Abstract
Phospho-N-acetyl-muramyl-pentapeptide translocase (translocase 1) catalyzes the first of a sequence of lipid-linked steps that ultimately assemble the peptidoglycan layer of the bacterial cell wall. This essential enzyme is the target of several natural product antibiotics and has recently been the focus of antimicrobial drug discovery programs. The catalytic mechanism of translocase 1 is believed to proceed via a covalent intermediate formed between phospho-N-acetyl-muramyl-pentapeptide and a nucleophilic amino acid residue. Amino acid sequence alignments of the translocase 1 family and members of the related transmembrane phosphosugar transferase superfamily revealed only three conserved residues that possess nucleophilic side chains: the aspartic acid residues D115, D116, and D267. Here we report the expression and partial purification of Escherichia coli translocase 1 as a C-terminal hexahistidine (C-His6) fusion protein. Three enzymes with the site-directed mutations D115N, D116N, and D267N were constructed, expressed, and purified as C-His6 fusions. Enzymatic analysis established that all three mutations eliminated translocase 1 activity, and this finding verified the essential role of these residues. By analogy with the structural environment of the double aspartate motif found in prenyl transferases, we propose a model whereby D115 and D116 chelate a magnesium ion that coordinates with the pyrophosphate bridge of the UDP-N-acetyl-muramyl-pentapeptide substrate and in which D267 therefore fulfills the role of the translocase 1 active-site nucleophile.
Enzymes involved in the assembly of the peptidoglycan layer of bacterial cell walls represent important targets for antibacterial chemotherapy (15, 49). The study of this class of enzymes and the search for selective inhibitors are likely to lead to the development of new chemotherapeutic agents, which are urgently needed to combat antimicrobial drug resistance, the threat of which has recently been highlighted by the acquisition of resistance by methicillin-resistant Staphylococcus aureus to vancomycin (43).
Peptidoglycan consists of a β-1,4-linked N-acetyl-glucosamine-N-acetyl-muramyl-pentapeptide (GlcNAc-MurNAc-pentapeptide) polymer, assembled from cytoplasmic precursors UDP-MurNAc-l-Ala-γ-d-Glu-X-d-Ala-d-Ala (UDP-MurNAc-pentapeptide; X, l-Lys or meso-diaminopimelic acid [meso-DAP]) and UDP-GlcNAc (15, 49). Central to this process is the transfer of the phospho-MurNAc-pentapeptide to a lipid carrier, undecaprenyl phosphate. The resulting lipid-linked intermediate (undecaprenyl-pyrophosphoryl-MurNAc-pentapeptide; lipid intermediate 1) is then the precursor of a series of membrane-bound steps that lead to peptidoglycan formation.
The enzyme responsible for lipid 1 formation is phospho-MurNAc-pentapeptide translocase (EC 2.7.8.13; translocase 1 [23, 45]). The enzyme catalyzes the reversible transfer of phospho-MurNAc-pentapeptide from UMP to undecaprenyl phosphate and requires Mg2+ as a cofactor: undecaprenyl phosphate + UDP-MurNAc-pentapeptide →Mg2+ UMP + undecaprenyl-pyrophosphoryl-MurNAc-pentapeptide.
This type of reaction, namely, the transfer of a phosphosugar to a polyprenyl phosphate carrier, is catalyzed by a superfamily of prokaryotic and eukaryotic polyisoprenyl-phosphate N-acetyl hexosamine 1-phosphate transferases, of which translocase 1 is a member (5). Significant sequence similarity exists between translocase 1 and other bacterial members of this family, such as the GlcNAc1-phosphate transferase WecA and the N-acetyl fucose 1-phosphate transferase WbcO involved in lipopolysaccharide synthesis and the eukaryotic UDP-GlcNAc glucosamine:dolichol phosphate GlcNAc 1-phosphate transferases that catalyze the first step in N-linked glycoprotein biosynthesis (5, 16, 36, 49).
Translocase 1 is known to be an integral membrane protein. Some detail regarding arrangement of the translocase 1 polypeptide within the cytoplasmic membrane has been obtained by Bouhss et al. (10), who have used β-lactamase fusions to show that the positions of cytoplasmic loops of the Escherichia coli and S. aureus translocases are conserved. Translocase 1 is encoded by the mraY gene positioned at 2 min in the E. coli genome between the UDP-MurNAc-l-Ala-γ-d-Glu-meso-DAP:d-alanyl alanine ligase gene (murF) and the UDP-MurNAc-Ala:γ-d-Glu ligase gene (murE) in a cluster of cell wall and cell division genes (27), an arrangement reflected throughout many bacterial genomes (http://www.tigr.org). Although it has previously been held that translocase 1 is strictly limited to the prokaryotes, recent evidence suggests the existence of a translocase 1 homologue in Arabidopsis thaliana (41).
Gene knockout experiments have demonstrated conclusively that the mraY gene is essential to cell viability in both E. coli and the gram-positive pathogen Streptococcus pneumoniae and is therefore an attractive target for antimicrobial drug development (6, 11, 14, 48). Several nucleoside natural product antibiotics that contain the uridine moiety of the natural substrate of translocase 1 have been shown to be potent inhibitors of this enzyme, namely, the mureidomycins, the liposidomycins, tunicamycin, and, most recently, the muramycins (12, 13, 28, 29, 32, 37). The latter class of compounds are growth inhibitory towards both gram-positive and gram-negative pathogens (37), whereas the mureidomycins show selective activity against Pseudomonas species (30).
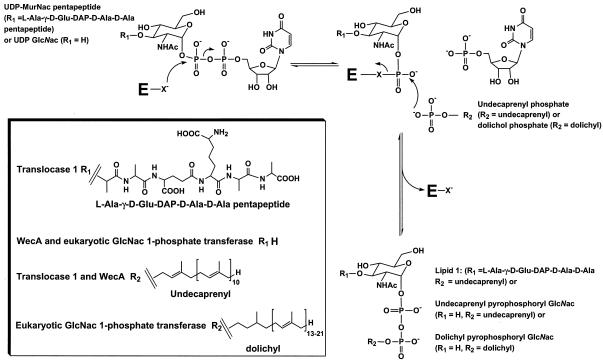
It is therefore very likely that these nucleoside antibiotics target in vivo the active site of translocase 1 (12, 32). Further, relief by Mg2+ of the inhibition of E. coli translocase 1 by nucleoside analogues of mureidomycin A suggests that the antibiotic binds (in part) to the same site occupied by the metal ion in the enzyme active site (21, 25). Therefore, an understanding of the function of the active site of this enzyme is essential to the development of inhibitors of translocase 1. Using isotope exchange in studies with S. aureus translocase 1, Heydanek et al. showed that the enzyme-catalyzed reaction is a two-step process involving an attack of an active-site nucleophile upon the α-P of the UDP-MurNAc-pentapeptide to give a MurNAc-pentapeptide-phosphoenzyme intermediate and free UMP, followed by an attack of undecaprenyl phosphate on the MurNAc-pentapeptide-phosphoenzyme intermediate, which results in regeneration of the free enzyme and formation of lipid 1 (23) (Fig. 1).
FIG. 1.
Reaction mechanism of translocase 1, WecA, and eukaryotic GlcNAc 1-phosphate transferase. E represents enzyme; X− denotes an enzyme active-site nucleophile. R1 represents l-Ala-γ-d-Glu-DAP-d-Ala-d-Ala in the case of translocase 1 and H in the case of both WecA and eukaryotic GlcNAc 1-phosphate transferase. R2 is the polyprenyl chain of the lipid substrate, which is undecaprenyl for translocase 1 and WecA and dolichyl for eukaryotic GlcNAc 1-phosphate transferase. NHAc is NHCOCH3.
Further characterization of structure and enzymatic function of translocase 1 has, however, been severely hampered by problems encountered with the overexpression and purification of the enzyme. Modest overexpression and detergent extraction of enzymatically active E. coli translocase 1 have been reported previously (13); however, further attempts to purify the enzyme were not successful, and thus there has been no characterization of the amino acids involved in translocase 1 substrate binding or catalysis nor identification of any potential active-site nucleophile implicated by the experiments of Heydanek et al. (23).
In this paper, therefore, we report the identification of three strictly conserved aspartate residues (D115, D116, and D267) that may perform these roles and demonstrate their essential function in E. coli translocase 1 catalysis by enzymatic characterization of C-terminal His6 (C-His6)-tagged translocase 1 species generated by site-directed mutagenesis of D115, D116, and D267 into asparagines. We use these results to suggest a model of the catalytic apparatus of this essential antibiotic target.
MATERIALS AND METHODS
Chemicals.
Restriction enzymes, T4 DNA ligase, and primers were from Invitrogen. DNA purification kits were from Qiagen. The triazine dyes brown H-4RD, blue H-ERD, green HE-4BDA, navy H-ER, red HE-3B, turquoise H-A, yellow HE-R, brown MX-5BR, blue MX-2G, orange MX-2R, red MX-5B, turquoise MX-G, blue H-GR, green H-4G, red H-7G, yellow H-5G, yellow M-8G, yellow MX-4R, red H-8BN, yellow HE-3G, blue 3-GA, brilliant red 3-BA, brilliant yellow 3G-P, brilliant blue R, brilliant orange 3R, brilliant violet 5R, and black B, immobilized on 5-ml columns of Sepharose 4B, were the generous gift of PanTherix Ltd. Immobilized cobalt metal affinity resin (Talon resin) was from Clontech. Undecaprenyl phosphate was from Larodan Fine Chemicals AB. Carbenicillin, 3-[(3-cholamidopropyl)-dimethylamino]-1-propanesulphonate (CHAPS), and isopropyl-β-d-thiogalactopyranoside (IPTG) were from Melford Laboratories Ltd. [3H]UDP-N-acetylglucosamine, polyvinylidene difluoride (PVDF) membranes, biotinylated molecular weight markers, horseradish peroxidase (HRP)-streptavidin conjugate, and ECL bioluminescence reagents were from Amersham. Mouse monoclonal anti-His6 antibody and Pwo DNA polymerase were from Roche, goat anti-mouse antibody conjugated to HRP was from Promega, and rabbit anti-maltose binding protein (anti-MBP) antibody and goat anti-rabbit antibody were from New England Biolabs, Hitchin, United Kingdom. Protein assay reagents were from Pierce Inc. UDP-MurNAc-l-Ala-γ-d-Glu-m-DAP-14C-d-Ala-14C-d-Ala was a gift of SmithKline Beecham Pharmaceuticals. UDP-MurNAc-pentapeptide was prepared as previously described (38). E. coli MurG was supplied by J. J. Li (University of Warwick). All other chemicals were from Sigma.
E. coli strains.
JM109 [recA1 endA1 gyrA96 thi hsdR17 (rK− mK+) relA supE44 Δ(lac-proAB) F′ traD36 proAB lacIqZΔM15] (49) was from C. G. Dowson (University of Warwick). DH5α [φdlacΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169] was from D. Boyle (Edinburgh University). C41(DE3), a derivative of BL21(DE3) [F′ ampT hsdS8 (r8m8)gal dcm DE3] (39) was from Avidis S. A. France. TB1 {F− ara Δ(lac-proAB) [φ80dlac Δ(lacZ)M15] rpsL(Strr) thi hsdR} was from New England Biolabs. MV1190 [Δ(lac-proAB) thi supE Δ(srl-recA)306::Tn10(Tetr) F′ traD36 proAB lacIqZΔM15] was from M. Black (SmithKline Beecham Pharmaceuticals).
Plasmids.
All plasmids were Ampr. pBROC525 is a derivative of pTrc99A (2) with the E. coli mraY gene, controlled by a trc promoter regulated by lacIq, inserted into the NcoI/BamH1 site (13). pMALc2E, an overexpression vector carrying a tac promoter, a multiple cloning site (MCS), lacIq, malE, and lacZα, was from New England Biolabs. pET21b, an overexpression vector with a T7 promoter and terminator, an MCS with a 3′ hexahistidine sequence, and lacIq, was from Novagen. pJFY3c, a derivative of pJF118EH (20) with the E. coli mraY gene, controlled by a tac promoter regulated by lacIq, inserted into the KpnI site (11), was from D. Boyle (Edinburgh University).
Construction and expression of proteins with site-directed mutations (33).
Plasmid pBROC525, containing the E. coli mraY gene, was digested on a large scale with SspI/EcoRI, and the required fragment (825 bp) was purified by preparative scale gel electrophoresis and electroelution. The partial mraY fragment was ligated with HincII/EcoRI-digested, purified M13mp18 (50) and used to transform E. coli MV1190 to give the recombinant clone M525. A stock of uracil-containing single-stranded M525 DNA was prepared as recommended in the Bio-Rad M13 Mutagene kit manual for use as a template in mutagenesis reactions. Mutagenic primers were as follows: D115N, 5′-GGC TTT GTT AAT GAT TAT CGC AAA G-3′; D116N, 5′-GGC TTT GTT GAT AAT TAT CGC AAA G-3′; and D267N, 5′-GTC TTA ATG GGC AAT GTA GGT TCG C-3′. Boldface letters represent mutated nucleotides.
In each case, 15 μl of stopped-mutagenesis-reaction mixture was used to transform cells of E. coli MV1190. For each mutagenesis reaction, five or more plaques were picked at random and used to infect 1.5-ml cultures of E. coli MV1190. Single-stranded DNA was purified from the culture supernatant and sequenced using an appropriate primer to screen for the required mutations.
For each mutant, 10 ml of E. coli MV1190 culture at an optical density at 600 nm of 0.3 was infected with phage stock and grown for several hours. Cells were collected, and replicative-form DNA was isolated. The 641-bp StyI/EcoRI fragment was purified, ligated with pBROC525 (5 μg) that had been digested with the same enzymes, and used to transform MV1190. Two clones were selected for DNA sequencing to verify the desired mutations. For each mutant, replicative-form DNA (3 μg) was digested with StyI/EcoRI and the 641-bp partial mraY fragments were purified. The MraY expression vector pBROC525 (13) (5 μg) was digested with the same enzymes, and the larger 4.7-kb fragment was purified. Mutant fragments were ligated with the vector fragment and used to transform E. coli JM109. Expression of native MraY proteins with site-directed mutations was carried out as previously described for JM109/pBRO525 (13). Activity of translocase 1 (solubilized in 50 mM Tris [pH 7.5] containing 0.5% Triton X-100) was assayed in 100 mM Tris (pH 7.5) containing 50 mM KCl and 25 mM MgCl2 by using the fluorescence enhancement assay described previously (13).
Construction of MraY-C-His6 and MraY-MBP expression vectors.
To construct an expression vector carrying an mraY gene with a 3′ sequence encoding a His6 peptide, a 1.1-kb fragment containing the E. coli mraY gene was amplified by PCR with Pwo DNA polymerase from pJFY3c by using the primers GGAATTCCA▾TATGTTAGTTTGGCTG and CCCGC▾TCGAGACGTACCTTCAGCGTT to add NdeI and XhoI restriction sites (indicated by ▾) to the 5′ and 3′ ends of the gene and eliminate the mraY-encoded stop codon. A product of the correct mass was obtained and was gel purified using a Qiagen gel purification kit and restricted in a double digest with NdeI and XhoI. pET21b vector was similarly restricted, and both insert and plasmid were purified on Qiagen spin columns, at which point they were ligated overnight with T4 DNA ligase. Ligation products were used to transform E. coli JM109, and positive transformants were isolated on Luria broth agar containing 50 μg of ampicillin/ml. Single colonies were screened by single-colony PCR (according to the pET system manual [Novagen]) using primers for the T7 promoter (TTAATACGACTCACTATAGGG) and T7 terminator (CTAGTTATTGCTCAGCGGT) sequences flanking the MCS of the vector. Clones carrying the recombinant mraY genes were sequenced to verify the predicted sequences of the mraY insert and the 5′ and 3′ junctions between the vector and the insert. One correct clone (pET21b/mraY) was then retained for expression of the translocase 1 protein.
The D115N, D116N, and D267N mutations of the mraY gene were amplified by PCR and cloned into pET21b to generate pET21b/D115N, pET21b/D116N, and pET21b/D267N MraY fusions with C-His6 sequences in a fashion identical to that employed for the wild-type mraY gene.
Protein assays.
Protein concentration was determined using the bicinchoninic acid method (44).
Preparation of E. coli membrane extracts.
An overnight culture (4 ml) of freshly transformed E. coli grown in Luria broth with 50 μg of carbenicillin/ml was used to inoculate 650 ml of the same medium at 37°C. Once the A600 had reached 0.7 to 1.0, protein expression was induced by the addition of 0.5 mM IPTG. All further steps were performed at ≤4°C. Cells were harvested by centrifugation at the times indicated below and washed once in 20 mM Tris-HCl, 1 mM MgCl2, and 2 mM β-mercaptoethanol adjusted to pH 7.5 (extraction buffer). Cell pastes were resuspended in 3 ml of extraction buffer plus 2.5 mg of lysozyme/g (wet weight) of cells/ml by shaking on ice for 30 min. Suspensions were then disrupted by sonication and centrifuged for 30 min at 10,000 × g. Supernatants were recentrifuged for 30 min at 100,000 × g. The resulting membrane pellets were washed for 60 min in extraction buffer and 1 M KCl, collected by centrifugation for 30 min at 100,000 × g, resuspended in extraction buffer, and then repelleted by centrifugation for 30 min at 100,000 × g.
The washed membranes were resuspended at 4 mg of protein/ml in extraction buffer plus 1.5% (wt/vol) CHAPS and 15% (vol/vol) glycerol and stirred for 30 min. The extracted membrane proteins were then isolated from any insoluble material by centrifugation for 30 min at 100,000 × g.
Translocase 1 assays. (i) Assay 1: formation of 14C-lipid 1 from [14C]UDP-MurNAc-pentapeptide.
Unless specified otherwise, assays followed the translocase 1-catalyzed transfer of [14C]phospho-MurNAc-pentapeptide from UDP-MurNAc-l-Ala-γ-d-Glu-m-DAP-14C-d-Ala-14C-d-Ala to undecaprenyl phosphate to form lipid 1 (46). Incubations were carried out at 37°C in 50 μl of 0.1 M Tris-Cl, pH 7.5, containing 25 mM MgCl2, 96.9 μM undecaprenyl phosphate, 30 μg of phosphatidylglycerol/ml, 3 μM (4.54 nCi/assay) [14C]UDP-MurNAc-pentapeptide, 2% (vol/vol) glycerol, and 0.5% (wt/vol) CHAPS. The lipid components of the assays were dried down and resolubilized by addition of the buffer and detergent. Assays were initiated by enzyme or buffer, and unless stated otherwise, mixtures were incubated for 1 h. Reactions were terminated by addition of 50 μl of 6 M pyridinium acetate, pH 4.5. Lipid products were purified by extraction with 100 μl of n-butanol and quantitated by liquid scintillation counting. Control experiments omitting undecaprenyl phosphate showed that rates of accumulation of n-butanol-soluble 14C were 0 to 12% of those obtained in the presence of undecaprenyl phosphate. Control experiments performed in the presence of mureidomycin A (10 μM) (13, 28, 29) resulted in a complete loss of undecaprenyl phosphate-dependent incorporation of 14C into the n-butanol phase.
(ii) Assay 2: coupling formation of lipid 1 from UDP-MurNAc-pentapeptide to 3H-lipid 2 synthesis with MurG and [3H]UDP-GlcNAc.
In assay 2, formation of lipid 1 by translocase 1 from UDP-MurNAc-pentapeptide and undecaprenyl phosphate was coupled to incorporation of [3H]GlcNAc into lipid 1 by MurG (51). Here, incubations were carried out at 37°C in 50 μl of 0.1 M Tris-Cl, 25 mM MgCl2 (pH 7.5) containing 96.9 μM undecaprenyl phosphate, 60 μg of phosphatidylglycerol/ml, 20 μM (45.4 nCi/assay) [3H]UDP-GlcNAc, 10 μM UDP-MurNAc-pentapeptide, 2% (vol/vol) glycerol, 0.5% (wt/vol) CHAPS, and 0.2 mg of E. coli MurG/ml. Assays were initiated by enzyme or buffer, and mixtures were incubated for 1 h. Mixtures for control experiments lacked UDP-MurNAc-pentapeptide. Incubations were stopped and the lipid products were quantitated as described for assay 1.
Hydroxylamine trapping experiments.
Membranes (3.5 ml, 8 mg of protein/ml) containing overexpressed E. coli translocase 1 were solubilized by addition of equal volumes of 50 mM Tris (pH 7.5), 2 mM β-mercaptoethanol, 1 mM MgCl2, 20% glycerol, and 6% CHAPS with stirring for 1 h at 4°C. Unsolubilized material was removed by centrifugation (100,000 × g for 30 min). The supernatant was collected, and 2 M MgCl2 (25 μl) and 2 M KCl (50 μl) were added, followed by [14C]UDP-MurNAc-pentapeptide (1.3 μCi; final concentration, 4 μM). After incubation of the reaction mixture for 1 min, the reaction was stopped by addition of acetone (2 ml at −20°C) and the sample was incubated at −20°C for 10 min. Precipitate was collected by centrifugation (12,000 × g for 10 min) and resuspended in 100 mM Tris, pH 7.0 (1 ml). Acetone (1 ml) was added, and the mixture was incubated at −20°C for 10 min. Precipitate was collected by centrifugation (13,500 rpm for 5 min in a microcentrifuge [Eppendorf]) and washed with a 1:1 complex of 100 mM Tris (pH 7.0) and acetone (1 ml). The final pellet was dissolved completely in 50 mM sodium phosphate (pH 7.0)-0.2 M NaCl-2% (wt/vol) sodium dodecyl sulfate (SDS) (450 μl). The sample was sonicated (3 min). Blue dextran (2 mg) dissolved in the same buffer (50 μl) was added to the sample as a calibrant for chromatography.
The sample was applied to a column of Sephadex G75 (73 by 1 cm) equilibrated with 50 mM sodium phosphate (pH 7.0), 0.2 M NaCl, and 2% SDS at 25°C. Elution from this column was at 10.7 ml/h with the same buffer. Fractions (5 ml) were collected, and radioactivity was measured by scintillation counting.
For experiment 2, the final pellet was extracted three times with a 2:1 complex of CHCl3 and methanol (1 ml each time). Extraction was promoted by sonication (3 min) on ice. For experiment 3, after the incubation for 1 min with [14C]UDP-MurNAc-pentapeptide, UMP (7.4 mg dissolved in 50 μl of water) was added to a final concentration of approximately 10 mM and the mixture was incubated for a further 1 min before precipitation and chromatography. For experiment 4, the pH was adjusted to 9.5 (by addition of 3 M NaOH), and 1.5 M hydroxylamine, pH 9.5 (140 μl; final concentration, 100 mM), was added. The sample was incubated at room temperature for 15 min before chromatography.
Triazine dye ligand chromatography.
A library of 28 triazine dyes immobilized on 5 ml of Sepharose 4B (see “Chemicals”) was equilibrated at 4°C with 50 ml of 50 mM Tris, 1 mM MgCl2, 2 mM β-mercaptoethanol, 10% (vol/vol) glycerol, 1.5% (wt/vol) CHAPS, and 30 μg of phosphatidylglycerol/ml adjusted to pH 7.5 (low-salt buffer). Each column was loaded with 0.61 mg of extracted membrane protein with translocase 1 activity of 89.1 pmol/min and washed with 10 ml of low-salt buffer followed by 10 ml of low-salt buffer containing 1 M KCl. Fluid from column washes was collected and dialyzed into low-salt buffer for determination of translocase 1 activity.
One column, Sepharose 4B-yellow HE-3G, was chosen for further purification trials. Therefore, fresh Sepharose 4B-yellow HE-3G was prepared (18).
A 70-ml column of Sepharose 4B-yellow HE-3G was equilibrated at 4°C with low-salt buffer and was then loaded with 7.4 mg of extracted membrane protein with translocase 1 activity of 415.4 pmol/min at 33 ml/h. The column was washed at the same flow rate with 200 ml of low-salt buffer followed by a gradient over 600 ml in low-salt buffer from 0 to 1 M KCl. Fractions (6 ml) were collected and dialyzed into low-salt buffer for determination of translocase 1 activity.
Immobilized cobalt column chromatography.
Extracted enzyme was dialyzed into 50 mM sodium phosphate, 0.3 M NaCl, 10% (vol/vol) glycerol, 1.5% (wt/vol) CHAPS, and 30 μg of phosphatidylglycerol/ml adjusted to pH 7.0 (phosphate buffer) overnight at 4°C. All subsequent steps were performed at this temperature. A 5-ml column of immobilized cobalt affinity Talon resin was equilibrated in phosphate buffer and loaded with the dialyzed protein at 1.2 ml/min. Elution from this column was then carried out at the same flow rate with 30 ml of phosphate buffer and then 30 ml of phosphate buffer supplemented with 0.15 M imidazole. Two-milliliter fractions were collected. Fractions were then dialyzed into low-salt buffer prior to enzyme assay.
SDS-PAGE, Western blotting, and protein sequencing. (i) SDS-PAGE analysis of protein.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with either 8.5% acrylamide for MBP fusion analysis or 12% acrylamide for all other experiments as described in reference 34. Gels were stained with 0.15% (wt/vol) Coomassie brilliant blue in a 5:1:4 complex of methanol, acetic acid, and water or with silver (42).
(ii) Western blotting.
To detect the MBP or C-His6 epitopes of fusion proteins fractionated by SDS-PAGE on gels calibrated with biotinylated molecular weight markers, proteins were electrophoretically transferred from the polyacrylamide gels onto PVDF membranes overnight in 39 mM glycine, 48 mM Tris, 0.037% (wt/vol) SDS, and 20% (vol/vol) methanol at 4°C. The resulting blot was then blocked with 10% (wt/vol) low-fat milk powder in phosphate-buffered saline (PBS) for 1 h at 25°C and washed three times for 20 min each in PBS-0.1% (vol/vol) polyoxyethylene sorbitan monolaurate (Tween 20; PBS-T). The blot was then probed for MBP or C-His6 tags with, respectively, a rabbit anti-MBP antibody at a dilution of 1 in 10,000 or 0.1 μg of mouse monoclonal anti-His6 antibody/ml in PBS-T for 1 h. Blots were washed three times for 20 min in PBS-T and then incubated for 1 h with HRP-linked anti-rabbit immunoglobulin G at a dilution of 1 in 2,000 or HRP-linked anti-mouse immunoglobulin G at a dilution of 1 in 2,500 to detect MBP or the C-His6 epitopes, respectively. Streptavidin conjugated to HRP was also added at this point at a dilution of 1 in 1,500 to detect the molecular weight markers. The blot was washed three times for 20 min each in PBS-T. Protein fusions were detected by HRP-mediated bioluminescence by using the enhanced chemiluminescence method of detection.
(iii) N-terminal sequence determination.
Protein species were fractionated by SDS-PAGE on gels that had been prerun according to the method described in reference 19 and then electroblotted in 10 mM 3-cyclohexylamino-1-propane sulfonic acid adjusted to pH 11.0 onto PVDF membranes. Protein species were visualized and subjected to N-terminal sequencing by automated Edman degradation as described in references 17 and 35.
RESULTS
Identification of potential catalytic residues in translocase 1 by sequence alignment.
Amino acid sequence alignments were used to identify candidates for the active-site nucleophile implicated by the experiments of Heydanek et al. (23). An amino acid acting as a catalytic residue within the translocase 1 active site (i) must be conserved among all bacterial sequences and among those of other members of the polyisoprenyl-phosphate N-acetyl hexosamine 1-phosphate transferase superfamily and (ii) must be orientated on the cytoplasmic face of the cell membrane for access to the cytoplasmic pool of peptidoglycan precursors. In order to identify such amino acids, a BLAST search (1) of the prokaryotic genomes on the Institute for Genomic Research website (http://www.tigr.org) was performed using the primary structure inferred from the E. coli mraY and wecA nucleotide sequences. The protein sequences were then aligned with those of a number of eukaryotic UDP-GlcNAc:dolichol phosphate N-acetyl-glucosamine 1-phosphate transferases by using CLUSTAL W (24) to identify residues that were uniformly conserved and therefore likely to be essential to the catalytic function of the enzyme. The final alignment was composed of translocase 1 sequences from 18 species, WecA sequences from 8 species, and eight eukaryotic phospho-GlcNAc transferase sequences.
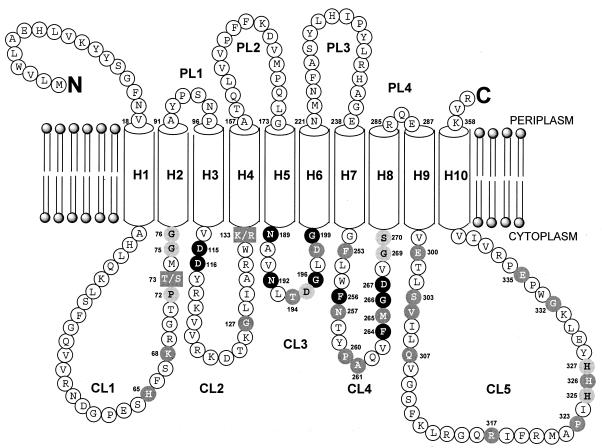
As illustrated in Fig. 2, only 12 amino acid residues are conserved in all prokaryotic and eukaryotic sequences: G112, D115 (replaced with E86 in Helicobacter pylori J99 WecA), D116, N189, N192, G196, G199, N257, F264, G266, D267, and G274 (E. coli numbering). Superposition of the 10 transmembrane domains of the E. coli and S. aureus translocase 1 polypeptides at the positions determined experimentally by Bouhss et al. (10) on the corresponding translocase 1 sequences suggested that these conserved residues were positioned on cytoplasmic loops CL2 to CL4 of the translocase 1 amino acid sequence (except G112 and C274), as illustrated in Fig. 2. However, of these residues, Asp-115, Asp-116 (in CL2), and Asp-267 (in CL4) were the only nucleophilic amino acids that could fulfill a catalytic role in the reaction catalyzed by translocase 1. The Asp-Asp pair at positions 115 and 116 is reminiscent of a DDXXD motif found in enzymes that bind pyrophosphate substrates, such as the prenyl transferases and terpene cyclases, where the Asp-Asp pair binds the Mg2+ cofactor, which in turn binds the pyrophosphate of the substrate (38, 46).
FIG. 2.
Membrane topology model of E. coli translocase 1 showing locations of conserved amino acid residues. The experimental model of translocase 1 reported in reference 10 is shown, superimposed onto which are conserved residues identified by amino acid sequence alignment. Residues conserved in all sequences are shown in black circles; residues conserved in translocase 1 and WecA sequences are shown in boldface in shaded circles; residues conserved in only translocase 1 sequences are shown in white type in shaded circles. Boxed residues 73 and 133 are those with conservative substitutions (T→S and K→R). CL1 to CL5 correspond to cytoplasmic loops 1 to 5, respectively, H1 to H10 correspond to transmembrane helices 1 to 10, respectively, and PL1 to PL4 denote periplasmic loops 1 to 4, respectively. N and C indicate the N and C termini of the protein.
Observation and trapping of a covalent intermediate.
If the active-site nucleophile in the translocase 1-catalyzed reaction is a conserved aspartic acid residue, then the covalent intermediate formed in the enzymatic reaction is an acyl phosphate, which in principle could be trapped using a reactive nucleophile such as hydroxylamine.
In order to examine the covalent intermediate formed in the translocase 1-catalyzed reaction, solubilized E. coli translocase 1 was incubated with UDP-MurNAc-l-Ala-γ-d-Glu-m-DAP-14C-d-Ala-14C-d-Ala (but no additional undecaprenyl phosphate) and the labeled enzyme was analyzed by Sephadex G75 gel filtration chromatography. Two new peaks of radioactivity were repeatedly observed: a small peak (peak 1; 80 to 125 cpm) in fractions 4 to 5 which coeluted with blue dextran and a larger, lower-molecular-weight peak (peak 2; 380 to 1,200 cpm) in fractions 10 to 11, as shown in Table 1. These peaks were followed by a larger peak containing [14C]UDP-MurNAc-pentapeptide. Extraction of the labeled enzyme with a 2:1 complex of chloroform and methanol prior to gel filtration had no effect upon peak 1 but gave a 60% reduction in counts for peak 2. Incubation of the labeled enzyme with 10 mM UMP prior to gel filtration led to the disappearance of counts for both peaks 1 and 2. These data imply that peak 2 corresponds to lipid-linked MurNAc-pentapeptide species whereas peak 1 corresponds to enzyme-linked MurNAc-pentapeptide. From the specific activity of the labeled substrate (46 nCi/nmol), the amount of labeled enzyme can be calculated to be approximately 1 pmol. If a 1:1 complex with protein is formed, this would correspond to 30 to 40 ng of labeled MraY in a total sample of 28 mg of membrane protein.
TABLE 1.
Sephadex G75 chromatography of 14C-labeled solubilized translocase 1a
| Expt | Exptl conditions | Radioactivityc (cpm) of:
|
|
|---|---|---|---|
| Peak 1 fractionsd | Peak 2 fractionse | ||
| 1 | Translocase 1 solubilized in 3% CHAPS | 110 | 380 |
| 2 | Repeat of expt 1 | 80 | 1,160 |
| Sample preextracted with 2:1 CHCl3-MeOHb | 80 (100%) | 460 (40%) | |
| 3 | Repeat of expt 1 | 125 | 720 |
| Sample pretreated with 10 mM UMP | 10 (8%) | 10 (4%) | |
| 4 | Repeat of expt 1 | 125 | 860 |
| Sample pretreated with 0.1 M hydroxylamine | 45 (36%) | 730 (85%) | |
Procedure described in Materials and Methods.
MeOH, methanol.
After subtraction of background (20 cpm). Percentages are relative to the values obtained in the repeats of experiment 1.
Enzyme-linked MurNAc-pentapeptide (fractions 4 and 5).
Lipid-linked MurNAc-pentapeptide (lipid 1; fractions, 10 and 11).
Treatment of the labeled enzyme with 0.1 M hydroxylamine, 0.2 M NaCl, and 2% SDS prior to gel filtration gave a 64% reduction in counts for peak 1 but only a 15% reduction in counts for peak 2. These data are consistent with the reaction of hydroxylamine with an acyl phosphate intermediate to release phospho-MurNAc-pentapeptide. Although the observed counts are low (due to the very low abundance of translocase 1), these experimental data support the existence of a covalent intermediate in the translocase 1-catalyzed reaction.
Partial purification of native E. coli translocase 1.
In order to characterize the effects of mutations in translocase 1, we required a source of purified native translocase 1 for comparison. Therefore, efforts were made to purify E. coli translocase 1 activity. We expressed translocase 1 in E. coli DH5α harboring pJFY3C carrying the mraY gene under the control of a tac promoter (11). Induction of expression with IPTG caused an immediate cessation of growth followed by slow cell lysis, preventing the continued expression of this protein beyond 90 min postinduction. There was no visible band corresponding to translocase 1 (predicted molecular mass of 38 kDa) upon SDS-PAGE. Nevertheless, the enzyme, extracted from membranes with 1.5% (wt/vol) CHAPS, was assessed for binding and elution of translocase 1 from a library of 28 5-ml columns of triazine dyes covalently linked to Sepharose 4B. Three immobilized dyes (navy HE-R, blue MX-2G, and yellow HE-3G) bound translocase 1 as judged by the absence of activity in the low-salt buffer wash fluid. Elution with 1 M KCl from these three columns gave 26, 16, and 65% of the applied translocase 1 activity, respectively, and thus yellow HE-3G was selected for further study.
Once it was established that the native enzyme could be partially purified by elution with salt, attempts were made to elute the enzyme with its substrate, UDP-MurNAc-pentapeptide, or its product, UMP. Both ligands, at 0.05 or 10 mM, respectively, failed to elute the enzyme from immobilized yellow HE-3G, although the enzyme could be recovered in a 68% yield by subsequent washing with 1 M KCl.
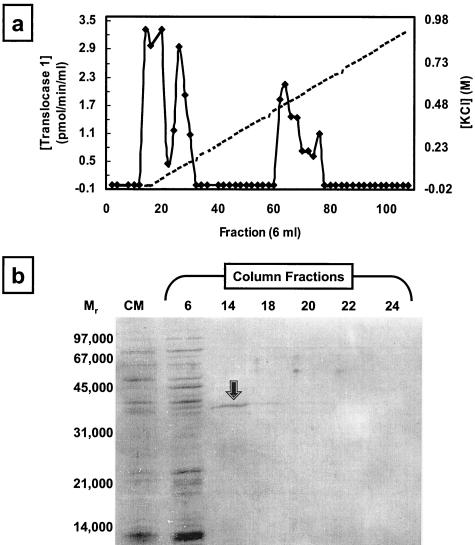
Purification of translocase 1 on Sepharose 4B-yellow HE-3G was then carried out on a larger scale: enzyme was eluted from a 70-ml column on an 8.6-column volume 0 to 1 M KCl gradient. Under these conditions, the enzyme activity was resolvable into three peaks, as shown in Fig. 3a. Analysis by silver-stained SDS-PAGE failed to detect any protein bands in the last two peaks of activity, indicating the very low abundance of translocase 1 after purification. In contrast, SDS-PAGE analysis of the first peak eluting at the beginning of the KCl gradient revealed the presence of a protein of 38 kDa (Fig. 3b), a mass similar to the anticipated mass of native translocase 1.
FIG. 3.
Purification of native translocase 1 by KCl gradient elution from yellow HE-3G immobilized on Sepharose 4B. (a) Purification of native E. coli translocase 1 from Sepharose 4B-yellow HE-3G. Translocase activity elution is represented by closed diamonds, and the 0 to 1 M KCl gradient is denoted by a dashed line. (b) SDS-PAGE analysis of fractions from the yellow HE-3G column. CM, CHAPS-extracted membranes (2 μg of protein loaded). The gel was silver stained. The arrow denotes the protein species in fraction 14 characterized further by N-terminal sequencing.
To determine the identity of the protein in the first peak of translocase 1 activity, fraction 14 was concentrated by lyophilization and resuspended in 0.4 ml of 0.1% (wt/vol) SDS. The protein was fractionated by SDS-PAGE and then blotted onto PVDF membrane and subjected to N-terminal sequencing. The N-terminal sequence DLNIKMIPG was obtained, which was identical to the sequence of E. coli penicillin binding protein 5 (9) but not that of E. coli translocase 1 (expected N terminus, MLVWLAEHL).
Expression and purification of E. coli translocase 1 as a fusion protein.
The inability to overexpress and purify the native enzyme led us to undertake construction of E. coli translocase 1 fusion proteins. Construction of an N-terminal MBP-translocase 1 fusion (see supplemental material at http://www.warwick.ac.uk/fac/sci/Chemistry/biochem) and expression in E. coli TB1 revealed no visible protein band at 81 kDa corresponding to the MBP-translocase 1 fusion. Western blotting with an anti-MBP antibody revealed a band at approximately 40 kDa, similar to the mass of MBP alone (see supplemental material at http://www.warwick.ac.uk/fac/sci/Chemistry/biochem), clearly showing that the MBP-translocase 1 fusion is unstable and susceptible to proteolysis. Likewise, attempts to generate an N-terminal His6-MraY fusion also gave no translocase 1 activity (data not shown).
A C-terminal His6-translocase 1 fusion was then constructed. The mraY gene was subcloned into pET21b to generate pET21b/mraY. The correct sequence of the gene was ascertained by sequencing as was the in-frame 5′ mraY initiator methionine codon and the 3′ pET21b-carried in-frame codons for Leu-Glu-His6-stop. The construct was used to transform the expression host E. coli C41(DE3), which has been shown to be tolerant to the expression of membrane proteins (40). Induction of this construct with IPTG at 37°C for 4 h led to inhibition of cell growth relative to that of the expression host transformed with empty pET21b. This inhibition of growth was similar to (but not as acute as) the retardation of growth encountered upon expression of the untagged enzyme from pJFY3c and was consistent with expression of a toxic recombinant protein.
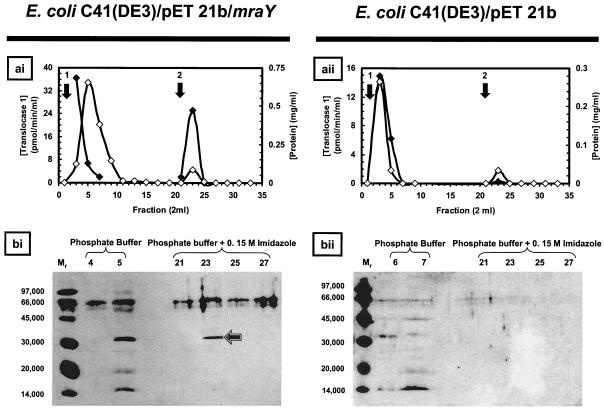
In order to confirm the expression of active translocase 1 from pET21b/mraY, membranes were prepared from E. coli C41(DE3) transformed with either pET21b/mraY or pET21b that had been induced with IPTG. These membranes were then extracted with a buffer containing 1.5% (wt/vol) CHAPS and 15% (wt/vol) glycerol. Radiochemical assays of these extracts revealed only a modest 1.4-fold increase in specific activity of translocase 1 over that in the empty vector control. Nevertheless, the presence of the C-His6 tag in the recombinant wild-type translocase 1 offered the potential of purification from the E. coli host-carried enzyme. Extracts of pET21b/mraY were applied to an immobilized cobalt column, and after washing of the column with buffer alone, protein was eluted with 0.15 M imidazole. Assays revealed two peaks of translocase 1 activity accounting for 43% of the activity that was loaded (Fig. 4ai). The first-peak fractions (fractions 1 to 5), with translocase 1 activity of ∼174 pmol/min (25% of the enzyme activity loaded), failed to bind to the column and eluted with phosphate buffer alone, while the second-peak fractions (fractions 21 to 25), with translocase 1 activity of 126 pmol/min (18% of the enzyme activity loaded), were eluted with phosphate buffer containing 0.15 M imidazole. In contrast, similar fractionation of membrane extracts from C41(DE3)/pET21b yielded translocase 1 activity of ∼98 pmol/min exclusively in the phosphate buffer wash fluid (Fig. ii, fractions 1 to 6). This activity accounted for 67% of the translocase 1 activity loaded and corresponded to host translocase 1, which does not bind to the affinity column. The eluted E. coli MraY-His6 fusion protein had a specific activity of 325 pmol/min/mg of protein (data from Fig. 4) compared with a specific activity of overexpressed native E. coli MraY of 1 to 2 nmol/min/mg (13); therefore, it appears that the His6 fusion protein has a lower specific activity than the native enzyme.
FIG. 4.
Purification of C-His6 translocase 1 from membrane extracts of C41(DE3). (ai and bi) Purification of C-His6-tagged translocase 1 from E. coli C41(DE3) transformed with pET21b/mraY by immobilized cobalt chromatography. (ai) Solubulized membrane protein (7.47 mg) with translocase 1 activity of 695.8 pmol/min was applied to a 5-ml column of immobilized cobalt. Arrow 1 denotes application of the phosphate buffer wash, and arrow 2 denotes application of 0.15 M imidazole in phosphate buffer. Filled diamonds, translocase 1 activity; empty diamonds, protein concentration. (bi) Western blot analysis of the column fractions containing purified C-His6 translocase 1 for hexahistidine-containing protein species. Lanes were loaded with 40 μl of the fractions specified in the figure. The arrow indicates the band corresponding to the purified C-His6-tagged translocase 1. The band at Mr of 66 in bound and unbound fractions was observed widely and is probably due to a biotin-containing E. coli protein stained as an artifact of the Western blot assay. (aii and bii) Chromatography of solubilized membrane protein from E. coli C41(DE3) transformed with pET21b by immobilized cobalt chromatography. (aii) Solubilized membrane protein (2.86 mg) with translocase 1 activity of 146.2 pmol/min was applied to a 5-ml column of immobilized cobalt. Arrow 1 denotes application of the phosphate buffer wash, and arrow 2 denotes application of 0.15 M imidazole in phosphate buffer. Filled diamonds, translocase 1 activity; empty diamonds, protein concentration. (bii) Western blot analysis of the column fractions for hexahistidine-containing protein species. Lanes were loaded with 40 μl of the fractions specified in the figure. Mr signifies lanes loaded with molecular weight markers.
To confirm expression and identification of the wild-type C-His6 translocase 1 protein, Western blotting was performed on fractions containing translocase 1 activity by using an anti-His6 antibody. For the recombinant pET21b/mraY construct, a band at an apparent molecular mass of 32 kDa was observed in fractions 5 (buffer wash) and 23 (0.15 M imidazole wash) which contained translocase 1 activity (Fig. i). For the pET21b control (Fig. ii), no band was observed using Western blotting. These data indicate that the translocase 1-His6 fusion protein is expressed, extracted from membranes, partially bound by the immobilized cobalt column, eluted by 0.15 M imidazole, and catalytically active. The wild-type translocase 1 was not bound by the cobalt column. Therefore, despite the low levels of expression of the recombinant tagged translocase 1 gene product, it is possible to separate recombinant translocase 1-His6 from host wild-type translocase 1 by using affinity chromatography. The apparent molecular mass of 32 kDa is lower than the predicted molecular mass of 39 kDa for the translocase 1 polypeptide. This discrepancy is typical of integral membrane proteins, the masses of which can often be underestimated by as much as 30 to 35% (22), and has been previously documented for β-lactamase fusions of translocase 1 (10). Silver staining of similar SDS-PAGE gels showed the presence of many protein bands, indicating that the MraY-His6 protein is present at low abundance, even after affinity purification.
Site-directed mutagenesis of Asp-115, Asp-116, and Asp-267 to asparagine.
The roles of the three aspartic acids identified above, namely, D115, D116, and D267, were investigated by replacement with asparagine by using site-directed mutagenesis. Site-directed mutations D115N, D116N, and D267N of mraY were prepared using the method of Kunkel (33). The mutated mraY genes were expressed first as the native MraY proteins in the pBROC525 expression vector previously described (13) and secondly as the MraY-His6 fusion proteins by subcloning into the pET21b vector and expression in C41(DE3).
(i) Expression of mutant MraY proteins in JM109/pBROC525.
The mutated mraY genes were subcloned into the pBROC525 expression vector described previously and expressed in E. coli JM109 (13). Membranes from each strain were prepared and extracted with buffer containing 0.5% Triton X-100. Translocase 1 activity was determined using the fluorescence enhancement assay described previously (13). As shown in Table 2, the D115N, D116N, and D267N mutant proteins showed 5.9, 3.7, and 9.1% of wild-type activity, respectively. The activity of background E. coli MraY (from the empty vector control is equivalent to 4 to 5% of wild-type activity. Since mraY is an essential gene (11), it is not possible to construct a strain lacking mraY in order to remove contaminating background MraY activity. Therefore, although each mutant protein appeared to have very low translocase 1 activity, it was not possible to infer the precise level of activity in each mutant protein. The ability to separate the MraY-His6 fusion protein from native MraY by affinity chromatography provided an opportunity to study more definitively the level of activity in each mutant protein.
TABLE 2.
Activity of the translocase 1 enzymes with site-directed mutations D115N, D116N, and D267N expressed as native MraY and as affinity-purified MraY-His6 fusions
| Enzyme | Sp acta (fluorescence units/min/mg) of native MraYb (% of wild-type activity) | Sp actc (pmol/min/mg) of MraY-His6 fusiond (% of wild-type activity)
|
|
|---|---|---|---|
| Assayed in 25 mM MgCl2 | Assayed in 100 mM MgCl2 | ||
| Empty vector | 0.42, 0.71 (4.2 ± 1.0) | 0.0 (0.0) | 0.0 (0.0) |
| Wild-type MraY | 13.5 (100) | 26.7 (100) | 17.0 (100) |
| D115N mutant | 0.79 (5.9) | 0.0 (0.0) | 0.0 (0.0) |
| D116N mutant | 0.50 (3.7) | 0.22 (0.82) | 0.0 (0.0) |
| D267N mutant | 1.23 (9.1) | 0.0 (0.0) | 0.7 (4.1) |
Assayed using the fluorescence enhancement assay as previously described (13).
Expressed in E. coli JM109/pBROC525 and assayed as solubilized membranes in 0.5% Triton X-100.
Assayed using a coupled MraY/MurG radiochemical assay (assay 2) as described in Materials and Methods. The specific activity of the wild-type MraY-His6 enzyme is lower than that shown in Fig. 4 due to the use of the coupled assay.
Expressed in E. coli C41(DE3)/pET21b and purified by cobalt affinity chromatography.
(ii) Expression and affinity elution of mutant MraY-His6 proteins in C41(DE3)/pET21b.
Each mutated mraY gene was subcloned into pET21b and expressed in E. coli C41(DE3). Prior to induction with IPTG, C41(DE3) strains carrying the pET21b mutation constructs grew as quickly as a control strain carrying an empty vector. However, upon induction of expression of the mutant translocases at 37°C by treatment with 0.5 mM IPTG, different effects on growth of the host were observed relative to the growth of the strain carrying an empty vector. Induction of the D115N mutant showed severe growth inhibition, similar to that by the native MraY-His6. Induction of the D116N mutant showed no apparent inhibition of growth compared to the growth of a C41/pET21b control (doubling time, 60 min), and the D267N mutant showed slight growth inhibition (doubling time, 80 min).
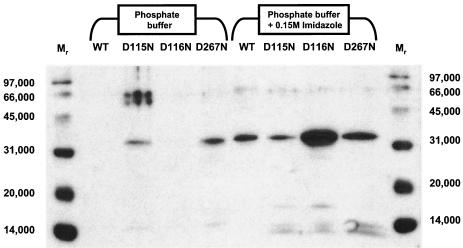
As was the case for expression of the wild-type translocase 1, no expression of protein of the correct mass could be detected by SDS-PAGE of membrane extracts followed by silver staining. The CHAPS-extractable membrane fractions from E. coli C41(DE3) expressing the C-His6 wild type and D115N, D116N, and D267N translocase 1 species, which contained 1.24, 3.81, 6.69, and 1.89 mg of protein, respectively, were applied to immobilized cobalt columns, which were washed with phosphate buffer, and then eluted with buffer containing 0.15 M imidazole. Analysis by Western blotting (Fig. 5) revealed bands at 32 kDa in the imidazole wash fluids corresponding to expression of the C-His6 wild type and D115N, D116N, and D267N mutant translocases. Additionally, a proportion of the D115N and D267N mutant proteins failed to bind to the column, being eluted by phosphate buffer alone (Fig. 5). The levels of protein expression for the three mutant proteins varied widely: D115N and D267N mutant proteins were expressed at levels comparable to the expression of the wild-type protein; however, expression of the D116N mutant protein was approximately an order of magnitude above that of the wild type (Fig. 5). These data implied that the mutant proteins could be expressed, solubilized, and separated from the host-encoded translocase 1.
FIG. 5.
Western blot analysis of cobalt column purification of C-His6-tagged translocases with site-directed mutations. Peak fractions from the phosphate buffer (first four sample lanes) and imidazole (second four sample lanes) washes of immobilized cobalt columns loaded with CHAPS-extracted membrane protein from E. coli C41(DE3) expressing wild-type translocase 1 and D115N, D116N, and D267N mutants were analyzed by SDS-PAGE and Western blotting. Protein (2.82 μg) was loaded in each lane. WT, wild-type translocase 1. Loading of mutant translocase 1 samples are as indicated above each lane. Mr signifies lanes loaded with molecular weight markers.
The translocase 1 activity of each affinity-purified mutant enzyme was determined using assay 2 and compared with the activity of affinity-purified wild-type MraY-His6 protein. Radiochemical assays performed on each eluted mutant enzyme (Table 2) failed to detect any translocase 1 activity (<20 cpm above background), whereas assay of the wild-type translocase 1-His6 enzyme showed 27 pmol/min/mg of protein (1,138 cpm). Therefore, any residual activity of the three mutant enzymes is <2% of the wild-type activity. Assays were repeated in the presence of 0.1 M MgCl2, a concentration fourfold higher than the standard assay concentration, to investigate whether the lack of activity was due to impairment of Mg2+ binding. Again, no significant activity could be detected in any of the three mutant enzymes.
DISCUSSION
The biochemical study of translocase 1 and its exploitation as an antibacterial target have been hindered to a large extent by the refractoriness of the MraY protein product to overexpression and purification. The two-step catalytic mechanism implied by isotope exchange experiments of Heydanek et al. with S. aureus translocase 1 (23) and the implied existence of an active-site nucleophile have not to date been investigated using purified protein.
In this paper we report the successful expression and partial purification of the C-His6-tagged form of the E. coli translocase 1 via expression in E. coli C41(DE3), a strain tolerant to the expression of membrane proteins (40). Similarly, other C-terminal tags such as Strep Tag II (Genosys Biotechnologies Inc.) have also proved useful for translocase 1 expression (51). Even with the use of C41(DE3), expression of full-length active translocase 1 was still partially cytotoxic, although it is unclear whether this resulted from elevated translocase 1 activity or distortion of the inner membrane of E. coli due to insertion of the recombinant protein. We have observed both with the native E. coli translocase 1 and the translocase 1-His6 fusion protein that two peaks of activity are observed upon purification, which implies that more than one form of the enzyme is present, perhaps due to the existence of more than one conformation or oligomeric state of the protein. The specific activity of the MraY-His6 fusion protein appears to be rather lower than that of native MraY as judged by the modest 1.4-fold enhancement of MraY activity upon overexpression and the specific activity of the affinity-purified protein of 325 pmol/min/mg of protein compared with a specific activity of overexpressed native E. coli MraY of 1 to 2 nmol/min/mg (13). However, the ability to fractionate recombinant translocase 1 from that of the expression host provided us with an opportunity to investigate in more detail the function of conserved residues via site-directed mutagenesis.
Sequence alignments of translocase 1 with WecA sequences and their eukaryotic homologues identified only three amino acid residues with nucleophilic side chains that are completely conserved in translocase 1 and its related prokaryotic and eukaryotic homologues: Asp-115, Asp-116, and Asp-267. These residues are candidates for the amino acid nucleophile supporting phosphotransfer catalysis in this superfamily of enzymes. In addition, as noted by Amer and Valvano (3), there is an HHH motif in translocase 1 sequences (residues 326 to 328 in the E. coli sequence), found as an HXH motif in WecA sequences (residues 279 and 281 in the E. coli sequence), that is completely absent in the eukaryotic enzymes. Finally, there are 12 further amino acid residues conserved in all prokaryotic sequences but not in eukaryotes. This may account for the selectivity of translocase 1 inhibition by mureidomycin A and liposidomycin B and the lack of selectivity of tunicamycin, which targets translocase 1, WecA, and eukaryotic GlcNAc phosphotransferases (12, 13, 16, 26, 28, 29, 31, 32).
In order to examine whether D115, D116, or D267 is essential for catalysis, we generated three mutant proteins with the site-directed mutations D115N, D116N, and D267N. The mutant proteins were expressed both as native MraY and as MraY-His6 fusion proteins, which in the latter case were partially purified by using a cobalt affinity column. The translocase 1 activity of the mutated MraY proteins was <10% of wild-type activity, with background MraY activity present at 4 to 5%. No catalytic activity could be detected in each of the MraY-His6 mutants by radiochemical assay, and this finding confirmed their lack of catalytic activity. The observation that the D115N mutant showed growth inhibition similar to that of wild-type translocase 1 upon induction suggests that there may have been some residual activity in this mutant: if so, then it was below the detection limit of the assay (approximately 2% that of the wild-type enzyme). The fact that each mutant could be recovered from E. coli membranes suggests that they were correctly folded and inserted into the membrane and were unlikely to have suffered any gross topological defects resulting from the mutagenesis. Our data are therefore consistent with the conclusion that D115, D116, and D267 are each required for the catalytic activity of translocase 1.
In view of the similarity to the DDXXD/N motif of the prenyl transferases, in which the Asp-Asp pair is involved in coordination of Mg2+ which itself is involved with pyrophosphate binding (39, 47), we speculate that D115 and D116 of E. coli MraY may chelate the Mg2+ cofactor, which in turn binds the pyrophosphate bridge of the UDP-MurNAc-pentapeptide. It was hoped that by assaying the D115N or D116N mutant proteins at high MgCl2 concentrations, some residual catalytic activity might be restored, but none was detected. Interestingly, Amer and Valvano (4) recently mutated the corresponding D90 and D91, but unfortunately not D217 (which respectively align with D115, D116, and D267 of translocase 1) to glycine or glutamic acid in E. coli WecA and found that the substitutions DD90/91GG and DD90/91EE result in 30% residual WecA activity. They further reported that WecA with the DD90/91EE mutation shows increased activity at higher concentrations of MgCl2, suggesting that these residues may interact with Mg2+ (4).
A model which is consistent with these data is shown in Fig. 6. In this model, D115 and D116 ligate the Mg2+ cofactor and D267 acts as the catalytic nucleophile (labeled X in Fig. 1). The consequences of mutating D115 and D116 in MraY are more deleterious than those observed upon mutation of D90 and D91 in WecA (4), implying some differences in the Mg2+ binding sites of the two enzymes, which do have different affinities for MgCl2 (optimum activity at 3 mM MgCl2 for WecA [4] and at 25 mM for MraY [13]). Recent studies on the mechanism of action of the inhibitor mureidomycin A indicate that the amino terminus of the antibiotic binds in place of the magnesium cofactor (21, 25); therefore, the characterization of this interaction may be of importance for the design of future translocase 1 inhibitors.
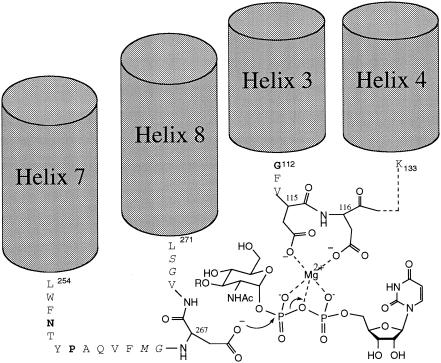
FIG. 6.
Proposed model for active site of MraY. Asp-115 and Asp-116 are proposed to be involved in binding of the pyrophosphate bridge of UDP-MurNAc-pentapeptide-lipid 1, while Asp-267 is proposed to act as the catalytic nucleophile. The positioning of cytoplasmic loop 2 (by helices 3 and 4) and loop 4 (by helices 7 and 8) is indicated. NHAc, NHCOCH3; RO, lactyl-pentapeptide. Bold indicates conserved residues.
Using β-lactamase fusions, Bouhss et al. (10) showed that all of the completely conserved amino acid residues are found on the cytoplasmic loops of translocase 1. Therefore, we suggest that the transmembrane helices of this enzyme act as a scaffold to position the cytoplasmic loops, forming the active site of the enzyme on the cytoplasmic face of the membrane and ensuring the optimal configuration of D115, D116, and D267 for catalysis. This proposal is supported by the observation that the number and predicted positions of transmembrane helices are conserved throughout all of the translocase 1 sequences that we have analyzed in silico (data not shown) and that Bouhss et al. (10) have analyzed in vivo.
An understanding of the structure and function of this enzyme will assist in further elucidation of the roles of these essential amino acids in catalysis and in the exploitation of translocase 1, known to be the molecular target for nucleoside antibiotic natural products (12, 13, 28-30, 32, 37) and for the antibacterial E protein from bacteriophage φX174 (7, 8), as an antibacterial target.
Acknowledgments
We thank the Wellcome Trust (grant 054393), BBSRC (grant B14699), EPSRC, and SmithKline Beecham Pharmaceuticals for financial support.
We also thank R. Southgate, J. Lonsdale, M. Burnham, and M. Black (SKB Pharmaceuticals) for advice, help, and provision of [14C]-UDP-MurNAc-pentapeptide and C. A. Gentle and D. Short for supporting practical work. We gratefully acknowledge the provision of molecular biology facilities by C. G. Dowson (University of Warwick). Finally, we thank D. Boyle (University of Edinburgh) for the pJFY3C expression plasmid and PanTherix Limited for the dye column library.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Amann, E., B. Ochs, and K. J. Abel. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 30:301-315. [DOI] [PubMed] [Google Scholar]
- 3.Amer, A. O., and M. A. Valvano. 2001. Conserved amino acid residues found in a predicted cytosolic domain of the lipopolysaccharide biosynthetic protein WecA are implicated in the recognition of UDP-N-acetyl glucosamine. Microbiology 147:3015-3025. [DOI] [PubMed] [Google Scholar]
- 4.Amer, A. O., and M. A. Valvano. 2002. Conserved aspartic acids are essential for the enzymic activity of the WecA protein initiating the biosynthesis of O-specific lipopolysaccharide and enterobacterial common antigen in Escherichia coli. Microbiology 148:571-582. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, M. S., S. S. Eveland, and N. P. J. Price. 2000. Conserved cytoplasmic motifs that distinguish sub-groups of the polyprenol phosphate:N-acetylhexosamine-1-phosphate transferase family. FEMS Microbiol. Lett. 191:169-175. [DOI] [PubMed] [Google Scholar]
- 6.Barbosa, M. D. F. S., H. O. Ross, M. C. Hillman, R. P. Meade, M. G. Kurilla, and D. L. Pompliano. 2002. A multitarget assay for inhibitors of membrane associated steps of peptidoglycan biosynthesis. Anal. Biochem. 306:17-22. [DOI] [PubMed] [Google Scholar]
- 7.Bernhardt, T. G., W. D. Roof, and R. Young. 2000. Genetic evidence that the bacteriophage φX174 lysis protein inhibits cell wall synthesis. Proc. Natl. Acad. Sci. USA 97:4297-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhardt, T. G., D. K. Struck, and R. Y. Young. 2001. The lysis protein E of φX174 is a specific inhibitor of the MraY-catalyzed step in peptidoglycan synthesis. J. Biol. Chem. 276:6093-6097. [DOI] [PubMed] [Google Scholar]
- 9.Blattner, F. R., G. Plunkett, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 10.Bouhss, A., D. Mengin-Lecreulx, D. Le Beller, and J. van Heijenoort. 1999. Topological analysis of the MraY protein catalysing the first membrane step of peptidoglycan synthesis. Mol. Microbiol. 34:576-585. [DOI] [PubMed] [Google Scholar]
- 11.Boyle, D. S., and W. D. Donachie. 1988. MraY is an essential gene for cell growth in Escherichia coli. J. Bacteriol. 180:6429-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandish, P. E., K.-I. Kimura, M. Inukai, R. Southgate, J. T. Lonsdale, and T. D. H. Bugg. 1996. Modes of action of tunicamycin, liposidomycin B and mureidomycin A: inhibition of phospho-N-acetylmuramyl-pentapeptide translocase from Escherichia coli. Antimicrob. Agents Chemother. 40:1640-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandish, P. E., M. K. Burnham, J. T. Lonsdale, R. Southgate, M. Inukai, and T. D. H. Bugg. 1996. Slow binding inhibition of phospho-N-acetylmuramyl-pentapeptide translocase (Escherichia coli) by mureidomycin A. J. Biol. Chem. 271:7609-7614. [DOI] [PubMed] [Google Scholar]
- 14.Branstrom, A. A., S. Midha, C. B. Longley, K. Han, E. R. Baizman, and H. R. Axelrod. 2000. Assay for identification of inhibitors for bacterial MraY translocase or MurG transferase. Anal. Biochem. 280:315-319. [DOI] [PubMed] [Google Scholar]
- 15.Bugg, T. D. H. 1999. Bacterial peptidoglycan biosynthesis and its inhibition, p. 241-294. In M. Pinto (ed.), Comprehensive natural products chemistry, vol. 3. Elsevier, Oxford, United Kingdom. [Google Scholar]
- 16.Bugg, T. D. H., and P. E. Brandish. 1994. From peptidoglycan to glycoproteins: common features of lipid-linked oligosaccharide biosynthesis. FEMS Microbiol. Lett. 119:255-262. [DOI] [PubMed] [Google Scholar]
- 17.Child, C. J., J. B. Spencer, P. Bhogal, and P. M. Shoolingin-Jordan. 1996. Structural similarities between 6-methylsalicylic acid synthase from Penicillium patulum and vertebrate type I fatty acid synthase: evidence from thiol modification studies. Biochemistry 35:12267-12274. [DOI] [PubMed] [Google Scholar]
- 18.Dean, P. D. G., and D. H. Watson. 1979. Protein purification using immobilised triazine dyes. J. Chromatogr. 165:301-319. [DOI] [PubMed] [Google Scholar]
- 19.Dunbar, B., and S. B. Wilson. 1994. A buffer exchange procedure giving enhanced resolution to polyacrylamide gels pre-run for protein sequencing. Anal. Biochem. 216:227-228. [DOI] [PubMed] [Google Scholar]
- 20.Fürste, J. P., W. Pansegrau, R. Frank, H. Blöcker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 21.Gentle, C. A., S. A. Harrison, M. Inukai, and T. D. H. Bugg. 1999. Structure-function studies on nucleoside antibiotic mureidomycin A: synthesis of 5′-functionalised uridine models. J. Chem. Soc. Perkin Trans. I 10:1287-1294. [Google Scholar]
- 22.Henderson, P. J. F., C. K. Hoyle, and A. Ward. 2000. Expression, purification and properties of multidrug efflux proteins. Biochem. Soc. Trans. 28:513-517. [PubMed] [Google Scholar]
- 23.Heydanek, M. G., W. G. Struve, and F. C. Neuhaus. 1969. On the initial stages of peptidoglycan synthesis. III. Kinetics and uncoupling of phospho-N-acetylmuramyl-pentapeptide translocase (uridine 5′-phosphate). Biochemistry 8:1214-1221. [DOI] [PubMed] [Google Scholar]
- 24.Higgins, D., J. Thompson, T. Gibson, J. D. Thompson, D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard, N. I., and T. D. H. Bugg. 2003. Synthesis and activity of 5′-uridinyl dipeptide analogues mimicking the amino terminal peptide chain of nucleoside antibiotic mureidomycin A. Bioorg. Med. Chem. 11:3083-3099. [DOI] [PubMed] [Google Scholar]
- 26.Hyland, S. A., and M. S. Anderson. 2003. A high-throughput solid phase extraction assay capable of measuring polyprenol phosphate: sugar-1-phosphate transferases as exemplified by WecA, MraY and MurG proteins. Anal. Biochem. 317:156-164. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda, M., M. Wachi, H. K. Jung, F. Ishino, and M. Matsuhashi. 1991. The Escherichia coli mraY gene encoding undecaprenyl-phosphate phospho-N-acetylmuramoyl-pentapeptide translocase. J. Bacteriol. 173:1021-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inukai, M., F. Isono, and A. Takatsuki. 1993. Selective inhibition of the bacterial translocase reaction in peptidoglycan synthesis by mureidomycins. Antimicrob. Agents Chemother. 37:980-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isono, F., and M. Inukai. 1991. Mureidomycin A, a new inhibitor of bacterial peptidoglycan synthesis. Antimicrob. Agents Chemother. 35:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isono, F., T. Katayama, M. Inukai, and T. Haneishi. 1989. Mureidomycins A-D, novel nucleoside antibiotics with sphaeroplast forming activity. III. Biological properties. J. Antibiot. 42:674-679. [DOI] [PubMed] [Google Scholar]
- 31.Kimura, K., N. Miyata, G. Kawanishi, Y. Kamio, K. Izaki, and K. Isono. 1989. Liposidomycin C inhibits phospo-N-acetylmuramylpentapeptide transferase in peptidoglycan synthesis of Escherichia coli Y-10. Agric. Biol. Chem. 53:1811-1815. [Google Scholar]
- 32.Kimura, K. I., and T. D. H. Bugg. 2003. Recent advances in antimicrobial nucleoside antibiotics targeting cell wall biosynthesis. Nat. Prod. Rep. 20:252-273. [DOI] [PubMed] [Google Scholar]
- 33.Kunkel, T. A. 1985. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. USA 82:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 35.LeGendre, N., and P. Matsudaria. 1988. Direct protein microsequencing from Immobilon-P transfer membrane. BioTechniques 6:154-159. [PubMed] [Google Scholar]
- 36.Lehrman, M. A. 1994. A family of UDP-GlcNAc/MurNAc:polyisoprenol-P GlcNAc/MurNAc-1-P transferases. Glycobiology 4:768-771. [DOI] [PubMed] [Google Scholar]
- 37.Lin, Y.-I., L. Zhong, G. D. Francisco, L. A. McDonald, R. A. Davis, G. Singh, Y. Yang, and T. S. Mansour. 2002. Muramycins, novel peptidoglycan biosynthesis inhibitors: semisynthesis and SAR of their derivatives. Bioorg. Med. Chem. Lett. 12:2341-2344. [DOI] [PubMed] [Google Scholar]
- 38.Lugtenberg, E. J., L. De Haas-Menger, and W. H. Ruyters. 1972. Murein synthesis and identification of cell wall precursors of temperature-sensitive lysis mutants of Escherichia coli. J. Bacteriol. 109:326-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrero, P. F., C. D. Poulter, and P. A. Edwards. 1992. Effects of site-directed mutagenesis of the highly conserved aspartate residues in domain II of farnesyl diphosphate synthase activity. J. Biol. Chem. 267:21873-21878. [PubMed] [Google Scholar]
- 40.Miroux, B., and J. E. Walker. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260:289-298. [DOI] [PubMed] [Google Scholar]
- 41.Mondego, J. M. C., J. L. Simoes-Araujo, D. E. de Oliveira, and M. Alves-Ferreira. 2003. A gene similar to bacterial translocase 1 (mraY) identified by cDNA-AFLP is expressed during flower bud development of Arabidopsis thaliana. Plant Science 164:323-331. [Google Scholar]
- 42.Morrissey, J. H. 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307-310. [DOI] [PubMed] [Google Scholar]
- 43.Pearson, H. 2002. “Superbug” hurdles key drug barrier. Nature 418:469. [DOI] [PubMed] [Google Scholar]
- 44.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 45.Struve, W. G., R. K. Sinha, and F. C. Neuhaus. 1966. On the initial stage in peptidoglycan synthesis: phospho-N-acetylmuramyl-pentapeptide translocase (uridine monophosphate). Biochemistry 5:82-93. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka, H., R. Oiwa, S. Matsukura, and S. Omura. 1979. Amphomycin inhibits phospho-N-acetylmuramyl-pentapeptide translocase in peptidoglycan synthesis of Bacillus. Biochem. Biophys. Res. Commun. 86:906-908. [DOI] [PubMed] [Google Scholar]
- 47.Tarshis, L. C., M. Yan, C. D. Poulter, and J. C. Sacchettini. 1994. Crystal structure of recombinant farnesyl diphosphate synthase at 2.6-Å resolution. Biochemistry 33:10871-10877. [DOI] [PubMed] [Google Scholar]
- 48.Thanassi, J. A., S. L. Hartmann-Neumann, T. J. Dougherty, B. A. Dougherty, and M. J. Pucci. 2002. Identification of 113 conserved essential genes using a high throughput gene disruption system in Streptococcus pneumoniae. Nucleic Acids Res. 30:3152-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Heijenoort, J. 2001. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat. Prod. Rep. 18:503-519. [DOI] [PubMed] [Google Scholar]
- 50.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 51.Zawadzke, L. E., P. Wu, L. Cook, L. Fan, M. Casperson, M. Kishnani, D. Calambur, S. J. Hofstead, and R. Padmanabha. 2003. Targetting the MraY and MurG bacterial enzymes for antimicrobial therapeutic intervention. Anal. Biochem. 314:243-252. [DOI] [PubMed] [Google Scholar]