Abstract
Vibrio cholerae is the causal organism of the cholera epidemic, which is mostly prevalent in developing and underdeveloped countries. However, incidences of cholera in developed countries are also alarming. Because of the emergence of new drug-resistant strains, even though several generic drugs and vaccines have been developed over time, Vibrio infections remain a global health problem that appeals for the development of novel drugs and vaccines against the pathogen. Here, applying comparative proteomic and reverse vaccinology approaches to the exoproteome and secretome of the pathogen, we have identified three candidate targets (ompU, uppP and yajC) for most of the pathogenic Vibrio strains. Two targets (uppP and yajC) are novel to Vibrio, and two targets (uppP and ompU) can be used to develop both drugs and vaccines (dual targets) against broad spectrum Vibrio serotypes. Using our novel computational approach, we have identified three peptide vaccine candidates that have high potential to induce both B- and T-cell-mediated immune responses from our identified two dual targets. These two targets were modeled and subjected to virtual screening against natural compounds derived from Piper betel. Seven compounds were identified first time from Piper betel to be highly effective to render the function of these targets to identify them as emerging potential drugs against Vibrio. Our preliminary validation suggests that these identified peptide vaccines and betel compounds are highly effective against Vibrio cholerae. Currently we are exhaustively validating these targets, candidate peptide vaccines, and betel derived lead compounds against a number of Vibrio species.
Introduction
Vibrio cholerae is a noninvasive gram-negative bacterium that causes water borne disease cholera, which is characterized by profuse watery diarrhea and vomiting [1]. The severity of the diarrhea and vomiting causes rapid dehydration and electrolyte imbalance that leads to death. The V. cholerae O395 strain is a classical O1 serotype strain responsible for cholera epidemics in Asian countries, and the non-O1 sero-group Vibrio cholerae O139 has been implicated as the causative agent of sporadic cases of gastro-enteritis and extra-intestinal infections [2], [3]. Both of the strains have been reported to cause significant numbers of morbidities [4], [5]. Although considerable research is ongoing to develop new drugs and vaccines and many antibiotics are already used to treat cholera, the infection remains frequently uncontrolled because of emerging antibiotic resistance of the pathogen [6], [7], [8]. Therefore, novel drugs and vaccines must be developed to tackle the Vibrio infection and transmission.
The identification of antigenic and virulence factors is paramount in developing antibiotics against a pathogen. In most cases, exomembrane (surface exposed) and secretary proteins exhibit antigenicity and virulence and are therefore suitable for targeting. Similarly, in the post-genomics era, computational approaches for the identification of genomic targets [9] and the use of reverse vaccinology [10] are becoming popular for rapid identification of novel targets to develop both drugs and vaccines against any given pathogen.
The present study aims to identify broad spectrum and novel drug and vaccine targets for a number of Vibrio strains, including V. cholerae strains O395 and O139; to design peptide vaccines; and to identify lead natural compounds first time from Piper betel, a well-known plant with medicinal value, to make use of these targets.
Materials and Methods
Drug and Vaccine Target Prioritization Parameters
Target prioritization in pathogenic microorganisms is accomplished in various ways [11]. Among these important prioritization considerations are subcellular localization, non-host homolog essential genes, core pathogen genes, pathogenic island association, involvement in the pathogen’s unique metabolic pathways, druggability, availability of 3D structural information, and low molecular weight of the target protein (≤110 kDa) [9], [11]–[14]. The exoproteome and secretome are good source of targets for developing vaccines and drugs (dual targets) [9], [10]. Therefore, we first screened the exoproteome and secretome of the pathogen for potential targets, followed by the application of other prioritization parameters to identify targets.
Screening of the Exoproteome and Secretome and Target Identification
We applied the classical reverse vaccinology strategy [10] and a modified method of subtractive proteomics [15], [16] to identify candidate drug vaccine targets in V. cholerae strain O395 and other Vibrio serotypes. In brief, the Vibrio cholera O395 proteome, which consists of 3875 proteins, was screened using CELLO [17], PSLpred [18], PSORTb [19], SOSUI-GramN [20], and SurfG+ [21] to identify the exomembrane and secreted proteins. Thereafter, the essential, non-human homolog Vibrio proteins (putative targets) from the pool of exoproteome and secretome were identified using the Database of Essential Genes (DEG) [22] and NCBI BLASTp [23], as described by Barh et al., 2011 [15]. Selected non-human homolog essential Vibrio proteins were then checked for their pathway involvement, and the best targets were selected based on the involvements of these targets in the unique essential bacterial metabolic pathways and another twelve criteria as described by Barh et al., 2011 [15] for target selection. The final list of identified targets was then checked for their presence in different Vibrio strains and related species using NCBI prokaryotic genome BLASTp.
Additional Evaluation of the Essentiality Parameters of Targets
The DEG-based essentiality of the identified targets was further validated using sequence-based computational approaches: (i) strand-bias; (ii) codon adaptation index (CAI); (iii) patterns of enzyme classes distributed, and (iv) clusters of orthologous groups (COG) of proteins. Essential genes are mostly located on leading strands and show strand bias [24]. We used Ori-Finder [25] to check the replication origin- and replication termini-based determination of strand-bias and the localization of the identified target genes in leading or lagging strands. CAI values are reported as one of the measures to evaluate essential genes, with a CAI >0.5 indicative of significant essentiality [26]. We used ACUA software [26] to calculate the CAI values of our identified targets. The distribution of enzyme classes of the targets was determined with BRENDA [27] and UniProtKB [28]. The targets were also examined for their bias toward COG functional subcategories for essentiality as per the findings of Lin et al., 2010 [29].
Evaluation of Prioritization Parameters of Targets
We further checked the identified targets for their molecular weight if they are of ≤110 kDa using UniProtKB [28]. The druggability of the targets was determined using the DrugBank database [30]. The amino acid sequences of identified potential targets were aligned using BLASTp with a cutoff E-value = 0.01 against the DrugBank -listed targets for which specific compounds are available in the database. The availability of 3D structural information of targets was verified with PDB [31]. When structures were not available, a homology modeling or threading approach was performed and verified with various 3D modeling parameters (see 3D modeling of targets section).
Additional Prioritization Parameters of Targets by PPIs and Hostpathogen Interactions
Target identification based on host-pathogen interactions has been implemented in many organisms, including M. tuberculosis [32]. Therefore, to verify the reliability of our identified targets, we searched for protein-protein interactions (PPIs) among the identified targets and also host-pathogen interactions. All V. cholera O395 targets were selected to make PPI networks using VisAnt [33]. Further, KEGG pathways [34] were incorporated into the PPI networks and analyzed for their involvement in bacterial pathogenesis and essential pathways. To identify host-pathogen interactions, 20,000 experimentally-validated host-pathogen interactions for 24 pathogens were downloaded from the PathoSystems Resource Integration Center (PATRIC) database [35]. In PATRIC, Vibrio-specific host-pathogen interaction data are not available. Therefore, we used sequences from pathogens listed in PATRIC that are 90% homologous to our identified Vibrio targets to determine interactions and interacting human counterparts. The interacting human counterparts were also analyzed for their involvement in key biological processes and pathways involved in host response to infection, such as immunity and apoptosis, and examined whether they are key nodes in those pathways using the Search Tool for the Retrieval of Interacting Genes (STRING) [36] and the Database for Annotation, Visualization and Integrated Discovery (DAVID) [37]. Targets that are involved in bacterial pathogenesis or essential pathways and interact with key molecules in host response pathways are generally more effective targets.
Prediction of Antigenic B-cell Derived T-cell Epitopes
Once the targets are finalized, the novel strategy of epitope designing as described by Barh et al., 2011 [16] was applied to design peptide vaccines from the vaccine targets. Briefly, the secreted and exomembrane proteins were checked for antigenicity using the VaxiJen v2.0 server (threshold = 0.4, ACC output) [38], and thereafter, their virulences were predicted using VirulentPred [39]. Proteins that were antigenic according to VirulentPred and showed an antigenicity score >0.5 in VaxiJen were selected. The exomembrane sequences of each virulent protein commonly derived from VaxiJen and VirulentPred analysis were determined by TMHMM v2.0 [40]. The BCPreds server [41] was used for B-cell epitope prediction (cutoff >0.8, 20-mer epitopes) and epitope sequences were matched with surface-exposed sequences of corresponding proteins. The surface-exposed B-cell epitope sequences were further checked for antigenicity using VaxiJen, and the best epitopes were selected for T-cell epitope prediction using ProPred [42] and ProPred I [43]. QSAR-based simulation analysis of each T-cell epitope was performed by MHCPred v.2 [44] and VaxiJen to detect half maximal (50%) inhibitory concentration (IC50) and antigenicity, respectively. For a second level confirmation, the selected T-cell epitopes were further screened by T-epitope designer [45], and epitopes were selected that showed binding affinity to ≥80% of HLA molecules, including the A*0201, A*0204, and B*2705, DRB1*0101 and DRB1*0401 alleles that are most common in the human population. Finally, epitopes that bound more than 13 MHC molecules in ProPred and ProPred-I with less than 100 nM IC50 for DRB1*0101 in MHCPred v2.0 and that bound ≥80% of HLA molecules in T-epitope designer were selected for fold-level topology analysis to select the best epitopes.
3D Modeling of Targets and Topology Analysis of Epitopes
For topology analysis of the identified epitopes and for virtual screening, the target proteins were modeled. The Phyre2 server [46] was used for homology modeling, and the threading approach was performed using the I-TASSER server [47]. The homology-based models were validated using the Structure Analysis and Verification Server (SAVS) Vs.4 (http://services.mbi.ucla.edu/SAVES/), and threading-based models were based on confidence scores (C-score range −5.0 to +2.0) and TM-scores of the resultant protein models. Further loop refinement of threading-based models was done by the ModLoop server [48], and finally, structure verification was performed by ERRAT plot version 2.0 [49], RAMPAGE [50], and the Dali server [51]. The localization and positioning of the epitopes within the folded proteins were analyzed using Pepitope server [52].
Ligand Library Preparation and Virtual Screening
Piper betel, one of the economic crops of West Bengal, India, is reported to have various medicinal and antimicrobial properties. However, no specific compound from this plant has so far been tested for antibacterial property. We collected 128 natural compounds of Piper betel from published literature to construct our ligand library. The library was also enriched with 35 well known antibiotics that are used to treat cholera with an aim to compare the efficacy of betel compounds with these antibiotics. The catalytic pockets of identified targets were determined using Molegro Virtual Docker (MVD) [53], CASTp [54], Pocket-Finder [55], and Active Site Prediction Server [56]. GOLD 4.1.2 software [57] was used for virtual screening. The best five betel derived ligands and antibiotics based on GOLD fitness scores and negative binding energy were selected and further validated using RMSD and MolDock scores in Molegro Virtual Docker 4.2.0 screening. The efficacy of top five betel compounds in respect to the top five antibiotics were determined based on GOLD fitness and Molegro Virtual Docker scores.
Preliminary Validation of Epitopes using IEDB and Betel Compounds against V. cholerae O1 Inaba
We preliminary validated the identified candidate peptide vaccines using the Immune Epitope Database (IEDB) [58]. One of the identified candidate betel compounds was also checked for its anti-Vibrio properties against V. cholerae O1 Inaba. The bacteria were maintained in Mueller-Hinton (MH) Broth, placed on a shaker incubator and grown at 37°C for 16–18 h, to reach the logarithmic phase. After that, bacterial cultures were adjusted to an absorbance of 0.1 at 600 nm (1×107 UFC/ml) to test the bactericidal activity of the candidate betel compound by two methods. a) Disk diffusion method: MH agar plates were prepared and spreaded with 1×107 UFC of bacterial cultures, and then sensi-disks (Ampicillin and Chloramphenicol) and disks impregnated with the betel compound (dissolved in water, at concentrations of 20, 40, 60, 80, 100, 200 and 300 mM); were placed on MH agar plates and were incubated at 37°C for 24 h. Finally, the zone inhibition was measured by using a Vernier caliper. To test the comparative efficacy of the candidate betel compound in respect to conventional anti-Vibrio antibiotics, we performed b) Colony-forming units (CFU/ml) assay: Here, 1×107 UFC/ml of bacterial suspension were resuspended in tubes containing MH broth, alone (control for bacterial growth) or incubated with 100 µg/ml of Chloramphenicol (control for bacterial inhibition), or with 20, 40, 60, 80, and 100 mM of candidate betel compound. After that, tubes were incubated at 37°C for 0, 20, 40, 60 and 80 min in shaking. Finally, the number of viable bacteria was counted each time by obtaining the CFU/ml from serial 10-fold dilutions prepared in MH broth and plating onto MH agar.
Results and Discussion
Genome Screening and Target Identification
We identified 513 membrane (160 from Ch-I and 353 from Ch-II) and 317 secreted (113 from Ch-I and 204 from Ch-II) proteins for a total of 830 proteins based on our exoproteome and secretome analysis of V. cholerae strain O395. The V. cholerae strain O395 proteome consists of 3875 proteins; therefore, 13.2% and 8.18% of proteins of the entire Vibrio proteome constitutes exoproteome and secretome, respectively. DEG-based essential gene analysis revealed only 178 essential proteins (119 exomembrane and 59 secreted) out of the total 830. Only 10 essential proteins (7 exomembrane and 3 secreted) were found to be non-human homologs and therefore probable targets (Table S1).
As shown in Table S2, among these 10 proteins, 3 are hypothetical (VC0395_0360, VC0395_A1375, and VC0395_A2856). The antigenicity and virulence analysis showed that 9 out of these 10 proteins are antigenic and that 7 are virulent. All 10 proteins were further analyzed for their involvement in the pathogen’s essential unique pathways using the KEGG pathway database. The three hypothetical proteins and LysE did not show any pathway involvement and therefore were removed from the analysis. fadL-3 (Long chain fatty acid transport protein) does not show any vital role in any bacterial essential pathway and was therefore also eliminated. rodA (rod shape determining protein) is involved in the regulation of cell shape processes [59] and is essential for Vibrio; however, it is not an essential gene for S. aureus [60] and also did not provide any T-cell epitopes in further analysis.
Cell membrane-localized TatC (sec-independent translocase protein) was identified as an interesting target in Vibrio. TatC is a virulent protein and is involved in pathways such as membrane transport and the bacterial secretion system. TatC has been reported as a target in M. leprae [61] and Klebsiella pneumonia MGH78578 [62]. However, according to the AEROPATH Target Database (http://aeropath.lifesci.dundee.ac.uk/), in P. aeruginosa, TatC is not an essential gene and it also did not generate any B-cell derived T-cell epitopes in further analysis.
The secreted protein ompU/VC0395_A0162 (Outer membrane protein ompU) was found to be an important target as it is involved in the V. Cholerae pathogenic cycle. ompU is involved in host cell invasion during Vibrio infections [63], and for pathogenic Vibrio harveyi SF-1, it is reported as a candidate subunit and DNA vaccine [64].
The second most important target is membrane-localized yajC/VC0395_A0472 (Preprotein translocase subunit yajC), which is involved in the bacterial secretion system, a vital pathway for bacterial survival. The C. botulinum yajC is reported as a putative target [65] and is also listed as a target in M. leprae, M. tuberculosis, and Wolbachia endosymbiont of Brugia malayi in the TDR Targets Database [66]. Our analysis also showed that both of these proteins from Vibrio are exomembrane/secreted, antigenic, and highly virulent and are therefore suitable for vaccine and drug design where yajC is a novel candidate target for Vibrio (Table S2).
Apart from these two vaccine targets, the third important target we identified is the membrane bound enzyme uppP/VC0395_A0054 (Undecaprenyl pyrophosphate phosphatase) because of its vital role in the bacterial-specific peptidoglycan biosynthesis pathway and its involvement in cell wall biosynthesis. uppP is reported as an antibiotic resistant gene [67] and is also a listed target for M. Leprae and M. tuberculosis in the TDR Targets Database [66]. However, we are reporting uppP for the first time as a target in Vibrio, therefore it is a novel target for this pathogen.
We used 21 Vibro species (both pathogenic and non-pathogenic) available in NCBI and when we searched these three targets (ompU, yajC, and uppP) for their presence among these Vibrio species using comparative BLASTp in NCBI server, we found that all three targets are present in 12 species, including the virulent strains Vibrio anguillarum 775, Vibrio cholerae O1 biovar El Tor str. N16961, Vibrio splendidus LGP32, Vibrio cholerae O395, and Vibrio harveyi, and the non-virulent strain Vibrio fischeri ES114. Therefore, all of these selected targets can be used for broad-spectrum drug and vaccine design for a number of Vibrio serotypes (Table S2).
Additional Evaluation of the Essentiality Parameters of Targets
The identified targets ompU, yajC, and uppP were further verified with additional parameters for essentiality in the pathogen genome. Essential enzymes are better targets [15], and most of the essential enzymes belong to the following enzyme classes: transferases, oxidoreductases, ligases, hydrolases, lyases, and isomerases [68]. Among the three targets, uppP (EC = 3.6.1.27) is a hydrolase and therefore meets the criteria to be an essential gene. The other two proteins (the secreted protein ompU and the preprotein translocase subunit yajC) are not enzymes; thus, additional analyses for essentiality were done using a combination of strand-bias, CAI, and COG-bias analysis. The strand-bias analysis showed that these three targets are located in the leading strand and that the codon adaptation indexes (CAI) are 0.63, 0.58, and 0.80, respectively, for ompU, yajC, and uppP, satisfying the cutoff value of >0.5 for being an essential gene. Previous reports have suggested that the essential genes of M. ulcerans belong to COG subcategories E, H, J. D, N, V and M [68]. Our identified targets ompU, yajC, and uppP, respectively, belong to M (cell envelope and membrane biogenesis), N (cell motility and secretion), and V (cellular processes and signaling) categories. Therefore, these three targets are essential as per the COG-bias analysis also.
Evaluation of the Prioritization Parameters of Targets
Proteins with molecular weight ≤110 kDa are proposed to be effective targets [68]. The molecular weights of Yajc, uppP, and ompU are 11.9 kDa, 29,3 kDa, and 37,7 kDa, respectively; therefore, these proteins are of the low molecular weight. This parameter is highly desirable for a target so that the target can be easily purified for further validation [69]. Targets are preferably druggable [70], and 3D structure is required for in silico drug discovery by modeling, virtual screening, and druggability analysis. The druggability of these three targets was first tested using a DrugBank search to determine if specific compounds are available against these targets. The results showed that only ompU is potentially druggable by small molecules such as N-(6,7,9,10,17,18,20,21-octahydrodibenzo[b,k] [1], [4], [7], [10], [13], [16]hexaoxacyclooctadecin-2-yl) acetamide, Dodecane, and (Hydroxyethyloxy)Tri(Ethyloxy)Octane, N-Octyl-2-Hydroxyethyl Sulfoxide. However, the E-values were high. No molecule was found to target Yajc and uppP in DrugBank. The druggability analysis using DrugBank was negative, potentially because of the novel nature of these identified targets, the non-availability of their 3D structures in PDB, and no previous study on their druggability aspects. Therefore, in this study, we attempted to model these three targets and further tested for druggability using virtual screening.
PPIs and Host Pathogen Interactions
We used all 10 initially identified targets, including the hypothetical proteins, to make PPI networks of the targets in V. cholera O395. The phylogenetic analysis and domain fusion-based PPI networks show that with the exception of VC0395_0360 (putative hydrolase/Hypothetical) and LysE, all targets interact with other Vibrio proteins. The KEGG-based analysis of the PPI networks reveals that the selected targets ompU, yajC, and uppP are involved in the V. cholera pathogenic cycle, bacterial secretion system, and peptidoglycan biosynthesis pathways, respectively. All of these pathways are unique to bacteria and involved in pathogenesis. Therefore, the PPIs-based analysis also supports our selected final three targets.
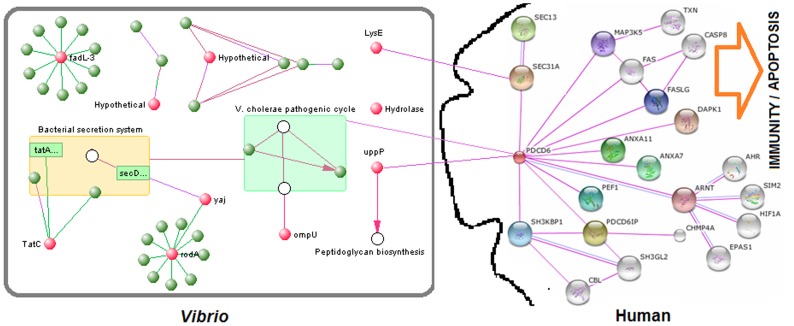
Our host-pathogen interaction analysis revealed that only uppP and LysE have host protein interacting counterparts. Our selected target uppP, which is involved in the peptidoglycan biosynthesis pathway, directly interacts with or binds to the PDCD6 (Programmed cell death 6) protein of the human host. The gene enrichment, pathway, and centrality analyses show that PDCD6 is a key molecule in host immunity and the apoptosis pathway ( Figure 1 ). The other target, LysE, interacts with the host SEC31A (Protein transport protein SEC31A). SEC31A is also involved in immunity and apoptosis in the host but is not a key molecule in these pathways. LysE is also not involved in any bacterial pathogenesis pathway, and the exclusion of LysE from the final list of targets is therefore justified. Although the two selected targets ompU and yajC do not directly interact with any host protein, the network analysis showed that the pathways in which these two proteins are involved (the bacterial secretion system and the V. cholerae pathogenic cycle) are interlinked and that some proteins in the V. cholerae pathogenic cycle interact with PDCD6 ( Figure 1 ). Therefore, these two selected targets (ompU and yajC) indirectly interact with PDCD6, leading us to the observation that all of our final selected targets (ompU, yajC, and uppP) interact with PDCD6 and modulate host response in terms of modulation of immunity and the apoptosis pathway in the host.
Figure 1. Protein-protein and host-pathogen interactions among ten preliminary identified Vibrio targets.
The interactions demonstrate that the finally selected three targets (ompU, yajC, and uppP) are involved in Vibrio pathogenesis and modulate host response (immunity and apoptosis) by interacting with the host protein PDCD6.
3D Modeling
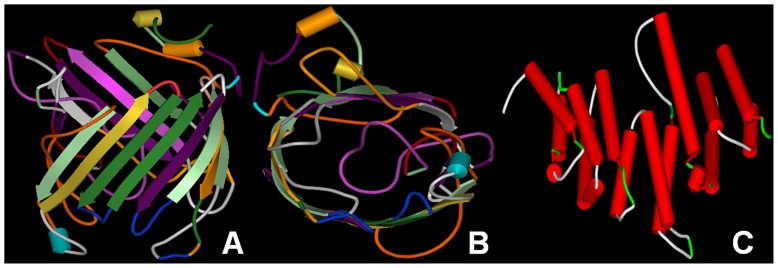
We first attempted to model ompU, yajC, and uppP using the Phyre 2 server. However, the attempt failed because of unavailability of the proper template. We therefore developed threading-based 3D structure models of these proteins. We were able to model ompU and uppP using I-TASSER; however, we could not model the yajC protein using this approach. Models were validated using the RAMPAGE, ERRAT plot, and Dali servers. Models were found to satisfy all criteria (Table S3, A-E). The 3D models of uppP and ompU are represented in Figure 2 .
Figure 2. 3D models of Vibrio targets constructed using threading approaches.
A) Front view of ompU, B) Side view of ompU, and C) Front view of uppP.
Epitope Design
Antigenicity and cell-exposed sequences
A good epitope should be cell exposed and antigenic. Therefore, these three targets ompU, yajC, and uppP were first analyzed using VaxiJen and then by TMHMM. The antigenicity scores of these three proteins were found to be 0.766, 0.744 and 0.484, respectively, for ompU, yajC, and uppP; therefore, they are all highly antigenic (Table S2, column-4). The TMHMM-based exomembrane region for ompU is 1–350 amino acids and is therefore fully exposed to the outside of the membrane. The cell-exposed amino acid sequences of uppP are 30–84, 132–156 and 206–219, and for yajC, the sequence is 1–14 (Table S4, column 6).
Antigenic B-cell epitope-derived T-cell epitopes
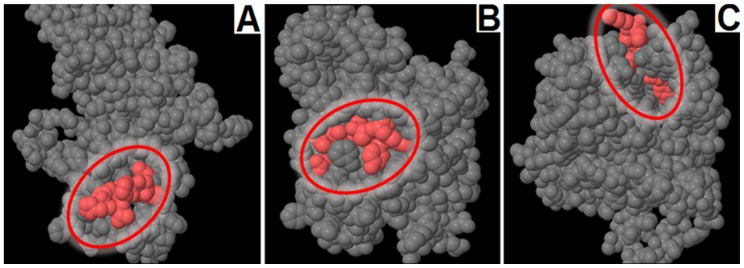
Using the approach described above, we identified one B-cell epitope from yajC, two from uppP, and thirteen from ompU (Table S4, column 2). However, when we analyzed for the presence of T-cell epitopes within these B-cell epitopes according to our selected criteria, yajC did not produce any T-cell epitope. ompU generated two (“VTETNAAKY” and “YNNAETAKK”) and uppP only one (“VTSGEPVHS”) epitope satisfying all of our criteria (Table S5). The entire protein sequence of uppP is non-virulent, but this single epitope is highly virulent and antigenic. Therefore, the uppP protein is a candidate novel vaccine target for Vibrio. The Pepitope analysis also showed that all of the identified T-cell epitopes are of the exomembrane topology within their corresponding folded proteins ( Figure 3 ).
Figure 3. Pepitope analysis of identified T-cell epitopes for their exomembrane topology (colored in red) within the corresponding folded proteins.
A) The “VTSGEPVHS” epitope of uppP, B) the “VTETNAAKY” epitope of ompU, and C) the “YNNAETAKK” epitope of ompU.
Drug Target and Virtual Screening
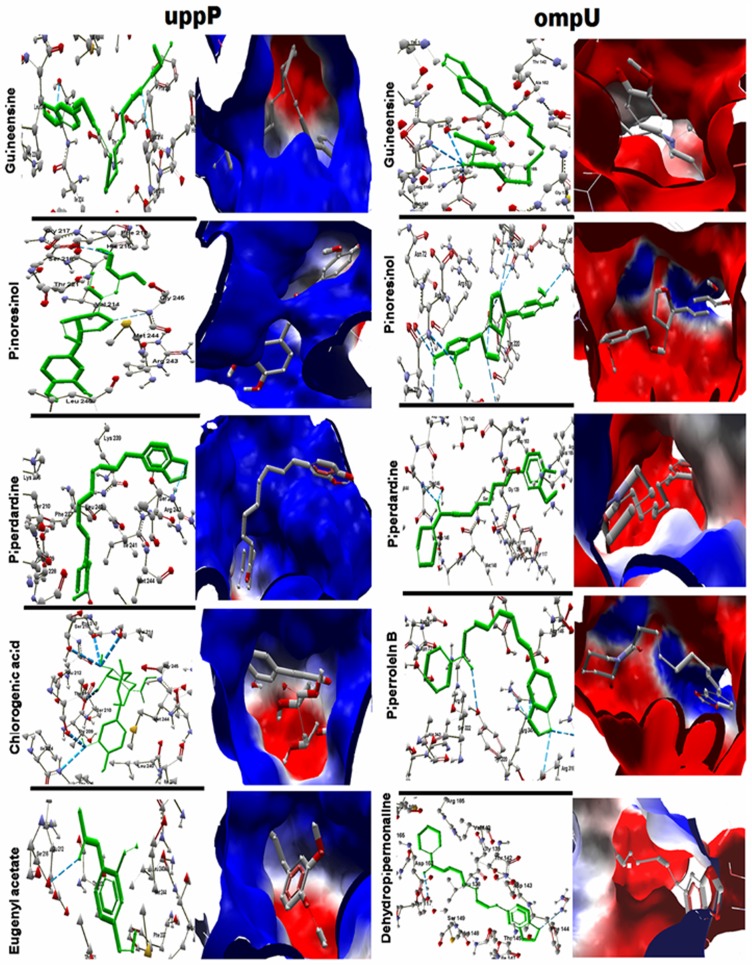
Since ompU is a secreted and uppP is an exomembrane protein, they are also suitable drug targets. The Piper betel leaf is used in folk medicine for treatment of several situations [72], and the leaf extracts are experimentally shown to be useful as antimicrobial [73], anti-leishmanial [74], antimalarial [75], anti-filarial [76], anti-fungal [77], anti-allergic [78], immunomodulator [79], gastroprotective [80], antioxidant [81], and anti-inflammatory [82] agents. We performed literature mining and collected 128 active phytochemicals from betel leaf and used them to screen against these two targets. The docking was done against the best cavity according to the Molegro virtual docker (MVD), CASTp, Pocketfinder, and Active Site Prediction Server (Table S6). The docking results based on the GOLD fitness score and Moldock score show that Guineesine, Pinoresinol, and Piperdardine can bind and render the activities of both the targets with high specificity. Apart from these three common compounds, Dehydropipernonaline and Piperrolein B were found to be effective on ompU and Chlorogenic acid and Eugenyl acetate on uppP ( Figure 4 , Table S7A). Several other betel compounds such as Piperardine and Peridine are also found to be effective against these targets however their GOLD fitness and Moldock scores are less. It should also be noted from the docking results that the Piper betel compounds are superior to the conventional antibiotics that are prescribed for the treatment of cholera in inhibiting these two targets (Table S7B).
Figure 4. The best seven Piper betel compounds that may render activities of Vibrio targets ompU and uppP.
GOLD fitness and Moldock scores were considered to select the compounds. Guineesine, Pinoresinol, and Piperdardine inhibit both targets. Dehydropipernonaline and Piperrolein B are effective on ompU. Chlorogenic acid and Eugenyl acetate are good ligands for uppP.
Validation of Epitopes and Betel Compounds
Among the identified three candidate peptide vaccines, we found ompU derived “VTETNAAKY” is 80% identical to an experimentally validated linear peptide vaccine derived from adhesin P1 of Mycoplasma pneumoniae M129 [71]. However, we could not get any similar peptide in IEDB for other two identified epitopes ((ompU derived “YNNAETAKK” and uppP based “VTSGEPVHS”), perhaps due to unavailability of similar peptides in IEDB or because of their novelty as candidate vaccines.
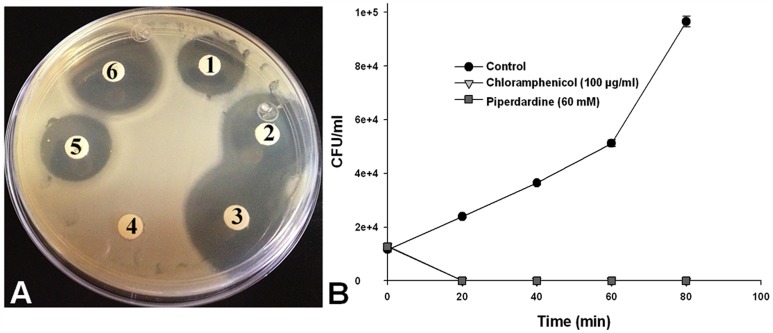
Piperdardine was used in this preliminary validation. This betel compound is found to be highly effective against V. cholerae O1 Inaba and the effect is concentration-dependent ( Figure 5A ). While we tested Piperdardine for its efficacy in respect to Chloramphenicol using growth kinetics assay, we observed that 60 mM of Piperdardine was able to inhibit V. cholerae O1 Inaba growth similar to100 µg/ml of Chloramphenicol treatment ( Figure 5B ). Form these assays; it’s also evident that the anti-Vibrio efficacy of Piperdardine is better than that of Chloramphenicol, although Piperdardine requires a higher concentration. In this study, we did not check the target specificity of Piperdardine in V. cholerae O1 Inaba. However, currently we are conducting in-depth validations and target specificities of all identified betel compounds against a number of Vibrio species.
Figure 5. Anti-Vibrio activity of Piperdardine.
A). Growth inhibition effects Piperdardine, Ampicillin, and Chloranphenicol on V. Cholerae O1 Inaba growth as per the disk diffusion method. 1) 100 mM, 2) 200 mM, and 3) 300 mM Piperdardine; 4) water; 5) Ampicillin (10 µg); and 6) Chloranphenicol (30 µg). The zones of inhibition (mm) around disks containing Piperdardine are concentration-dependent: 1) 19.3±0.03; 2) 26.23±0.1; 3) 28.65±0.16. Controls: 4) 0±0; 5) 18.51±0.16; and 6) 29.47±0.16. B). Effects on Piperdardine and Chloranphenicol on V. Cholerae O1 Inaba growth as per the Colony-forming units (CFU/ml) assay. As per the method described in the text, 60 mM of Piperdardine (squares) shows anti-Vibrio effect similar to 100 µg/ml of Chloramphenicol (triangles).
Conclusion
In summary, in this analysis, we have identified ompU, uppP, and yajC from the Vibrio cholerae strain O395 secretome and membrane proteome as novel targets that can be useful in designing broad-spectrum peptide vaccines or drugs against most of the virulent strains of the pathogen. YNNAETAKK and VTETNAAKY from ompU and VTSGEPVHS from uppP were found to be effective candidate peptide vaccines. Piper betel-derived Piperdardine, Pinoresinaol, and Guineensine can target both ompU and uppP, whereas Dehydropipernonaline and Piperrolein B are specific inhibitors of ompU and Eugenyl acetate and Chlorogenic acid are specific to uppP. Most of these compounds show better efficacy than the currently-used anti-Vibrio drugs in our in silico analysis. Our validation results first time demonstrate that Piperdardine exhibits anti-Vibrio effects in a dose dependent manner and 60 mM of Piperdardine is having similar anti-Vibrio effect as 100 µg/ml of Chloramphenicol has. We are currently validating all of our identified targets, candidate peptide vaccines, and betel derived lead compounds against most of the Vibrio strains and serotypes available.
Supporting Information
Final statistics of membrane and secreted essential proteins. The proteome of the Vibrio cholerae strain O395 was screened using CELLO, PSLpred, PSORTb, SOSUI-GramN, and SurfG+ to identify the membrane proteome and secretome. The genome contains a total of 3998 genes encoding 3875 proteins. The essentialities of these membrane and secreted proteins were determined by DEG-based BLASTp. The cutoff values for bit score, E-value, and percentage of identity at the amino acid level, respectively, were ≥100, E = 0.0001, and ≥40%. A total of 178 essential proteins were identified in which 119 are membrane located and 59 are secreted. Essential non-host homologs of the pathogen were identified using NCBI Human BLASTp with default parameters. A total of 10 (7 membrane and 3 secreted) essential non-host homologs was found.
(DOC)
Features of the identified 10 targets in V. Cholerae . Ten V. cholerae O395 targets were selected based on subtraction proteomics. VC0395_0360 and VC0395_0374 are located in Chromosome-I (Ch-I), whereas the other eight targets are located in Chromosome-II (Ch-II). Column-1 and Column-3, respectively, represent locus tags and target names. The blue-colored (ompU, uppP and yajC) meet all conditions for good targets and may be used for broad-spectrum drug and vaccine designing. These three targets are also common to twelve Vibrio species. Column 4 represents the COG categories. Column 5 provides detailed annotation of the corresponding Vibrio target. Column 6 provides the information on Virulence based on VirulentPred. VaxiJen-based antigenicity of the target Vibrio protein is provided in Column 7. Column 8 provides PARTIC and other analysis-based host proteins that interact with the corresponding targets. Columns 9–29 represent Vibrio strains/species tested for having identical targets in their genome/proteome based on homology. X represents absence of the target and √ represents presence. The last column represents the BLAST results of corresponding Vibrio targets with the human genome/proteome, and all targets show non-homology.
(DOC)
A) Template and structure selection for modeling. Because the homology-based approaches for 3D modeling failed, we performed modeling using a threading approach. The three target proteins were submitted to the I-TASSER server, and we observed that the C-score (−5, 2) and TM score were in acceptable ranges. (i) Template selection for modeling Column-1 (The rank of templates) represents the top ten threading templates used by I-TASSER. Ident1 (Column-3) is the percentage sequence identity of the templates in the threading-aligned region with the query sequence. The Ident2 (Column-4) is the percentage sequence identity of the entire template with the query sequence. Coverage (Column-5) represents the coverage of the threading alignment and is equal to the number of aligned residues divided by the length of the query protein. Column-6 represents the normalized Z-score of the threading alignments. Alignment with a normalized Z-score >1 indicates a good alignment. (ii) Target protein structure selection B) Energy of the protein-modeled structures The modeled structures were subjected to energy minimization. We performed energy minimization in the Swiss PDB Viewer and then checked using RAMPAGE and ERRAT plot. The energies of these two proteins were as follows. C) RAMPAGE results To validate the stereochemical properties of the two targets’ modeled proteins, we used the RAMPAGE server. The expected percentages for residues in the favored region, allowed region, and outliers region are 98%, 2% and 0%, respectively. Our results demonstrated that the parameters of our modeled proteins are close to these cutoff values, and the models are therefore acceptable. D) ERRAT plot results for ompU and uppP. To further examine the non-bonded interaction of atoms in the models of the two targets, we used the Erraplot server. This server provides the quality factor of the modeled structure. Good, high-resolution structures generally produce quality factor values of approximately 95% or higher. For lower resolutions (2.5 to 3A), the average overall quality factor is approximately 91%. The following ERRAT plot criteria clearly show that our modeled proteins are of high quality. E) Validation of structures using the Dali server To provide strong support of the modeled structure, we performed structure-structure alignment in the Dali server and examined the function. We observed Z-scores of 2 that were greater than the threshold for a good alignment for both of the modeled proteins. Therefore, the models are acceptable for further structure-based in silico analysis.
(DOC)
Identification of B-cell epitopes. As described in the methods, the amino acid sequences of yajC, uppP and ompU were subjected to the BCPreds server for B-cell epitope identification. The BCPreds and VaxiJen scores and the transmembrane topology for the selected B-cell epitopes from each target are listed in this table.
(DOC)
Selected B-cell epitope-derived T-cell epitopes and their properties. The method is adopted as described by Barh et al., 2010 [16]. The final selected epitopes are highlighted in red.
(DOC)
Active residues of ompU and uppP in the best cavity. We predicted the active residues for the largest cavity from Molegro Virtual Docker (MVD), and we verified our predictions with Cast-P, Pocketfinder and Active site prediction server. All predictions were in good agreement with the predicted result of MVD. However, in uppP, we observe a Histidine residue that is well known for ligand specification.
(DOC)
Virtual screening for uppP and ompU . The docking was performed as described in the methods. The top five ligands were selected based on their GOLD fitness score, MolDock score and RMSD. A ligand with a GOLD fitness score >25 is considered to be a good ligand. Similarly, the standard RMSD ranges from 0 to 4. Apart from electrostatic and hydrophobic interactions, more than 2 H-bonds indicate the ligand stability in the docked position.
(DOC)
Acknowledgments
We thank Erika Acosta-Smith for her efficient technical support while we carrying out microbial experiments.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sack DA, Sack RB, Nair GB, Siddique AK (2004) Cholera. Lancet 363 (9404): 223–233. [DOI] [PubMed] [Google Scholar]
- 2. Shimada T, Sakazaki R (1977) Additional serovars and inter-O antigenic relationships of V. cholerae. Jpn J Med Sci Biol. 30 (5): 275–277. [DOI] [PubMed] [Google Scholar]
- 3. Faruque SM, Albert MJ, Mekalanos JJ (1998) Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 62 (4): 1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siddique AK, Zaman K, Baqui AH, Akram K, Mutsuddy P, et al. (1992) Cholera epidemics in Bangladesh: 1985–1991. J Diarrhoeal Dis Res. 10 (2): 79–86. [PubMed] [Google Scholar]
- 5. Siddique AK, Akram K, Zaman K, Mutsuddy P, Eusof A, et al. (1996) Vibrio cholerae O139: how great is the threat of a pandemic? Trop Med Int Health. 1 (3): 393–398. [DOI] [PubMed] [Google Scholar]
- 6. Fazil MH, Singh DV (2011) Vibrio cholerae infection, novel drug targets and phage therapy. Future Microbiol. 6 (10): 1199–1208. [DOI] [PubMed] [Google Scholar]
- 7. Mandal S, Mandal MD, Pal NK (2011) Cholera: a great global concern. Asian Pac J Trop Med. 4 (7): 573–580. [DOI] [PubMed] [Google Scholar]
- 8. Tran HD, Alam M, Trung NV, Kinh NV, Nguyen HH, et al. (2012) Multi-drug resistant Vibrio cholerae O1 variant El Tor isolated in northern Vietnam between 2007 and 2010. J Med Microbiol. 61(3): 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barh D, Tiwari S, Jain N, Ali A, Santos AR, et al. (2011) In silico subtractive genomics for target identification in human bacterial pathogens. Drug Dev Res. 72: 162–177. [Google Scholar]
- 10. Pizza M, Scarlato V, Masignani V, Giuliani MM, Aricò B, et al. (2000) Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287 (5459): 1816–1820. [DOI] [PubMed] [Google Scholar]
- 11. Agüero F, Al-Lazikani B, Aslett M, Berriman M, Buckner FS, et al. (2008) Genomic-scale prioritization of drug targets: the TDR Targets database. Nat Rev Drug Discov 7: 900–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caffrey CR, Rohwer A, Oellien F, Marhöfer RJ, Braschi S, et al. (2009) A comparative chemogenomics strategy to predict potential drug targets in the metazoan pathogen, Schistosoma mansoni. PLoS One 4(2): e4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crowther GJ, Shanmugam D, Carmona SJ, Doyle MA, Hertz-Fowler C, et al. (2010) Identification of attractive drug targets in neglected-disease pathogens using an in silico approach. PLoS Negl Trop Dis 4: e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abadio AK, Kioshima ES, Teixeira MM, Martins NF, Maigret B, et al. (2011) Comparative genomics allowed the identification of drug targets against human fungal pathogens. BMC Genomics 12: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barh D, Jain N, Tiwari S, Parida BP, D’Afonseca V, et al. (2011) A novel comparative genomics analysis for common drug and vaccine targets in Corynebacterium pseudotuberculosis and other CMN group of human pathogens. Chem Biol Drug. 78 (1): 73–84. [DOI] [PubMed] [Google Scholar]
- 16. Barh D, Misra AN, Kumar A, Azevedo V (2010) A novel strategy of epitope design in Neisseria gonorrhoeae. Bioinformation 5 (2): 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu CS, Lin CJ, Hwang JK (2004) Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 13 (5): 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhasin M, Garg A, Raghava GP (2005) PSLpred: prediction of subcellular localization of bacterial proteins. Bioinformatics 21: 2522–2524. [DOI] [PubMed] [Google Scholar]
- 19. Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, et al. (2005) PSORTb v.2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21: 617–623. [DOI] [PubMed] [Google Scholar]
- 20. Imai K, Asakawa N, Tsuji T, Akazawa F, Ino A, et al. (2008) SOSUI-GramN: high performance prediction for sub-cellular localization of proteins in Gram-negative bacteria. Bioinformation 2: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barinov A, Loux V, Hammani A, Nicolas P, Langella P, et al. (2009) Prediction of surface exposed proteins in Streptococcuspyogenes, with a potential application to other Gram-positive bacteria. Proteomics 9: 61–73. [DOI] [PubMed] [Google Scholar]
- 22. Zhang R, Lin Y (2009) DEG 5.0, a database of essential genes in both prokaryotes and eukaryotes. Nucleic Acids Res. 37: D455–D458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gish W, States JD (1993) Identification of protein coding regions by database similarity search. Nat Genet. 3: 266–272. [DOI] [PubMed] [Google Scholar]
- 24. Rocha EP, Danchin A (2003) Essentiality, not expressiveness, drives gene-strand bias in bacteria. Nat Genet 34: 377–378. [DOI] [PubMed] [Google Scholar]
- 25. Gao F, Zhang CT (2008) Ori-Finder: a web-based system for finding oriCs in unannotated bacterial genomes. BMC Bioinformatics 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vetrivel U, Arunkumar V, Dorairaj S (2007) ACUA: a software tool for automated codon usage analysis. Bioinformation 2: 62–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schomburg I, Chang A, Schomburg D (2002) BRENDA, enzyme data and metabolic information.Nucleic Acids Res. 30(1): 47–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magrane M, Consortium U (2011) UniProt Knowledgebase: a hub of integrated protein data. Database (Oxford). 2011: bar009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin Y, Gao F, Zhang CT (2010) Functionality of essential genes drives gene strand-bias in bacterial genomes. Biochem Biophys Res Commun 396: 472–476. [DOI] [PubMed] [Google Scholar]
- 30.Knox C, Law V, Jewison T, Liu P, Ly S, et al.. (2011) DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs.Nucleic Acids Res. 39(Database issue): D1035–41. [DOI] [PMC free article] [PubMed]
- 31. Kouranov A, Xie L, de la Cruz J, Chen L, Westbrook J, et al. (2006) The RCSB PDB information portal for structural genomics. Nucleic Acids Res. 34: D302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin MY, Ottenhoff TH (2008) Host-pathogen interactions in latent Mycobacterium tuberculosis infection: identification of new targets for tuberculosis intervention. Endocr Metab Immune Disord Drug Targets. 8 (1): 15–29. [DOI] [PubMed] [Google Scholar]
- 33. Hu Z, Hung JH, Wang Y, Chang YC, Huang CL, et al. (2009) VisANT 3.5: multi-scale network visualization, analysis and inference based on the gene ontology. Nucleic Acids Res. 37: W115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gillespie JJ, Wattam AR, Cammer SA, Gabbard JL, Shukla MP, et al. (2011) PATRIC: the comprehensive bacterial bioinformatics resource with a focus on human pathogenic species. Infect. Immun. 79: 4286–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, et al. (2011) The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39: D561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc 4 (1): 44–57. [DOI] [PubMed] [Google Scholar]
- 38. Doytchinova IA, Flower DR (2007) VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garg A, Gupta D (2008) VirulentPred: a SVM based prediction method for virulent proteins in bacterial pathogens. BMC Bioinformatics 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krogh A, Larsson B, von Heijne G, Sonnhammer EL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305(3): 567–580. [DOI] [PubMed] [Google Scholar]
- 41. El-Manzalawy Y, Dobbs D, Honavar V (2008) Predicting linear B-cell epitopes using string kernels. J Mol Recognit. 21(4): 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singh H, Raghava GP (2001) ProPred: prediction of HLA-DR binding sites. Bioinformatics 17(12): 1236–7. [DOI] [PubMed] [Google Scholar]
- 43. Singh H, Raghava GP (2003) ProPred1: prediction of promiscuous MHC Class-I binding sites. Bioinformatics 19(8): 1009–1014. [DOI] [PubMed] [Google Scholar]
- 44. Guan P, Doytchinova IA, Zygouri C, Flower DR (2003) MHCPred: A server for quantitative prediction of peptide-MHC binding, Nucleic Acids Res. 31(13): 3621–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kangueane P, Sakharkar MK (2005) T-Epitope Designer: A HLA-peptide binding prediction server. Bioinformation 1(1): 21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kelley LA, Sternberg MJ (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 4(3): 363–371. [DOI] [PubMed] [Google Scholar]
- 47. Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 5(4): 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fiser A, Sali A (2003) ModLoop: automated modeling of loops in protein structures. Bioinformatics 19(18): 2500–2501. [DOI] [PubMed] [Google Scholar]
- 49. Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 2(9): 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lovell SC, Davis IW, Arendall WB 3rd, de Bakker PI, Word JM, et al (2003) Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins 50(3): 437–450. [DOI] [PubMed] [Google Scholar]
- 51. Holm L, Rosenström P (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res. 38: W545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mayrose I, Penn O, Erez E, Rubinstein ND, Shlomi T, et al. (2007) Pepitope: epitope mapping from affinity-selected peptides. Bioinformatics 23(23): 3244–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomsen R, Christensen MH (2006) MolDock: A New Technique for High-Accuracy Molecular Docking. J Med Chem. 49(11): 3315–3321. [DOI] [PubMed] [Google Scholar]
- 54. Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, et al. (2006) CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues.Nucleic Acids Res. 34: W116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hendlich M, Rippmann F, Barnickel G (1997) LIGSITE: automatic and efficient detection of potential small molecule-binding sites in proteins. J Mol Graph Model. 15(6): 359–363. [DOI] [PubMed] [Google Scholar]
- 56. Singh T, Biswas D, Jayaram B (2011) AADS–an automated active site identification, docking, and scoring protocol for protein targets based on physicochemical descriptors. J Chem Inf Model. 51(10): 2515–2527. [DOI] [PubMed] [Google Scholar]
- 57. Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD (2003) Improved protein-ligand docking using GOLD. Proteins 52(4): 609–623. [DOI] [PubMed] [Google Scholar]
- 58. Kim Y, Ponomarenko J, Zhu Z, Tamang D, Wang P, et al. (2012) Immune epitope database analysis resource. Nucleic Acids Res. 40: W525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hunt A, Rawlins JP, Thomaides HB, Errington J (2006) Functional analysis of 11 putative essential genes in Bacillus subtilis. Microbiology 152(10): 2895–2907. [DOI] [PubMed] [Google Scholar]
- 60. Chaudhuri RR, Allen AG, Owen PJ, Shalom G, Stone K, et al. (2009) Comprehensive identification of essential Staphylococcus aureus genes using Transposon-Mediated Differential Hybridisation (TMDH). BMC Genomics 10: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shanmugam A, Natarajan J (2010) Computational genome analyses of metabolic enzymes in Mycobacterium leprae for drug target identification. Bioinformation 4(9): 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Georrge JN, Umrania V (2011) In silico identification of putative drug targets in Klebsiella pneumonia MGH78578. Indian Journal of Biotechnology 10: 432–439. [Google Scholar]
- 63. Duperthuy M, Schmitt P, Garzón E, Caro A, Rosa RD, et al. (2011) Use of ompU porins for attachment and invasion of Crassostrea gigas immune cells by the oyster pathogen Vibrio splendidus. Proc Natl Acad Sci U S A 108(7): 2993–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sperandio V, Bailey C, Girón JA, DiRita VJ, Silveira WD, et al. (1996) Cloning and characterization of the gene encoding the ompU outer membrane protein of Vibrio cholerae.Infect Immun. 64(12): 5406–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reddy GK, Rao KN, Prasad PR (2011) Identification of drug and vaccine targets in Clostridium botulinum-A by the approach in-silico subtractive genomics. International Journal of Pharmaceutical Studies and Research 2: 48–54. [Google Scholar]
- 66. Magariños MP, Carmona SJ, Crowther GJ, Ralph SA, Roos DS, et al. (2012) TDR Targets: a chemogenomics resource for neglected diseases. Nucleic Acids Res. 40: D1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu B, Pop M (2009) ARDB-Antibiotic Resistance Genes Database. Nucleic Acids Res. 37: D443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Butt AM, Nasrullah I, Tahir S, Tong Y (2012) Comparative Genomics Analysis of Mycobacterium ulcerans for the Identification of Putative Essential Genes and Therapeutic Candidates. PLoS One. 7(8): e43080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Duffield M, Cooper I, McAlister E, Bayliss M, Ford D, et al. (2010) Predicting conserved essential genes in bacteria: in silico identification of putative drug targets. Mol Biosyst 6: 2482–2489. [DOI] [PubMed] [Google Scholar]
- 70. Keller TH, Pichota A, Yin Z (2006) A practical view of ‘druggability’. Curr Opin Chem Biol 10: 357–361. [DOI] [PubMed] [Google Scholar]
- 71. Dallo SF, Su CJ, Horton JR, Baseman JB (1998) Identification of P1 gene domain containing epitope(s) mediating Mycoplasma pneumoniae cytoadherence. J Exp Med. 167(2): 718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Valentão P, Gonçalves RF, Belo C, de Pinho PG, Andrade PB, et al. (2010) Improving the knowledge on Piper betle: targeted metabolite analysis and effect on acetylcholinesterase. J Sep Sci. 33(20): 3168–3176. [DOI] [PubMed] [Google Scholar]
- 73. Ali I, Khan FG, Suri KA, Gupta BD, Satti NK, et al. (2010) In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann Clin Microbiol Antimicrob. 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sarkar A, Sen R, Saha P, Ganguly S, Mandal G, et al. (2008) An ethanolic extract of leaves of Piper betle (Paan) Linn mediates its antileishmanial activity via apoptosis. Parasitol Res. 102(6): 1249–1255. [DOI] [PubMed] [Google Scholar]
- 75. Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Amran AA, Mahmud R (2010) Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules. 16(1): 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Singh M, Shakya S, Soni VK, Dangi A, Kumar N, et al. (2009) The n-hexane and chloroform fractions of Piper betle L. trigger different arms of immune responses in BALB/c mice and exhibit antifilarial activity against human lymphatic filarid Brugia malayi. Int Immunopharmacol b96: 716–28. [DOI] [PubMed] [Google Scholar]
- 77. Trakranrungsie N, Chatchawanchonteera A, Khunkitti W (2008) Ethnoveterinary study for antidermatophytic activity of Piper betle, Alpinia galanga and Allium ascalonicum extracts in vitro. Res Vet Sci. 84(1): 80–84. [DOI] [PubMed] [Google Scholar]
- 78. Wirotesangthong M, Inagaki N, Tanaka H, Thanakijcharoenpath W, Nagai H (2008) Inhibitory effects of Piper betle on production of allergic mediators by bone marrow-derived mast cells and lung epithelial cells. Int Immunopharmacol. 8(3): 453–457. [DOI] [PubMed] [Google Scholar]
- 79. Kanjwani DG, Marathe TP, Chiplunkar SV, Sathaye SS (2008) Evaluation of immunomodulatory activity of methanolic extract of Piper betel. Scand J Immunol. 67(6): 589–593. [DOI] [PubMed] [Google Scholar]
- 80. Majumdar B, Ray Chaudhuri SG, Ray A, Bandyopadhyay SK (2003) Effect of ethanol extract of Piper betle Linn leaf on healing of NSAID-induced experimental ulcer–a novel role of free radical scavenging action. Indian J Exp Biol. 41(4): 311–315. [PubMed] [Google Scholar]
- 81. Dasgupta N, De B (2004) Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 88: 219–224. [Google Scholar]
- 82. Ganguly S, Mula S, Chattopadhyay S, Chatterjee M (2007) An ethanol extract of Piper betle Linn. mediates its anti-inflammatory activity via down-regulation of nitric oxide. J Pharm Pharmacol. 59(5): 711–718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Final statistics of membrane and secreted essential proteins. The proteome of the Vibrio cholerae strain O395 was screened using CELLO, PSLpred, PSORTb, SOSUI-GramN, and SurfG+ to identify the membrane proteome and secretome. The genome contains a total of 3998 genes encoding 3875 proteins. The essentialities of these membrane and secreted proteins were determined by DEG-based BLASTp. The cutoff values for bit score, E-value, and percentage of identity at the amino acid level, respectively, were ≥100, E = 0.0001, and ≥40%. A total of 178 essential proteins were identified in which 119 are membrane located and 59 are secreted. Essential non-host homologs of the pathogen were identified using NCBI Human BLASTp with default parameters. A total of 10 (7 membrane and 3 secreted) essential non-host homologs was found.
(DOC)
Features of the identified 10 targets in V. Cholerae . Ten V. cholerae O395 targets were selected based on subtraction proteomics. VC0395_0360 and VC0395_0374 are located in Chromosome-I (Ch-I), whereas the other eight targets are located in Chromosome-II (Ch-II). Column-1 and Column-3, respectively, represent locus tags and target names. The blue-colored (ompU, uppP and yajC) meet all conditions for good targets and may be used for broad-spectrum drug and vaccine designing. These three targets are also common to twelve Vibrio species. Column 4 represents the COG categories. Column 5 provides detailed annotation of the corresponding Vibrio target. Column 6 provides the information on Virulence based on VirulentPred. VaxiJen-based antigenicity of the target Vibrio protein is provided in Column 7. Column 8 provides PARTIC and other analysis-based host proteins that interact with the corresponding targets. Columns 9–29 represent Vibrio strains/species tested for having identical targets in their genome/proteome based on homology. X represents absence of the target and √ represents presence. The last column represents the BLAST results of corresponding Vibrio targets with the human genome/proteome, and all targets show non-homology.
(DOC)
A) Template and structure selection for modeling. Because the homology-based approaches for 3D modeling failed, we performed modeling using a threading approach. The three target proteins were submitted to the I-TASSER server, and we observed that the C-score (−5, 2) and TM score were in acceptable ranges. (i) Template selection for modeling Column-1 (The rank of templates) represents the top ten threading templates used by I-TASSER. Ident1 (Column-3) is the percentage sequence identity of the templates in the threading-aligned region with the query sequence. The Ident2 (Column-4) is the percentage sequence identity of the entire template with the query sequence. Coverage (Column-5) represents the coverage of the threading alignment and is equal to the number of aligned residues divided by the length of the query protein. Column-6 represents the normalized Z-score of the threading alignments. Alignment with a normalized Z-score >1 indicates a good alignment. (ii) Target protein structure selection B) Energy of the protein-modeled structures The modeled structures were subjected to energy minimization. We performed energy minimization in the Swiss PDB Viewer and then checked using RAMPAGE and ERRAT plot. The energies of these two proteins were as follows. C) RAMPAGE results To validate the stereochemical properties of the two targets’ modeled proteins, we used the RAMPAGE server. The expected percentages for residues in the favored region, allowed region, and outliers region are 98%, 2% and 0%, respectively. Our results demonstrated that the parameters of our modeled proteins are close to these cutoff values, and the models are therefore acceptable. D) ERRAT plot results for ompU and uppP. To further examine the non-bonded interaction of atoms in the models of the two targets, we used the Erraplot server. This server provides the quality factor of the modeled structure. Good, high-resolution structures generally produce quality factor values of approximately 95% or higher. For lower resolutions (2.5 to 3A), the average overall quality factor is approximately 91%. The following ERRAT plot criteria clearly show that our modeled proteins are of high quality. E) Validation of structures using the Dali server To provide strong support of the modeled structure, we performed structure-structure alignment in the Dali server and examined the function. We observed Z-scores of 2 that were greater than the threshold for a good alignment for both of the modeled proteins. Therefore, the models are acceptable for further structure-based in silico analysis.
(DOC)
Identification of B-cell epitopes. As described in the methods, the amino acid sequences of yajC, uppP and ompU were subjected to the BCPreds server for B-cell epitope identification. The BCPreds and VaxiJen scores and the transmembrane topology for the selected B-cell epitopes from each target are listed in this table.
(DOC)
Selected B-cell epitope-derived T-cell epitopes and their properties. The method is adopted as described by Barh et al., 2010 [16]. The final selected epitopes are highlighted in red.
(DOC)
Active residues of ompU and uppP in the best cavity. We predicted the active residues for the largest cavity from Molegro Virtual Docker (MVD), and we verified our predictions with Cast-P, Pocketfinder and Active site prediction server. All predictions were in good agreement with the predicted result of MVD. However, in uppP, we observe a Histidine residue that is well known for ligand specification.
(DOC)
Virtual screening for uppP and ompU . The docking was performed as described in the methods. The top five ligands were selected based on their GOLD fitness score, MolDock score and RMSD. A ligand with a GOLD fitness score >25 is considered to be a good ligand. Similarly, the standard RMSD ranges from 0 to 4. Apart from electrostatic and hydrophobic interactions, more than 2 H-bonds indicate the ligand stability in the docked position.
(DOC)