 and PAP2 (Production of Anthocyanin Pigment 1 and 2, [39]) are two MYB genes that mediate the JA-dependent anthocyanin biosynthesis. It has been reported that the induction of both genes by JA is also dependent of COI1 [37]. In response to MeJA, coi1-40 maintained PAP1 (Figure 6C) and displayed reduced levels of PAP2 (Figure 6D). However, both displayed higher expression levels than in coi1-1. The expression of two further genes was also assessed, MYC2 and VSP1. MYC2 is a central transcription factor for most JA-induced responses and VSP1 (Vegetative Storage Protein 1, [40]) is a specific marker induced by JA. In both we found a stronger difference between these alleles. There is a certain amount of JA signalling that goes through coi1-40 inducing the expression of MYC2 (Figure 6E) and VSP1 (Figure 6F) to considerable levels, which could explain some of the phenotype differences between coi1-1 and coi1-40.
Figure 6. Allele specific molecular phenotypes of coi1-40.
Col-0, coi1-1, and coi1-40 plants were grown both in mock and 50 µM MeJA plates. RNA was extracted 10 days after germination, and transcript levels for the following genes were measured by RT-qPCR: (A) ASA1; (B) COI1; (C) PAP1; (D) PAP2; (E) MYC2; (F) VSP1. The levels of expression are normalized to three reference genes and to the level of Col-0 in mock.
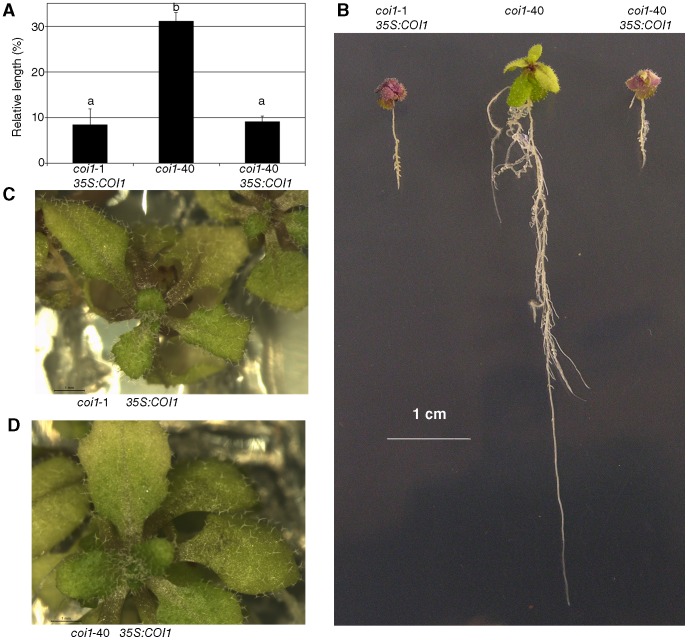
We complemented the coi1-40 mutation by crossing it with a transgenic line that express COI1-Flag under the control of the promoter 35S [41]. The coi1-40 35S:COI1-Flag behaved as the control coi1-1 35S:COI1-Flag homozygous line for relative length of the root (Figure 7A and B) and number of trichomes (Figure 7C and 7D) demonstrating that these phenotypes are caused by the mutation in coi1-40.
Figure 7. Complementation of coi1-40.
Plants of coi1-40, coi1-1 35S:COI1 and 35S:COI1 in coi1-40 background were grown in plates supplemented with and without MeJA as is described in Figure 4. (A) Length of primary root. The plants were grown with and without 50 µM MeJA. At 10 days old, the lengths of the roots were measured in both conditions, and their ratio (MeJA treated divided by mock treated) expressed as a percentage. (B) Lateral root phenotype. Picture showing the phenotype of the three lines, coi1-40, coi1-1 35S:COI1, coi1-40 35S:COI 20 days post germination. Both lines, coi1-1 35S:COI1 and coi1-40 35S:COI show similar phenotype and opposite to that shown in the control coi1-40. (C) and (D) Trichome phenotype. Plants were grown in media with 10 µM MeJA. No difference in the number of trichomes was found between coi1-1 35S:COI1 (C) and coi1-40 35S:COI (D). The picture shows the plants when the fifth true leaf emerged.
Discussion
A NahG extragenic suppressor
Resistance against biotrophs in plants depends largely on SA accumulation [42]. As NahG plants have reduced levels of SA, this provides a background where mutations that enhance the resistance in an SA independent manner are easily recognizable. Here we report the screening of a NahG mutagenised line, which resulted in the identification of intragenic (mutations in the NahG transgene itself), and extragenic mutations. An extragenic mutant, coi1-40, was fully characterized, and shown to be more resistant against biotrophic pathogens than the parental lines both in the presence or absence of NahG (Figure 1A).
coi1-40 is an interesting and informative allele by itself, as described below. However, our initial objective was to analyze the interaction between SA-dependent and independent branches of the resistance response. coi1-40 was found in our screen because it suppresses the susceptibility of NahG (Figure 1A). This suppression is produced by two mechanisms. First, the insensibility of the mutant to coronatine depletes the advantage that this chemical gives to the pathogen [31]. Second, since the steady-state level of PR1 protein in coi1-40 is almost undetectable but is strongly induced upon Pto inoculation, (Figure 1B), we speculate that coi1-40 increases the sensitivity to pathogen signals that trigger resistance. As coi1-40 was identified as an allele of COI1, mutating coi1 would potentially increase sensitivity to SA, since in wild type plants there is negative crosstalk between JA and SA [43]. The same increased sensitivity is shown with respect to BTH (Figure 3D).
Therefore, one of the mechanisms of coi1-40 suppressing NahG susceptibility is SA-independent (coronatine is no longer a virulence factor), but the other mechanism is SA-dependent (enhanced perception of SA). The two incompatible (Figure 1C and 1D) and the two non-host interactions (Figure 1E and 1F) were not affected by coi1-40. Therefore, increased sensitivity to SA and lack of coronatine recognition conferred by coi1-40 mutants has no effect on these interactions although it showed enhanced basal resistance. On the other hand, the response to P. cucumerina in coi1-40 was severely compromised (Figure 2) pointing to an impairment in the JA-disease resistance against necrotrophic pathogens. Interestingly, the response to P. cucumerina also shows the negative regulation from SA to JA. Thus, when the leaves were sampled two weeks after inoculation (Figure 2B), coi1-40 NahG were slightly less susceptible than coi1-40, likely due to the reduced perception of SA in NahG, that leads to the increased perception of JA by a weak allele of COI1.
coi1-40 differentiates phenotypes related to JA in roots
coi1-40 shares several phenotypes with other JA mutants such as jin1 or coi1-1. Thus, like coi1-1 [34], coi1-40 is fully susceptible to P. cucumerina (Figure 2). When exogenously applied, MeJA is able to induce a small resistance against subsequent Pto infections [27]. coi1-40 does not trigger this resistance (Figure 3A), nor SAR (Figure 3B, [44]), although it has an enhanced perception of SA and its analogues (Figure 3D, [45]).
There are certain differences between the phenotypes induced by the coi1-40 and coi1-1 alleles (Table 1). Four of these phenotypes indicate that coi1-40 is a hypomorph, i.e. intermediate between Col-0 and the coi1-1 null mutant. These unrelated phenotypes include the relative length of roots growing in MeJA plates, the senescence induced by JA, the production of carotenoids induced by JA, and the fertility. While two are produced by exogenous application of MeJA, two are responding to endogenous levels. While two are on sterile plates, two are in soil. From the mentioned phenotypes, it is tempting to merely assign a weak or leaky character to coi1-40, however, there are three phenotypes in which coi1-40 behaves as a hypermorph; lateral root growth, trichome development and SIS.
Table 1. Summary of the phenotypes that differentiate the coi1-40 and coi1-1 alleles.
| Genotypes | ||||
| Phenotypes | Col-0 | coi1-40 | coi1-40×coi1-1 F1 | coi1-1 |
| Root lenght1 | + | ++ | ++ | +++ |
| Senescence2 | +++ | ++ | n.d. | + |
| Carotenoids1 | +++ | ++ | n.d. | + |
| Fertility | +++ | ++ | ++3 | 0 |
| Lateral roots1 | + | +++ | ++++ | ++ |
| Trichomes1 | ++ | +++ | +++ | + |
| SIS | + | ++ | n.d. | 0 [10] |
Measured in plants grown on MeJA supplemented plates;
Senescence induced by MeJA.
Amount of seeds estimated. n.d.: not determined.
The development of lateral roots is orchestrated by the distribution of auxins (basipetal in the root and acropetal in the leaf [46]). Auxin application stimulates the formation of lateral roots [47], while inhibitors of auxins prevent the formation of them [46]. Therefore, the increased number of lateral roots in coi1-40 may be brought about by an increase in production or perception of auxins. The gene ASA1 (ANTHRANILATE SYNTHASE α1) is an auxin biosynthesis gene responsible for lateral root formation in the presence of JA [35]. Expression analysis has shown that the expression of ASA1 is COI1 dependent (Figure 6A, [35]). Therefore, ASA1 could act to integrate JA and auxin signalling. While the line of argument for auxins being involved in the formation of lateral roots in coi1-40 is appealing, this could not be verified experimentally. Thus, ASA1 was not induced by MeJA in coi1-40 (Figure 6A), nor was there any apparent phenotype of the coi1-40 mutant in 2,4-D plates (data not shown).
coi1-40 produces more trichomes in response to JA than the wild type
Arabidopsis responds to wounding or MeJA applications by increasing the number of trichomes in the newly formed leaves [48]. Surprisingly, not all mutants in JA signalling are defective in trichome response to JA [38]. In coi1-40 this response to JA is hypermorphic (Figure 5D and E). The production of trichomes in Arabidopsis involves a complex genetic model, including Glabra3 (GL3, [49]), among other genes. JA induces the expression of GL3, setting in motion the formation of trichomes [38]. In coi1-40, the levels of GL3 are not altered upon MeJA treatment (data not shown).
SIS
Pto grows better in coi1-40 plants where SAR has been triggered, which could indicate that, like other mutants in JA signalling, coi1-40 is negatively affected in SAR and displays a hypermorphic SIS [10]. Although the initial observation of SIS was obtained from incubation with a virulent strain, there is evidence that an avirulent strain can also trigger SIS [10]. While in a wild type plant this effect would be overcome by SAR, coi1-40 allows separating these two opposing tendencies, favouring SIS (Figure 3B).
Behaviour of F1s
The lateral root and trichome phenotypes are dominant with respect to coi1-1; one copy of coi1-40 increases both the number of lateral roots and trichomes if the other allele is coi1-1 (Figure 4D and Figure S7). This fact implies that any explanation of the mentioned phenotypes by secondary EMS mutations in NahGCW is highly unlikely, since all the phenotypes are dependent on the locus COI1. In addition, these phenotypes cosegregate perfectly in a dominant fashion with a molecular marker for coi1-40 in an F2 segregating family of coi1-1×coi1-40. Similarly, in the cross coi1-40×Col-0, the lateral root phenotype cosegregates perfectly in a recessive fashion with the same marker. In contrast, the lateral root phenotype was not seen in F2 populations from NahGCW×Col-0, or NahGCW×Laer-0, (data not shown).
coi1-40 and other coi1 alleles
coi1-40 shares certain phenotypes with coi1-20 [50]. This allele is also resistant to Pto, and induces PR1 strongly upon inoculation. However, coi1-20 is male sterile, and the double coi1-20 NahG does not suppress NahG susceptibility [50]. Other alleles like coi1-15 and 18 are also male sterile. The mutations in coi1-15 and 18 are frameshifts that introduce stop codons [51] while the mutation in coi1-20 is unknown. coi1-16 is fertile at temperatures below 20°C; however, root growth inhibition and JA-responsive promoter activity are not restored at lower temperatures [52]. Recently, coi1-16 was used to recover loci that suppress the ABA signalling pathway, since coi1-16 is also hypersensitive to ABA in seed germination [53]. The molecular lesion in coi1-16 results in a change of leucine to phenylalanine in the leucine-rich repeat. Lately, two more alleles, coi1-21 and coi1-22 were described as fertile and impaired in JA signalling [54]. Both alleles have a mutation in the leucine-rich repeat and suppressed the rar1 phenotype in the resistance triggered by RPM1 [54]. The mutation in coi1-40 induces an amino acid substitution in the F-box domain of the protein [51], a domain in which no previous mutations have been found. In a transgenic line the very same amino acid was changed from glutamic acid to alanine [55]. These COI1E22A plants did not perceive JA and were male-sterile. The phenotype of lateral roots and trichomes of COI1E22A was not reported, and seeds for that line are no longer available (Dr. Xie, personal communication), so the only phenotype that we can compare is the fertility of the pollen. Since coi1-40 is fertile (Figure 5C), the change to lysine does not inactivate completely the F-Box, as the change to alanine does [55]. We believe that this partial function of the F-Box is responsible of the modular behaviour of coi1-40, a theory that is discussed below.
A modular model for COI1 function
We propose two models to explain the disparity of phenotypes for coi1-40. The first one implies that it is a weak allele that retains some function. The difference in phenotypes would be a question of thresholds; some phenotypes are fully functional with the level of signal transduced by coi1-40, while others are no longer functional. For example, JA inhibits root growth (Figures 4A and B), and at the same time induces lateral root initiation [35]. If one of the phenotypes (inhibition of root growth) requires a high level of signal to occur, and the other (lateral root initiation) requires a low level threshold, a hypermorphic phenotype in an intermediate coi1 allele would be observed. Since there are a number of coi1 weak alleles, and none are reported to have hypermorphic phenotypes, this hypothesis is unlikely.
A second and simpler explanation would be a modular or selective function for COI1. In this model, coi1-40 would be impaired in some interactions, but others would function near or above wild type levels. Mechanistically, it implies that the mutated F-box is still functional in some interactions, while all other alleles described encode premature stop codons, or changes in the LRRs. However, the expression of JA-induced genes in coi1-40 does not provide a strong argument in favour of this hypothesis, although it is worth mentioning that in three out of six genes in mock conditions, the expression levels of the genes in coi1-40 are higher than in Col-0 (Figure 6). This fact could be interpreted as an enhanced response to the endogenous levels of JA (explaining the phenotype of lateral roots), but this response is not observed with exogenous MeJA.
Trichome induction is dependent on COI1 and independent of MYC2; therefore there are other components in the JA signal transduction (e.g. MYBs, [8]). The same coi1-40 protein that leads to MYC2 related phenotypes could interact in a stronger fashion with the other components. However, COI1 does not interact directly with MYC2, but the JAZ proteins (Jasmonate-Zim domain, [56]) are the required link. COI1 interacts with ASK1 to form the SCFCOI1 complex [8] that leads to the degradation of the JAZ proteins [56]. COI1 binds ASK1 through its F-box domain, while it binds to JAZ proteins through its leucine-rich repeat domain. Since the JAZs are repressors, their degradation allows MYC2 and others to promote the response to JA. In this model coi1-40 would interact with ASK1, and this complex would not promote the degradation of the JAZ proteins that are repressing MYC2, but it would degrade other JAZs. Interestingly, a knock out line of JAZ1 also showed an inhibition of the main root in media supplemented with MeJA and a significant increase in the number of lateral roots [57]. We propose that in the presence of JA, coi1-40 degrades some JAZs while stabilizing others, and each one of the JAZs has its own specific interactions.
Materials and Methods
Plant Growth and Inoculation
Arabidopsis thaliana was sown and grown as described [45]. Plants were grown in controlled environment rooms (CER) with days of 8 h at 21°C, 150 µmol m−2 s−1 and nights of 16 h at 19°C. For long day experiments, plants were also grown in a CER with the same conditions, except with 16 h of light and 8 h of darkness. The following genotypes were used: npr1 [58], ndr1 [59], sid2 [60], cpr1 [14], cpr5 [15], dnd1 [16], lsd1 [17], rpm1 [23], rps2 [24], nho1 [61], ocp3 [26], coi1-1 [34], and jin1 [28]. The treatments, inoculations, and sampling started 30 minutes after the initiation of the artificial day to ensure reproducibility. Pseudomonas syringae pv. tomato DC3000 (Pto) was maintained as described [62]. Pto was used with the pVSP61 plasmid containing avrRpm1 [62], avrRpt2 [22], or an empty vector. Pto(cfa −) [31], Pseudomonas syringae pv. phaseolicola isolate NPS3121, and Pseudomonas syringae pv. tabaci were obtained from Dr. Jeff Dangl (UNC, Chapel Hill, NC, USA). The bacteria were grown, inoculated and measured as described [63]. Systemic Acquired Resistance was performed as reported by [64], inoculating leaves with both incompatible and compatible pathogens using a blunt syringe. Plectosphaerella cucumerina was provided by Brigitte Mauch-Mani (University of Neuchatel, Switzerland), and used as described [65]. For all the experiments, at least three independent treatments were performed (three independent sets of plants sown and treated on different dates).
Chemical Treatments
Benzothiadiazole (BTH, CGA 245704), in the form of a commercial product (Bion® 50 WG, a gift from Syngenta Agro S.A., Spain) was prepared in water for each treatment and applied with a household sprayer. The BTH treatments were done as described in [45] and [66]. Briefly, plants were treated with either mock or 350 µM BTH four times during two weeks, starting when the plants were one week old. Then, the fresh weight of each genotype was recorded in both treatments and expressed as percentage of fresh weight of mock-treated plants.
JA-related phenotypes
For in vitro culture, plants were grown in Johnson's media [67] with 1 mM KH2PO4. When indicated, the plates were supplemented with either 10 or 50 µM MeJA (Duchefa, Haarlem, The Netherlands), depending on the experiment. The length of the roots was measured with ImageJ software (MIH, Bethesda, MD, USA), and the number of lateral roots, with the help of a magnifying glass. Only lateral roots longer than 0.2 mm were counted. When measuring the effect of MeJA on Pto growth, MeJA was applied by spray at 100 µM in 0.1% DMSO (SIGMA, St. Louis, MO, USA) and 0.02% Silwet L-77 (Crompton Europe Ltd, Evesham, UK) one day before Pto inoculation [68]. Senescence induced by MeJA was measured as described by [36]. For carotenoid measurements, the protocol described by [69] was followed. In order to quantify the amount of seeds produced per plant, eight coi1-40 and eight wild type plants were selected by molecular marker analysis from an F2 backcross with Col-0. Eight coi1-1 plants were also selected from an F2 population segregating for this mutation. Plants were grown in long day conditions, and when the first fruit had matured, the aerial part was covered with a paper bag to avoid loss of seeds. Once the plant had senesced, the seeds were cleaned and weighed. The number of trichomes on the fifth true leaf of 14-day-old plants grown on plates with 10 µM MeJA was determined with the aid of a magnifying glass as described by [38]. The pictures of trichomes were taken with a JSM-5410 scanning electron microscope (JEOL, Tokyo, Japan) in the Electron Microscopy Service (Universidad Politécnica de Valencia, Spain).
Western Blot
Immunodetection of PR1 protein was carried out as described [19], using an Amersham ECL Plus Western Blotting Detection Reagent (GE HealthCare, Little Chalfont, UK). The second antibody was a 1∶25,000 dilution of Anti-Rabbit IgG HRP Conjugate (Promega, Madison, WI, USA). Chemiluminescent signals were detected using a LA-3000 Luminescent Image Analyzer (Fujifilm Life Science, Stamford, USA). Immunodetection of the large subunit of RuBisCO was accomplished with a 1∶200,000 dilution of a RuBisCO antibody (a gift of Dr. Luis Cañas, IBMCP) and then as mentioned before for the rest of the detection. The amount of signal was quantified with Photoshop (Adobe Photoshop CS4, San Jose, CA, USA).
Mutagenesis, screening, and mapping
Once the screening conditions were established, seeds of NahGCW were mutagenized with 0.15% ethyl methanesulfonate (M0880, SIGMA) for 8 hr, and M2 seed collected from ∼100 M1 plants. For the screening, 15-day-old M2 plants were spray inoculated with Pto at an OD600 of 0.1. One week later, the inoculation was repeated, and the evaluation took place one week after the second inoculation. To confirm the mutants, the M3 of isolated M2 were similarly inoculated, starting at 28 days after germination. Under these conditions, NahG plants either die or are severely affected, while wild type plants look unaffected. For mapping, coi1-40 was crossed with the ecotype Laer-0, and, in the segregating F2, plants were selected by the phenotype of the mutant. CAPS [70] and SSLP [71] markers were used from TAIR [72].
COI1 was sequenced by specific primers (Table S1). coi1-40 can be detected by the primers coi1-40F and coi1-40R, (Table S1) followed by digestion with TaqI (Fermentas, Madrid, Spain). For the determination of intragenic vs. extragenic mutations, an F1 was obtained between the suppressor and Col-0. If no susceptible plants segregated in 50–100 F2 plants, the molecular lesion was interpreted as being in the NahG gene itself, and therefore the suppressor was considered to be intragenic. Conversely, if in the F2 plants appeared that were as susceptible as NahGCW and as resistant as Col-0, the suppressor was labelled as extragenic.
RT-qPCR
Total RNA from 10-day-old plants grown on media with or without 50 µM MeJA was extracted with Trizol (Invitrogen, Barcelona, Spain), following the manufacturer's instructions. The details of the RT-qPCR (MIQE data) are provided as Methods S1.
Supporting Information
Sequencing primers of coi1-40 and molecular markers.
(PDF)
Proof of concept of the NahG suppressor screen. Three accessions (Col-0, Ws-1, and Laer-0), five single mutants (NahGCW (this work), cpr1 [14], cpr5 [15], lsd1 [17], and dnd1 [16]) and double mutant combinations with NahGCW were spray-inoculated with Pseudomonas syringae pv. tomato isolate DC3000 (Pto) at an OD600 of 0.1 at 28 days after germination and again one week later. The pictures were taken one week after the second inoculation.
(TIF)
Characterization of NahG intragenic suppressors. (A) Resistance and allelelism test of intragenic suppressors. The resistance (R) of M3 intragenic suppressor plants, the non-complementation of the intragenic suppressors with Col-0, the resistance (R) evaluation of the F1 and F2, and the allelism test between these suppressors was checked. For this purpose, four-weeks-old plants (the number indicated as “n”) were challenged as in Figure S1. (B) Quantification of growth of Pto. Plants were inoculated as in Figure S1, and the growth of Pto quantified as described in Methods. Note that with the first 40 mutants, the screen is by no means saturated. The intragenic suppressors form an internal control, since the screen has been sensitive enough to detect 12 reversions to wild type of a single locus. Assuming a Poisson distribution and the extreme scenario that all the extragenic suppressors belong to different complementation groups, the average ratio of alleles per complementation group of 1.38 implies that in the first 40 mutants there would be a maximum of 25% complementation groups not present [73].
(TIF)
Salicylic acid content of coi1- 40. Both free and total (free plus conjugated) Salicylic Acid is reported for 28 day-old unchallenged plants. Three samples of 100 mg leaves were frozen in liquid nitrogen. Salicylic acid measurements were performed with the biosensor Acinetobacter sp. ADPWH_lux as described ([74], [75]). The SA levels of Ws-0 are similar to those in Col-0 (data not shown).
(TIF)
Dry weight of roots growing with and without JA. The plants were grown as described in Figure 4A, with and without 50 µM MeJA. At 17 days-old, the dry weight of the roots was measured in both conditions, and their ratio (MeJA treated divided by mock treated) expressed as a percentage. The dry weight was determined after drying the roots for 48 h at 65°C. jin1 and coi1-1 mutants were used as controls.
(TIF)
Mutation in coi1 -40 and comparison of COI1 and TIR1 related F-box proteins from Arabidopsis. Amino acid sequences of COI1, TIR1 and five other TIR1-related F-box proteins from Arabidopsis (AFB) were aligned using CLUSTALW ([76]). Identical residues in all five AFBs and TIR1 are denoted in yellow and the substitution in the amino acid 22 responsible of the coi1-40 phenotype is denoted in red (glutamic acid (E) for lysine (K)).
(TIF)
Details of the trichomes in two coi1 alleles. SEM pictures of leaf epidermal trichomes of the Arabidopsis mutants coi1-1 (A and B) and coi1-40 (C and D). coi1-40 trichomes show bigger base cells, wider stem and more papillae along the trichome surface than the coi1-1 mutant (scale bar for A and C is 300 µm, scale bar for B and D is 40 µm).
(TIF)
Analysis of the F1 between coi1 -1 and coi1 -40. coi1-40, coi1-1 and its F1 were tested as described in Figure 4 and 5, for: (A) Lateral roots in plates with 50 µM MeJA. At 14 days old, the number of lateral roots longer than 0.2 mm was counted with the help of a magnifying glass. Note that there is a synergistic effect in the F1, with more lateral roots than its parents. (B) Trichomes in plates with 10 µM MeJA. When the fifth true leaf emerged, the number of trichomes was counted with the help of a magnifying glass.
(TIF)
MIQE data of the RT-qPCRs presented.
(PDF)
Acknowledgments
We are grateful to Syngenta for providing BTH and to Dr. Luis Cañas (IBMCP) for providing the anti-RuBisCo. Thanks to Amparo Cuéllar for her help sorting out the suppressors. Thanks also to Dr. Pablo Vera and Dr. Roberto Solano for their helpful comments; and to Dr Diane Saunders for carefully reading the final manuscript.
Funding Statement
This work was supported by grant BIO201018896 from “Ministerio de Economia y Competitividad” (MINECO) of Spain and by grant ACOMP/2012/105 from “Generalitat Valenciana” to PT, a JAE-CSIC Fellowship to JVC, a FPI-MINECO to AD, and Fellowships from the European Molecular Biology Organization and the Human Frontier Science Program to BBHW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vlot AC, Dempsey DMA, Klessig DF (2009) Salicylic Acid, a Multifaceted Hormone to Combat Disease. Ann Rev Phytopathology 47: 177–206. [DOI] [PubMed] [Google Scholar]
- 2. Mauch F, Mauch-Mani B, Gaille C, Kull B, Haas D, et al. (2001) Manipulation of salicylate content in Arabidopsis thaliana by the expression of an engineered bacterial salicylate synthase. Plant J 25: 67–77. [DOI] [PubMed] [Google Scholar]
- 3. Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, et al. (1993) Requirement for salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756. [DOI] [PubMed] [Google Scholar]
- 4. Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, et al. (1994) A Central Role of Salicylic Acid in Plant Disease Resistance. Science 266: 1247–1250. [DOI] [PubMed] [Google Scholar]
- 5. Lawton K, Weymann K, Friedrich L, Vernooij B, Uknes S, et al. (1995) Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol Plant Microbe Interact 8: 863–870. [DOI] [PubMed] [Google Scholar]
- 6. Ross AF (1961) Systemic acquired resistance induced by localized virus infections in plants. Virology 14: 340–358. [DOI] [PubMed] [Google Scholar]
- 7. Pieterse CMJ, Van Loon LC (1999) Salicylic acid-independent plant defence pathwways. Trends Plant Sci 4: 52–58. [DOI] [PubMed] [Google Scholar]
- 8. Fonseca S, Chico JM, Solano R (2009) The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr Opin Plant Biol 12: 539–547. [DOI] [PubMed] [Google Scholar]
- 9. Ton J, De Vos M, Robben C, Buchala A, Metraux JP, et al. (2002) Characterization of Arabidopsis enhanced disease susceptibility mutants that are affected in systemically induced resistance. Plant J 29: 11–21. [DOI] [PubMed] [Google Scholar]
- 10. Cui J, Bahrami AK, Pringle EG, Hernandez-Guzman G, Bender CL, et al. (2005) Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci, USA 102: 1791–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10: 372–379. [DOI] [PubMed] [Google Scholar]
- 12. Garcion C, Lohmann A, Lamodiere E, Catinot J, Buchala A, et al. (2008) Characterization and Biological Function of the ISOCHORISMATE SYNTHASE2 Gene of Arabidopsis. Plant Physiol 147: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tornero P, Chao RA, Luthin WN, Goff SA, Dangl JL (2002) Large scale structure-function analysis of the Arabidopsis RPM1 disease resistance protein. Plant Cell 14: 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, et al. (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X (1997) The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 9: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu I-C, Parker J, Bent AF (1998) Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci, USA 95: 7819–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dietrich RA, Delaney TP, Uknes SJ, Ward EJ, Ryals JA, et al. (1994) Arabidopsis mutants simulating disease resistance response. Cell 77: 565–578. [DOI] [PubMed] [Google Scholar]
- 18. Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62: 3321–3338. [DOI] [PubMed] [Google Scholar]
- 19. Wang D, Weaver ND, Kesarwani M, Dong X (2005) Induction of protein secretory pathway is required for systemic acquired resistance. Science 308: 1036–1040. [DOI] [PubMed] [Google Scholar]
- 20. Delaney T, Uknes S, Vernooij B, Friedrich L, Weymann K, et al. (1994) A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250. [DOI] [PubMed] [Google Scholar]
- 21. Ritter C, Dangl JL (1995) The avrRpm1 gene of Pseudomonas syringae pv. maculicola is required for virulence on Arabidopsis. Mol Plant Microbe Interact 8: 444–453. [DOI] [PubMed] [Google Scholar]
- 22. Debener T, Lehnackers H, Arnold M, Dangl JL (1991) Identification and molecular mapping of a single Arabidopsis thaliana locus determining resistance to a phytopathogenic Pseudomonas syringae isolate. Plant J 1: 289–302. [DOI] [PubMed] [Google Scholar]
- 23. Grant MR, Godiard L, Straube E, Ashfield T, Lewald J, et al. (1995) Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269: 843–846. [DOI] [PubMed] [Google Scholar]
- 24. Mindrinos M, Katagiri F, Yu G-L, Ausubel FM (1994) The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78: 1089–1099. [DOI] [PubMed] [Google Scholar]
- 25. Robert-Seilaniantz A, Navarro L, Bari R, Jones JD (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10: 372–379. [DOI] [PubMed] [Google Scholar]
- 26. Coego A, Ramirez V, Gil MJ, Flors V, Mauch-Mani B, et al. (2005) An Arabidopsis homeodomain transcription factor, OVEREXPRESSOR OF CATIONIC PEROXIDASE 3, mediates resistance to infection by necrotrophic pathogens. Plant Cell 17: 2123–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pieterse CM, van Wees SC, van Pelt JA, Knoester M, Laan R, et al. (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berger S, Bell E, Mullet JE (1996) Two Methyl Jasmonate-Insensitive Mutants Show Altered Expression of AtVsp in Response to Methyl Jasmonate and Wounding. Plant Physiol 111: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Attaran E, Zeier TE, Griebel T, Zeier J (2009) Methyl Salicylate Production and Jasmonate Signaling Are Not Essential for Systemic Acquired Resistance in Arabidopsis. Plant Cell 21: 954–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan J, Zhang C, Gu M, Bai Z, Zhang W, et al. (2009) The Arabidopsis CORONATINE INSENSITIVE1 Protein Is a Jasmonate Receptor. Plant Cell 21: 2220–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mittal S, Davis KR (1995) Role of the phytotoxin Coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Mol Plant Microbe Interact 8: 165–171. [DOI] [PubMed] [Google Scholar]
- 32. Genoud T, Metraux JP (1999) Crosstalk in plant cell signaling: structure and function of the genetic network. Trends Plant Sci 4: 503–507. [DOI] [PubMed] [Google Scholar]
- 33. Lawton KA, Friedrich L, Hunt M, Weymann K, Delaney T, et al. (1996) Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J 10: 71–82. [DOI] [PubMed] [Google Scholar]
- 34. Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis Mutants Selected for Resistance to the Phytotoxin Coronatine Are Male Sterile, Insensitive to Methyl Jasmonate, and Resistant to a Bacterial Pathogen. Plant Cell 6: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun J, Xu Y, Ye S, Jiang H, Chen Q, et al. (2009) Arabidopsis ASA1 Is Important for Jasmonate-Mediated Regulation of Auxin Biosynthesis and Transport during Lateral Root Formation. Plant Cell 21: 1495–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128: 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shan X, Zhang Y, Peng W, Wang Z, Xie D (2009) Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J Exp Bot 60: 3849–3860. [DOI] [PubMed] [Google Scholar]
- 38. Yoshida Y, Sano R, Wada T, Takabayashi J, Okada K (2009) Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis. Development 136: 1039–1048. [DOI] [PubMed] [Google Scholar]
- 39. Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12: 2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berger S, Bell E, Sadka A, Mullet JE (1995) Arabidopsis thaliana Atvsp is homologous to soybean VspA and VspB, genes encoding vegetative storage protein acid phosphatases, and is regulated similarly by methyl jasmonate, wounding, sugars, light and phosphate. Plant Mol Biol 27: 933–942. [DOI] [PubMed] [Google Scholar]
- 41. Feng S, Ma L, Wang X, Xie D, Dinesh-Kumar SP, et al. (2003) The COP9 signalosome interacts physically with SCF COI1 and modulates jasmonate responses. Plant Cell 15: 1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nawrath C, Métraux JP, Genoud T (2005) Chemical signals in plant resistance: salicylic acid. . In: Tuzun S, Bent E, editors. Multigenic and Induced Systemic Resistance in Plants. Dordrecht, Netherlands.: Springer US. pp. pp. 143–165.
- 43. Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5: 325–331. [DOI] [PubMed] [Google Scholar]
- 44. Truman W, Bennett MH, Kubigsteltig I, Turnbull C, Grant M (2007) Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc Natl Acad Sci USA 104: 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Canet JV, Dobón A, Ibáñez F, Perales L, Tornero P (2010) Resistance and biomass in Arabidopsis: a new model for Salicylic Acid perception. Plant Biotech J 8: 126–141. [DOI] [PubMed] [Google Scholar]
- 46. Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, et al. (2001) Auxin Transport Promotes Arabidopsis Lateral Root Initiation. Plant Cell 13: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Celenza JL, Grisafi PL, Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes & Development 9: 2131–2142. [DOI] [PubMed] [Google Scholar]
- 48. Traw MB, Bergelson J (2003) Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol 133: 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Payne CT, Zhang F, Lloyd AM (2000) GL3 encodes a bHLH protein that regulates trichome development in arabidopsis through interaction with GL1 and TTG1. Genetics 156: 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, et al. (2001) Resistance to Pseudomonas sringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation. Plant J 26: 509–522. [DOI] [PubMed] [Google Scholar]
- 51. Xie D-X, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094. [DOI] [PubMed] [Google Scholar]
- 52. Ellis C, Turner JG (2002) A conditionally fertile coi1 allele indicates cross-talk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings. Planta 215: 549–556. [DOI] [PubMed] [Google Scholar]
- 53. Fernandez-Arbaizar A, Regalado JJ, Lorenzo O (2012) Isolation and characterization of novel mutant loci suppressing the ABA hypersensitivity of the Arabidopsis coronatine insensitive 1–16 (coi1-16) mutant during germination and seedling growth. Plant Cell Physiol 53: 53–63. [DOI] [PubMed] [Google Scholar]
- 54. He Y, Chung E-H, Hubert DA, Tornero P, Dangl JL (2012) Specific Missense Alleles of the Arabidopsis Jasmonic Acid Co-Receptor COI1 Regulate Innate Immune Receptor Accumulation and Function. PLoS Genet 8: e1003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu L, Liu F, Lechner E, Genschik P, Crosby WL, et al. (2002) The SCFCOI1 Ubiquitin-Ligase Complexes Are Required for Jasmonate Response in Arabidopsis. Plant Cell 14: 1919–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671. [DOI] [PubMed] [Google Scholar]
- 57. Grunewald W, Vanholme B, Pauwels L, Plovie E, Inze D, et al. (2009) Expression of the Arabidopsis jasmonate signalling repressor JAZ1/TIFY10A is stimulated by auxin. EMBO Rep 10: 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63. [DOI] [PubMed] [Google Scholar]
- 59. Century KS, Holub EB, Staskawicz BJ (1995) NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc Natl Acad Sci USA 92: 6597–6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565. [DOI] [PubMed] [Google Scholar]
- 61. Lu M, Tang X, Zhou JM (2001) Arabidopsis NHO1 Is Required for General Resistance against Pseudomonas Bacteria. Plant Cell 13: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ritter C, Dangl JL (1996) Interference between two specific pathogen recognition events mediated by distinct plant disease resistance genes. Plant Cell 8: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tornero P, Dangl JL (2001) A high throughput method for quantifying growth of phytopathogenic bacteria in Arabidopsis thaliana . Plant J 28: 475–481. [DOI] [PubMed] [Google Scholar]
- 64. Macho AP, Guevara CM, Tornero P, Ruiz-Albert J, Beuzon CR (2010) The Pseudomonas syringae effector protein HopZ1a suppresses effector-triggered immunity. New Phytol 187: 1018–1033. [DOI] [PubMed] [Google Scholar]
- 65. Ton J, Mauch-Mani B (2004) Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38: 119–130. [DOI] [PubMed] [Google Scholar]
- 66. Canet JV, Dobón A, Roig A, Tornero P (2010) Structure-Function Analysis of npr1 Alleles in Arabidopsis Reveals a Role for its Paralogs in the Perception of Salicylic Acid. Plant, Cell & Environ 33: 1911–1922. [DOI] [PubMed] [Google Scholar]
- 67. Johnson CM, Stout PR, Broyer TC, Carlton AB (1957) Comparative chlorine requirements of different plant species. Plant and Soil 8: 337–353. [Google Scholar]
- 68. Dobón A, Canet JV, Perales L, Tornero P (2011) Quantitative genetic analysis of salicylic acid perception in Arabidopsis. Planta 234: 671–684. [DOI] [PubMed] [Google Scholar]
- 69. Mehrtens F, Kranz H, Bednarek P, Weisshaar B (2005) The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol 138: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4: 403–410. [DOI] [PubMed] [Google Scholar]
- 71. Bell CJ, Ecker JR (1994) Assignment of 30 Microsatellite Loci to the Linkage Map of Arabidopsis. Genomics 19: 137–144. [DOI] [PubMed] [Google Scholar]
- 72. Swarbreck D, Wilks C, Lamesch P, Berardini TZ, Garcia-Hernandez M, et al. (2008) The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucl Acids Res 36: D1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jürgens G, Mayer U, Torres Ruiz RA, Berleth T, Mísera S (1991) Genetic analysis of pattern formation in the Arabidopsis embryo. Development (Supplement 1) : 27–38.
- 74. Huang WE, Wang H, Zheng H, Huang L, Singer AC, et al. (2005) Chromosomally located gene fusions constructed in Acinetobacter sp. ADP1 for the detection of salicylate. Environ Microbiol 7: 1339–1348. [DOI] [PubMed] [Google Scholar]
- 75. Defraia CT, Schmelz EA, Mou Z (2008) A rapid biosensor-based method for quantification of free and glucose-conjugated salicylic acid. Plant Methods 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, et al. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31: 3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequencing primers of coi1-40 and molecular markers.
(PDF)
Proof of concept of the NahG suppressor screen. Three accessions (Col-0, Ws-1, and Laer-0), five single mutants (NahGCW (this work), cpr1 [14], cpr5 [15], lsd1 [17], and dnd1 [16]) and double mutant combinations with NahGCW were spray-inoculated with Pseudomonas syringae pv. tomato isolate DC3000 (Pto) at an OD600 of 0.1 at 28 days after germination and again one week later. The pictures were taken one week after the second inoculation.
(TIF)
Characterization of NahG intragenic suppressors. (A) Resistance and allelelism test of intragenic suppressors. The resistance (R) of M3 intragenic suppressor plants, the non-complementation of the intragenic suppressors with Col-0, the resistance (R) evaluation of the F1 and F2, and the allelism test between these suppressors was checked. For this purpose, four-weeks-old plants (the number indicated as “n”) were challenged as in Figure S1. (B) Quantification of growth of Pto. Plants were inoculated as in Figure S1, and the growth of Pto quantified as described in Methods. Note that with the first 40 mutants, the screen is by no means saturated. The intragenic suppressors form an internal control, since the screen has been sensitive enough to detect 12 reversions to wild type of a single locus. Assuming a Poisson distribution and the extreme scenario that all the extragenic suppressors belong to different complementation groups, the average ratio of alleles per complementation group of 1.38 implies that in the first 40 mutants there would be a maximum of 25% complementation groups not present [73].
(TIF)
Salicylic acid content of coi1- 40. Both free and total (free plus conjugated) Salicylic Acid is reported for 28 day-old unchallenged plants. Three samples of 100 mg leaves were frozen in liquid nitrogen. Salicylic acid measurements were performed with the biosensor Acinetobacter sp. ADPWH_lux as described ([74], [75]). The SA levels of Ws-0 are similar to those in Col-0 (data not shown).
(TIF)
Dry weight of roots growing with and without JA. The plants were grown as described in Figure 4A, with and without 50 µM MeJA. At 17 days-old, the dry weight of the roots was measured in both conditions, and their ratio (MeJA treated divided by mock treated) expressed as a percentage. The dry weight was determined after drying the roots for 48 h at 65°C. jin1 and coi1-1 mutants were used as controls.
(TIF)
Mutation in coi1 -40 and comparison of COI1 and TIR1 related F-box proteins from Arabidopsis. Amino acid sequences of COI1, TIR1 and five other TIR1-related F-box proteins from Arabidopsis (AFB) were aligned using CLUSTALW ([76]). Identical residues in all five AFBs and TIR1 are denoted in yellow and the substitution in the amino acid 22 responsible of the coi1-40 phenotype is denoted in red (glutamic acid (E) for lysine (K)).
(TIF)
Details of the trichomes in two coi1 alleles. SEM pictures of leaf epidermal trichomes of the Arabidopsis mutants coi1-1 (A and B) and coi1-40 (C and D). coi1-40 trichomes show bigger base cells, wider stem and more papillae along the trichome surface than the coi1-1 mutant (scale bar for A and C is 300 µm, scale bar for B and D is 40 µm).
(TIF)
Analysis of the F1 between coi1 -1 and coi1 -40. coi1-40, coi1-1 and its F1 were tested as described in Figure 4 and 5, for: (A) Lateral roots in plates with 50 µM MeJA. At 14 days old, the number of lateral roots longer than 0.2 mm was counted with the help of a magnifying glass. Note that there is a synergistic effect in the F1, with more lateral roots than its parents. (B) Trichomes in plates with 10 µM MeJA. When the fifth true leaf emerged, the number of trichomes was counted with the help of a magnifying glass.
(TIF)
MIQE data of the RT-qPCRs presented.
(PDF)