Abstract
We experimentally identified the activities of six predicted heptosyltransferases in Actinobacillus pleuropneumoniae genome serotype 5b strain L20 and serotype 3 strain JL03. The initial identification was based on a bioinformatic analysis of the amino acid similarity between these putative heptosyltrasferases with others of known function from enteric bacteria and Aeromonas. The putative functions of all the Actinobacillus pleuropneumoniae heptosyltrasferases were determined by using surrogate LPS acceptor molecules from well-defined A. hydrophyla AH-3 and A. salmonicida A450 mutants. Our results show that heptosyltransferases APL_0981 and APJL_1001 are responsible for the transfer of the terminal outer core D-glycero-D-manno-heptose (D,D-Hep) residue although they are not currently included in the CAZY glycosyltransferase 9 family. The WahF heptosyltransferase group signature sequence [S(T/S)(GA)XXH] differs from the heptosyltransferases consensus signature sequence [D(TS)(GA)XXH], because of the substitution of D261 for S261, being unique.
Introduction
Actinobacillus pleuropneumoniae is a non-motile Gram-negative bacterium causing porcine pleuropneumonia, a highly contagious respiratory disease transmitted through aerosols or close contact with infected animals including asymptomatic carriers [1]. This disease is often fatal and characterized by hemorrhagic, fibrinous and necrotic lung lesions; the clinical features ranging from acute to chronic [2], and it is an important cause economic losses worldwide in the porcine industry [2].
To date, two biovars have been described on the basis of NAD growth dependence; and fifteen serotypes based on capsular antigens [3], [4]; the disease can be caused by any serotype, although differences in virulence have been described [5], [6].
Studies aimed at the identification of A. pleuropneumoniae virulence factors showed that, as in other pathogenic bacteria, there is an array of these factors. However, the most relevant one appears to be the Apx toxins [5]. Other possible virulence factors include the capsule [7], [8], [9], outer membrane proteins involved in iron uptake [1], [10], [11], and lipopolysaccharides (LPS) [12], [13]. Biofilm formation [14], autotransporter adhesion[15], and autotransporter protease synthesis [16] have been also described to contribute to the pathogenicity of this bacterium.
The LPS appears to play a role in virulence in different stages of the A. pleuropneumoniae infection, including adhesions to lower respiratory tract, induction of lesions, and persistence in the upper respiratory tract reviewed in [17]. In addition the LPS molecules appear to interact with ApxI and ApxII toxins [18].
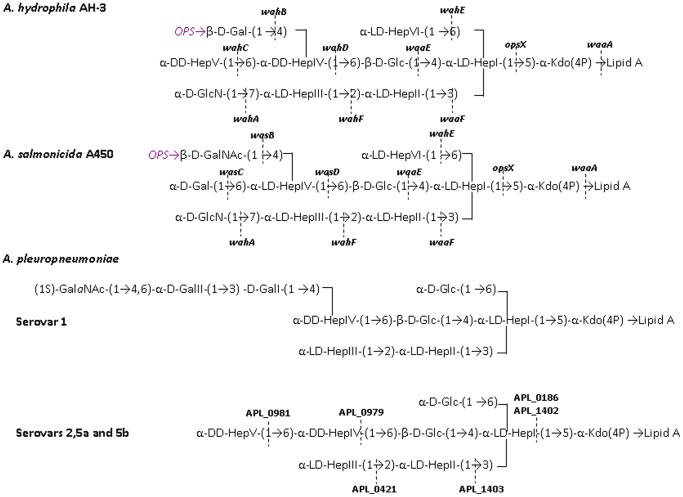
The LPS of A. pleuropneumoniae consist of three domains: an endotoxic glycolipid (lipid A), an O-polysaccharide chain (O-PS or O-antigen), and an intervening core oligosaccharide (core-OS). The structures the O-PS present in fourteen out of the fifteen capsular serotypes have been determined [19], [20], [21]. By contrast, the core LPS structure has been elucidated only for representative strains of A. pleuropneumoniae i.e., serotypes 1 (strain 4074), 2 (strain 4226), 5a (strain K17), and 5b (strain L20) [22]. All these strains share a common heptasaccharide, but differ in substitutions at D,D-HepIV residue (Figure 1). The common heptasaccharide includes three L-glycero-D-manno-heptose (L,D-Hep) and one D-glycero-D-manno heptose (D,D-Hep) residues. Relative to serotype 1, an additional D,D-HepV residue was found in serotypes 2, 5a, and 5b (Figure 1).
Figure 1. LPS core chemical structures for A. hydrophila [24], A. salmonicida [33], and A. pleuropneumoniae serotypes 1, 5a, and 5b [22].
The genes involved in core LPS biosynthesis are shown for A. hydrophila and A. salmonicida [25], [33].
Until now only one A. pleuropneumoniae heptosyltransferase was identified for a strain belonging to the serotype 1 [23]. Since the common heptasaccharide present in the core OS of the A. pleuropneumoniae studied strains is highly similar to the core OS of A. hydrophila [24] (Figure 1) we decided to use well defined core mutants of A- hydrophila AH-3 [25] producing surrogate acceptor LPS molecules to identify the heptosyltransferases involved in the core OS biosynthesis of genome strains belonging to serotypes 3 and 5b.
Results
Bioinformatic and phylogenetic analysis
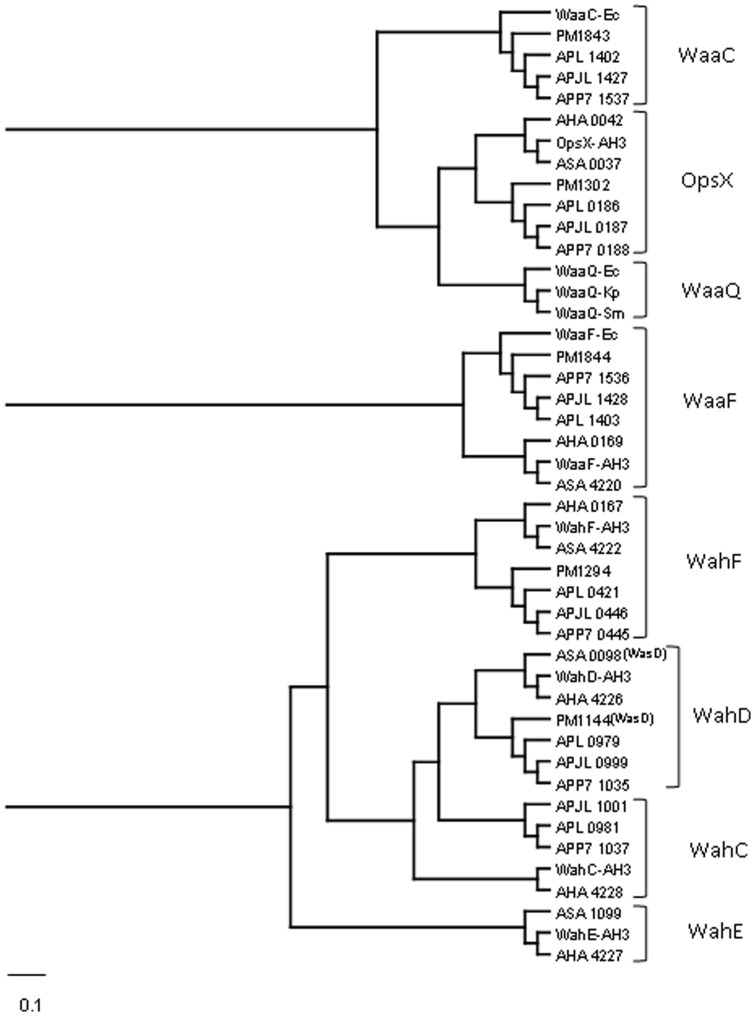
The core LPS structures of A. pleuropneumoniae studied strains contain between four and five heptose residues (Figure 1) requiring an equal number of heptosyltransferases. These heptosyltransferases were predicted on the basis of the nature of the substrate heptose, either L,D-Hep or D,D-Hep, and the linkage to the corresponding substrate core residue. In order to attribute putative functions we performed a bioinformatic analysis based on the alignment (Clustal W) of heptosyltransferases whose function is experimentally proved with those of A. pleuropneumoniae strains L20 (serotype 5b) [26], JL03 (serotype 3) [27], and AP76 (serotype 7) [28]. After this alignment, we performed a phylogenetic analysis using the same heptosyltransferases whose function is experimentally proved and the ones obtained with A. pleuropneumoniae strains. The cladogram obtained is shown in Figure 2 as an indicative of the similarity degree among these proteins.
Figure 2. Cladogram obtained running the phylogenetic inference software Protpars from the PHYLIP package version 3.5c as indicated in Materials and Methods section.
The separation and the large of the lanes are relative to the similarity degree according to the program used (the scale bar indicates an evolutionary distance of 0.1 aminoacid substitutions per position).The cladogram shows the similarity relationship among putative heptosyltransferases from A. pleunopneumoniae strains L20, JL03, and AP76 (APL, APJL, and APP7, respectively), A. hydrophila AH3 and ATCC7966T strains (AH-3 and AHA, respectively), A. salmonicida A450 strain (ASA), E. coli (Ec), K. pneumoniae (Kp), S. marcescens (Sm), and P. multocida (PM).
In the Carbohydrate-Active EnZymes database (CAZy) (http://www.cazy.org) five glycosyltransferases (APL_0186, APL_0421, APL_0979, APL_1402, and APL_1403) belonging to family GT9, that includes known heptosyltransferases, are reported for the genome strain L20 (serotype 5b) [26]. Similarly five homologues of these heptosyltransferases are found in A. pleuropneumoniae genome strains JL03 (serotype 3) [27] and AP76 (serotype 7) [28]. The presence of these heptosyltransferases is in apparent agreement with the five heptose residues present in the L20 strain core LPS (3 L,D-Hep and 2 D,D-Hep) [23] (Figure 1). However, it should be noted that APL_0186 (OpsX) and APL_1402 (WaaC) correspond to two different versions of heptosyltransferase I.
OpsX and WaaC transfer the first L,D-Hep residue to phosphorylated and unphosphorylated Kdo, respectively [25], [29], [30]. Indeed enzymes (APL_0904 and APJL_0916) for the phosphorylation at the 4-OH position of Kdo are also present in strain L20 or JL03, respectively. As expected these two heptosyltransferases, on the basis of amino acid similarity, cluster in two different branches when analyzed using Clustal W (Figure 2). Aeromonas hydrophila strains ATCC 7966 and AH-3 and A. salmonicida A450 contain only the OpsX version (AHA_0042, OpsX-AH3 and ASA-0037) and as expected their core LPS oligosaccharides only contain phosphorylated Kdo [24]. The genome strain Pm70 of Pasteurella multocida contains both versions of heptosyltransferase I and analysis of the core LPS of strains Pm70 and VP161has shown that their core LPS contains two major oligosaccharides only differing in their Kdo phosphorylation [31], [32]. No phosphorylated Kdo was reported in the core LPS of the four A. pleuropneumoniae strains studied [32] probably because in this study the core fraction was obtained by acid hydrolysis that could remove the phosphate from Kdo.
The same Clustal W analysis shows that known heptosyltransferases from Aeromonas hydrophyla and salmonicida transferring L,D-Hep II (WaaF) and L,D-Hep III (WahF) cluster with putative heptosyltransferases from A. pleuropneumoniae L20 (APL_1403 and APL_0421), and Pasteurella multocida Pm70 (Figure 2) in agreement with the presence of a common trisaccharide of L,D-Hep in the inner core of these bacteria (Figure 1).
A fourth cluster encompasses A. hydrophila WahD (AHA_4226) and A. salmonicida WasD (ASA_0098) with A. pleuropneumoniae APL_0979 and P. multocida PM1144. WahD and WasD transfer a D,D-Hep and L,D-Hep residue attached by an α 1,6 linkage to the β-D-Glc residue in A. hydrophila and A. salmonicida, respectively [25], [33]. Thus, they should recognize a very similar LPS substrate only differing in the donor sugar, either ADP-D,D-Hep or ADP-L,D-Hep. The presence of the disaccharide α-D.D-Hep (1,6) β-D-Glc and α-L.D-Hep (1,6) β-D-Glc in A. pleuropneumoniae (serotypes 1, 2, 5a and 5b) and P. multocida, respectively, suggest that APL_0970 and PM1144 are homologues of WahD and WasD.
Two proximal clusters includes WahC from A. hydrophila and APL_0981, APLJ_1001, and APP7_1037 from A. pleuropneumoniae genomic strains L20 (serotype 5b), J03 (serotype 3) and AP76 (serotype 7). WahC has been shown to be the heptosyltransferase responsible for the transfer of the terminal D,D-Hep residue of the A. hydrophila AH-3 core LPS [25] (Figure 1) and although their counterparts from A. pleuropneumoniae are actually included in a non-classified group of glycosyltransferases (GTnc, http://www.cazy.org) the present analysis could suggest a common function for all these proteins.
As a control for this analysis we used the WahE proteins from A. hydrophila and salmonicida involved in the transfer of a L,D-Hep residue by an α 1,6 linkage to L,D-Hep I [25], [33] (Figure 1). As shown in Figure 2 no similar proteins from A. pleuropneumoniae or P. multocida fall in this cluster.
Functional identification of the two versions of A. pleuropneumoniae heptosyltransferase I
In order to unambiguously identify the functions of the above putative heptosyltransferases we decided to use LPS from several A. hydrophila AH-3 strain mutants as a source of surrogate LPS acceptor molecules. These mutants were previously constructed by either KmR insertion or internal in-frame deletion in such a way to avoid polar effects on downstream genes [25].
First, we established the function of APL_0186 by its expression under the control of the PARA promoter (pBAD33-Gm-APL_0186). The core LPS from A. hydrophila AH-3ΔopsX mutant only contains a phosphorylated Kdo residue and is devoid of O-antigen [25]. The pBAD33-Gm-APL_0186 was introduced into A. hydrophila AH-3ΔopsX mutant and LPS from this strain was analyzed by SDS-PAGE after growth in inducing conditions. The APL_0186 expression plasmid fully complemented the opsX mutation leading to the production of full length LPS including O-antigen (Figure 3A, lane 3). The same result was obtained when using APJL_0187 expression plasmid from A. pleuropneumoniae strain JL03 (serotype 3) (Figure 3A, lane 4). These results strongly suggest that APL_0186 and APJL_0187 correspond to the OpsX version of heptosyltransferase I. By contrast, neither APL_1402 nor APJL_1427 were able to modify the LPS migration pattern of A. hydrophila AH-3ΔopsX mutant (Figure 3A, lanes 5 and 6), suggesting that they could correspond to the WaaC version of heptosyltransferase I. In agreement with this hypothesis, expression of APL_1402 and APJL_1427 in K. pneumoniae 52145ΔwaaC [34] were able to restore the full length K. pneumoniae 52145 core LPS and O-antigen production (Figure 3B, lanes 3 and 4). These results establish that A. pleuropneumoniae contains the two versions of heptosyltransferase I.
Figure 3. LPS analysis by SDS-PAGE.
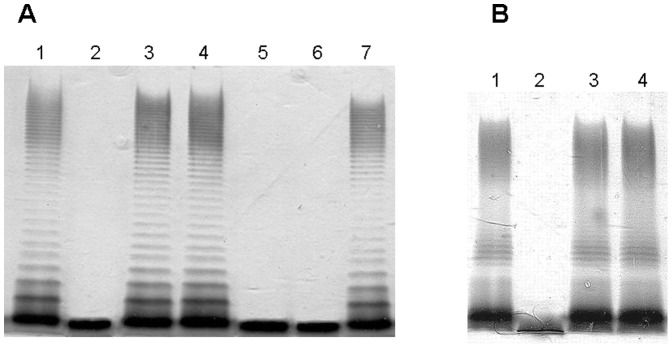
(A) LPS samples from A. hydrophila AH-3 (lanes 1 and 7), AH-3ΔopsX (lane 2), AH-3ΔopsX (pBAD33-Gm-APL_0186) (lane 3), AH-3ΔopsX (pBAD33-Gm-APJL_0187) (lane 4), AH-3ΔopsX (pBAD33-Gm-APL_1402) (lane 5), and AH-3ΔopsX (pBAD33-Gm-APJL_1427) (lane 6). (B) LPS samples from K. pneumoniae 52145 (lane 1), 52145ΔwaaC (lane 2), 52145ΔwaaC (pBAD33-Gm-APL_1402) (lane 3), and 52145ΔwaaC (pBAD33-Gm-APJL_1427) (lane 4).
Heptosyltransferases II and III
The LPS from A. hydrophila AH-3ΔwaaF is devoid of O-antigen and contains a trisaccharide core [α-L,D-Hep-(1→6)-α-L,D-Hep-(1→5)-Kdo-P] [25]. This core structure is similar to the one expected from a A. pleuropneumoniae waaF mutant [α-L,D-Hep-(1→5)-Kdo-P] and it was expected that the putative WaaF enzymes APL_1403 and APJL_1428 would be able to recognized the A. hydrophila AH-3ΔwaaF core. As expected, expression of APL_1403 (pBAD33-Gm-APL_1403) or APJL_1428 (pBAD33-Gm-APJL_1428) into A. hydrophila AH-3ΔwaaF resulted in the production of full length A. hydrophila AH-3 core including O-antigen (Figure 4, lanes 3 and 4). By contrast, expression of APL_0421 or APJL_0446 in this genetic background did not modify the mutant A. hydrophila AH-3ΔwaaF core LPS (Figure 4, lanes 5 and 6).
Figure 4. SDS-PAGE analysis of LPS prepared from A. hydrophila AH-3 (lane 1), AH-3.
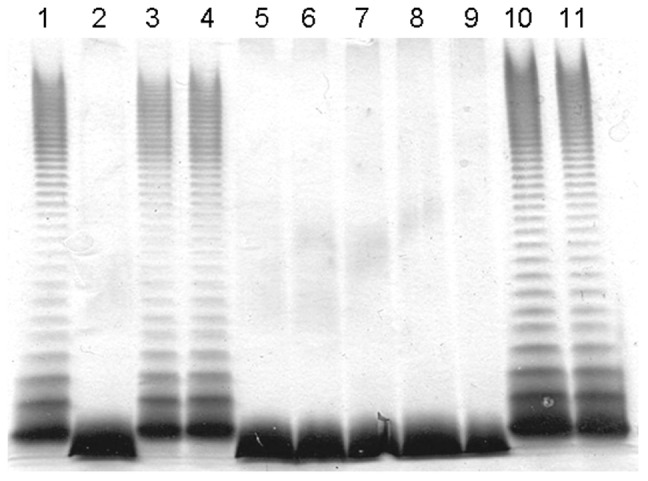
ΔwaaF (lane 2), AH-3ΔwaaF (pBAD33-Gm-APL_1403) (lane 3), AH-3ΔwaaF (pBAD33-Gm-APJL_1428) (lane 4), AH-3ΔwaaF (pBAD33-Gm-APL_0421) (lane 5), AH-3ΔwaaF (pBAD33-Gm-APJL_0446) (lane 6), AH-3ΔwahF (lane 7), AH-3ΔwahF (pBAD33-Gm-APL_1403) (lane 8), AH-3ΔwahF (pBAD33-Gm-APJL_1428) (lane 9), AH-3ΔwahF (pBAD33-Gm-APL_0421) (lane 10), and AH-3ΔwahF (pBAD33-Gm- APJL_0446) (lane 11).
On the other hand, both APL_0421 or APJL_0446 were able to restore wild type AH-3 core when expressed into A. hydrophila AH-3ΔwahF mutant (Figure 4, lanes 10 and 11) but not APL_1403 and APJL_1428 (Figure 4, lanes 8 and 9), suggesting that the A. pleuropneumoniae heptosyltransferase III recognize the A. hydrophila AH-3Δ wahF mutant core [α-L,D-Hep-(1→6)-[α-L,D-Hep-(1→3]-α-L,D-Hep-(1→5)-Kdo-P] [26]. Thus, these results unambiguously allow assigning heptosyltransferase II and III functions to APL_1403 (APJL_1428) and APL_0421 (APJL_0446), respectively.
WahD
As mentioned above, the WahD from A. hydrophila AH-3 is involved in the transfer of the D,D-Hep residue linked to Glc by an α 1,6 linkage while WasD from A. salmonicida transfer the L,D-Hep to Glc using the same linkage (Figure 1) [25], [33]. As seen in Figure 2 these proteins cluster together and APL_0979 and APJL_0999 are more similar to Pasteurella multocida PM1144 than to Aeromonas WahD or WasD. It is difficult to infer the function solely from the protein clustering because the only expected difference between WahD and WasD homologues should be in the region recognizing either ADP-D,D,Hep or ADP-L,D-Hep. From the known core LPS structures of A. pleuropneumoniae (Figure 1) it should be expected that both APL_0979 and APJL_0999 are homologues of WahD. If this hypothesis is correct, then expression of APL_0979 and APJL_0999 in A. hydrophila AH-3ΔwahD mutant should lead to the generation of full core LPS (Figure 5A, lanes 3 and 4). In addition, expression of APL_0979 and APJL_0999 in A. salmonicida A450ΔwasD mutant did not result in full core complementation (Figure 5B, lanes 3 and 4) while P. multocida PM1114 expressed in A. salmonicida A450ΔwasD did complement the mutant core LPS phenotype (Figure 5B, lane 5), while no such complementation was seen when it was expressed in A. hydrophila AH-3ΔwahD mutant (Figure 5A, lane 5).
Figure 5. LPS analysis by SDS-PAGE.
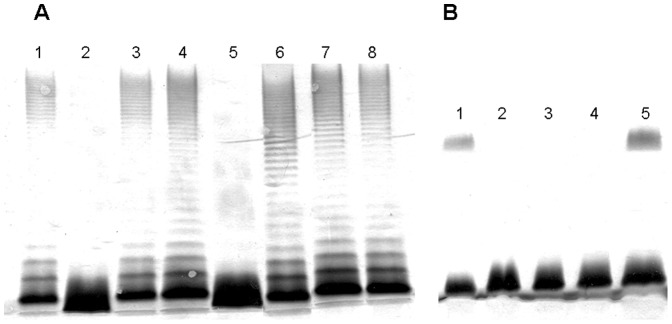
(A) LPS samples from A. hydrophila AH-3 (lane 1), AH-3ΔwahD (lane 2), AH-3ΔwahD (pBAD33-Gm-APL_0979) (lane 3), AH-3ΔwahD (pBAD33-Gm-APJL_0999) (lane 4), AH-3ΔwahD (pBAD33-Gm-PM1114) (lane 5), AH-3ΔwahC (lane 6), AH-3ΔwahC (pBAD33-Gm-APL_0981) (lane 7), and AH-3ΔwahC (pBAD33-Gm-APJL_1001) (lane 8). (B) LPS samples from A. salmonicida A450 (lane 1), A450ΔwasD (lane 2), A450ΔwasD (pBAD33-Gm-APL_0979) (lane 3), A450ΔwasD (pBAD33-Gm-APJL_0999) (lane 4), and A450 ΔwasD (pBAD33-Gm-PM1114) (lane 5).
To further confirm the function of APL_0979 and APJL_0999, LPS from A. hydrophila AH-3ΔwahD, A. hydrophila AH-3ΔwahD + pBAD33-Gm-APL_0979, and A. hydrophila AH-3ΔwahD + pBAD33-Gm-APJL_0999, were isolated. These LPS samples were hydrolyzed by mild-acid treatment and the core OS fraction isolated by chromatography. MS analysis of the OS fraction from A. hydrophila AH-3ΔwahD showed a major signal at m/z 1311.44 corresponding to a core OS (Hex1HexN1Hep4Kdo1) consistent with the loss of the outer-core trisaccharide fragment α-D,D-Hep-(1→6)-[β-D-Gal-(1→4)]-α-D,D-Hep from the full core LPS (Table 1). By contrast, MS analysis of the OS fraction from A. hydrophila AH-3ΔwahD + pBAD33-Gm-APJL_0999 showed a major signal at m/z 1857.617 (Table 1) in agreement with a full length AH-3 core (Hex2HexN1Hep6Kdo1) (Table 1) [25]. The second major signal at m/z 1503.498 was assigned to a core OS devoid of the terminal D,D-Hep and D-Gal from the wild type AH-3 core OS (Table 1). Similar results were obtained when analyzing the OS fraction from A. hydrophila AH-3ΔwahD + pBAD33-Gm-APL_0979 (data not shown). Taken together, these data allow to attribute a heptosyltransferase function involved in the transfer of the first D,D-Hep residue of the outer-core in A. pleuropneumoniae to APL-0979, and APJL_0999.
Table 1. Negative ion charge deconvoluted electrospray ionization mass spectral data of the core oligosaccharides released by mild acid hydrolysis from LPS of A. hydrohila AH-3ΔwahD, A. hydrohila AH-3ΔwahD (PBAD33-Gm-APJL_0999), A. hydrohila AH-3ΔwahC, and A. hydrohila AH-3ΔwahC (pBAD33-Gm-APJL_1001).
| Strain | Molecular mass (Da) | Proposed composition | |
| observed | calculateda | ||
| AH-3ΔwahD | 1311.44 | 1311.43 | anh-Kdo, 4 Hep, Hex, HexN |
| AH-3ΔwahD +APJL_ 0999 | 1937.59 | 1937.58 | anh-Kdo, 6 Hep, 2 Hex, HexN, P |
| 1857.62 | 1857.61 | anh-Kdo, 6 Hep, 2 Hex, HexN | |
| 1695.57 | 1695.56 | anh-Kdo, 6 Hep, Hex, HexN | |
| 1503.50 | 1503.50 | anh-Kdo, 5 Hep, Hex, HexN | |
| AH-3ΔwahC | 1665.58 | 1665.55 | anh-Kdo, 5 Hep, 2 Hex, HexN |
| 1503.53 | 1503.50 | anh-Kdo 5 Hep, Hex, HexN | |
| 1311.46 | 1311.43 | anh-Kdo, 4 Hep, Hex, HexN | |
| AH-3ΔwahC + APJL_1001 | 1937.60 | 1937.58 | anh-Kdo, 6 Hep, 2 Hex, HexN, P |
| 1857.63 | 1857.61 | anh-Kdo, 6 Hep, 2 Hex, HexN | |
| 1695.57 | 1695.56 | anh-Kdo, 6 Hep, Hex, HexN | |
| 1503.50 | 1503.50 | anh-Kdo, 5 Hep, Hex, HexN | |
Average mass units (in daltons) used for calculation of molecular masses based on the proposed composition, as follows: anh-Kdo, 202.048; Hep, 192.063; Hex, 162.053; HexN, 161.069; and P, 79.966.
WahC
The A. hydrophila AH-3 WahC is responsible for the transfer of the terminal D,D-Hep residue of the AH-3 core LPS [25], and shows significant similarity to A. pleuropneumoniae APL_0981 and APJL_1001. No homologous protein from A. salmonicida was detected (Figure 2) in agreement with the presence of terminal D,D-Hep in A. hydrophila but not in A. salmonicida (Figure 1). A major difference between these two groups of proteins is that while the AH-3 and ATCC 7966 WahC are assigned to the GT9 family in the CAZY classification of glycosyltransferases, the two A. pleuropneumoniae proteins are actually grouped as non-classified glycosyltransferases on the basis of weak similarity to established glycosyltransferase families. However, in the Kegg database, both of these proteins are shown as having a glycosyltransferase family 9 (heptosyltransferase) motif. On the basis of the presence of a terminal D,D-Hep in the A. pleuropneumoniae core OS of serotype 2, 5a, and 5b (genome strain L20) [22], we hypothesized that APL_0981 would be a WahC homologue. Furthermore, APL-0981 is identical to APP7_1037 (serotype 7), and shows only one amino acid residue different when compared with APJL_1001 (serotype 3) for which the core LPS structure remains unknown.
To test the above hypothesis APL_0981 and APJL_1001 were introduced and expressed into A. hydrophila AH-3ΔwahC. As previously shown, the LPS of the AH-3 mutant still contains O-antigen (Figure 5, lane 6) and its core OS was devoid of the terminal D,D-Hep residue as demonstrated by MS analysis of its core OS fraction (Table 1) and methylation analysis [25]. A. hydrophila AH-3ΔwahC harboring pBAD33-Gm-APL_0981 or APJL_1001 recover full length core LPS since no differences in mobility of the lipidA-core band could be detected among LPS isolated from these strains and wild type AH-3 (Figure 5, lanes 6, 7, and 8). Furthermore, MS analysis of the core LPS fraction isolated from A. hydrophila AH-3ΔwahC harboring pBAD33-Gm-APJL_1001 gave a major signal a m/z 1857.63 corresponding to full length core OS (Hex2HexN1Hep6Kdo1), other signals were attributed to Hex1HexN1Hep6Kdo1 (m/z 1695.573) and Hex1HexN1Hep5Kdo1 (m/z 1503.495) (Table 1). Similar results were obtained with the core OS fraction from A. hydrophila
AH-3ΔwahC harboring pBAD33-Gm-APL_0981 (data not shown). These results, strongly suggests that A. pleuropneumoniae APL_0981 and APJL_1001 are functional homologues of A. hydrophila WahD and are responsible for the transfer of the terminal outer-core D,D-Hep residue (Figure 1).
Discussion
The bioinformatic and phylogenetic analysis reported here shows that four L,D-heptosyltransferases (WaaC, OpsX, WaaF, and WaaQ) can be grouped on the basis of their amino acid similarity (Figure 2 and Table 2), while the heptosyltransferase WahE would constitute a more distant group. The two other L,D-heptosyltransferases WahF and WasD (ASA 4222) appear to be related to D,D-heptosyltransferases (WahD and WahC) (see cladogram in Figure 2). It is not clear that differences between residues found to be important in WaaC with those in other heptosyltransferase are specifically significant for the catalytic function and interaction with ADP-L,D-Hep of E. coli heptosyltranferase I (WaaC) [35]. The key residues for the catalytic function and interaction with ADP- L,D-Hep were D13, K192, E222, D261, and H266, the last two (D261and H266) present in the proposed heptosylytansferases signature sequence D261(T/S)(A/G)XXH266. These conserved residues were shown to interact with ADP-2-deoxy-2-fluoro-heptose [35]. The only residues that were found conserved in all the heptosyltransferases analyzed in this study (Table 2) were D13 and H266. The K192 was found to interact with the O5 and O6 atoms of the heptose, and it was present in L,D- heptosyltransferases WaaC, OpsX, WaaF, and WaaQ. It has been suggested that in D,D-heptosyltransferases the K192 could be substituted by other residues as by R in Helicobacter pylori [35], [36]. This is also the case for D,D-heptosyltransferases WahD and WahC where K192 is substituted by R or N or R, respectively. We also found K192 in all the analyzed L,D-heptosyltransferases except WahF with N or T substituting K192 (Table 2). The E. coli WaaC E222 residue, which interacts with the O2 and O3 residues of the heptose [35], was found in heptosyltransferases WahE, WahF, and WahD, being substituted by D in WaaQ and by other residues in OpsX, WaaF, WasD, and WahC (Table 2). E. coli WaaC D261 and H266 residues were previously shown to interact with O3 of the heptose and in addition H266 contacts with the 2’’ fluorine of the ADP-2-deoxy-2-fluoro-heptose [35]. They are found in all the heptosyltransferases analyzed in this work with the exception of WahF where D261 is substituted by S (Table 2). The WahF signature [S(T/S)(G/A)XXH] is unique because of the change in the heptosyltransferases consensus signature sequence [D(T/S)(G/A)XXH]. Minor differences were also observed for the signature sequences of D,D-heptosyltransferases WahD [D(T/S)(S/A)XXH] and WahC [D(T/G)(G/S)XXH]. All these results are summarized in Table 2.
Table 2. The presence of E. coli WaaC enzyme key residues for the catalytic function and interaction with ADP- L,D-Hep [35] in well characterized heptosyltransferases in different Gram-negative bacteria (Enterobacteriaceae and Aeromonadaceae). Comparison of the identified E. coli WaaC enzyme key residues based in amino acid alignment.
| Enzyme | Linkage | Amino acid positiona | ||||||||
| 13 | 192 | 222 | 261 | 262 | 263 | 264 | 265 | 266 | ||
| WaaC | α-LD-HepI-(1→5)-Kdo | D | K | E | D | T/S | G/A | X | X | H |
| OpsX | α-LD-HepI-(1→5)-Kdo(4P) | D | K | L/Y/R | D | T/S | G/A | X | X | H |
| WaaF | α-LD-HepII-(1→3)-α-LD-HepI | D | K | K/H/V/S | D | T/S | G/A | X | X | H |
| WahE | α-LD-HepIV-(1→6)-α-LD-HepI | D | K | E | D | T/S | G/A | X | X | H |
| WaaQ | α-LD-HepIII-(1→7)-α-LD-HepII | D | K | D | D | T/S | G/A | X | X | H |
| WahF | α-LD-HepIII-(1→2)-α-LD-HepII | D | N/T | E | S | T/S | G/A | X | X | H |
| WasD | α-LD-HepV-(1→6)-β-D-Glc | D | K | E/A | D | T/S | S/A | X | X | H |
| WahD | α-DD-HepV-(1→6)-β-D-Glc | D | R | E | D | T/S | S/A | X | X | H |
| WahC | α-DD-Hep-(1→6)-α-DD-Hep | D | N/R | K/P | D | T/G | G/S | X | X | H |
Amino acid position referred to E. coli K12 [35].
This analysis stresses the importance of experimental identification of the function of hypothetical heptosyltransferases. Two approaches are possible; one is based on the construction of individual mutations and analysis of the core LPS defects [25]; and the second one, uses surrogate LPS molecules to test the function of individual heptosyltransferases. The first approach can be difficult in some bacterial species and has the problem that the conclusions are based on a negative feature represented by the shortage of the wild type core, in addition sometimes the interpretation of data is complicated by the presence of different core OS in the mutant strains. The second approach requires having surrogate LPS molecules highly similar to the predicted heptosyltransferase LPS acceptor molecule. The LPS surrogate acceptors would be provided by previously obtained mutants from other bacteria, where the acceptor (mutant strains) should be constructed in such a way that there are no downstream effects. The use of surrogate LPS molecules allows for a positive trait: the addition of a new residue to surrogate core LPS, and if adequate the analysis is facilitated because the modified surrogate core LPS will acquire also the corresponding O-antigen domain (Figures 3, 4, and 5) and further confirmed by composition and MS analysis (Table 1).
In this work we show the feasibility of this approach by identifying the A. pleuropneumoniae heptosyltransferases from strains L20 and JL03. The same functions are expected from the homologous proteins from strain AP76 (Figure 2). Furthermore, our results based in the use of surrogate LPS acceptor from an A. hydrophila ΔwahC mutant (Figure 5, lanes 6, 7, and 8) and MS analysis (Table 1) allow to establish unambiguously an heptosyltransferase function for APL_0981 and APJL_1001 although these two proteins are not included in the glycosyltransferase GT9 family in the CAZY classification. These two proteins are involved in the addition of the terminal D,D-Hep residue of the A. pleuropneumoniae core LPS (Figure 1), and then besides the sequence similarity should be included as GT9 family members.
There is an increasing interest in the development of antivirulence agents and one of the potentially interesting targets for them is the WaaC heptosyltransferase I [37]. Our and previous analyses of the heptosyltransferases involved in LPS biosynthesis clearly show that the most conserved region corresponds to the residues involved in catalytic function and interaction with the ADP-L,D-Hep. Thus, the search for antivirulent inhibitors of the WaaC and other heptosyltransferases interacting with these residues appears be the best way to proceed.
Materials and Methods
Bacterial strains, plasmids, and growth conditions
Bacterial strains, and plasmids used in this study are listed in Table 3. Aeromonas strains were routinely grown on tryptic soy broth (TSB) or tryptic soy agar (TSA) at 30°C unless stated otherwise. A. pleuropneumoniae strains were grown on Brain Herat Infusion (BHI) at 37°C and E. coli strains were grown on Luria-Bertani (LB) Miller broth and LB Miller agar at 37°C [38]. Kanamycin (50 µg/ml), rifampicin (100 µg/ml), or gentamycin (20 µg/ml) were added to the different media.
Table 3. Bacterial strains and plasmids used.
| Strain or plasmid | Relevant characteristics | Reference or source |
| Strains | ||
| A. pleuropneumoniae | ||
| L20 | Wild-type, capsular serotype 5b | U.A.B.a |
| JL03 | Wild.type, capsular serotype 3 | U.A.B. |
| E. coli | ||
| LMG194 | F- ΔlacX74 galE galK thi rpsL ΔphoA (PvuII) Δara714 leu::Tn10 | [48] |
| HB101 | F- mcrB mrr hsdS20 (rB - mB -) recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20 (SmR) glnV44 λ- | [49] |
| K. pneumoniae | ||
| 52145ΔwaaC | In frame deletion waaC mutant | [34] |
| A. hydrophila | ||
| AH-3 | Wild-type, O34 | [50] |
| AH-3ΔwahD | wahD Km::Tn5; RifR KmR | [25] b |
| AH-ΔopsX | opsX (waaC a) Km::Tn5; RifR KmR | [25] |
| AH-3ΔwahC | In frame deletion mutant ΔwahC | [25] |
| AH-3ΔwaaF | In frame deletion mutant ΔwaaF | [25] |
| AH-3ΔwahF | wahF Km::Tn5; RifR KmR insertion mutant | [25] |
| A. salmonicida | ||
| A450 | [33] | |
| A450ΔwasD | wasD (wahD-likea) Km::Tn5; RifR KmR insertion mutant | [33] |
| Plasmids | ||
| pRK2073 | Conjugation helper plasmid, SpcR | [51] |
| pBAD33-Gm | Arabinose-inducible expression vector, GmR | [33] |
| pBAD33-Gm-APL_0186 | pBAD33-Gm with APL_0186 from strain L20 | This study |
| pBAD33-Gm-APL_0421 | pBAD33-Gm with APL_0421 from strain L20 | This study |
| pBAD33-Gm-APL_0979 | pBAD33-Gm with APL_0979 from strain L20 | This study |
| pBAD33-Gm-APL_0981 | pBAD33-Gm with APL_0981 from strain L20 | This study |
| pBAD33-Gm-APL_1402 | pBAD33-Gm with APL_1402 from strain L20 | This study |
| pBAD33-Gm-APL_1403 | pBAD33-Gm with APL_1403 from strain L20 | This study |
| pBAD33-Gm-APJL_0187 | pBAD33-Gm with APJL_0187 from strain JL03 | This study |
| pBAD33-Gm-APJL_0446 | pBAD33-Gm with APJL_0446 from strain JL03 | This study |
| pBAD33-Gm-APJL_0999 | pBAD33-Gm with APJL_0999 from strain JL03 | This study |
| pBAD33-Gm-APJL_1001 | pBAD33-Gm with APJL_1001 from strain JL03 | This study |
| pBAD33-Gm-APJL_1427 | pBAD33-Gm with APJL_1427 from strain JL03 | This study |
| pBAD33-Gm-APJL_1428 | pBAD33-Gm with APJL_1428 from strain JL03 | This study |
Universidad Autónoma Barcelona, Spain
Original nomenclature [25]
General DNA methods and computer analysis of sequence data
General DNA manipulations were done essentially as described [39]. DNA restriction endonucleases, T4 DNA ligase, E. coli DNA polymerase (Klenow fragment), and alkaline phosphatase were used as recommended by the suppliers. Deduced amino acid sequences used from different Enterobacteriaceae and Aeromonadaceae were obtained from the GenBank, EMBL, and SwissProt databases. ClustalW [40] was used for multiple-sequence alignments with a gap open penalty of 10, gap extension penalty of 0.05, no weight transition, with hydrophilic gaps and GPSNDQERK hydrophilic residues for proteins, and BLOSUM as selected weight matrix.
Plasmid constructions and mutant complementation studies
For complementation studies, the A. pleuropneumoniae genes (APL_0186, APL_0421, APL_0979, APL_0981, APL_1402, APL_1403, APJL_0187, APJL_0446, APJL_0999, APJL_1001, APJL_1427, and APJL_1428) were PCR-amplified by using specific primer pairs (Table 4) and ligated to the plasmid pBAD33-Gm. The plasmid constructions were transformed into E. coli LMG194 by electroporation, plated on gentamycin LB agar plates and incubated at 37°C. Plasmids with the amplified genes were independently transferred into the corresponding mutants by triparental mating using the mobilizing strain HB101/pRK2073. Transconjugants were selected on plates containing gentamycin and rifampicin and confirmed by PCR. Each gene was expressed from the arabinose-inducible and glucose-repressible pBAD33 promoter. Repression from the araC promoter was achieved by growth in medium containing 0.2% (w/v) D-glucose, and induction was obtained by adding l-arabinose to a final concentration of 0.2% (w/v). The cultures were grown for 18 h at 30°C in TSB medium supplemented with gentamycin and 0.2% glucose, diluted 1∶100 in fresh medium (without glucose) and grown until they reached A 600 nm of about 0.2. Then l-arabinose was added, and the cultures were grown for another 2 h. Repressed controls were maintained in glucose-containing medium.
Table 4. Primers used to amplify and subclone individual genes in pBAD33-Gm.
| Primersa,b | Amplified Fragment |
| opsX (APL_0186 and APJL_0187) | |
| AXf: 5’-TGCTCTAGATAATCAGACCGAACCCACGC-3’ | |
| AXr: 5′-CCCAAGCTTCGGCCGGGGTGAGTAATA-3’ | AXf-AXr (1243 bp) |
| wahF (APL_0421 and APJL_0446) | |
| AF2f: 5’-TGCTCTAGATACCGAGCAAGCGGTCAAAT-3’ | |
| AF2r: 5’-CCCAAGCTTGACTTGCCATTTTAACAGGCTTT-3’ | AF2f-AF2r (1185 bp) |
| wahD (APL_0979 and APJL_0999) | |
| ADf: 5’-TGCTCTAGATTAGTCCCGGCTCGGAAATC-3’ | |
| ADr: 5’-CTGCAGTGCCCCAGCTCTTGTTCAAT-3’ | ADf-ADr (1219 bp) |
| wahC (APL_0981 and APJL_1001) | |
| AC2f: 5’-TGCTCTAGATGTGCGCATGGATTTTACGG-3’ | |
| AC2r: 5’-CTGCAGTGTCCTGGATTCAAGCGGTC-3’ | AC2f-AC2r (1155 bp) |
| waaC (APL_1402) | |
| AC11f: C: 5’-TGCTCTAGACGACCGCTTGTTCGTATTCAT-3’ | |
| AC11r: D: 5’-CCCAAGCTTGCAAGCCTCTAATGCAGGAAC-3’ | AC11f-AC11r (1198 bp) |
| waaC (APJL_1427) | |
| AC12f: 5’-TGCTCTAGATGCAGGTTTAGACCGCTTG-3’ | |
| AC12r: 5’-CCCAAGCTTTCACACGCGGTTTGCG-3’ | AC12f-AC12r (1167 bp) |
| waaF (APL_1403 and APJL_1428) | |
| AF1f: 5’-TGCTCTAGATGACCGCTTGTTATTTTAGGGA-3’ | |
| AF1r: 5’-CTGCAGTGACCGCTTGCAATTAGCCT-3’ | AF1f-AF1r (1082 bp) |
Bold, underlined, and italic letters XbaI, HindIII, and PstI restriction sites, respectively.
The PCR amplified products were digested with the indicated restriction enzymes and ligated to XbaI-HindIII or XbaI-PstI digested pBAD33-Gm.
LPS isolation and SDS-PAGE
For screening purposes LPS was obtained after proteinase K digestion of whole cells [41]. LPS samples were separated by SDS-PAGE or N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine (Tricine)-SDS-PAGE [42], [43] and visualized by silver staining as previously described [41], [44]. For large-scale isolation LPS was extracted from cells grown in TSB at 30°C. Cells were dried and suspended in 25 mM Tris-HCl buffer containing 2 mM CaCl2 pH 7.63 (10 mL g -1) were treated at 37°C with RNAse, DNAse (24 h, 1 mg/g each), and then with Proteinase K (36 h, 1 mg/g). The suspension was dialyzed and lyophilized. The phenol/chloroform/light petroleum ether method [45] was used for strains producing rough LPS, while the phenol/water procedure [46] was used for the strains producing the O antigen domain (smooth LPS).
Preparation of oligosaccharide fraction
A portion of the LPS (∼50 mg) from each strain was heated with aqueous 2% HOAc (6 mL) at 100 °C for 45 min. The precipitate was removed by centrifugation (13,000 g, 20 min) and the supernatant fractionated on a column (56×2.6 cm) of Sephadex G-50 (S) in 0.05 M pyridinium acetate buffer pH 4.5 with monitoring using a differential refractometer (Knauer, Germany).
Mass spectrometry
Electrospray ionization mass spectra were run in the negative ion mode using a micrOTOF II instrument (Bruker Daltonics, USA). Mass spectra were acquired using standard experimental sequences as provided by the manufacturer. Samples (∼50 ng µL-1) were dissolved in a 1∶1 (v/v) water-acetonitrile mixture and sprayed at a flow rate of 3 µL min-1. Capillary entrance voltage was set to 4.5 kV and exit voltage to −150 V; drying gas temperature was 180°C. The spectra showing several charge states for each component were charge deconvoluted, and mass numbers given refer to monoisotopic molecular masses.
Phylogenetic analysis
The analysis was performed by running the phylogenetic inference software Protpars from the PHYLIP (Phylogeny Inference Package) package version 3.5c (Department of Genetics University of Washington, Seattle) [47], using heptosyltransferases whose function is experimentally proved from enteric bacteria, Aeromonas or Pasteurella multocida with those of A. pleuropneumoniae strains L20 (serotype 5b) [26], JL03 (serotype 3) [27], and AP76 (serotype 7) [28]. The cladogram is indicative by the separation and the large of the lanes from the values obtained running the program (the scale bar indicates an evolutionary distance of 0.1 aminoacid substitutions per position).
Acknowledgments
We also thank Maite Polo for her technical assistance, Sofiya N. Senchenkova and Anna Kondakova for help with mass spectrometry, and Marta Cerdá (CReSA, UAB, Spain) for kindly providing Actinobacillus strains.
Funding Statement
This work was supported by Plan Nacional de I+D+i grant (Ministerio de Educación, Ciencia y Deporte) and from Generalitat de Catalunya (Centre de Referència en Biotecnologia). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bosse J T, Janson H, Sheehan BJ, Beddek AJ, Rycroft, et al. (2002) Actinobacillus pleuropneumoniae : pathobiology and pathogenesis of infection. Microbes Infect 4: 225–235. [DOI] [PubMed] [Google Scholar]
- 2.Gottschak M, Taylor DJ (2006) Actinobacillus pleuropneumoniae. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of swine, 9th ed. Blackwell Publishing, Ames: Iowa. pp. 563–576. [Google Scholar]
- 3. Dubreuil JD, Jacques M, Mittal KR, Gottschalk M (2000) Actinobacillus pleuropneumoniae surface polysaccharides: their role in diagnosis and immunogenicity. Anim. Health Res Rev 1: 73–93. [DOI] [PubMed] [Google Scholar]
- 4. Blackall PJ, Klaasen HL, van den Bosch H, Kuhnert P, Frey J (2002) Proposal of a new serovar of Actinobacillus pleuropneumoniae: serovar 15. Vet Microbiol 84 47–52. [DOI] [PubMed] [Google Scholar]
- 5. Frey J (1995) Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol 3: 257–261. [DOI] [PubMed] [Google Scholar]
- 6. Jacobsen MJ, Nielsen JP, Nielsen R (1996) Comparison of virulence of different Actinobacillus pleuropneumoniae serotypes and biotypes using an aerosol infection model. Vet Microbiol 49: 159–168. [DOI] [PubMed] [Google Scholar]
- 7. Ward CK, Inzana TJ (1994) Resistance of Actinobacillus pleuropneumoniae to bactericidal antibody and complement is mediated by capsular polysaccharide and blocking antibody specific for lipopolysaccharide. J Immunol 153: 2110–2121. [PubMed] [Google Scholar]
- 8. Ward CK, Lawrence ML, Veit HP, Inzana TJ (1998) Cloning and mutagenesis of a serotype-specific DNA region involved in encapsulation and virulence of Actinobacillus pleuropneumoniae serotype 5a: concomitant expression of serotype 5a and 1 capsular polysaccharides in recombinant A. pleuropneumoniae serotype 1. Infect Immun 66: 3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rioux S, Galarneau C, Harel J, Kobisch M, Frey J, et al. (2000) Isolation and characterization of a capsule-deficient mutant of Actinobacillus pleuropneumoniae serotype 1. Microb Pathog 28: 279–289. [DOI] [PubMed] [Google Scholar]
- 10. Haesebrouck F, Chiers K, Van Overbeke I, Ducatelle R (1997) Actinobacillus pleuropneumoniae infections in pigs: the role of virulence factors in pathogenesis and protection. Vet Microbiol 58: 239–249. [DOI] [PubMed] [Google Scholar]
- 11. Jacques M (2004) Surface polysaccharides and iron-uptake systems of Actinobacillus pleuropneumoniae . Can J Vet Res 68: 81–85. [PMC free article] [PubMed] [Google Scholar]
- 12. Jacques M (1996) Role of lipo-oligosaccharides and lipopolysaccharides in bacterial adherence. Trends Microbiol 4: 408–410. [DOI] [PubMed] [Google Scholar]
- 13. Jacques M, Paradis SE (1998) Adhesin–receptor interactions in Pasteurellaceae . FEMS Microbiol Rev 22: 45–59. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan JB, Mulks MH (2005) Biofilm formation is prevalent among field isolates of Actinobacillus pleuropneumoniae . Vet Microbiol 108: 89–94. [DOI] [PubMed] [Google Scholar]
- 15. Auger E, Deslandes V, Ramjeet M, Contreras I, Nash JH, et al. (2009) Host-pathogen interactions of Actinobacillus pleuropneumoniae with porcine lung and tracheal epithelial cells. Infect Immun 77: 1426–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baltes N, Buettner FF, Gerlach GF (2007) Selective capture of transcribed sequences (SCOTS) of Actinobacillus pleuropneumoniae in the chronic stage of disease reveals an HlyX-regulated autotransporter protein. Vet Microbiol 123: 110–121. [DOI] [PubMed] [Google Scholar]
- 17. Chiers K, De Waele T, Pasmans F, Ducatelle R, Haesebrouck F (2010) Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet Res 41 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramjeet M, Cox AD, Hancock MA, Mourez M, Labrie J, et al. (2008) Mutation in the LPS outer core biosynthesis gene, galU, affects LPS interaction with the RTX toxins ApxI and ApxII and citolytic activity of Actinobacillus pleuropneumoniae . Mol Microbiol 70: 221–235. [DOI] [PubMed] [Google Scholar]
- 19.Perry MB, Altman E, Brisson J-R, Beynon LM, Richards JC (1990) Structural characteristics of the antigenic capsular polysaccharides and lipopolysaccharides involved in the serological classification of Actinobacillus (Haemophilus) pleuropneumoniae strains. Serodiagn Immunother Infect Dis 4: 299–308. [Google Scholar]
- 20. Perry MB, McLean LL (2004) Structural characterization of the antigenic O-polysaccharide in the lipopolysaccharide produced by Actinobacillus pleuropneumoniae serotype 14. Carbohydr Res 339: 1399–1402. [DOI] [PubMed] [Google Scholar]
- 21. McLean LL, Perry MB, Vinogradov E (2004) Characterization of the antigènic polysaccharide O chain and the capsular polysaccharide produced by Actinobacillus pleuropneumoniae serotype 13. Infect Immun 72: 5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michael FS, Brisson J-R, Larocque S, Monteiro M, Li J, et al. (2004) Structural analysis of the lipopolysaccharide derived core oligosaccharides of Actinobacillus pleuropneumoniae serotypes 1, 2, 5a, and the genome strain 5b. Carbohydr Res 339: 1973–1984. [DOI] [PubMed] [Google Scholar]
- 23. Ramjeet M, Deslandes V, Michael FS, Cox AD, Kobisch M, et al. (2005) Truncation of the lipopolysaccharide outer core affects susceptibility to antimicrobial peptides and virulence of Actinobacillus pleuropneumoniae serotype 1. J Biol Chem 280: 39104–39114. [DOI] [PubMed] [Google Scholar]
- 24. Knirel YA, Vinogradov E, Jiménez N, Merino S, Tomás JM (2004) Structural studies of the R-type lipopolysaccharide of Aeromonas hydrophila . Carbohydr Res 339: 787–793. [DOI] [PubMed] [Google Scholar]
- 25. Jiménez N, Canals R, Lacasta A, Kondakova AN. Lindner B, et al. (2008) Molecular analysis of three Aeromonas hydrophila AH-3 (serotype O34) lipopolysaccharide core biosynthesis gene clusters. J Bacteriol 190: 3176–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Foote SJ, Bossé JT, Bouevitch AB, Langford PR, Young NM, et al. (2008) The complete genome sequence of Actinobacillus pleuropneumoniae L20 (serotype 5b). J Bacteriol 190: 1495–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Z, Zhou Y, Li L, Zhou R, Xiao S, et al. (2008) Genome biology of Actinobacillus pleuropneumoniae JL03, an isoalate of serotype 3 prevalent in China. PLoS One 3: e1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhan B, Angen Ø, Hedegaard J, Bendixen C, Panitz F (2010) Draft genome sequences of Actinobacillus pleuropneumoniae serotypes 2 and 6. J Bacteriol 192: 5846–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gronow S, Brabetz W, Lindner B, Brade H (2005) OpsX from Haemophilus influenzae represents a novel type of heptosyltransferase I in lipopolysaccharide biosynthesis. J Bacteriol 187: 6242–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harper M, Boyce JD, Cox AD, Michael FS, Wilkie IW, et al. (2007) Pasteurella multocida expresses two lipopolysaccharide glycoforms simultaneously, but only a single form is required for virulence: identification of two acceptor-specific heptosyl I transferases. Infect Immun 75: 3885–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michael FS, Li J, Vinogradov E, Li J, Cox AD (2005) Structural analysis of the lipopolysaccharide from Pasteurella multocida genome strain Pm70 and identification of the putative lipopolysaccharide glycosyltranferases. Glycobiology 15: 323–333. [DOI] [PubMed] [Google Scholar]
- 32. Michael FS, Li J, Vinogradov E, Larocque S, Harper M, et al. (2005) Structural analysis of the lipopolysaccharide of Pasteurella multocida strain VP161: identification of both Kdo-P and Kdo-Kdo species in the lipopolysaccharide. Carbohydr Res 340: 59–68. [DOI] [PubMed] [Google Scholar]
- 33. Jiménez N, Lacasta A, Vilches S, Reyes M, Vazquez J, et al. (2009) Genetics and proteomics of Aeromonas salmonicida lipopolysaccharide core biosynthesis. J Bacteriol 191: 2228–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Izquierdo L, Coderch N, Piqué N, Bedini E, Corsaro MM, et al. (2003) The Klebsiella pneumoniae wabG gene: its role in the biosynthesis of the core lipopolysaccharide and virulence. J Bacteriol 185: 7213–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grizot S, Salem M, Vongsouthi V, Durand L, Moreau F, et al. (2006) Structure of the Escherichia coli heptosyltransferase WaaC: binary complex with ADP and ADP-2-deoxy-2-fluoro heptose. J Mol Biol 363: 383–394. [DOI] [PubMed] [Google Scholar]
- 36. Hiratsuka K, Logan SM, Conlan JW, Chandan V, Aubry A, et al. (2005) Identification of a D-glycero-D-manno-heptosyltransferase from Helicobacter pylori . J Bacteriol 187: 5156–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moreau F, Desroy N, Genevard JM, Vongsouthi V, Gerusz V, et al. (2008) Discovery of new Gram-negative antivirulence drugs: structure and properties of novel E. coli WaaC inhibitors. Bioorg Med Chem Lett 18: 4022–4026. [DOI] [PubMed] [Google Scholar]
- 38.Miller JH (1972) Experiments in molecular genetics.Cold Spring Harbor Laboratory Press, Cold Spring Harbor New York.
- 39.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: A laboratory manual, second Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor New York.
- 40. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hitchcock PJ, Brown TM (1983) Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol 154: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lesse AJ, Campagnari AA, Bittner WE, Apicella MA (1990) Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods 126: 109–117. [DOI] [PubMed] [Google Scholar]
- 43. Pradel E, Schnaitman CA (1991) Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J Bacteriol 173: 6428–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsai CM, Frasch CE (1982) A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem 119: 115–119. [DOI] [PubMed] [Google Scholar]
- 45. Galanos C, Lüderitz O, Westphal O (1969) A new method for the extraction of R lipopolysaccharides. Eur J Biochem 9: 245–249. [DOI] [PubMed] [Google Scholar]
- 46. Westphal O, Jann K (1965) Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr Chem 5: 83–89. [Google Scholar]
- 47. Felsenstein J (1992) Estimating effective population size from samples of sequences: a bootstrap MonteCarlo integration methods. Genet Res 60: 209–220. [DOI] [PubMed] [Google Scholar]
- 48. Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177: 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boyer HW, Roulland-Dussoix DA (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli . J Mol Biol 41: 459–472. [DOI] [PubMed] [Google Scholar]
- 50. Merino S, Camprubí S, Tomás JM (1992) Effect of growth temperature on outer-membrane components and virulence of Aeromonas hydrophila strains of serotype O:34. Infect Immun 60: 4343–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Canals R, Jiménez N, Vilches S, Regué M, Merino S, et al. (2006) The UDP N-acetylgalactosamine 4-epimerase is essential for mesophilic Aeromonas serotype O:34 virulence. Infect Immun 74: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]