Abstract
Bacillus subtilis possesses five osmotically regulated transporters (Opu) for the uptake of various compatible solutes for osmoprotective purposes. We have now found that compatible solutes also function as thermoprotectants for B. subtilis. Low concentrations of glycine betaine enhanced the growth of the B. subtilis wild-type strain JH642 at its maximal growth temperature (52°C) but did not allow an extension of the upper growth limit. A similar enhancement in the growth of B. subtilis was also observed by the addition of several other compatible solutes that are structurally related to glycine betaine or by the addition of proline. Each of these compatible solutes was taken up under heat stress by the cell through the same Opu transporters that are used for their acquisition under osmostress conditions. Northern blot analysis revealed a moderate increase in transcription of the structural genes for each of the Opu transport systems in cells that were propagated at 52°C. In contrast, the uptake level of radiolabeled glycine betaine was very low under high-temperature growth conditions but nevertheless allowed the buildup of an intracellular glycine betaine pool comparable to that found in cells grown at 37°C in the absence of salt stress. Although exogenously added glutamate has only a limited osmoprotective potential for B. subtilis, it was found to be a very effective thermoprotectant. Collectively, our data demonstrate thermoprotection by a variety of compatible solutes in B. subtilis, thus ascribing a new physiological function for this class of compounds in this microorganism and broadening the physiological role of the known osmoprotectant uptake systems (Opu).
Although some microorganisms contain water-selective channels, or aquaporins, in their cytoplasmic membranes (20), bacteria cannot actively transport water in or out of the cell. Their intracellular water content is therefore determined by purely osmotic processes. When the osmolality in their habitat fluctuates and water passes through the cytoplasmic membrane, microorganisms must actively adjust their intracellular solute pool to prevent dehydration under hypertonic growth conditions and bursting under hypotonic circumstances (13). Most bacteria accomplish this task through the accumulation of ions, in particular K+, and specific organic osmolytes at high osmolalities (26, 30, 38, 61, 63), and they expel these compounds through mechanosensitive channels when the external osmolality drops (4, 9, 48, 60).
A common response of many Bacteria and Archaea to high-osmolality growth conditions is the high-level accumulation, either through synthesis or uptake from the environment, of organic osmolytes, the so-called compatible solutes (26, 27, 30, 38, 50, 61, 63). These compounds are highly congruous with cellular functions and can be accumulated up to molar concentrations in the cytoplasm without disturbing essential cellular processes and the functioning of cell components (19, 42). Consequently, the high-level accumulation of compatible solutes makes a major contribution to curbing the outflow of water from the cell under hypertonic growth conditions (49) and to maintaining turgor within physiological acceptable boundaries under these circumstances (65).
Many microorganisms are able to acquire preformed compatible solutes from environmental sources through high-affinity transport systems (17, 26, 46, 57). Five osmotically regulated osmoprotectant uptake systems (OpuA to OpuE) operate in the gram-positive soil bacterium Bacillus subtilis (16, 17, 38) which collectively allow the uptake of 13 preformed compatible solutes or the biosynthetic precursor of glycine betaine (16). The uptake and high-level intracellular accumulation of compatible solutes, in particular glycine betaine, provide effective osmoprotection to the B. subtilis cell and allow its growth over a wide range of external salinities (10). In addition to the effective acquisition of preformed compatible solutes, B. subtilis can also synthesize glycine betaine from the precursor choline via the GbsB and GbsA enzymes (10, 11); choline is taken up by the cell via the OpuB and OpuC ABC transport systems (36). In the absence of preformed compatible solutes in the growth medium, B. subtilis adjusts its intracellular solute pool through the de novo synthesis of the compatible solute proline (45, 64). Osmoregulatory proline biosynthesis involves sequential reactions of the ProJ, ProA, and ProH enzymes (7, 16; J. Brill and E. Bremer, unpublished results). It is thus apparent that the uptake and synthesis of compatible solutes play a central role in the cellular adaptation of B. subtilis to high-osmolality environments (16, 17).
Compatible solutes also function as protein stabilizers both in vitro (22, 25, 39, 43) and in vivo (15, 21, 28). This aspect of the physiological function of these compounds is generally explained in terms of the preferential exclusion model (3, 12). The protein-stabilizing properties of compatible solutes are likely to explain the protective functions of these compounds against heat stress in both the Bacteria and the Archaea (14, 21, 23, 41, 50, 54). Compatible solutes have also been shown to serve as chill protectants for the human pathogen Listeria monocytogenes (5, 40) and for B. subtilis (18).
The function of compatible solutes as osmoprotectants has been firmly established for B. subtilis, and the molecular details of the uptake and synthesis of these compounds have already been studied in quite some detail (16, 17). We have now asked whether the acquisition of preformed compatible solutes or their synthesis also plays a role in the adaptation of this soil microorganism to elevated growth temperatures. B. subtilis is well suited for such an investigation because a comprehensive set of mutants that disrupt the transporters for compatible solutes (Opu) (16) or the biosynthetic routes for proline (J. Brill and E. Bremer, unpublished results) and glycine betaine (11) are available. We report here that glycine betaine and several structurally related compatible solutes serve as effective thermoprotectants for B. subtilis and demonstrate that the previously characterized osmoregulated Opu transporters are required for their acquisition under elevated growth temperatures.
MATERIALS AND METHODS
Bacterial strains.
The B. subtilis strains used for this study are all derivatives of the wild-type strain JH642 (trpC2 pheA1; BGSC 1A96), a kind gift of J. Hoch, La Jolla, Calif., and are listed in Table 1.
TABLE 1.
B. subtilis strains
| Straina | Relevant genotype | Reference or source |
|---|---|---|
| JH642 | trpC2 pheA1 | J. Hoch, BGSC1A96 |
| BLOB9 | Δ(opuE::tet)1 | 62 |
| JBB5 | Δ(gbsAB::neo)2 | 11 |
| JSB8 | Δ(proHJ::neo)1 | J. Brill |
| RMKB20 | Δ(opuA::erm)4 opuC-20::Tn10(spc)Δ(opuD::neo)2 | 36 |
| RMKB22 | Δ(opuA::erm)4 opuB-20::Tn10(spc) Δ(opuD::neo)2 | 36 |
| RMKB24 | Δ(opuA::erm)4 Δ(opuBD::tet)23 opuC-20::Tn10(spc) Δ(opuD::neo)2 | 36 |
| RMKB33 | Δ(opuA::erm)4 Δ(opuBD::tet)23 opuC-20::Tn10(spc) | 36 |
| RMKB34 | Δ(opuBD::tet)23 opuC-20::Tn10(spc) Δ(opuD::neo)2 | 36 |
All strains are derivatives of JH642.
Media, chemicals, and growth conditions.
B. subtilis strains were routinely maintained and propagated on Luria-Bertani agar plates. For all growth experiments, Spizizen's minimal medium (SMM), with 0.5% (wt/vol) glucose as the carbon source, l-tryptophan (20 mg liter−1), l-phenylalanine (18 mg liter−1), and a solution of trace elements (31), served as a chemically defined medium. The osmolality of growth media was increased by the addition of NaCl from a 5 M stock solution. Growth of the cultures was monitored by measuring the optical density at 578 nm (OD578). All cultures were grown aerobically at either 37 or 52°C in a shaking water bath set at 220 rpm. For the determination of growth curves at 37°C, 75-ml portions of prewarmed SMM were inoculated with an exponentially growing preculture to an optical density of 0.1. For the determination of growth curves at 52°C, 75-ml portions of SMM (prewarmed to 37°C) were inoculated with exponentially growing cultures (pregrown at 37°C) to an OD578 of 0.1 and were heated within 15 min to 52°C in a water bath.
Glycine betaine, choline chloride, γ-butyrobetaine, and carnitine were purchased from Sigma (Deisenhofen, Germany). Proline betaine (stachydrinehydrochloride) was synthesized by Extrasynthese (Genay, France). Ectoine was purchased from BIOMOL (Hamburg, Germany). Crotonobetaine was a kind gift from J. Brass (Lonza AG, Visp, Switzerland). Dimethylsulfoniumpropionate (DMSP) was kindly provided by T. Hansen (University of Groningen, Groningen, The Netherlands). Homobetaine and choline-O-sulfate were synthesized by G. Nau-Wagner (Marburg, Germany). Radiolabeled [1-14C]glycine betaine (55 mCi mmol−1) was purchased from American Radiolabeled Chemicals Inc. (St. Louis, Mo.), and radiolabeled [1-14C]glutamate (45 mCi mmol−1) was obtained from DuPont (Boston, Mass.).
Transport assays and determination of the glycine betaine and glutamate pools.
The initial transport rates of [1-14C]glycine betaine and [1-14C]glutamate were measured in exponentially growing cells of B. subtilis strain JH642. Cells were grown to an OD578 of 0.5 to 1 at either 37 or 52°C. [1-14C]glycine betaine (10 μM) or [1-14C]glutamate (60 μM) was added to 2 ml of cells, and at different time points, 300-μl aliquots were passed through 0.45-μm-pore-size nitrocellulose filters (Schleicher and Schuell, Dassel, Germany) and the filters were then washed with 20 ml of SMM of the same osmolality. The radioactivity remaining on the filters was determined by liquid scintillation counting. For the determination of the glycine betaine and glutamate pools in B. subtilis cells, SMM containing either 1 mM radiolabeled glycine betaine or 1 mM radiolabeled glutamate was inoculated with exponentially growing cells to an OD of 0.1 and grown at the given temperature to an OD578 of 0.5. Three-hundred-microliter aliquots of the cell suspension were then sucked onto a 0.45-μm-pore-size nitrocellulose filter, and the cells were washed with 20 ml of SMM of the same osmolality as that used for originally growing the cells. The radioactivity remaining on the filters was then determined by liquid scintillation counting. The intracellular solute concentrations of glycine betaine and glutamate in B. subtilis were calculated by using a cell volume of 6.7 μl per mg of cell protein (S. Moses, E. P. Bakker, and E. Bremer, unpublished results).
Northern blot analysis.
Total RNAs were isolated from B. subtilis log-phase cells (OD578, 0.5 to 1) grown either at 37 or 52°C by the acid-phenol method (2, 44). Various amounts of total RNA were separated on a denaturing 1.4% agarose gel, transferred onto a nylon membrane (NY13N; Schleicher and Schuell), and hybridized with digoxigenin-labeled single-strand antisense RNA probes specific for the transcript of interest. Probes for the various opu genes, proHJ, and proBA were generated by in vitro transcription with a Strip EZTM RNA T7 kit (Ambion, Austin, Tex.). Templates for the transcription reaction were generated by PCR with synthetic oligonucleotides that carried an artificially added T7 promoter sequence at the 5′ end of the reverse primer. RNA-RNA hybridization was performed at 68°C in a hybridization solution containing 50% formamide, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 2% blocking reagent (Roche Diagnostics, Mannheim, Germany), 0.1% N-lauroylsarcosine, and 7% sodium dodecyl sulfate. The filters were washed according to standard procedures (52) and the transcripts were detected with chemiluminescent ECF-Vista (Amersham Biosciences, Freiburg, Germany) as the substrate in a Storm 860 phosphorimager (Amersham Biosciences).
Primer extension analysis.
For primer extension analysis of the proHJ operon, the total RNA was isolated by the acid-phenol method from exponentially growing (OD578, 0.5 to 1) B. subtilis cells grown at 37°C with and without 0.6 M NaCl or at 52°C. A reverse transcriptase reaction was set up with 20 μg of total RNA and 2 pmol of a synthetic proHJ-specific oligonucleotide (5′-GGCTACTTTCTTTTGATCAAAGATCGG-3′) labeled at its 5′ end with the infrared dye IRD-800 (MWG Biotech, Ebersberg, Germany). The B. subtilis total RNA, the proHJ-specific oligonucleotide, and the reverse transcriptase buffer (Promega, Mannheim, Germany) were mixed in a volume of 20 μl, heated to 75°C for 3 min, and then allowed to slowly cool to 42°C. One microliter of avian myeloblastosis virus reverse transcriptase (Promega) and 1 μl of an 8 mM nucleotide solution were added, and the reaction mixture was incubated for 1 h at 42°C. The cDNA was precipitated by the addition of ice-cold ethanol and was resuspended in stop-buffer solution from a Thermo Sequenase DYEnamic Direct cycle sequencing kit (Amersham Biosciences). In parallel, a sequencing reaction (53) was carried out with the same proHJ-specific primer and the proHJ+ plasmid pJS13 (J. Brill and E. Bremer, unpublished results) as the template. The primer extension and DNA sequencing reactions were separated side by side on a sequencing gel in a LI-COR DNA sequencer (MWG Biotech) to determine the exact position of the 5′ end of the proHJ mRNA.
HPLC determination of the proline and glutamate pools of B. subtilis.
For a quantitative high-performance liquid chromatography (HPLC) analysis of the amino acids glutamate and proline in B. subtilis, cells were grown in SMM at either 37 or 52°C to an OD578 of 1 to 1.5. Cells were harvested by centrifugation (3,000 × g) and subsequently lyophilized. The dry weight of the cell pellets was determined, and cells were extracted according to a method described by Bligh and Dyer (8). The cells were homogenized by ultrasound in 500 μl of extraction mixture (methanol-chloroform-water at 10:5:4 [vol/vol/vol]) and were shaken vigorously for 30 min. Subsequently, equal volumes (130 μl) of chloroform and water were added and shaking was continued for another 30 min. Phase separation was enhanced by centrifugation in an Eppendorf table-top centrifuge; the upper aqueous phase was recovered and evaporated to dryness at 55°C. The dried residue was resuspended in 200 μl of water prior to a reaction with 9-fluorenyl-methoxycarbonyl chloride (FMOC). Samples and standards were allowed to react with FMOC according to the precolumn derivatization protocol with FMOC and 1-aminoadamantane (E. Grom, Application Service, Rottenburg-Hailfingen, Germany), with minor modifications. We added 40 μl of the sample or standard of a suitable concentration (5 to 220 μM) to 40 μl of internal standard (50 μM taurine) in 0.5 M sodium borate buffer (pH 7.7), followed by the addition of 80 μl of FMOC reagent II (E. Grom). The vial was vortexed for 45 s. For removal of the excess of the fluorescent dye FMOC, 100 μl of 1-aminoadamantane solution (40 mM in acetone-borate buffer at a 1:1 ratio [vol/vol]) was added and left to react for at least 45 s. Afterwards, 140 μl of solvent A (20% acetonitrile and 0.5% tetrahydrofurane in 50 mM sodium acetate buffer, pH 4.6) was added and the sample was then loaded onto a reversed-phase column (125- by 4-mm LiChroCart cartridge [E. Grom] packed with 4 μm of Supersphere 60 RP-8 [Merck, Darmstadt, Germany]) by an automated injection system. The solvent system employed for amino acid separation consisted of solvent A and solvent B (80% acetonitrile in sodium acetate buffer, pH 4.6). The chromatographic separation of amino acids was performed at a flow rate of 1.25 ml min−1 at 45°C, using a solvent gradient established between solvents A and B. Fluorescently labeled amino acids were detected by a fluorescence detector (model 821-FP; Jasco, Gross Umstadt, Germany) set at an excitation wavelength of 254 nm and an emission wavelength of 316 nm. The quantitation of proline and glutamate was done with Pyramid 2000 software (Axxiom, Moorpark, Calif.).
RESULTS
Thermoprotection of B. subtilis by glycine betaine.
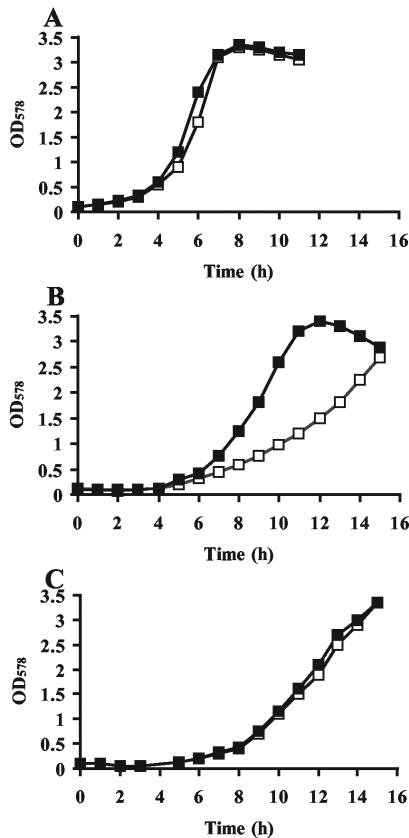
Compatible solutes have a well-established role as osmoprotectants (17, 30, 63), but recently these compounds were also shown to confer thermoprotection both in microorganisms (21, 23) and in plants (1, 24). To test whether the potent osmoprotectant glycine betaine (10) could serve as a thermoprotectant in B. subtilis, we first tested the maximal growth temperature of the B. subtilis wild-type strain JH642 in a chemically defined minimal medium (SMM). This strain was able to grow at 52°C, but clearly at a reduced growth rate compared to that at 37°C (Fig. 1A and B). There was no growth of JH642 when it was cultured at 53°C (data not shown). The addition of 1 mM glycine betaine had no appreciable effect on the growth of strain JH642 cultured at 37°C (Fig. 1A), but a clear thermoprotective function of glycine betaine was noticeable at 52°C (Fig. 1B). At this temperature, the lag phase of the cells grown in the presence of 1 mM glycine betaine was shortened compared to that of cells grown without this compound. The doubling time (td) of the JH642 culture was decreased from 3.4 h without glycine betaine to 1.7 h with glycine betaine at 52°C (Fig. 1B). Hence, the compatible solute glycine betaine serves as a thermoprotectant for B. subtilis. We also tested whether the addition of 1 mM glycine betaine would increase the upper temperature limit of growth of B. subtilis, but we found that this was not the case. Strain JH642 was not able to grow at 53°C in SMM, regardless of whether glycine betaine (1 mM) was present or not (data not shown).
FIG. 1.
Effect of glycine betaine on the growth of B. subtilis at 37 and 52°C. Cells of the B. subtilis wild-type strain JH642 (A and B) and the mutant strain RMKB24 [Δ(opuA::erm)4 Δ(opuBD::tet)23 opuC-20::Tn10(spc) Δ(opuD::neo)2] (C) were grown in the presence (closed squares) and absence (open squares) of 1 mM glycine betaine at 37°C (A) and 52°C (B and C). Growth was monitored over time by measuring the OD578.
Glycine betaine is taken up under heat stress via the OpuA, OpuC, and OpuD transporters.
At a high salinity, B. subtilis employs three transport systems (OpuA, OpuC, and OpuD) for the acquisition of glycine betaine for osmoprotective purposes (35-37). To investigate whether these transporters are also responsible for the uptake of glycine betaine under heat stress, we grew strain RMKB24 (OpuA− OpuB− OpuC− OpuD−), which lacks all of these transporters, at 52°C in the presence and absence of 1 mM glycine betaine. In contrast to the case for the parent strain, JH642 (Fig. 1B), glycine betaine was unable to protect strain RMKB24 from the detrimental effects of high temperature (Fig. 1C). It is thus apparent that B. subtilis employs already characterized glycine betaine transport systems for thermoprotective purposes and that no new glycine betaine uptake system beyond those of the described OpuA, OpuC, and OpuD transporters is operational at 52°C.
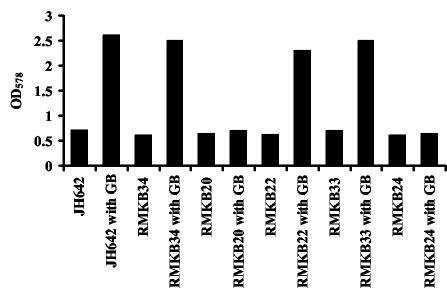
To test which of the known glycine betaine transport systems (35-37) was responsible for glycine betaine uptake under heat stress, we used the following isogenic set of B. subtilis mutants that express only one of the glycine betaine transporters: RMKB34 (OpuA+), RMKB22 (OpuC+), and RMKB33 (OpuD+) (Table 1). Each of these strains was protected from heat stress at 52°C (Fig. 2), but no such heat stress protection was noticeable when strain RMKB20 (OpuB+), or as already noted (Fig. 1C), RMKB24 (OpuA− OpuB− OpuC− OpuD−) was used for this experiment (Fig. 2). Hence, each of the glycine betaine transporters involved in the acquisition of glycine betaine as an osmoprotectant is also involved in glycine betaine acquisition at high temperatures.
FIG. 2.
Glycine betaine is taken up under heat stress by the OpuA, OpuC, and OpuD transporters. Cells of the B. subtilis wild-type strain JH642 and its mutant derivatives were grown at 52°C in SMM containing 1 mM glycine betaine (GB). Seventy-five-milliliter cultures were inoculated to an OD578 of 0.1 and their growth yields were measured after 13 h. The following B. subtilis strains were used for this experiment: JH642 (OpuA+ OpuB+ OpuC+ OpuD+), RMKB34 (OpuA+ OpuB− OpuC− OpuD−), RMKB20 (OpuA− OpuB+ OpuC− OpuD−), RMKB22 (OpuA− OpuB− OpuC+ OpuD−), RMKB33 (OpuA− OpuB− OpuC− OpuD+), and RMKB24 (OpuA− OpuB− OpuC− OpuD−).
Low concentrations of glycine betaine are sufficient for thermoprotection.
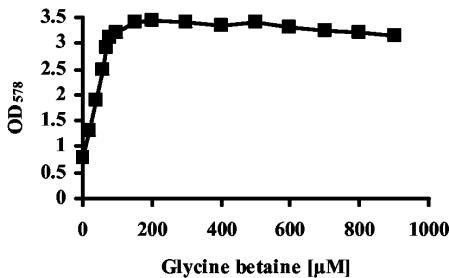
To test the concentration dependence of thermoprotection by glycine betaine, we grew the wild-type strain JH642 in SMM at 52°C in the absence or presence of various low concentrations of this compatible solute and monitored growth yields after 13 h (Fig. 3). A thermoprotective effect of glycine betaine was already noticeable when 20 μM glycine betaine was added to the culture, and half-maximal thermoprotection was achieved by the addition of 50 μM glycine betaine. Hence, very low concentrations of glycine betaine are sufficient to exert a clear thermoprotective effect on the growth of B. subtilis.
FIG. 3.
Low concentrations of glycine betaine are sufficient for thermoprotection. Cells of the B. subtilis wild-type strain JH642 were grown at 52°C in medium (SMM) containing different concentrations of glycine betaine. Twenty-milliliter cultures were inoculated to an OD578 of 0.1 and their growth yields were measured after 13 h.
A variety of compatible solutes serve as heat protectants for B. subtilis.
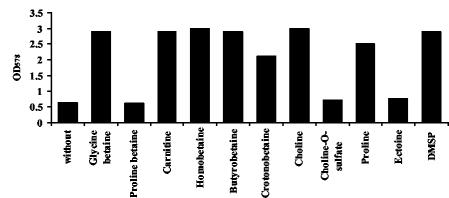
In addition to glycine betaine, B. subtilis is known to acquire a variety of preformed compatible solutes from environmental sources for osmoprotective purposes (16, 33, 34, 37, 47). This soil bacterium can also synthesize glycine betaine from the precursor choline under osmostress conditions via the GbsA and GbsB enzymes (10, 11). To test whether the known osmoprotectants of B. subtilis also could serve as heat protectants, we grew strain JH642 at 52°C in the presence of 1 mM glycine betaine, homobetaine, proline betaine, carnitine, butyrobetaine, crotonobetaine, choline-O-sulfate, proline, ectoine, DMSP, or choline (Fig. 4). With the notable exceptions of ectoine, choline-O-sulfate, and proline betaine, each of these compatible solutes protected B. subtilis strain JH642 from the detrimental effects of high temperature.
FIG. 4.
Thermoprotective effect of various compatible solutes. Cells of the wild-type strain JH642 were grown in the absence or presence of a 1 mM concentration of various compatible solutes at 52°C. Seventy-five-milliliter cultures were inoculated to an OD578 of 0.1 and their growth yields were measured after 12 h.
Choline functions as the precursor for glycine betaine and exerts osmoprotection, but it does not possess an osmoprotective function per se (10, 11, 36). To test whether the thermoprotective effect of choline was dependent on its GbsA- and GbsB-mediated enzymatic conversion to glycine betaine (11), we grew the Δ(gbsAB::neo)2 strain JBB5 in the presence of either glycine betaine or choline. In this strain, choline did not function as a thermoprotectant, although glycine betaine did (data not shown). Consequently, the thermoprotective effect of choline on the growth of B. subtilis was dependent on its conversion into glycine betaine.
We tested whether the above identified thermoprotectants (Fig. 4) were also taken up by the same transport systems that are used by B. subtilis for their acquisition under osmostress conditions (16). For these experiments, we again used an isogenic set of mutants that express only one of the osmoprotectant uptake systems (Table 1). Homobetaine and DMSP were taken up through both OpuA and OpuC; carnitine, butyrobetaine, and crotonobetaine were solely taken up through OpuC; choline was acquired through OpuB and OpuC; and proline was transported into the cell via OpuE (data not shown). Hence, the transport profile of the compatible solutes is exactly the same for thermoprotection as for osmoprotection (16). In no case did we detect an additional uptake activity for these compatible solutes at 52°C, since there was no thermoprotection (with the exception of proline) in an OpuA− OpuB− OpuC− OpuD− OpuE+ strain (RMKB24) and the thermoprotective effect of proline was lost in an OpuE− strain (BLOB9) (data not shown).
Northern blot analyses of the opu transcripts under heat stress.
The opuA, opuB, and opuC loci are genetically organized as osmotically inducible operons (36, 37; G. Nau-Wagner and E. Bremer, unpublished results). opuD is cotranscribed with ytfP (a locus of unknown function located upstream of opuD) from one promoter that is not osmotically regulated and another, positioned immediately upstream of opuD, that is osmotically controlled and SigB dependent (F. Spiegelhalter and E. Bremer, unpublished results). The opuE gene is cotranscribed with the downstream sapB gene; their osmoregulated transcription stems from a SigA-dependent promoter and an additional SigB-dependent promoter positioned just upstream of opuE (58, 62).
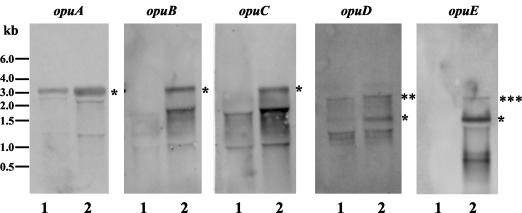
To test whether growth at high temperatures induces the transcription of the various opu genes, we performed Northern blot experiments. The total RNA was isolated from cells grown at either 37 or 52°C, separated on a denaturing 1.4% agarose gel, blotted onto a nylon membrane, and separately reacted with single-stranded antisense RNA probes that were specific for the opuA, opuB, opuC, opuD, or opuE mRNA. In each case, we detected a moderate up-regulation of the various opu transcripts (Fig. 5).
FIG. 5.
Northern blot analyses of the transcripts of different opu genes at 37 and 52°C. The total RNA was isolated from exponentially growing (OD578 = 0.5 to 1) B. subtilis cells that were cultivated either at 37 or 52°C. Equal amounts of the obtained RNA (1 μg for the detection of the opuA transcripts and 4 μg for the detection of the opuB, opuC, opuD, and opuE transcripts) were separated on denaturing agarose gels and subjected to Northern blot analyses using single-stranded antisense RNA probes specific for opuA, opuB, opuC, opuD, and opuE. Lanes 1, RNA isolated from cells grown at 37°C; lanes 2, RNA isolated from cells grown at 52°C. In all blots, the detected transcript comprising the whole transcriptional unit is marked with an asterisk. **, the transcript detected probably resulted from a readthrough of the gene found upstream (ytfP) of opuD; ***, the detected transcript includes a gene (sapB) located downstream of opuE. In each of these Northern blots, we detected additional bands that are likely a result of mRNA degradation.
Influence of heat stress on glycine betaine transport and its intracellular pool in B. subtilis.
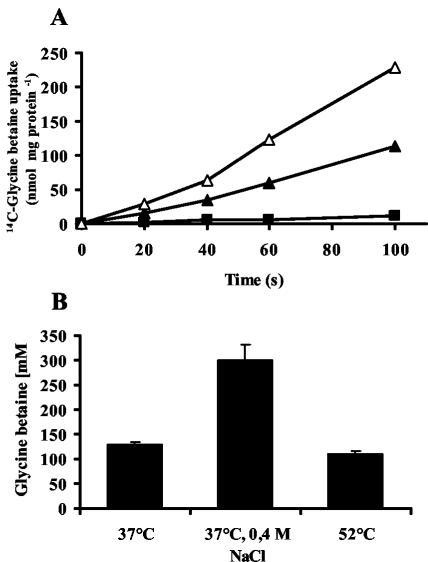
Since the transcription of the various opu genes from B. subtilis is induced upon heat stress, we tested whether the uptake of glycine betaine also increased under this growth condition. The uptake of radiolabeled glycine betaine was measured in cells grown at 37°C, 37°C with 0.4 M NaCl (because the glycine betaine transport rate is known to be induced by osmostress [37]), and 52°C. The uptake of glycine betaine in strain JH642 (OpuA+ OpuC+ OpuD+) under these conditions is a reflection of the joint contributions of the OpuA, OpuC, and OpuD transporters (35-37). The uptake of glycine betaine occurred at a rate of 63 nmol min−1 mg of protein−1 in cells that were grown in SMM at 37°C (Fig. 6A) and increased to 128 nmol min−1 mg of protein−1 in the culture of JH642 that was grown in SMM in the presence of 0.4 M salt (Fig. 6A). The overall uptake of glycine betaine was very low in cells that were cultured in SMM at 52°C and occurred at a rate of 7 nmol min−1 mg of protein−1 (Fig. 6A). This low glycine betaine uptake rate of the cultures grown at 52°C is surprising in view of the fact that the transcription of the opuA, opuC, and opuD loci is increased at this growth temperature in comparison to the mRNA levels detected at 37°C (Fig. 5).
FIG. 6.
Glycine betaine uptake and its intracellular pool in cells grown at 37°C, 37°C with 0.4 M NaCl, and 52°C. (A) For determination of the initial transport rate of radiolabeled glycine betaine under different temperature and salinity conditions, cells were propagated at 37°C (closed triangles), 37°C with 0.4 M NaCl (open triangles), and 52°C (closed squares) to an OD578 of approximately 0.5. These cells were then assayed for glycine betaine uptake at a final substrate concentration of 10 μM. (B) For determination of the intracellular glycine betaine pool under the same growth conditions, cells were inoculated to an OD578 of 0.1 and grown in the presence of 1 mM radiolabeled glycine betaine at 37°C, 37°C with 0.4 M NaCl, and 52°C. After the cells reached an OD578 of 0.5, the amount of radiolabeled glycine betaine taken up by the cells was determined by scintillation counting, and their glycine betaine contents were calculated by using an intracellular volume of 6.7 μl mg of cell protein−1.
To assess the size of the intracellular glycine betaine pool of cells grown at various temperatures and salinities, we measured the glycine betaine content. Cells were inoculated to an OD578 of 0.1 in medium containing 1 mM radiolabeled glycine betaine and were allowed to grow to an OD578 of 0.5 at either 37°C, 37°C with 0.4 M NaCl, or 52°C. After the cells had reached this optical density, samples were removed from the cultures and used to determine the radiolabeled glycine betaine content. Using a cell volume of 6.7 μl mg of cell protein−1 for B. subtilis (S. Moses, E. P. Bakker, and E. Bremer, unpublished results), we calculated a glycine betaine content of 130 ± 5 mM glycine betaine for cells that were grown at 37°C. As expected, osmotic stress increased the glycine betaine pool, and we found 300 ± 30 mM glycine betaine in cells grown at 37°C in the presence of 0.4 M NaCl. Cells that were subjected to heat stress at 52°C contained 110 ± 4 mM glycine betaine, a cellular level similar to that of unstressed cells that were propagated at 37°C (Fig. 6B). Hence, despite the strongly reduced glycine betaine transport rate found at 52°C (Fig. 6A), B. subtilis is capable of accumulating a substantial glycine betaine pool, but the intracellular level of this compatible solute is not increased in comparison to cells grown at 37°C (Fig. 6).
The pool of endogenously synthesized compatible solute proline is not increased during heat stress.
It is known that Salmonella enterica serovar Typhimurium produces increased levels of the compatible solute trehalose when it is challenged by heat stress (23). Proline serves as the primary endogenously synthesized osmoprotectant for B. subtilis (64), which uses an osmotically responsive synthesis pathway that is dependent on the sequential reactions of the ProJ, ProA, and ProH enzymes (J. Brill and E. Bremer, unpublished results) and that uses glutamate as the precursor molecule (7). The transcription of the proHJ operon is up-regulated in response to osmotic stress, whereas the transcription of the proBA gene cluster is not responsive to this environmental cue (J. Brill and E. Bremer, unpublished results).
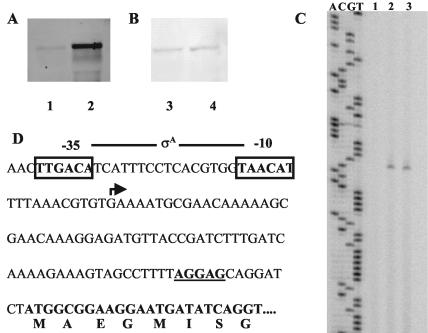
To test whether the transcription of the proHJ and proBA genes is induced upon cultivation of the cells at 52°C, we performed Northern blot experiments with cells that were grown at either 37 or 52°C. Transcription of the proHJ operon was clearly induced upon growth of the cells at 52°C (Fig. 7A), whereas that of the proBA gene cluster was not responsive to heat stress (Fig. 7B). This situation is therefore comparable to that found under osmostress conditions (J. Brill and E. Bremer, unpublished results). The osmostress-responsive transcription of the proHJ operon depends on a SigA-type promoter (Fig. 7D) (J. Brill and E. Bremer, unpublished results). To test whether the same promoter was also involved in mediating the heat stress induction of the proHJ genes, we performed a primer extension analysis. The total RNA was isolated from cells that were grown either at 37°C, at 37°C with 0.6 M NaCl, or at 52°C in the absence of salt and were used for a reverse transcription reaction with a proH-specific primer. As observed previously, the proHJ promoter responded to salt stress with increased transcription (Fig. 7C), and we now found that the same promoter is also responsive to heat stress (Fig. 7C).
FIG. 7.
Northern blot analyses of the proHJ and proBA genes under heat stress and mapping of the transcriptional start of proHJ. (A and B) The total RNA was isolated from B. subtilis cells grown at 37°C (lanes 1 and 3) or 52°C (lanes 2 and 4). Samples (4 μg for the detection of proHJ and 10 μg for the detection of proBA) of the total RNA were separated in a denaturing agarose gel, blotted onto a nylon membrane, and hybridized with digoxigenin-labeled single-stranded antisense probes specific for proHJ (A) and proBA (B). (C) Total RNAs were prepared from cells grown at 37°C (lane 1), 37°C with 0.6 M NaCl (lane 2), and 52°C (lane 3), and 20 μg of each was used for a reverse transcription reaction with a proH-specific oligonucleotide. The same oligonucleotide was used for a sequencing reaction to size the cDNA. (D) Nucleotide sequence of the proHJ promoter region. The transcription initiation site is indicated by an arrow and the −10 and −35 sequences are boxed. The putative ribosome-binding site is printed in bold and underlined. The part of the coding sequence of proH shown here is printed in bold.
Since the genes (proHJ) for the enzymes that are centrally involved in proline biosynthesis under osmostress conditions are also induced upon heat stress (Fig. 7), we measured the proline contents of cells grown at 52°C and also determined the pool for the biosynthetic precursor (glutamate) of proline by HPLC analysis. Cells of strain JH642 were grown at 37 and 52°C to an OD578 of 1 to 1.5 and were harvested; the soluble components were extracted and the extract was used for HPLC analysis. The growth temperature did not significantly influence the glutamate content of the cells: cells grown at 37°C had a glutamate content of 378 ± 7 μM g of dry weight−1, while cells grown at 52°C had a glutamate content of 352 ± 5 μM g of dry weight−1. The proline content of cells grown at 37°C was about 5 μM mg of dry weight−1, whereas there was no detectable proline in cells grown at 52°C. Hence, despite the increased transcription of the proHJ genes at high temperatures, there was no concomitant increase in cellular proline content.
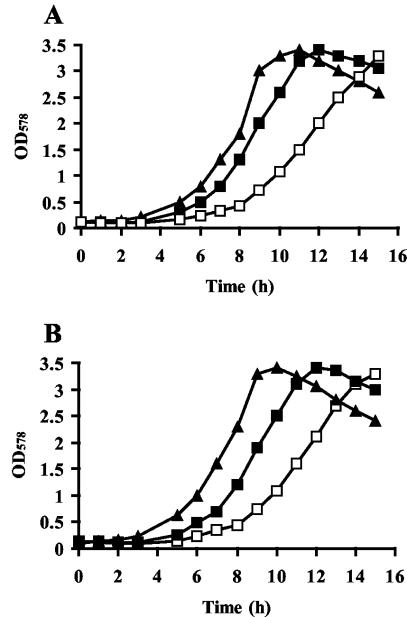
A derivative of strain JH642 that carries a gene disruption mutation in the proHJ genes, strain JSB8, shows severely retarded growth in high-osmolality media in comparison to its proHJ+ parent since it cannot synthesize the large amounts of proline required for osmoprotection (J. Brill and E. Bremer, unpublished results). To test whether such a mutation in proHJ would influence growth at a high temperature as well, we propagated strains JH642 and JSB8 in parallel at 52°C. There was no difference in the growth rates of the strains at this elevated temperature (Fig. 8).
FIG. 8.
Thermoprotection of B. subtilis by glutamate. Cells of the B. subtilis wild-type strain JH642 (A) and the proJH mutant strain JSB8 (B) were inoculated to an OD578 of 0.1 and incubated at 52°C in the absence (open squares) and presence of 1 mM glycine betaine (closed squares) and 1 mM glutamate (triangles). Growth was monitored over time.
Glutamate is an effective thermoprotectant for B. subtilis.
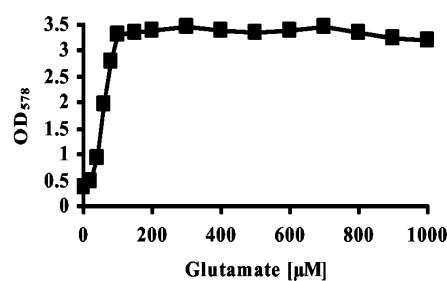
We also investigated whether exogenously added glutamate would enhance the growth of B. subtilis at a high temperature. Glutamate has only a modest osmoprotective effect in B. subtilis (A. D. Kerres, M. Dolezal, and E. Bremer, unpublished results), but we found that it can effectively protect strain JH642 from growth retardation at high temperatures (Fig. 8A). Its thermoprotective effect is even slightly stronger than that mediated by glycine betaine. The presence of 1 mM glutamate in the growth medium decreased the lag phase of cells that were grown at a high temperature and decreased the td from 3.4 to 1.6 h (Fig. 8A). As observed with glycine betaine (Fig. 3), the addition of very low concentrations of glutamate to the heat-stressed cells substantially increased the growth yield within a defined time frame (11 h) (Fig. 9).
FIG. 9.
Low concentrations of glutamate are sufficient for thermoprotection. Cells of the B. subtilis wild-type strain JH642 were grown at 52°C in medium (SMM) containing different concentrations of glutamate. Twenty-milliliter cultures were inoculated to an OD578 of 0.1 and their growth yields were monitored after 11 h.
Since glutamate serves as the precursor for proline biosynthesis in B. subtilis (7), we wondered whether the thermoprotective effect of glutamate was dependent on its ProH- and ProJ-mediated enzymatic conversion into proline. We therefore monitored the thermoprotective effect of glutamate in strain JSB8 [Δ(proHJ::neo)2] and found that this amino acid was still as thermoprotective as in the wild-type strain (Fig. 8B).
Thermoprotection by glutamate does not depend on an increased cellular pool of this amino acid.
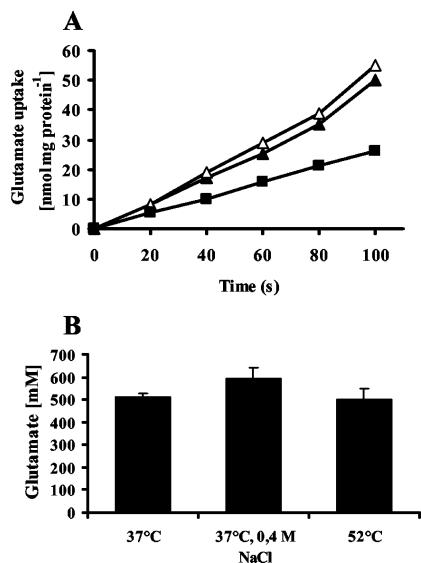
Since exogenously provided glutamate exerts a thermoprotective effect on the growth of B. subtilis, we tested whether there was an increase in glutamate transport in heat-stressed cells. The rate of glutamate transport was determined for cells (OD578 = 0.5) that were grown at 37°C, 37°C with 0.4 M NaCl, and 52°C (Fig. 10B). An increased salinity had no significant effect on the rate of glutamate uptake at 37°C, and glutamate transport was decreased in cells propagated at 52°C. Cells grown without osmotic stress took up glutamate at a rate of 30 nmol min−1 mg of protein−1, while cells grown in the presence of 0.4 M NaCl show a transport rate of 27 nmol min−1 mg of protein−1. At 52°C, the transport rate dropped to 15 nmol min−1 mg of protein−1 (Fig. 10A).
FIG. 10.
Determination of glutamate transport and its intracellular pool under different growth conditions. (A) Cells of the wild-type strain JH642 were grown at 37°C (closed triangles), 37°C with 0.4 M NaCl (open triangles), and 52°C (closed squares) to mid-exponential phase (OD578, 0.5 to 0.8) and were assayed for radiolabeled glutamate uptake at a final concentration of 60 μM. (B) Cells of the B. subtilis wild-type strain JH642 were inoculated to an OD578 of 0.1 in medium containing 1 mM radiolabeled glutamate. Cells were grown at 37°C, 37°C with 0.4 M NaCl, and 52°C. At an OD578 of 0.5, the amount of radiolabeled glutamate taken up by the cells was determined by scintillation counting and the intracellular glutamate pool was calculated by using an intracellular volume of 6.7 μl mg of cell protein−1.
We also determined the intracellular pool of glutamate. Cells were grown in the presence of 1 mM radiolabeled glutamate at 37°C, 37°C with 0.4 M NaCl, and 52°C. At an OD578 of 0.5, the amount of accumulated radiolabeled glutamate was determined and its intracellular concentration was calculated by using a cell volume of 6.7 μl mg of protein−1 (S. Moses and E. Bremer, unpublished results). Cells grown at 37°C accumulated 510 ± 15 mM glutamate. The increase in the salinity of the growth medium had only a very moderate effect on the glutamate pool, since these cells accumulated glutamate to a level of 595 ± 48 mM. Despite the reduced rate of glutamate uptake found in cells grown at 52°C (Fig. 10A), the glutamate pool was comparable (500 ± 47 mM) to that measured in cells grown at 37°C (Fig. 10B).
DISCUSSION
In its natural habitats, B. subtilis is exposed to sudden increases in growth temperature, and it has therefore developed sophisticated cellular adaptation reactions, collectively known as the heat shock response, that help it to refold temperature-damaged proteins through chaperones and to degrade thermally denatured polypeptides through proteases (55). While the cellular adaptation reactions of B. subtilis to heat shock have already been investigated at the molecular level in considerable detail, less attention has been paid to the continued growth of B. subtilis at high temperatures. We have demonstrated here that the addition of low concentrations of glycine betaine (Fig. 3) and other compatible solutes provide thermoprotection to B. subtilis in a chemically defined minimal medium by shortening the lag phase and by enhancing the growth rate at 52°C (Fig. 1B). However, these compounds do not extend the maximal growth temperature of B. subtilis, since there was no growth of the B. subtilis wild-type strain JH642 at 53°C.
Because exogenously provided compatible solutes afford thermoprotection, those cells that can acquire these compounds from exogenous sources will have a selective growth advantage at high temperatures. A set of five transport systems (OpuA to OpuE) for compatible solutes in B. subtilis that are responsible for the uptake of these compounds under conditions of high osmolality were previously characterized (35-37, 62). We found in this study that these Opu transporters are also responsible for compatible solute acquisition at continued elevated growth temperatures (52°C). There is no thermoprotection by compatible solutes in a mutant strain that lacks all known Opu transporters (Fig. 1C). Likewise, the recently observed chill protection of B. subtilis by exogenously provided glycine betaine (18) and structurally related compatible solutes also depends on the Opu uptake systems (T. Hoffmann and E. Bremer, unpublished results). Collectively, these data therefore ascribe a physiological function to these transporters not only for cellular osmoprotection (16, 17), but also for cellular adaptation to continued growth at low (18) and high (this study) temperatures.
Previous experiments have shown that the transcription of the structural genes for the various Opu transporters (OpuA to OpuE) is increased in response to increases in the external salinity, resulting in the enhanced uptake of compatible solutes under high-osmolality growth conditions (36, 37, 58, 62; F. Spiegelhalter and E. Bremer, unpublished results). Our Northern blot analyses showed that there is also a moderate up-regulation in the transcription of each of the opu genes in response to cell growth at a high temperature (52°C) in comparison to the level of transcription detected in cells propagated at 37°C in the absence of osmotic stress (Fig. 5). In contrast to the situation found in osmotically stressed cells, this increase in transcription is not reflected in the level of glycine betaine transport (35, 37) in heat-stressed B. subtilis cells (Fig. 6). The rate of glycine betaine uptake in the B. subtilis wild-type strain JH642 at 52°C (7 nmol min−1 mg of protein−1) was 9-fold lower than that observed in cells grown at 37°C in the absence of osmotic stress (63 nmol min−1 mg of protein−1) and 18-fold lower than that found in cells propagated at 37°C in the presence of 0.4 M NaCl (128 nmol min−1 mg of protein−1) (Fig. 6). We speculate that the low rate of glycine betaine transport observed in heat-stressed cells is attributable to substantial damage of the transporters for this compatible solute when B. subtilis is grown at the cutting edge of its maximal growth temperature (52°C).
Although the rate of glycine betaine uptake in heat-stressed cells is substantially lower than that for cells propagated at 37°C, heat-stressed B. subtilis cells eventually accumulate a glycine betaine pool (110 mM) that is comparable to that accumulated by non-heat-stressed cells (130 mM) (Fig. 6). Our determination of the glycine betaine pool in cells grown at 37°C is in very good agreement with that found by Whatmore et al. (175 mM in B. subtilis cells grown at 25°C) (64). The glycine betaine pool in heat-stressed cells is considerably smaller than that found in cells moderately osmotically stressed with 0.4 M NaCl (300 mM) and substantially smaller than that accumulated by truly osmotically stressed B. subtilis cells (1.3 M glycine betaine in cells propagated in a minimal medium containing 1 M NaCl) (S. Moses, E. P. Bakker, and E. Bremer, unpublished results). Hence, in contrast to the case for osmotically stressed cells, a moderate glycine betaine pool is apparently sufficient to provide thermoprotection to B. subtilis cells. A small pool size (40 to 50 mM) for glycine betaine was also found by Caldas et al. (21) to be sufficient for thermoprotection of Escherichia coli cells by this compatible solute.
As shown in Fig. 2 for glycine betaine, each individual Opu transporter retains the same substrate specificity for compatible solutes under high growth temperatures that it exhibits under conditions of high osmolality (16). Most of the tested compatible solutes known to function as osmoprotectants for B. subtilis (10, 16, 33-35, 47, 62) also serve as effective thermoprotectants (Fig. 4). There are three notable exceptions, ectoine, choline-O-sulfate, and proline betaine. The lack of thermoprotection by ectoine can probably be explained by the very low affinity (Ki = 1.5 mM) of the sole uptake system (OpuC) for this compound in B. subtilis cells (33). Choline-O-sulfate is also accumulated via OpuC. As an osmoprotectant it is as effective as glycine betaine, and it is efficiently taken up in salt-stressed B. subtilis cells, with a Km value of approximately 4 μM and a maximum rate of transport (Vmax) of approximately 45 nmol min−1 mg of protein−1, yet it does not serve as a thermoprotectant (Fig. 4). This compatible solute does not function as a chill protectant either, although it is accumulated by B. subtilis cells grown at a low temperature (15°C) (T. Hoffmann and E. Bremer, unpublished results). The lack of thermoprotection by proline betaine in B. subtilis (Fig. 4) is quite puzzling, since we have recently shown that this compatible solute provides effective thermoprotection to the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus (G. Holtmann and E. Bremer, unpublished results). The lack of thermoprotection by the osmoprotectants choline-O-sulfate and proline betaine exemplifies the fact that without detailed physiological tests one cannot simply ascribe both a thermoprotective and osmoprotective function to the same compatible solute for a given microorganism. The exact biophysical explanation for these functional differences is unclear at present.
Like E. coli (59), S. enterica serovar Typhimurium accumulates the compatible solute trehalose in response to osmotic stress. Canovas et al. (23) recently reported that the increased synthesis of this disaccharide provides thermoprotection to S. enterica serovar Typhimurium, and a thermoprotective effect of this sugar was also ascribed to the increased thermotolerance of stationary-phase E. coli cells (32). These observations raised the question of whether the amount of proline, the compatible solute synthesized by B. subtilis in response to osmotic stress (45, 64), was also increased in response to growth at high temperatures. We found that this was not the case. Fully consistent with this observation, we noted that a mutant that is entirely defective in osmoregulatory proline biosynthesis [Δ(proHJ)] does not have a growth disadvantage at 52°C (Fig. 8). However, we detected in heat-stressed B. subtilis cells a substantial increase in the amount of proHJ mRNA (Fig. 7A) that is transcribed from the same SigA-dependent promoter that directs proHJ production under high-osmolality growth conditions (Fig. 7C). A possible explanation for this phenomenon might be that the proline biosynthetic enzymes are damaged in heat-stressed cells and that the cell tries to compensate for this by increasing the level of transcription of the proHJ operon.
Exogenously provided glutamate has only a moderate osmoprotective effect in B. subtilis (A. D. Kerres, M. Dolezal, and E. Bremer, unpublished results), but we found that it served as a very effective thermoprotectant. Its thermoprotective capacity exceeds even that of the compatible solute glycine betaine (Fig. 8). The thermoprotection of B. subtilis by glutamate does not depend on its function as the precursor for proline biosynthesis (7), since a mutant [Δ(proHJ)] defective in osmoregulatory proline production was protected from the detrimental effects of high temperature as well as its proHJ+ parent strain, JH642 (Fig. 8). As was observed for exogenously provided glycine betaine (Fig. 6), the pool size of glutamate did not increase in thermally stressed B. subtilis cells (Fig. 10). Internally accumulated glutamate might serve a stabilizing function for thermolabile proteins in vivo; such a function was recently ascribed to this amino acid through in vitro analysis (29). This amino acid also specifically activates the chaperone ClpB in E. coli, resulting in an increased efficiency of chaperone-mediated protein disaggregation (29). Alternatively, the thermoprotective effects of glutamate might be a reflection of its central role in cellular metabolism (6). Ron and Davis (51) observed that in E. coli, one of the first processes to be inhibited by growth at a high temperature (42°C) is methionine biosynthesis. Supplementation of the growth medium with methionine rescues this growth defect and increases the maximal growth temperature of E. coli to 43 to 44°C. In view of the mentioned data on methionine biosynthesis in E. coli (51), it appears possible that the thermoprotective function of glutamate for B. subtilis is connected to the supplementation of a thermolabile biosynthesis route.
The osmoprotective effects of compatible solutes appear to be largely dependent on their ability to curb the outflow of water from the cell (49) and to stabilize turgor under high-osmolality growth conditions (65). Consequently, compatible solutes need to be amassed by the cell either through synthesis or transport processes to exceedingly high intracellular concentrations to maintain an appropriate water balance in the osmotically challenged cell (17, 26, 30). Such large pool sizes of compatible solutes appear not to be necessary for their functioning as thermoprotectants (reference 21 and this study). We consider it likely that the thermoprotective effect of compatible solutes reflects their ability to stabilize proteins both in vitro (14, 22, 28, 29, 39, 43, 56) and in vivo (15, 21, 28). The data presented here on the thermoprotection of B. subtilis by compatible solutes and previous studies on thermoprotection by these compounds in E. coli (21, 32) and on their chill-protective function in L. monocytogenes (40) and B. subtilis (18) demonstrate that the physiological functions of compatible solutes extend beyond their traditionally recognized role in osmoprotection.
Acknowledgments
We thank J. Brass, T. Hansen, M. Jebbar, and G. Nau-Wagner for providing us with various compatible solutes and J. Brill for bacterial strains. We appreciate the help of V. Koogle in editing the manuscript.
Financial support for this study was provided by the Deutsche Forschungsgemeinschaft through SFB-395, the Graduiertenkolleg “Proteinfunktion auf atomarer Ebene,” the European Union (contract IAC4-CT-2000-30041), the Fonds der chemischen Industrie, and the Max-Plack Institute for Terrestrial Microbiology (Marburg, Germany).
REFERENCES
- 1.Alia, H. Hayashi, A. Sakamoto, and N. Murata. 1998. Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant J. 16:155-161. [DOI] [PubMed] [Google Scholar]
- 2.Ambulos, N. P., Jr., E. J. Duvall, and P. S. Lovett. 1987. The mRNA for an inducible chloramphenicol acetyltransferase gene is cleaved into discrete fragments in Bacillus subtilis. J. Bacteriol. 169:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa, T., and S. N. Timasheff. 1985. The stabilization of proteins by osmolytes. Biochem. J. 47:411-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batiza, A. F., I. Rayment, and C. Kung. 1999. Channel gate! Tension, leak and disclosure. Struct. Fold Des. 7:R99-R103. [DOI] [PubMed] [Google Scholar]
- 5.Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. [DOI] [PubMed] [Google Scholar]
- 6.Belitsky, B. R. 2002. Biosynthesis of amino acids of the glutamate and aspartate families, alanine and polyamines, p. 203-231. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, D.C.
- 7.Belitsky, B. R., J. Brill, E. Bremer, and A. L. Sonenshein. 2001. Multiple genes for the last step of proline biosynthesis in Bacillus subtilis. J. Bacteriol. 183:4389-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 9.Blount, P., and P. C. Moe. 1999. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 7:420-424. [DOI] [PubMed] [Google Scholar]
- 10.Boch, J., B. Kempf, and E. Bremer. 1994. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 176:5364-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boch, J., B. Kempf, R. Schmid, and E. Bremer. 1996. Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: characterization of the gbsAB genes. J. Bacteriol. 178:5121-5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolen, D. W., and I. V. Baskakov. 2001. The osmophobic effect: natural selection of a thermodynamic force in protein folding. J. Mol. Biol. 310:955-963. [DOI] [PubMed] [Google Scholar]
- 13.Booth, I. R., and P. Louis. 1999. Managing hyperosmotic stress: aquaporins and mechanosensitive channels in Escherichia coli. Curr. Opin. Microbiol. 2:166-169. [DOI] [PubMed] [Google Scholar]
- 14.Borges, N., A. Ramos, N. D. Raven, R. J. Sharp, and H. Santos. 2002. Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 6:209-216. [DOI] [PubMed] [Google Scholar]
- 15.Bourot, S., O. Sire, A. Trautwetter, T. Touze, L. F. Wu, C. Blanco, and T. Bernard. 2000. Glycine betaine-assisted protein folding in a lysA mutant of Escherichia coli. J. Biol. Chem. 275:1050-1056. [DOI] [PubMed] [Google Scholar]
- 16.Bremer, E. 2002. Adaptation to changing osmolarity, p. 385-391. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, D.C.
- 17.Bremer, E., and R. Krämer. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria, p. 79-97. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 18.Brigulla, M., T. Hoffmann, A. Krisp, A. Völker, E. Bremer, and U. Volker. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation. J. Bacteriol. 185:4305-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown, A. D. 1976. Microbial water stress. Bacteriol. Rev. 40:803-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calamita, G. 2000. The Escherichia coli aquaporin-Z water channel. Mol. Microbiol. 37:254-262. [DOI] [PubMed] [Google Scholar]
- 21.Caldas, T., N. Demont-Caulet, A. Ghazi, and G. Richarme. 1999. Thermoprotection by glycine betaine and choline. Microbiology 145:2543-2548. [DOI] [PubMed] [Google Scholar]
- 22.Canovas, D., N. Borges, C. Vargas, A. Ventosa, J. J. Nieto, and H. Santos. 1999. Role of N-γ-acetyldiaminobutyrate as an enzyme stabilizer and an intermediate in the biosynthesis of hydroxyectoine. Appl. Environ. Microbiol. 65:3774-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canovas, D., S. A. Fletcher, M. Hayashi, and L. N. Csonka. 2001. Role of trehalose in growth at high temperature of Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:3365-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen, T. H., and N. Murata. 2002. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 5:250-257. [DOI] [PubMed] [Google Scholar]
- 25.Courtenay, E. S., M. W. Capp, C. F. Anderson, and M. T. Record, Jr. 2000. Vapor pressure osmometry studies of osmolyte-protein interactions: implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry 39:4455-4471. [DOI] [PubMed] [Google Scholar]
- 26.Csonka, L. N., and W. Epstein. 1996. Osmoregulation, p. 1210-1223. In F. C. Neidhard, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella. Cellular and molecular biology, vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 27.da Costa, M. S., H. Santos, and E. A. Galinski. 1998. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv. Biochem. Eng. Biotechnol. 61:117-153. [DOI] [PubMed] [Google Scholar]
- 28.Diamant, S., N. Eliahu, D. Rosenthal, and P. Goloubinoff. 2001. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276:39586-39591. [DOI] [PubMed] [Google Scholar]
- 29.Diamant, S., D. Rosenthal, A. Azem, N. Eliahu, A. P. Ben-Zvi, and P. Goloubinoff. 2003. Dicarboxylic amino acids and glycine-betaine regulate chaperone-mediated protein-disaggregation under stress. Mol. Microbiol. 49:401-410. [DOI] [PubMed] [Google Scholar]
- 30.Galinski, E. A., and H. G. Trüper. 1994. Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol. Rev. 15:95-108. [Google Scholar]
- 31.Harwood, C. R., and A. R. Archibald. 1990. Growth maintenance and general techniques, p. 1-26. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. Wiley & Sons Ltd., Chichester, United Kingdom.
- 32.Hengge-Aronis, R., W. Klein, R. Lange, M. Rimmele, and W. Boos. 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J. Bacteriol. 173:7918-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jebbar, M., C. von Blohn, and E. Bremer. 1997. Ectoine functions as an osmoprotectant in Bacillus subtilis and is accumulated via the ABC-transport system OpuC. FEMS Microbiol. Lett. 154:325-330. [Google Scholar]
- 34.Kappes, R., and E. Bremer. 1998. Response of Bacillus subtilis to high osmolarity: uptake of carnitine, crotonobetaine and γ-butyrobetaine via the ABC transport system OpuC. Microbiology 144:83-90. [DOI] [PubMed] [Google Scholar]
- 35.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappes, R. M., B. Kempf, S. Kneip, J. Boch, J. Gade, J. Meier-Wagner, and E. Bremer. 1999. Two evolutionarily closely related ABC-transporters mediate the uptake of choline for synthesis of the osmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol. 32:203-216. [DOI] [PubMed] [Google Scholar]
- 37.Kempf, B., and E. Bremer. 1995. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J. Biol. Chem. 270:16701-16713. [DOI] [PubMed] [Google Scholar]
- 38.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 39.Knapp, S., R. Ladenstein, and E. A. Galinski. 1999. Extrinsic protein stabilization by the naturally occurring osmolytes beta-hydroxyectoine and betaine. Extremophiles 3:191-198. [DOI] [PubMed] [Google Scholar]
- 40.Ko, R., L. T. Smith, and G. M. Smith. 1994. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 176:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamosa, P., A. Burke, R. Peist, R. Huber, M. Y. Liu, G. Silva, C. Rodrigues-Pousada, J. LeGall, C. Maycock, and H. Santos. 2000. Thermostabilization of proteins by diglycerol phosphate, a new compatible solute from the hyperthermophile Archaeoglobus fulgidus. Appl. Environ. Microbiol. 66:1974-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Rudulier, D., A. R. Strøm, A. M. Dandekar, L. T. Smith, and R. C. Valentine. 1984. Molecular biology of osmoregulation. Science 224:1064-1068. [DOI] [PubMed] [Google Scholar]
- 43.Lippert, K., and A. A. Galinski. 1992. Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 37:61-65. [Google Scholar]
- 44.Majumdar, D., Y. J. Avissar, and J. H. Wyche. 1991. Simultaneous and rapid isolation of bacterial and eukaryotic DNA and RNA: a new approach for isolating DNA. BioTechniques 11:94-101. [PubMed] [Google Scholar]
- 45.Measures, J. C. 1975. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature 257:398-400. [DOI] [PubMed] [Google Scholar]
- 46.Morbach, S., and R. Krämer. 2002. Body shaping under water stress: osmosensing and osmoregulation of solute transport in bacteria. Chembiochemistry 3:384-397. [DOI] [PubMed] [Google Scholar]
- 47.Nau-Wagner, G., J. Boch, J. A. Le Good, and E. Bremer. 1999. High-affinity transport of choline-O-sulfate and its use as a compatible solute in Bacillus subtilis. Appl. Environ. Microbiol. 65:560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pivetti, C. D., M. R. Yen, S. Miller, W. Busch, Y. H. Tseng, I. R. Booth, and M. H. Saier, Jr. 2003. Two families of mechanosensitive channel proteins. Microbiol. Mol. Biol. Rev. 67:66-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Record, M. T., Jr., E. S. Courtenay, D. S. Cayley, and H. J. Guttman. 1998. Responses of E. coli to osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem. Sci. 23:143-148. [DOI] [PubMed] [Google Scholar]
- 50.Roessler, M., and V. Müller. 2001. Osmoadaptation in bacteria and archaea: common principles and differences. Environ. Microbiol. 3:742-754. [DOI] [PubMed] [Google Scholar]
- 51.Ron, E. Z., and B. D. Davis. 1971. Growth rate of Escherichia coli at elevated temperatures: limitation by methionine. J. Bacteriol. 107:391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook, J., E. F. Fritsch, and T. E. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 53.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos, H., and M. S. da Costa. 2002. Compatible solutes of organisms that live in hot saline environments. Environ. Microbiol. 4:501-509. [DOI] [PubMed] [Google Scholar]
- 55.Schumann, W., M. Hecker, and T. Msadek. 2002. Regulation and function of heat-inducible genes in Bacillus subtilis, p. 359-368. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, D.C.
- 56.Shima, S., D. A. Herault, A. Berkessel, and R. K. Thauer. 1998. Activation and thermostabilization effects of cyclic 2,3-diphosphoglycerate on enzymes from the hyperthermophilic Methanopyrus kandleri. Arch. Microbiol. 170:469-472. [DOI] [PubMed] [Google Scholar]
- 57.Sleator, R. D., and C. Hill. 2002. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 26:49-71. [DOI] [PubMed] [Google Scholar]
- 58.Spiegelhalter, F., and E. Bremer. 1998. Osmoregulation of the opuE proline transport gene from Bacillus subtilis—contributions of the σA- and σB-dependent stress-responsive promoters. Mol. Microbiol. 29:285-296. [DOI] [PubMed] [Google Scholar]
- 59.Strøm, A. R., and I. Kaasen. 1993. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol. Microbiol. 8:205-210. [DOI] [PubMed] [Google Scholar]
- 60.Sukharev, S. I., P. Blount, B. Martinac, and C. Kung. 1997. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu. Rev. Physiol. 59:633-657. [DOI] [PubMed] [Google Scholar]
- 61.Ventosa, A., J. J. Nieto, and A. Oren. 1998. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 62:504-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Blohn, C., B. Kempf, R. M. Kappes, and E. Bremer. 1997. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol. Microbiol. 25:175-187. [DOI] [PubMed] [Google Scholar]
- 63.Welsh, D. T. 2000. Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol. Rev. 24:263-290. [DOI] [PubMed] [Google Scholar]
- 64.Whatmore, A. M., J. A. Chudek, and R. H. Reed. 1990. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J. Gen. Microbiol. 136:2527-2535. [DOI] [PubMed] [Google Scholar]
- 65.Wood, J. M. 1999. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63:230-262. [DOI] [PMC free article] [PubMed] [Google Scholar]