Abstract
The interaction between S-layer protein SbsB and the secondary cell wall polymer (SCWP) of Geobacillus stearothermophilus PV72/p2 was investigated by real-time surface plasmon resonance biosensor technology. The SCWP is an acidic polysaccharide that contains N-acetylglucosamine, N-acetylmannosamine, and pyruvic acid. For interaction studies, recombinant SbsB (rSbsB) and two truncated forms consisting of either the S-layer-like homology (SLH) domain (3SLH) or the residual part of SbsB were used. Independent of the setup, the data showed that the SLH domain was exclusively responsible for SCWP binding. The interaction was found to be highly specific, since neither the peptidoglycan nor SCWPs from other organisms nor other polysaccharides were recognized. Data analysis from that setup in which 3SLH was immobilized on a sensor chip and SCWP represented the soluble analyte was done in accordance with a model that describes binding of a bivalent analyte to a fixed ligand in terms of an overall affinity for all binding sites. The measured data revealed the presence of at least two binding sites on a single SCWP molecule with a distance of about 14 nm and an overall Kd of 7.7 × 10−7 M. Analysis of data from the inverted setup in which the SCWP was immobilized on a sensor chip was done in accordance with an extension of the heterogeneous-ligand model, which indicated the existence of three binding sites with low (Kd = 2.6 × 10−5 M), medium (Kd = 6.1 × 10−8 M), and high (Kd = 6.7 × 10−11 M) affinities. Since in this setup 3SLH was the soluble analyte and the presence of small amounts of oligomers in even monomeric protein solutions cannot be excluded, the high-affinity binding site may result from avidity effects caused by binding of at least dimeric 3SLH. Solution competition assays performed with both setups confirmed the specificity of the protein-carbohydrate interaction investigated.
In many bacteria and archaea, crystalline cell surface layers (S-layers) represent the outermost cell envelope component (37, 42). S-layers are composed of identical species of protein or glycoprotein subunits that are arranged in monomolecular crystalline arrays and cover the entire cell surface during all stages of cell growth and division. S-layer lattices show oblique (p1, p2), square (p4), or hexagonal (p3, p6) symmetry and possess pores of uniform size and morphology. Individual S-layer subunits interact with each other and with the supporting cell envelope components through noncovalent forces. After removal of the disrupting agent used for their isolation, extracted S-layer (glyco)proteins frequently maintain the ability to recrystallize in suspension or on various phase interfaces. This first-order self-assembly process leads to lattices that are identical to those observed on intact cells (33, 36, 41).
On the basis of sequence comparisons, the existence of a cell wall-targeting domain on surface proteins, including S-layer proteins and cell-associated exoenzymes of gram-positive bacteria, or outer membrane proteins of gram-negative bacteria has been suggested (7, 22). Modules of ∼55 amino acids containing 10 to 15 conserved residues were identified and referred to as S-layer-like homology (SLH) motifs. An SLH domain can be composed of either a single SLH motif or three repeating SLH motifs, which occur at the very beginning or at the very end of the mature protein (7, 21). Affinity studies revealed that SLH domains mediate binding of S-layer proteins, as well as cell-associated exoenzymes and exoproteins, to the rigid cell wall layer in gram-positive bacteria (2, 3, 5, 9, 12, 18, 24). In S-layer proteins, typically three SLH motifs were found in the amino-terminal region and there is strong evidence that secondary cell wall polymers (SCWPs) rather than peptidoglycan itself serve as anchoring structures for SLH domains (2, 3, 5, 12, 18, 24, 25, 34, 39).
In the present report, the nature of the basic interaction in the mechanism anchoring an S-layer protein to the rigid cell wall layer was systematically investigated by real-time surface plasmon resonance (SPR) biosensor technology. For that purpose, S-layer protein SbsB and the corresponding SCWP of Geobacillus stearothermophilus PV72/p2 (formerly Bacillus stearothermophilus PV72/p2) (29), an oxygen-induced variant strain of G. stearothermophilus PV72/p6 (38), were used as a model system. After cleavage of a 31-amino-acid-long signal peptide from the preprotein, mature SbsB is an 889-amino-acid-long polypeptide (equivalent to residues 32 to 920 of the preprotein sequence) with a theoretical relative molecular weight (Mr) of 94,745.5. SbsB carries three typical SLH motifs on its N-terminal part and self-assembles into an oblique lattice with p1 symmetry and the lattice constants a = 10.4 nm, b = 7.9 nm, and γ = 81° (30). The SCWP recognized by SbsB is an acidic polymer that is mainly composed of N-acetylglucosamine and N-acetylmannosamine in a molar ratio of 2:1 (39) and contains pyruvic acid residues. By gel permeation chromatography (GPC), the Mr of SCWP was estimated to be about 2.4 × 104 (34). The polymer chains are covalently linked to the peptidoglycan backbone.
To gain insight into the basic interaction between an S-layer protein and an SCWP, recombinant SbsB (rSbsB), two complementary truncated versions thereof, and isolated native and chemically modified SCWP were used for SPR biosensor measurements. The two truncated forms of SbsB were 3SLH, the SLH domain of the mature SbsB itself corresponding to the three SLH motifs (residues 32 to 209 of the preprotein sequence), and Δ3SLH-SbsB, an SbsB truncation missing the three SLH motifs (residues 208 to 920 of the preprotein sequence). Both complementary experimental setups in which either the rSbsB forms or the SCWP was immobilized on a sensor surface were investigated.
MATERIALS AND METHODS
Preparation of rSbsB, 3SLH, and Δ3SLH-SbsB.
For production of rSbsB and its truncated forms 3SLH and Δ3SLH-SbsB, expression vectors were constructed by inserting the appropriate PCR fragments into the vector pET-28a(+) (Novagen). Chromosomal DNA of G. stearothermophilus PV72/p2, which was grown in continuous culture (34), was prepared with a genomic DNA isolation kit (QIAGEN) and used as a template for PCR. Restriction sites flanking the gene fragments were generated by using PCR primers with 5′ extensions (forward primers, NcoI; reverse primers, BamHI or XhoI). The following oligonucleotides were used as PCR primers (restriction sites are italicized, start and reverse complementary stop codons are underlined, and complementary regions are in boldface type) with F2 and R1 for the production of rSbsB, F3 and R1 for the production of Δ3SLH-SbsB, and F2 and R3SLH for the production of 3SLH: F2, 5′-CGGAATTCCATGGCAAGCTTCACAGATGTTGC-3′; F3, 5′-CGGAATTCCATGGAAGTAACTGCGGTTAATTCG-3′; R1, 5′-GCGCGGATCCTTATTTTGTCACAGTCACATTGAC-3′; R3SLH, 5′-GACCGCTCGAGTTATACTTCAACTATTTCTGGCACTG-3′.
All PCR fragments were amplified with proofreading Pwo DNA polymerase (Roche). PCRs and cloning steps were performed in accordance with standard procedures (35). Gene expression was carried out as described in the pET System Manual (Novagen) by using Escherichia coli strain BL21(DE3) for rSbsB and Δ3SLH-SbsB and E. coli HMS174(DE3) for 3SLH-carrying expression vectors. The recombinant proteins were isolated from the host cells and purified as previously described (14). The 3SLH that accumulated in the soluble fraction of the lysed E. coli cells was precipitated with ammonium sulfate (45 to 65% of saturation). After centrifugation at 17,700 × g for 30 min at 4°C, the pellet was dissolved in aqua purificata (A. purif.; Milli-Q grade; resistance, >18.2 MΩ/cm) and dialyzed against A. purif. for 1 h and for 2 h against 50 mM Tris/HCl buffer (pH 7.2) containing 150 mM NaCl. 3SLH was then purified by GPC with a Superdex 200 column (Amersham Biosciences). Fractions from the peak eluting with the expected Mr for 3SLH were combined and dialyzed against A. purif. at 4°C for 18 h. The purity of the recombinant proteins was checked by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis. Electron microscopic investigations were performed as described previously (14).
Isolation of SCWP and chemical modifications.
G. stearothermophilus PV72/p2 was grown, peptidoglycan-containing sacculi were prepared, and SCWP was isolated therefrom in accordance with published procedures (34, 39). For comparison, SCWPs of B. sphaericus CCM 2177 and G. stearothermophilus PV72/p6, which were obtained as previously reported (6, 12), as well as dextran (Mr, ∼104; Sigma), carboxymethyl dextran sodium salt (CM-dextran) (Mr, ∼1.2 × 104; Fluka), dextran sulfate sodium salt (Mr, ∼104; Sigma), and heparin sodium salt (from porcine intestinal mucosa; Mr, ∼6,000; Sigma) were used.
Weak acid hydrolysis of SCWP was performed with 1% acetic acid under nitrogen at a carbohydrate concentration of 1 mg/ml for 60 or 90 min at 110°C. The product of weak acid hydrolysis for 60 min was further N acetylated at a carbohydrate concentration of 5 mg/ml in 0.8 M sodium bicarbonate by reaction with 4-μl portions of acetic anhydride per ml (20 in total) for 5 h at 0°C. During the whole procedure, the pH was kept constant at a value of 8 by titration with 4 M sodium hydroxide solution. The presence of pyruvic acid was assayed as described in reference 25.
For covalent immobilization on a sensor chip, pyridyl disulfide-activated SCWP was prepared by introduction of carbohydrazide at its reducing end, followed by reaction with sulfosuccinimidyl 6-[3′-(2-pyridyldithio)-propionamido]hexanoate (Sulfo-LC-SPDP). For that purpose, 5 mg of SCWP was dissolved per ml of aqueous carbohydrazide (Fluka) solution (saturated at room temperature and adjusted to pH 6.2 with HCl) and heated for 15 min at 100°C. Two milligrams of sodium cyanoborohydride (Sigma) per ml was then added, and the mixture was incubated for 16 h at 90°C. After removal of reagents by extensive dialysis against A. purif., the solution was lyophilized and 10 mg of this product was reacted with 10 mg of Sulfo-LC-SPDP (Pierce) in 1 ml of 100 mM sodium phosphate buffer (pH 7.5) for 2 h at room temperature. The sediment formed was removed by centrifugation (16,000 × g, 5 min, 20°C), and the supernatant was dialyzed against A. purif. After lyophilization, pyridyl disulfide-activated SCWP was obtained.
SPR experiments.
SPR biosensor experiments were performed on a BIACORE 2000 instrument with research grade CM5 sensor chips, both from Biacore International AB. For all measurements and immobilization procedures, the temperature was set to 25°C. The running buffer was HEPES-buffered saline (HBS), which contained 150 mM NaCl, 10 mM HEPES (pH 7.2), 3 mM EDTA, 0.005% Tween 20, and 0.01% sodium azide. The latter was omitted during the immobilization procedures. If not otherwise indicated, the buffer flow rate was 10 μl/min. Experiments were generally performed in randomized order by passing pulses of 250 μl of sample solution over the active and reference flow cells. Surfaces were regenerated by 2-min pulses of 7 M guanidine hydrochloride (GHCl) in 50 mM Tris/HCl buffer (pH 7.4).
Preparation of sensor surfaces.
For immobilization of rSbsB or its truncated forms on the carboxymethylated dextran matrix of the CM5 sensor chip, the standard amine coupling method was used in accordance with the manufacturer's protocol. Optimized conditions for activation and immobilization, protein concentrations, buffer conditions for coupling of the samples to distinct flow cells, and the immobilization levels obtained are given in Table 1. Briefly, a solution of 0.2 M 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDC; Sigma) containing 0.05 M N-hydroxysuccinimide (Fluka) and a solution of the corresponding protein were sequentially passed over the surface for the indicated times at a flow rate of 5 μl/min, followed by a 7-min pulse of 1 M ethanolamine/HCl (pH 8.5).
TABLE 1.
Optimized conditions for immobilization of sample proteins on CM5 sensor chips by the amine coupling method
| Protein | Concn (μM) | Buffer | Activation time (min) | Immobilization time (min) | Immobilized amta (RU) |
|---|---|---|---|---|---|
| rSbsB | 3 | 10 mM acetate, pH 4.5 | 1 | 5 | 1,957 ± 553 (3) |
| rSbsB | 3 | 10 mM acetate, pH 4.5 | 5 | 5 | 6,184 ± 1,321 (4) |
| 3SLH | 20 | 5 mM acetate, pH 6.0 | 5 | 5 | 621 ± 16 (2) |
| Δ3SLH-SbsB | 3 | 10 mM acetate, pH 4.5 | 1 | 5 | 3,009 ± 650 (2) |
Immobilized amount remaining after five regeneration cycles with 7 M GHCl [mean ± SD (number of immobilizations)].
Immobilization of SCWP on a CM5 sensor chip was carried out by using the manufacturer's surface thiol method with pyridyl disulfide-activated SCWP. The corresponding injection sequence was an N-hydroxysuccinimide-EDC mixture (0.05 and 0.2 M, respectively) for 3 min, a cystamine dihydrochloride solution (100 mM in 100 mM sodium borate buffer, pH 8.5) for 5 min, a 1 M ethanolamine-HCl solution (pH 8.5) for 7 min, a 1,4-dithio-d,l-threitol solution (100 mM in 100 mM sodium borate buffer, pH 8.5) for 5 min, pyridyl disulfide-activated SCWP (2.5 mg/ml of HBS) for 3 (low-density surface) or 60 (high-density surface) min, and a 2-(2-pyridinyldithio)ethaneamine hydrochloride (Biacore International AB)-NaCl solution (20 mM in 100 mM sodium formiate buffer, pH 4.3, containing 1 M NaCl) for 4 min.
To generate reference surfaces, blank immobilizations in accordance with both procedures were carried out as described above, except that instead of solutions containing the ligands to couple, only the corresponding buffers were injected. A further control flow cell was prepared with SCWP of B. sphaericus CCM 2177, which had been activated and coupled analogously. After finishing the immobilizations, five regeneration steps with 7 M GHCl were performed to ensure removal of noncovalently bound ligands.
SPR analysis—binding experiments.
All protein samples except 3SLH were freshly prepared by dissolving the protein in GHCl solution (5 M in 50 mM Tris/HCl buffer, pH 7.4) and dialyzing the solution against A. purif. for 2 h. Formed self-assembly products were removed by centrifugation (16,000 × g, 5 min, 20°C), and the protein concentration in the clear supernatant, which, according to reference 13, contained monomers and oligomers, was determined by UV absorbance at 280 nm with sequence-derived extinction coefficients. With the exception of pH series, all protein solutions were diluted 9:10 with 10×-concentrated running buffer. All carbohydrate-containing sample solutions were prepared from products of lyophilization. Dilutions were done with running buffer.
Direct binding was assayed with exponential dilution series to the base 2 from 14 μM to 6.7 pM for rSbsB and Δ3SLH-SbsB, from 100 μM to 11.9 pM for 3SLH, and from 250 μg/ml to 1.9 ng/ml for SCWP. In competition experiments, exponential dilution series of 7.5 μM to 14.3 pM rSbsB or 3SLH containing SCWP at a constant 1 μg/ml or at 5 mg/ml to 9.3 pg/ml and 3 nM rSbsB or 3SLH were preincubated for 1 h and injected over surfaces with immobilized rSbsB and SCWP, respectively. For measurement of pH series, an aqueous rSbsB solution (10 nM) was diluted 1:2 with 2×-concentrated Britton-Robinson universal buffer I (4) at pHs 4.0 to 10.0 in pH increments of 0.5 immediately before injection. Experiments were performed by injecting universal buffer for 10 min into a Biacore 2000 instrument, directly followed by rSbsB solution in the corresponding buffer, for 25 min with the instrument's “coinject” command.
In the present study, the response at the time relative to the start of injection was always reported as the difference between the signals arising from the active and reference cells, which—if necessary—was additionally corrected for baseline drift and bulk refractive index contribution. Therefore, calibration was done with dilutions of 2×-concentrated HBS in A. purif. and 2% CM-dextran solution in HBS, respectively, analogously to the procedure described in references 8 and 10.
Data analysis.
Sensorgrams recorded in direct binding assays at a series of analyte concentrations were arranged to represent response surfaces in the time-concentration-response space to present them more clearly and simplify global analysis. Generally, the first 15 s of the wash-on and washout phases were omitted from the fits to avoid effects of sample dispersion (16). The “heterogeneous ligand-parallel reactions” model implemented in BIAevaluation software version 3.1 (Biacore International AB) was extended to analogously handle three parallel reactions, and all other approaches were modeled and tested by nonlinear least-squares curve fitting with Mathematica version 4.0 for Microsoft Windows (Wolfram Research, Inc.). In solution competition experiments, initial rates were used to derive the free analyte concentrations and were calculated as slopes (at t = 0 s) of linear or exponential [R = a · (1 − e−b · t) + c] fits to the sensorgrams at the early injection phases.
RESULTS
Expression of the genes encoding S-layer protein rSbsB and the truncated forms Δ3SLH-SbsB and 3SLH in E. coli.
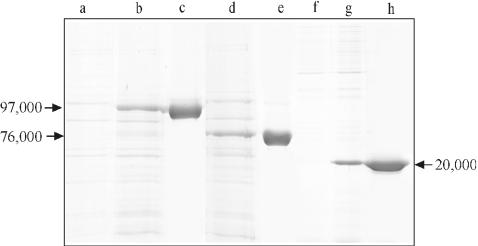
The plasmids carrying the PCR products encoding rSbsB or Δ3SLH-SbsB were established in E. coli BL21(DE3). After induction of expression by addition of isopropyl-β-d-thiogalactopyranoside (IPTG), additional high-molecular-mass protein bands were observed on SDS gels (Fig. 1). In the case of the entire sbsB gene, the estimated Mr of the band of the protein it encodes was 97,000 (Fig. 1, lane b) and corresponded exactly to the Mr of S-layer protein SbsB isolated from G. stearothermophilus PV72/p2 (38). When cultures of E. coli BL21(DE3) carrying the gene encoding Δ3SLH-SbsB were induced with IPTG, a pronounced protein band with an apparent Mr of 76,000 was detected on SDS gels (Fig. 1, lane d). This value was in accordance with the theoretical Mr of Δ3SLH-SbsB of 75,540. The plasmid carrying the PCR product encoding 3SLH was established in E. coli HMS 174(DE3). After induction of expression with IPTG, an additional protein band with an apparent Mr of 20,000 became visible on SDS gels (Fig. 1, lane g), which was close to the theoretical value of 19,715. On immunoblots, a strong cross-reaction was observed between these protein bands and the polyclonal rabbit antiserum raised against the S-layer protein of G. stearothermophilus PV72/p2 (data not shown). The final identification of rSbsB, Δ3SLH-SbsB, and 3SLH was accomplished by N-terminal sequencing, which led to ASFTD, EVTAV, and ASFTD, respectively. The apparent Mr on SDS gels and the results of immunoblotting and N-terminal sequencing confirmed the identities of the three different rSbsB forms.
FIG. 1.
SDS-PAGE analysis of SDS extracts from whole cells of E. coli BL21(DE3) before (lane a) and 5 h after (lanes b and d) induction of expression of the genes encoding rSbsB (lane b) and Δ3SLH-SbsB (lane d). In lanes f and g, SDS extracts from whole cells of E. coli HMS174(DE3) before (lane f) and 5 h after (lane g) induction of expression of the gene encoding 3SLH are shown. Lanes c, e, and h show purified rSbsB (c), Δ3SLH-SbsB (e), and 3SLH (h).
Investigation of the self-assembly properties of rSbsB, Δ3SLH-SbsB, and 3SLH.
To investigate whether rSbsB, Δ3SLH-SbsB, and 3SLH had assembled in the cytoplasm of the host cells, whole cells of E. coli BL21(DE3) and HMS174(DE3) carrying the constructs were fixed and embedded in Spurr resin and ultrathin sections were evaluated by transmission electron microscopy. In contrast to 3SLH, rSbsB and Δ3SLH-SbsB formed sheet-like structures in the cytoplasm of the expression host, which indicated the formation of self-assembly products (17, 26; data not shown). These findings were confirmed by SDS-PAGE analysis of samples collected during the isolation procedure, which revealed that rSbsB and Δ3SLH-SbsB had accumulated in the insoluble fraction of the lysed E. coli cells, whereas 3SLH was exclusively detected in the soluble fraction. After precipitation with ammonium sulfate, 3SLH was dissolved in A. purif., dialyzed against A. purif. and Tris/HCl buffer, and purified by GPC. Its elution as a single peak at the expected Mr of ∼20,000 indicated that 3SLH was monomeric. rSbsB and Δ3SLH-SbsB were extracted with 5 M GHCl from the insoluble fraction of the lysed host cells and purified by GPC under denaturing conditions. On SDS gels, each purified protein migrated as a single protein band with the expected Mr (Fig. 1, lanes c, e, and h).
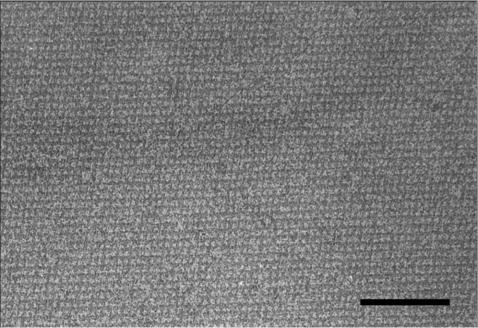
The ability to form self-assembly products was investigated by denaturing purified rSbsB and Δ3SLH-SbsB in 5 M GHCl and subsequent dialysis against A. purif. or solutions of monovalent or bivalent cations. As shown by negative staining, rSbsB and Δ3SLH-SbsB self-assembled into flat sheets exhibiting the oblique lattice with the lattice constants a = 10.4 nm, b = 7.9 nm, and γ = 81° (26, 30) (Fig. 2 and data not shown). These results confirmed data from a previous study showing that the SLH domain is not required for the self-assembly process (11).
FIG. 2.
Electron micrograph of a negatively stained preparation of self-assembly products formed by rSbsB. Bar, 100 nm.
Immobilization levels on sensor surfaces.
SCWP of G. stearothermophilus PV72/p2 was coupled to the cystamine-modified CM5 sensor surface as pyridyl disulfide-activated derivate by the surface thiol method, which led to 18 and 83 resonance units (RU) for low- and high-density surfaces, respectively. Immobilization of rSbsB, 3SLH, and Δ3SLH-SbsB on the CM-dextran layer of the CM5 sensor surface by the amine coupling method led to the levels shown in Table 1, which were obtained after five treatments with 7 M GHCl. In contrast to 3SLH, which was monomeric according to GPC, rSbsB and Δ3SLH-SbsB were capable of self-assembling into sheet-like structures (Fig. 2 and data not shown). For immobilization on a sensor surface and for interaction studies, solutions containing monomers and oligomers had to be prepared. For that purpose, dialysis of GHCl-extracted rSbsB and Δ3SLH-SbsB was interrupted at a distinct time point and self-assembly products were removed by centrifugation. The clear supernatants, which contained monomers and oligomers (13), were diluted to the desired protein concentrations and used in all further experiments.
Estimation of the Mr of SCWP.
The Mr of 2.4 × 104 published for the SCWP (34) was based on GPC. Since this method tends to overestimate the real Mrs of carbohydrate molecules containing pyruvic acid residues, the Mr was deduced from the response levels equivalent to the amount of rSbsB immobilized on the CM5 surface [Rimmob(rSbsB)] on the one hand and of the maximum binding capacity of this attached ligand for SCWP [Rmax(SCWP)] on the other hand. By applying high concentrations of SCWP, each binding site on the immobilized rSbsB can be expected to be saturated with one SCWP molecule. The following equation, which is an adapted form of the relationship given in reference 23, was used: Mr(SCWP) = Mr(SbsB) · GP · Rmax(SCWP)/[GC · Rimmob(rSbsB)]. Mr(SCWP) and Mr(rSbsB) denote the Mrs of SCWP and rSbsB, respectively. The proportionality factor 1,000 RU × mm2/ng (GP) or 833 RU × mm2/ng (GC) for proteins or carbohydrates, respectively, was derived from the refractive-index increments given in reference 31. An Mr of 10,338 ± 1,548 (mean ± standard deviation [SD]) for SCWP was calculated from five independent experiments and used in the subsequent analysis.
Specificity of carbohydrate recognition by rSbsB.
To investigate the specificity of the S-layer protein-SCWP interaction, various carbohydrate samples were passed over a sensor surface on which rSbsB had been immobilized. The compounds tested were glucose polymers with or without a negative charge (dextran, CM-dextran, dextran sulfate), the polyanionic glucosamine-containing glycan heparin, the SCWPs from B. sphaericus CCM 2177 and G. stearothermophilus PV72/p6 (6, 12), and the peptidoglycan and SCWP from G. stearothermophilus PV72/p2. Of all of the samples tested, only the SCWP from G. stearothermophilus PV72/p2 showed specific binding to rSbsB (Fig. 3 and data not shown). Furthermore, the SCWP from G. stearothermophilus PV72/p2 was investigated in carbohydrazide-modified form and after weak acid hydrolysis for 60 and 90 min, respectively, as well as after hydrolysis for 60 min and re-N-acetylation of amino sugars. From GPC, it was obvious that all of these modifications did not essentially alter the retention volume of the main peak, indicating that the Mr had not significantly changed. The results shown in Fig. 3 demonstrate that in comparison with the native SCWP (a), carbohydrazide modification of the reducing end (b) led to only a small decrease in binding activity. On the other hand, weak acid hydrolysis for 60 min at 110°C, which removed >90% of the N-acetyl residues but only about 50% of the pyruvic acid residues, caused a significant decrease in binding activity (c). N acetylation of such hydrolyzed samples had no influence on the binding characteristics observed (e), which led to the conclusion that the N-acetyl groups on the amino sugars are not required for the binding process. Samples subjected to weak acid hydrolysis for 90 min, which had lost the N-acetyl group and more than 90% of the pyruvic acid residues, did not bind any more (d). Together with the data from weak acid hydrolysis for 60 min and N acetylation, it became apparent that not the N-acetyl groups from the amino sugars but the pyruvic acid residues play a crucial role in the binding process.
FIG. 3.
Overlaid sensorgrams demonstrating the interaction of differently modified SCWP samples with rSbsB immobilized on a sensor chip. The SCWP was native (a), carbohydrazide modified (b), weak acid hydrolyzed for 60 (c) or 90 (d) min, or weak acid hydrolyzed for 60 min and N acetylated (e). The injection pulses lasted 0 to 1,500 s and are indicated by the gray bar at the abscissa of the plot. Responses are given relative to that of native SCWP. The injected concentration was 2 μg/ml in each case.
Direct measurement of SCWP binding to immobilized rSbsB, 3SLH, and Δ3SLH-SbsB.
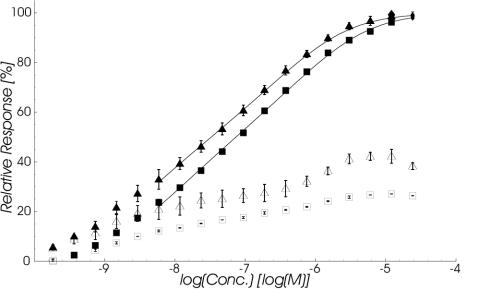
In the setup used for direct measurement of SCWP binding to immobilized rSbsB, 3SLH, and Δ3SLH-SbsB, exponential dilution series of SCWP solutions were injected at two different flow rates over sensor surfaces with immobilized rSbsB, 3SLH, or Δ3SLH-SbsB. Flow cells with blank immobilizations were used as a reference. As shown in Fig. 4, the SCWP specifically interacted with immobilized rSbsB and 3SLH in a concentration-dependent manner. In contrast, injections of SCWP over the Δ3SLH-SbsB surface resulted in no binding at any concentration (data not shown). Mean response surfaces for the interaction of SCWP with rSbsB and 3SLH measured at a flow rate of 10 μl/min are shown together with the SDs in Fig. 5.
FIG. 4.
Equilibrium binding isotherms for SCWP interacting with 3SLH (squares) or rSbsB (triangles) immobilized on a sensor surface as measured from the response levels at the end of the 25-min injection pulse (filled symbols) relative to saturation levels. Additionally, the corresponding values at the end of the 25-min washout phase are depicted (open symbols). Data were recorded at a flow rate of 10 μl/min and represent the mean ± SD of duplicate measurements on a single surface for 3SLH and on three surfaces with similar immobilization densities for rSbsB. The lines through the data points represent nonlinear least-square fits based on an avidity model for dimeric ligand binding to monomeric receptors (32).
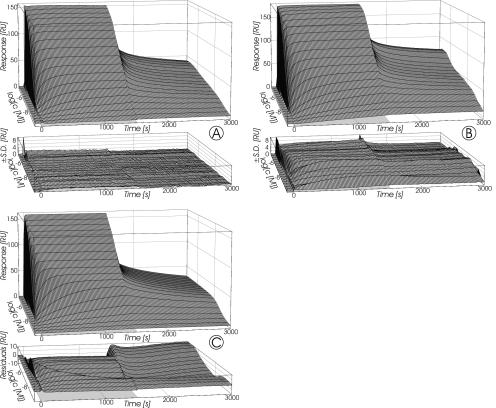
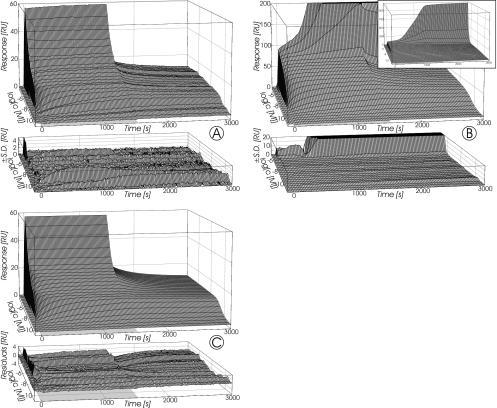
FIG. 5.
Response surfaces recorded for SCWP binding to immobilized 3SLH (A) and rSbsB (B) at a flow rate of 10 μl/min. The injection pulses lasted 0 to 1,500 s and are indicated by the light gray plane at the base of each plot. Grid lines were plotted at each injected concentration and every 20 s, respectively. Data represent the mean ± SD of duplicate measurements on a single surface for 3SLH and on three surfaces with similar immobilization densities for rSbsB. Panel C depicts the simulated response surface and corresponding residuals resulting from fitting of the model for bivalent binding to fixed ligands (27) to the data for SCWP binding to immobilized 3SLH (A).
The shape of the response surfaces in Fig. 5 shows that the interactions between immobilized 3SLH (A) or rSbsB (B) and soluble SCWP did not follow a simple Langmuir model. Furthermore, saturation of the immobilized proteins lasted over a concentration range of about 5 orders of magnitude (Fig. 4) and therefore Scatchard analysis (40) of the equilibrium responses also failed. Since natural polysaccharides, including SCWPs (12), are modularly structured, it is obvious that a model assuming 1:1 stoichiometry could not describe the data satisfactorily.
To analyze the data, we used the model of Müller et al. (27), which describes binding of bivalent analytes possessing only one kind of binding site to fixed ligands in terms of the intrinsic affinity of a single binding site. By coupling molecules to the CM-dextran matrix, they are fixed in space and thereby split into different populations. Ligands that are far apart from each other can only react monovalently, while others that are in close proximity can be bound simultaneously by a single analyte molecule. Binding of a bivalent analyte to a ligand able to participate in such a ternary complex is modeled as occurring in two steps, the second of which is driven by the local concentration of neighboring ligands. The contribution of bivalent binding is further quantified from the local distribution of the immobilized reaction partner in terms of the distance of two identical binding sites in the analyte molecule (its access radius [ra]) and is theoretically derived from stochastic considerations. The model of Müller et al. (27) was implemented in a way that the intrinsic kinetic rate constants for association (ka) and dissociation (kd), the ligand density—expressed as maximal response at saturation of ligand binding sites (Rmax)—and the ra were fitted globally to the entire data sets. Since the corrected relative response values directly reflected specific binding, no local parameters were introduced. For the necessary conversion of the response given in RU to molar concentrations, the generalized relationship [M] = [RU] · 103/(Mr · G · h) was used. In this equation, h denotes the height of the layer where the reaction takes place. This was set to 100 nm, which corresponds to the thickness of the CM-dextran matrix (20, 43). G is the proportionality factor for conversion between RU and nanograms per square millimeter. The value for carbohydrates (GC) was used as described above.
The results obtained by fitting the model to the data sets obtained for the interaction of SCWP with immobilized 3SLH are summarized in Table 2. The simulated response surface with the best-fit parameters for binding of the SCWP to 3SLH and the corresponding residuals are shown in Fig. 5C. The values of χ2 per degree of freedom (DF), as a measurement of the goodness of fit, show that, in the case of immobilized 3SLH, the model of Müller et al. (27) could reproduce key elements of the interaction with SCWP. Since data recorded at the higher flow rate of 50 μl/min were similar (Table 2), measurements were not essentially influenced by mass transport and rebinding effects. From the obtained ra as a geometric factor, it is predicted that at least two binding sites within a distance of about 14 nm exist on a single SCWP molecule. The calculated equilibrium dissociation constant (Kd) measured at flow rates of 10 and 50 μl/min was 7.7 × 10−7 M (Table 2). In the case of different binding sites, this value represents an overall Kd for all binding sites.
TABLE 2.
Intrinsic rate constants, ra, and goodness of fit expressed as χ2/DF for the interaction of SCWP with immobilized 3SLH calculated on the basis of the model for bivalent binding to fixed ligands (27)
| Flow rate (μl/min) | ka (M−1 · s−1) | kd (s−1) | Kd (M)a | ra (nm) | χ2/DF |
|---|---|---|---|---|---|
| 10 | 3.13 × 104 | 2.50 × 10−2 | 7.97 × 10−7 | 13.2 | 20.7 |
| 50 | 5.24 × 104 | 3.89 × 10−2 | 7.42 × 10−7 | 14.4 | 31.2 |
Equilibrium dissociation constant calculated as kd/ka.
In principle, rSbsB as a self-assembly systems is water insoluble. However, under certain conditions solutions containing monomers, as well as oligomeric forms, can be prepared (13, 39). From the coupling of such proteins, no random distribution but the formation of clusters in which molecules are fixed within a short distance of each other can be expected. Thus, an important requirement of the model of Müller et al. (27) was not fulfilled. A higher number of ligands in close proximity leads to a larger fraction of analyte, which can bind multivalently to the sensor chip. This avidity effect slows down the washout of the analyte dramatically. As can be seen in Fig. 4, in the case of immobilized rSbsB, the presence of clusters and avidity effects are clearly indicated by the higher SCWP levels remaining at the end of the washout phase. When the equilibrium binding isotherms (Fig. 4) were analyzed in accordance with the model of equilibrium dimerization of homogeneous monovalent receptors by divalent ligand (32), an increased cross-linking constant for immobilized rSbsB was obtained in comparison to that of 3SLH. This further indicated clustering of immobilized rSbsB and avidity effects.
Direct measurement of binding of rSbsB, 3SLH, and Δ3SLH-SbsB to immobilized SCWP.
In the complementary setup used for direct measurement of binding of rSbsB, 3SLH, and Δ3SLH-SbsB to immobilized SCWP, dilution series of rSbsB, 3SLH, and Δ3SLH-SbsB were injected over flow cells with immobilized SCWP at flow rates of 10 and 50 μl/min, respectively. Sensor surfaces with blank immobilizations, as well as analogously coupled SCWP of B. sphaericus CCM 2177 which were used as references, revealed that unspecific adsorption of all three sample proteins was negligible. Figure 6 demonstrates the response surfaces for 3SLH (A) and rSbsB (B) obtained at a flow rate of 10 μl/min on sensor surfaces with low immobilization densities of SCWP (18 RU). For both proteins, a pronounced dependence of specific binding on the concentration over a wide range of 6.7 pM to 14 μM rSbsB or 11.9 pM to 100 μM 3SLH without approaching surface saturation was observed. In contrast to rSbsB and 3SLH, Δ3SLH-SbsB did not bind to immobilized SCWP at all (data not shown).
FIG. 6.
Response surfaces recorded for binding of 3SLH (A) and rSbsB (B) to immobilized SCWP at a flow rate of 10 μl/min. The injection pulses lasted 0 to 1,500 s and are indicated by the gray plane at the base of each plot. Grid lines were plotted at each injected concentration and every 20 s, respectively. Data represent the mean ± SD of duplicate measurements on a single surface for 3SLH and on two surfaces with similar immobilization densities for rSbsB. Panel C depicts the simulated response surface and corresponding residuals resulting from fitting of the heterogeneous-ligand model for three parallel reactions to the data for 3SLH binding to immobilized SCWP (A).
Except for the highest concentrations of rSbsB (>2 μM), the common parts of the corresponding sensorgrams recorded for 3SLH and rSbsB at 10 and 50 μl/min were superimposable (data not shown), indicating that the measured response surfaces were not significantly influenced by mass transport limitations. For sample concentrations of less than about 1 μM, response surfaces for binding of 3SLH and rSbsB to the immobilized SCWP were nearly identical when the signals were appropriately scaled (Fig. 6A and B). This observation further supported the absence of diffusional limitations, since under the influence of such effects a different behavior for proteins with an almost fivefold difference in their Mrs would have been expected. At protein concentrations of greater than ∼1 μM, the sensorgrams differed markedly from each other (Fig. 6A and B). In the case of 3SLH, a further increase in the injected sample concentration resulted in a steep rise in the rapidly reached response levels during the wash-on phase, which quickly dropped back at the beginning of the washout phase to a relatively constant level, indicating at least a second process with a much faster off rate.
In contrast, for rSbsB, very different kinetic behavior was observed in the highest concentration range. The response values reached immediately after the start of injection were somewhat lower than those of 3SLH (e.g., after 5 and 10 s, −59 and −38%, respectively), but later the signal increased with time. The response levels reached during the injections were as high as 1,100 RU for the highest protein concentration used (14 μM) and stayed nearly constant during the following washout phases. Since a progressive rise without saturation requires a permanent increase of binding sites, this effect was attributed to the self-interaction of rSbsB molecules, namely, the crystallization process. By specific binding of rSbsB to immobilized SCWP, crystal nuclei were obviously generated that were fixed to the sensor surface and initiated further crystallization.
Concerning the concentration dependence of the response levels at the end of the wash-on phases, two sharp increases, which were separated by a slow-rise phase became visible for both 3SLH and rSbsB. This indicated the presence of at least two different kinds of binding sites at the immobilized SCWP. Binding data for 3SLH, which did not show any crystallization effects, were analyzed in terms of the heterogeneous ligand model implicated in the BIAevaluation software, which postulates two parallel reactions with independent pairs of rate constants. Since this model could not account for the slow-rise phase between the two sharp increases concerning the concentration dependence on the measured response surface, it was modified to handle three parallel reactions. As can be seen from Fig. 6C, the modified model allowed a fairly good fit to the data with the parameters given in Table 3. By applying this approach, the response surfaces obtained were interpreted in terms of three kinds of binding sites for 3SLH on the SCWP with low (Kd = 2.6 × 10−5 M), medium (Kd = 6.1 × 10−8 M), and high (Kd = 6.7 × 10−11 M) affinities. The values given represent mean Kd values determined at flow rates of 10 and 50 μl/min, which are given in Table 3.
TABLE 3.
Intrinsic rate constants and goodness of fit expressed as χ2/DF from fitting of the heterogeneous-ligand model for three parallel reactions to the data measured at the indicated flow rate for binding of 3SLH to SCWP immobilized on a sensor chip at a low ligand density
| Binding site | 10 μl/minb
|
50 μl/minc
|
||||
|---|---|---|---|---|---|---|
| ka (M−1 · s−1) | kd (s−1) | Kd (M)a | ka (M−1 · s−1) | kd (s−1) | Kd (M)a | |
| 1 | 1.77 × 106 | 9.08 × 10−5 | 5.13 × 10−11 | 2.89 × 106 | 2.36 × 10−4 | 8.17 × 10−11 |
| 2 | 4.20 × 104 | 2.69 × 10−3 | 6.40 × 10−8 | 6.42 × 104 | 3.70 × 10−3 | 5.76 × 10−8 |
| 3 | 4.22 × 104 | 1.17 | 2.77 × 10−5 | 1.73 × 104 | 4.09 × 10−1 | 2.36 × 10−5 |
Equilibrium dissociation constant calculated as ka/kd.
χ2/DF, 0.81.
χ2/DF, 0.45.
Examination of the affinity between rSbsB or 3SLH and SCWP by competitive inhibition assays.
To further investigate the protein-carbohydrate interaction in terms of specificity and affinity, competitive approaches were used. The solution competition method measures the equilibrium position of a bulk interaction with the SPR biosensor and thus restricts the information that can be gathered to its thermodynamics. Experiments were performed by preparing mixtures of SCWP with 3SLH or rSbsB in different ratios, keeping the concentration of either the protein or the glycan constant. The fraction of this reactant, which was contained freely in the equilibrated samples, was then determined for each case. For that purpose, mixtures were passed over a sensor surface with the corresponding interaction partner immobilized at a high density. Calculation of the equilibrium concentrations was done from initial slopes of recorded sensorgrams by using calibration curves obtained by injecting the same component solely over the identical sensor surface.
SCWP as a soluble competitor in solution competition assays.
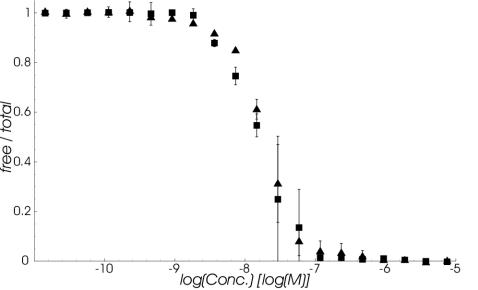
In the first experimental setup, the dependence of free 3SLH or rSbsB on the respective total SCWP concentration in mixtures containing a constant 3 nM protein concentration was determined on a high-density sensor surface with immobilized SCWP. Data resulting from this solution competition are demonstrated in Fig. 7. In the case of rSbsB, the observed SDs for duplicate measurements were somewhat higher than for 3SLH and the plateau at the lower SCWP concentrations was only vaguely visible. Since soluble SCWP could totally inhibit binding of 3SLH and rSbsB to the sensor surface, the data confirmed the specificity of the interaction investigated and that the binding sites on soluble 3SLH and rSbsB for soluble and immobilized SCWP were the same.
FIG. 7.
Dependence of the free-to-total 3SLH (squares) and rSbsB (triangles) ratios on the amount of SCWP added in equilibrated mixtures containing the corresponding sample at a constant 3 nM. The fraction of free protein in the solutions was calculated from the initial rates measured for its binding to a sensor surface with immobilized SCWP. Data represent the mean ± SD of duplicate measurements.
rSbsB or 3SLH as a soluble competitor in solution competition assays.
Furthermore, the experimental setup for solution competition was inverted. For that purpose, equilibrated mixtures containing SCWP at a constant 1 mg/liter and 3SLH or rSbsB at a series of concentrations were passed over a sensor chip with a high-density surface of immobilized rSbsB. Measured initial rates were used to evaluate the fraction of free SCWP in dependence on the total concentration of each protein. The results obtained, depicted in Fig. 8, clearly demonstrated that competition with soluble 3SLH and rSbsB could totally inhibit SCWP binding to the sensor surface. These findings again confirmed the specificity of the investigated interaction and that the binding sites on soluble SCWP for soluble and immobilized 3SLH and rSbsB are the same.
FIG. 8.
Dependence of the free-to-total SCWP ratio on the amount of 3SLH (squares) and rSbsB (triangles) added in equilibrated mixtures containing the glycan at a constant 1 mg/liter. The fraction of free SCWP in the solutions was calculated from the initial rates measured for its binding to a sensor surface with immobilized rSbsB. Data represent the mean ± SD of duplicate measurements.
pH dependence of the rSbsB-SCWP interaction.
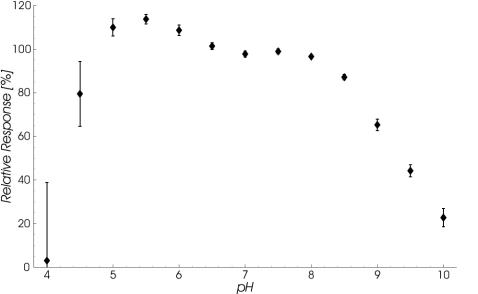
The interaction between rSbsB and its corresponding SCWP was further investigated under different pH conditions. Specific binding to a sensor surface with immobilized SCWP of a pH series containing 5 nM rSbsB was monitored. The response levels reached after 1,495 s of protein injections are depicted in Fig. 9 relative to the signal at pH 7.2 (running buffer) and reveal maximum binding at pH 5.5, with a sharp decline toward lower pH values and a pronounced shoulder around pH 7.5 in the opposite direction. The clear rise in the SDs for data points in the more acidic region relative to the observed maximum was attributed to the approach of the pH value to the isoelectric point of rSbsB and consequently to its decreasing solubility.
FIG. 9.
pH dependence of rSbsB binding to SCWP immobilized on a sensor surface. Response levels reached after 1,495 s of protein injection are plotted versus the pH of the solutions applied and relative to the corresponding signal at pH 7.2 (standard running buffer). Data represent the mean ± SD of four repeated measurements on three surfaces.
DISCUSSION
Various SLH domain-carrying S-layer proteins were found to recognize SCWPs covalently attached to the peptidoglycan as the proper anchoring structure in the rigid cell wall layer. This was concluded from observations that extraction of the SCWP from the cell wall fragments reduced or abolished binding of the corresponding SLH domain-carrying proteins (2, 3, 12, 18, 24, 34, 39). In the present study, the interaction between isolated SCWP and the S-layer protein SbsB of G. stearothermophilus PV72/p2 was investigated in detail by SPR-based real-time biosensor measurements with both experimental setups. This required attachment of either the polypeptide or the glycan component to the sensor chip. To achieve the latter, a method to introduce a free hydrazine residue at the reducing end of the polymer chains had to be developed, which enabled a wide range of modifications. Since only the modified reducing end was activated for covalent attachment, all polymer chains were bound to the biosensor matrix via the same group, which implies the advantage of a uniform orientation for subsequent interaction studies.
The SCWP was isolated, purified, and characterized in terms of its main constituents (34). An Mr of 2.4 × 104 had been estimated on the basis of GPC (34), which especially for acidic polysaccharides tends to overestimate the real Mr. Since the SPR signal is proportional to the mass increase on the sensor surface, the Mr can also be derived from biosensor data measured under particular conditions. Assuming one binding site per rSbsB molecule, the Mr of SCWP was calculated to be 1.03 × 104 from response levels equivalent to the amounts of immobilized protein and of the glycan saturating it. On the basis of this Mr, the estimated number of carbohydrazide molecules introduced per SCWP molecule during modification of the reducing ends of the polymer chains was about 1 (C. Mader, unpublished observation).
The mechanism of binding between S-layer proteins and SCWPs was thought to correspond to that occurring between polysaccharides and lectins (39). This includes highly specific recognition of the carbohydrate structures by the protein binding sites. To investigate this feature, the specificity of the S-layer protein for the glycan component was tested and the requirements of the carbohydrate for interaction were investigated. For that purpose, a sensor surface with immobilized rSbsB, together with appropriate reference cells, was used and binding of different sample compounds was checked. Because of the high isoelectric point of many SLH domains and the net negative charge of SCWPs (12, 25), it could be speculated that only an electrostatic mechanism formed the basis of the interaction, as was reported for heparin binding to selenoprotein P (1). Since neither dextrans carrying different degrees of net negative charges nor heparin, which, like the SCWP, is a glucosamine-containing polyanionic glycan, could bind to immobilized rSbsB, a simple charge interaction could be excluded. Furthermore, no interaction was observed between rSbsB and other SCWPs that were isolated from strains of another species or from strains belonging to the same species but having a different type of SCWP. Only the corresponding SCWP of G. stearothermophilus PV72/p2 and not even its peptidoglycan was bound by rSbsB. These results confirmed the previously deduced notion that the mechanism of recognition and binding of the SCWP and the N-terminal region of the S-layer protein is highly specific (39). Chemical modification of SCWP further revealed that the pyruvic acid residues play a crucial role in the binding process. It has previously been reported that in B. anthracis, pyruvylation of a peptidoglycan-associated cell wall polymer is a necessary modification for attachment of S-layer proteins EA1 and Sap to the bacterial cell surface (25). From the presence of proteins possessing SLH motifs, the presence of CsaB orthologs suspected of being involved in the addition of pyruvyl groups to peptidoglycan-associated polysaccharides, and the presence of pyruvate in the cell wall, it was concluded that the anchoring mechanism involving SLH domains and pyruvylated SCWPs is widespread among prokaryotes and has been conserved during evolution (25).
All of the measured biosensor data obtained in the present study consistently showed that Δ3SLH-SbsB lacking the three SLH motifs did not interact with SCWP, whereas the SCWP binding properties of 3SLH were essentially the same as those of rSbsB. Thus, the three SLH motifs formed a correctly folded and functional domain on its own, which proved to be solely responsible for SCWP recognition and binding.
The experimental data obtained showed the interaction between isolated SCWP and rSbsB or its SLH domain to be complex. To ensure the relevance of the recorded data, the experiments were performed in all possible setups considering the common precautions and controls (15, 19, 28).
The response surfaces obtained from direct binding of the SCWP to immobilized 3SLH and rSbsB, respectively, were characterized by an extremely wide concentration range of about 5 orders of magnitude necessary to approach saturation of the sensor surfaces (Fig. 5). Thereby, we noted no conspicuous steps but a continuous rise. Since in solution competition assays the response could be completely abolished by addition of 3SLH or rSbsB to the SCWP-containing sample solutions, the signals proved to arise specifically from the interaction investigated. Analysis of the measured data was attempted in terms of a model for definitely bivalent analytes possessing one kind of binding site, which therefore led to an overall Kd of 7.7 × 10−7 M for all binding sites. From this kinetic model, which assumes randomly immobilized ligands, a geometric parameter, namely, the ra, was estimated to be around 14 nm (Table 2), which denotes the distance between two binding sites within the same molecule. This is a plausible value, since the Mr for the SCWP corresponds to about 50 N-acetylhexosamine units, which will result in a length of about 25 nm if the polymer chains are linearly arranged and a contribution of 0.5 nm of each 1→4-linked hexopyranose is assumed. Evidence for coupling of preformed rSbsB oligomers during sensor surface preparation was derived from the imperfect description of the response surfaces by the model of Müller et al. (27).
Data recorded for the direct interaction of rSbsB with SCWP immobilized on a sensor chip provided strong evidence for nucleation of crystallization by initially bound rSbsB. From an analysis of data obtained with 3SLH that was done in accordance with an extension of the heterogeneous-ligand model, the existence of three different kinds of binding sites with low (Kd = 2.6 × 10−5 M), medium (Kd = 6.1 × 10−8 M), and high (Kd = 6.7 × 10−11 M) affinities was indicated. Since in solution competition assays the response could be completely abolished by addition of SCWP to the samples containing 3SLH or rSbsB, the signals proved to arise specifically from the investigated interaction. It has previously been reported that even supposedly monomeric protein solutions contain small amounts of multimeric aggregates that are difficult to detect but will dissociate very slowly and give misleadingly high affinities, as well as abnormal kinetics (44). According to this observation, the possibilities cannot be excluded that solutions of 3SLH contained small amounts of oligomers and that the high-affinity binding site resulted from avidity effects caused by binding of at least dimeric 3SLH.
As described above, analysis of data obtained from the inverted setup in which 3SLH was immobilized on a sensor chip was performed in accordance with a model for definitely bivalent analytes that assumes one kind of binding site and therefore led to an overall Kd value of 7.7 × 10−7 M for all binding sites. This value was in good agreement with the so far singularly reported Kd values for binding of SLH domains from S-layer proteins to cell wall fragments, which were in the range of 1.8 × 10−8 to 7.1 × 10−7 M when determined at 37°C (5).
To summarize, the SPR studies performed with both setups confirmed not only that the SLH domain of SbsB was exclusively responsible for SCWP binding but also the specificity of the protein-carbohydrate interaction investigated, since neither other SCWPs, nor other polysaccharides, nor the peptidoglycan was recognized. The measured data could be fitted to different models, which finally indicated the existence of at least two binding sites on a single SCWP molecule and allowed us to distinguish between binding sites with different affinities. Furthermore, it could be demonstrated that pyruvic acid residues play a crucial role in the binding process.
Acknowledgments
This work was supported by the Austrian Science Foundation, project P14689, and by the Competence Center “Biomolecular Therapeutics” (B.M.T.).
We thank A. Jungbauer for commenting on the manuscript.
REFERENCES
- 1.Arteel, G. E., S. Franken, J. Kappler, and H. Sies. 2000. Binding of selenoprotein P to heparin: characterization with surface plasmon resonance. Biol. Chem. 381:265-268. [DOI] [PubMed] [Google Scholar]
- 2.Beveridge, T. J., P. H. Pouwels, M. Sára, A. Kotiranta, K. Lounatmaa, K. Kari, E. Kerosuo, M. Haapasalo, E. M. Egelseer, I. Schocher, U. B. Sleytr, L. Morelli, M. L. Callegari, J. F. Nomellini, W. H. Bingle, J. Smit, E. Leibovitz, M. Lemaire, I. Miras, S. Salamitou, P. Beguin, H. Ohayon, P. Gounon, M. Matuschek, and S. F. Koval. 1997. Functions of S-layers. FEMS Microbiol. Rev. 20:99-149. [DOI] [PubMed] [Google Scholar]
- 3.Brechtel, E., and H. Bahl. 1999. In Thermoanaerobacter thermosulfurigenes EM1 S-layer homology domains do not attach to peptidoglycan. J. Bacteriol. 181:5017-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton, H. T. S., and R. A. Robinson. 1931. Universal buffer solutions and the dissociation constant of Veronal. J. Chem. Soc. 1931:1456-1462. [Google Scholar]
- 5.Chauvaux, S., M Matuschek, and P. Beguin. 1999. Distinct affinity of binding sites for S-layer homologous domains in Clostridium thermocellum and Bacillus anthracis cell envelopes. J. Bacteriol. 181:2455-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egelseer, E. M., K. Leitner, M. Jarosch, C. Hotzy, S. Zayni, U. B. Sleytr, and M. Sára. 1998. The S-layer proteins of two Bacillus stearothermophilus wild-type strains are bound via their N-terminal region to a secondary cell wall polymer of identical chemical composition. J. Bacteriol. 180:1488-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelhardt, H., and J. Peters. 1998. Structural research on surface layers: a focus on stability, surface layer homology domains, and surface layer-cell wall interactions. J. Struct. Biol. 124:276-302. [DOI] [PubMed] [Google Scholar]
- 8.Frostell-Karlsson, A., A. Remaeus, H. Roos, K. Andersson, P. Borg, M. Hämäläinen, and R. Karlsson. 2000. Biosensor analysis of the interaction between immobilized human serum albumin and drug compounds for prediction of human serum albumin binding levels. J. Med. Chem. 43:1986-1992. [DOI] [PubMed] [Google Scholar]
- 9.Fujino, T., P. Béguin, and J.-P. Aubert. 1993. Organization of a Clostridium thermocellum gene cluster encoding the cellulosomal scaffolding protein CipA and a protein possibly involved in attachment of the cellulosome to the cell surface. J. Bacteriol. 175:1891-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hämäläinen, M., and F. Markey. 1998. Sorting the sheep from the goats. BIA J. 2:12-16. [Google Scholar]
- 11.Howorka, S., M. Sára, Y. Wang, B. Kuen, U. B. Sleytr, W. Lubitz, and H. Bayley. 2000. Surface-accessible residues in the monomeric and assembled forms of a bacterial surface layer protein. J. Biol. Chem. 48:37876-37886. [DOI] [PubMed] [Google Scholar]
- 12.Ilk, N., P. Kosma, M. Puchberger, E. M. Egelseer, H. F. Mayer, and U. B. Sleytr. 1999. Structural and functional analyses of the secondary cell wall polymer of Bacillus sphaericus CCM 2177 that serves as an S-layer-specific anchor. J. Bacteriol. 181:7643-7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaenicke, R., R. Welsch, M. Sára, and U. B. Sleytr. 1985. Stability and self-assembly of the S-layer protein of the cell wall of Bacillus stearothermophilus. Biol. Chem. Hoppe-Seyler 366:663-670. [DOI] [PubMed] [Google Scholar]
- 14.Jarosch, M., E. M. Egelseer, C. Huber, D. Moll, D. Mattanovich U. B. Sleytr, and M. Sára. 2001. S-layer gene sbsC of Bacillus stearothermophilus ATCC 12980: molecular characterization and heterologous expression in Escherichia coli. Microbiology 147:1353-1363. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson, R., and A. Fält. 1997. Experimental design for kinetic analysis of protein-protein interactions with surface plasmon resonance biosensors. J. Immunol. Methods 200:121-133. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson, R., H. Roos, L. Fägerstam, and B. Persson. 1984. Kinetic and concentration analysis using BIA technology. Methods Companion Methods Enzymol. 6:99-110. [Google Scholar]
- 17.Kuen, B., A. Koch, E. Asenbauer, M. Sára, and W. Lubitz. 1997. Molecular characterization of the Bacillus stearothermophilus PV72 S-layer gene sbsB induced by oxidative stress. J. Bacteriol. 179:1664-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemaire, M., I. Miras, P. Gounon, and P. Beguin. 1998. Identification of a region responsible for binding to the cell wall within the S-layer protein of Clostridium thermocellum. Microbiology 144:211-217. [DOI] [PubMed] [Google Scholar]
- 19.Lipschultz, C. A., Y. Li, and S. Smith-Grill. 2000. Experimental design for analysis of complex kinetics using surface plasmon resonance. Methods 20:310-318. [DOI] [PubMed] [Google Scholar]
- 20.Löfas, S., and B. Johnsson. 1990. A novel hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands. J. Chem. Soc. Chem. Commun. 21:1526-1528. [Google Scholar]
- 21.Lupas, A. 1996. A circular permutation event in the evolution of the SLH domain? Mol. Microbiol. 20:897-898. [DOI] [PubMed] [Google Scholar]
- 22.Lupas, A., H. Engelhardt, J. Peters, U. Santarius, S. Volker, and W. Baumeister. 1994. Domain structure of the Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J. Bacteriol. 176:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mach, H., D. B. Volkin, C. J. Burke, C. R. Middaugh, R. J. Linhardt, J. R. Fromm, D. Loganathan, and L. Mattsson. 1993. Nature of the interaction of heparin with acidic fibroblast growth factor. Biochemistry 32:5480-5489. [DOI] [PubMed] [Google Scholar]
- 24.Mesnage, S., E. Tosi-Counture, and A. Fouet. 1999. Production and cell surface anchoring of functional fusions between SLH motifs of the Bacillus anthracis S-layer proteins and the Bacillus subtilis levansucrase. Mol. Microbiol. 31:927-936. [DOI] [PubMed] [Google Scholar]
- 25.Mesnage, S., T. Fontaine, T. Mignot, M. Delepierre, M. Mock, and A. Fouet. 2000. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 19:4473-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moll, D., C. Huber, B. Schlegel, D. Pum, U. B. Sleytr, and M. Sára. 2002. S-layer-streptavidin fusion proteins as template for nanopatterned molecular arrays. Proc. Natl. Acad. Sci. USA 99:14646-14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller, K. M., K. M. Arndt, and A. Plückthun. 1998. Model and simulation of multivalent binding to fixed ligands. Anal. Biochem. 261:149-158. [DOI] [PubMed] [Google Scholar]
- 28.Myszka, D. G. 1997. Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Curr. Opin. Biotechnol. 8:50-57. [DOI] [PubMed] [Google Scholar]
- 29.Nazina, T. N., T. P., Tourova, A. B. Poltaraus, E. V. Novikova, A. A. Grigoryan, A. E. Ivanova, A. M. Lysenko, W. Petrunyaka, G. A. Osipov, S. S. Belyaev, and M. V. Ivanov. 2001. Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int. J. Syst. Evol. Microbiol. 51:433-446. [DOI] [PubMed] [Google Scholar]
- 30.Neubauer, A., E. Györvary, D. Pum, M. Sára, and U. B. Sleytr. 2000. Investigation of the orientation of supramolecular protein structures (S-layers) on silicon, gold, and lipid films by scanning force microscopy. PTB-Ber. F 39:118-123. [Google Scholar]
- 31.Nice, E. C., and B. Catimel. 1999. Instrumental biosensors: new perspectives for the analysis of biomolecular interactions. Bioessays 21:339-352. [DOI] [PubMed] [Google Scholar]
- 32.Perelson, A. S. 1984. Some mathematical models of receptor clustering by multivalent ligands, p. 223-276. In A. S. Perelson, C. DeLisi, and F. W. Wiegel (ed.), Cell surface dynamics: concepts and models. Marcel Dekker, Inc., New York, N.Y.
- 33.Pum, D., and U. B. Sleytr. 1999. The application of bacterial S-layers in molecular nanotechnology. Trends Biotechnol. 17:8-12. [Google Scholar]
- 34.Ries, W., C. Hotzy, I. Schocher, U. B. Sleytr, and M. Sára. 1997. Evidence that the N-terminal part of the S-layer protein from Bacillus stearothermophilus PV72/p2 recognizes a secondary cell wall polymer. J. Bacteriol. 179:3892-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Sára, M., and U. B. Sleytr. 1996. Crystalline bacterial cell surface layers (S-layers): from cell structure to biomimetics. Prog. Biophys. Mol. Biol. 65:83-111. [DOI] [PubMed] [Google Scholar]
- 37.Sára, M., and U. B. Sleytr. 2000. S-layer proteins. J. Bacteriol. 182:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sára, M., B. Kuen, H. F. Mayer, F. Mandl, K. C. Schuster, and U. B. Sleytr. 1996. Dynamics in oxygen-induced changes in S-layer protein synthesis from Bacillus stearothermophilus PV72 and the S-layer-deficient variant T5 in continuous culture and studies of the cell wall composition. J. Bacteriol. 178:2108-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sára, M., C. Dekitsch, H. F. Mayer, E. M. Egelseer, and U. B. Sleytr. 1998. Influence of the secondary cell wall polymer on the reassembly, recrystallization, and stability properties of the S-layer protein from Bacillus stearothermophilus PV72/p2. J. Bacteriol. 180:4146-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scatchard, G. 1949. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 51:660-672. [Google Scholar]
- 41.Sleytr, U. B., M. Sára, and D. Pum. 2000. Crystalline bacterial cell surface layers (S-layers): a versatile self-assembly system, p. 177-213. In A. Ciferri (ed.), Supramolecular polymerization. Marcel Dekker, Inc., New York, N.Y.
- 42.Sleytr, U. B., P. Messner, D. Pum, and M. Sára (ed.). 1996. Crystalline bacterial cell surface proteins. R. G. Landes Company/Academic Press, Austin, Tex.
- 43.Stenberg, E., B. Persson, H. Roos, and C. Urbaniczky. 1991. Quantitative determination of surface concentration of protein with surface plasmon resonance using radiolabeled proteins. J. Colloid Interface Sci. 143:513-526. [Google Scholar]
- 44.Van der Merwe, A., and A. N. Barclay. 1994. Transient intercellular adhesion: the importance of weak protein-protein interactions. Trends Biochem. Sci. 19:354-358. [DOI] [PubMed] [Google Scholar]