Abstract
Several studies have highlighted differences in the resistances of various mouse strains to intravenous (i.v.) infection with Yersinia enterocolitica. In particular, differences in resistance and immunological response between BALB/c and C57BL/6 mouse strains have been determined. Following i.v infection, C57BL/6 mice are more resistant to Y. enterocolitica than are BALB/c mice. However, because Y. enterocolitica is typically a food-borne pathogen, the oral route of infection more accurately reflects the natural route of infection. Therefore, it was of interest to ascertain if the differences in resistance between mouse strains observed for an i.v. infection can be recapitulated following an oral infection. C57BL/6j, BALB/cj, and 129X1/Svj mouse strains presented no differences in 50% lethal dose (LD50) following oral infection with Y. enterocolitica. Subsequent analysis of cytokine levels, bacterial colonization and immune cell populations following oral infection confirmed characteristics previously described following i.v. Y. enterocolitica infection. All tissues analyzed from each mouse strain demonstrated a polarized Th1 cytokine profile and inflammatory cell influx throughout a 7-day course of infection. This immune response was present in all tissues and increased as bacterial colonization progressed. The lack of a differing LD50 phenotype and common trends in immunological response among the three mouse strains tested suggests that oral infection is a useful model for studying the host response to Y. enterocolitica infection.
The genus Yersinia contains three species of human pathogenic bacteria (13). Y. enterocolitica and Y. pseudotuberculosis are food-borne pathogens which cause a variety of symptoms and pathologies. Following ingestion, intestinal colonization occurs in the underlying lymphoid tissue of the small intestine, the Peyer's patches (PP), and the mesenteric lymph nodes (MLN). Y. enterocolitica infection typically results in a self-limiting gastroenteritis and/or mesenteric lymphadenitis; however, systemic infection of deeper tissues, such as the spleen and liver, may occur in the setting of immunosuppression or iron overload (35).
Carter and Collins established a mouse model of Y. enterocolitica infection in 1975 (14, 15). Subsequent studies based on this model have been instrumental in outlining the overall process of infection. Orally administered Y. enterocolitica can survive the low-pH environment of the stomach at least in part due to the expression of a urease (19, 22). Next, the bacteria enter the small intestine, where they bind to the intestinal epithelium (23). The bacteria then travel out of the lumen, eventually colonizing the underlying lymphoid tissues of the small intestine (14, 16). Once in the PP and MLN, the bacteria are able to replicate and survive. At the same time, the host mounts an inflammatory response characterized by an influx of neutrophils and macrophages. (7, 31) Subsequent disseminated infection may occur, resulting in neutrophil and macrophage infiltration, bacterial microcolony formation, microabscesses, and granuloma lesion formation occurring primarily in the MLN, liver, and spleen (6, 7, 31).
Several studies have highlighted differences in the resistance of various mouse strains to intravenous (i.v.) infection with Y. enterocolitica. BALB/cBy, BALB.B, Swiss, C3H/HeN, A, SWR, and DBA/2 mouse strains are more susceptible to i.v. infection than are C57BL/6 mice (26). For these mouse strains, the i.v. 50% lethal dose (LD50) is 3 × 102 to 6 × 102, while the C57BL/6 strain is more resistant, with an i.v. LD50 of 2 × 105 which is 667-fold higher than that for the BALB/cBy LD50 of 3 × 102. BALB/cBy parentally crossed with C57BL/6 produces an F1 progeny with an intermediate level of resistance (LD50 = 3 × 104), suggesting that the level of resistance is genetically linked. C57BL/6 mice were also shown to have decreased bacterial colonization in the PP compared to BALB/cBy mice following oral infection. In a similar study by Autenrieth et al., the i.v. LD50 for C57BL/6 mice was 2 × 104, 40-fold higher than that for the BALB/c mice which was of 5 × 102 (2). The above studies went on to demonstrate that resistance to i.v. Y. enterocolitica infection is not linked to the Salmonella resistance locus, Ity, or to the H-2 locus (26). Further inbreeding tentatively identified a potential resistance gene carried by C57BL/6 but not BALB/c mice (25). The ES-1 locus encodes an esterase of unknown function; however, the overall level of resistance appears to be a multigenic trait.
Although the above studies have elucidated a number of details concerning the host response to i.v.-administered Y. enterocolitica, naturally acquired Y. enterocolitica infection typically occurs following ingestion and uptake into the gastrointestinal tract, not direct injection into the bloodstream. Therefore, the primary goal of this study was to determine if the differences observed between mouse strains when using the i.v.-infection model were paralleled when using an oral-infection model. Resistance to orally administered Y. enterocolitica was assessed in C57BL/6j, BALB/cj, and 129X1/Svj inbred mouse strains. These strains were chosen due to their historical use in the study of Y. enterocolitica and bacterial pathogenesis in general. We analyzed LD50s, levels of five representative Th1/Th2 cytokines (tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], interleukin-2 [IL-2], IL-5, and IL-4), 11 different cell populations, and colonization and associated pathology of various tissues for each mouse strain on days 1, 3, and 7 postinfection. This information will be useful for studies utilizing the oral model and for comparison with data obtained from studies using alternate routes of infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Y. enterocolitica strain used in this study, JB580v, is a restriction-minus derivative of a virulent American strain, 8081v, serogroup O:8 Nalr (28). JB580v was grown at 26°C overnight with aeration in Luria-Bertani (LB) broth or on LB agar plates supplemented with 20 μg of nalidixic acid (Sigma Chemicals, St. Louis, Mo.) per ml. Actual numbers of CFU administered were determined by serial dilutions of the overnight culture followed by plating on LB agar plates containing 20 μg of nalidixic acid per ml.
Mice.
Virus-free C57BL/6j, BALB/cj, and 129X1/Svj mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). The animals were housed in plastic cages with wood shavings in a high-efficiency particulate air-filtered barrier unit maintained at 25°C with alternating 12-h periods of light and dark. The mice had drinking water at all times and were fed ad libitum with an autoclaved rodent pellet diet. They were infected orally with 0.1 ml of the indicated doses of bacteria via a 21-gauge feeding tube attached to a 1.0-ml syringe or i.v. through the tail vein with 0.1 ml of bacteria resuspended in phosphate-buffered saline (PBS). The Washington University Animal Studies Committee approved all animal experiments.
LD50, survival curves, and kinetics analysis.
Six groups of five to six mice from each genetic background were infected either orally or i.v. with successive 10-fold dilutions of the bacterial suspension (oral, 104 to 109 bacteria; i.v., 101 to 105 bacteria). The mice were monitored twice daily for a 14-day period. This analysis was done in duplicate in two independent experiments for each mouse strain. For kinetic analysis, mice were sacrificed by CO2 asphyxiation at various times postinfection (1, 3, or 7 days) following infection with 1 × 108 to 8 × 108 bacteria in a volume of 100 μl. Tissues (PP, MLN, and spleen) were surgically removed and weighed. The bacterial load recovered from the infected organs was determined by plating serial dilutions of the macerated tissues on LB plates containing 20 μg of nalidixic acid per ml to select for Nalr Y. enterocolitica. Results are reported as CFU per gram of tissue (between 2 and 110 mg for PP, between 5 and 182 mg for MLN, and between 14 and 1415 mg for spleens). Kinetic analysis was done in duplicate as two independent experiments for each mouse strain.
Histopathology.
Mice from each genetic background were orally infected with 1 × 108 to 8 × 108 CFU. Uninfected mice (day 0) and mice infected for 1, 3, or 7 days were sacrificed by CO2 asphyxiation. The small intestine, MLN, and spleen were removed. The lumen of the small intestine was flushed with PBS, and all tissues were fixed in either 10% neutral buffered formaldehyde or Bouin's fixative prior to being embedded in paraffin, sectioned, and stained with hematoxylin and eosin or with Y. enterocolitica specific antibody (20). The sections were scored for increased numbers of macrophages and neutrophils, granuloma formation, inflammatory infiltrates, necrosis, and bacterial colonization. A minimum of five mice per time point were examined.
Single-cell suspension preparation.
Four mice from each genetic background were sacrificed by CO2 asphyxiation on day 0, 1, 3, and 7 postinfection, and their spleens, MLN, and PP were surgically removed and placed in ice-cold HBSS+ (Ca2+- and Mg2+-free Hanks' balanced salt solution [HBSS; BioWhittaker, Walkersville, Md.] supplemented with 1% fetal calf serum [FCS] [HyClone, Logan, Utah] and 50 U of penicillin per ml-50 mg of streptomycin per ml [both from BioWhittaker]). The tissues were ground between sterile frosted glass slides, and the homogenates were incubated for 30 min at 37°C under 5% CO2 and humidity in RPMI medium (BioWhittaker) supplemented with 10% FCS, 2 mM Glutamax (alynyl-l-glutamine [Invitrogen, Carlsbad, Calif.]), 50 U of penicillin per ml-50 mg of streptomycin per ml, 1 mg of collagenase D (Sigma Chemicals) per ml, and 1 U of DNase (Fisher Scientific, Pittsburgh, Pa.) per ml. Cell suspensions were filtered through 40-μm-pore-size filters (BD Labware, Franklin Lakes, N.J.), washed twice with ice-cold HBSS+, resuspended in the above tissue culture medium without DNase or collagenase D and allowed to recover for 1.5 h at 37°C under 5% CO2 and humidity. Red blood cells were lysed from spleen samples by incubating the cell suspensions for 5 min at room temperature in 17 μM Tris-140 μM NH4Cl followed by two washes in ice-cold HBSS+. Cells were resuspended in the above RPMI tissue culture medium without collagenase D and DNase. Cell viability and number were assessed by trypan blue exclusion.
Flow cytometry.
Single-cell suspensions obtained as described above were resuspended in PBS with 0.05% (wt/vol) bovine serum albumin fraction V (Sigma Chemicals), and FC block (2.4G2; BD Pharmingen, San Diego, Calif.) at 2 × 107 cells ml−1. Then 106 cells were stained with marker-specific fluorophore (fluorescein-5-isothiocyanate [FITC] or phycoerythrin [PE])-conjugated antibodies (Abs), biotin-conjugated Abs, or the appropriate isotype control Abs for 30 min on ice. Cellular suspensions were then washed twice in PBS containing 1% bovine serum albumin fraction V and, where appropriate, stained with appropriate fluorophore-conjugated secondary Abs, streptavidin-PE, or streptavidin-FITC for 30 min on ice. The cells were washed twice as described above and fixed with 1% paraformaldehyde in PBS. Flow cytometric analysis was done on a triple-laser flow cytometer (FACS calibur; BD Biosciences, Mountain View, Calif.), and analysis was performed using the CellQuest program (BD Biosciences) and WinMDI v2.8 (Scripps Research Institute, La Jolla, Calif.). Dead cells were excluded based on forward and side light scatter. For the analysis of lymphocyte subpopulations, 10,000 cells from the lymphocyte population (as determined by forward versus side scatter) were analyzed for the expression of lymphocyte markers. For the analysis of nonlymphocyte subpopulations, 10,000 cells from a gate surrounding the lymphocyte population and extending to the edge of the side scatter were analyzed.
The following Abs and secondary staining reagents were used for flow cytometric studies: biotin-conjugated rat anti-mouse Ly-6G (Gr-1: RB6-8C5), biotin-conjugated hamster anti-mouse T-cell receptor (TCR) β chain (H57-597), PE-conjugated rat anti-mouse CD19 (1D3), FITC-conjugated rat anti-mouse LY-49G2 (4D11), PE-conjugated hamster anti-mouse CD11c (HL3), PE-conjugated hamster anti-mouse γδ T-cell receptor (GL3), FITC-conjugated rat anti-mouse CD4 (RM4-5), FITC-conjugated rat anti-mouse CD8a (53-6.7), PE-conjugated rat anti-mouse CD25 (PC61), PE-conjugated rat anti-mouse CD62L (MEL-14), FITC-conjugated hamster anti-mouse CD3ɛ chain (145-2C11), and appropriate isotype control Abs, streptavidin-FITC, streptavidin-PE (all from BD Pharmingen), and biotin-conjugated rat anti-mouse BM8 (Novus Biologicals, Littleton, Colo.).
Preparation of bone marrow-derived macrophages (BMM).
Three mice from each genetic background were sacrificed and had both femurs surgically removed and collected into 10 ml of ice-cold Dulbecco modified Eagle Medium (BioWhittaker) supplemented with 10% FCS, 2 mM Glutamax, and 50 U of penicillin per ml-50 mg of streptomycin per ml. The end of each femur was removed using sterile scissors, and the bone marrow was flushed into a bacterial petri dish using a 26-gauge syringe and 10 ml of ice-cold cell culture medium. Single-cell suspensions were made by repeatedly pipetting the cells through a 10-ml pipette. The cells were incubated at 37°C under 5% CO2 and humidity for 8 days at a density of 107 cells in bacterial petri dishes containing 10 ml of medium as described above supplemented with 20% L-cell medium. The cells were given 10 ml of fresh supplemented medium after 4 days. On day 8, visual inspection of the cells revealed a confluent monolayer of adherent cells. Nonadherent cells were removed by washing twice with room temperature PBS. Adherent cells were removed by incubation with 10 ml of ice-cold PBS-1.0 mM EDTA (pH 7.4) for 10 min. The cells were then resuspended in medium free of penicillin and streptomycin and supplemented with 20% L-cell medium and distributed into 96-well dishes at 105 cells per well.
In vitro lymphocyte stimulation.
BMM were loaded with antigen by overnight incubation with 10 μg of heat-killed (95°C for 30 min) Y. enterocolitica (HKY) organisms per ml (7). For determination of cytokine production, 2.5 × 105 cells from each cell suspension described above (PP, MLN, and spleens) from each mouse strain on days 0, 1, 3, and 7 were cocultured with the HKY-stimulated BMM for 48 h. Supernatants were collected and frozen at −80°C for subsequent cytokine analysis. The stimulation was done in duplicate in two independent experiments for each mouse strain.
Cytokine analysis.
Levels of IFN-γ, TNF-α, IL-5, IL-4, and IL-2 were determined using the mouse Th1/Th2 cytokine cytometric bead array kit (BD Biosciences). Culture supernatants (50-μl volumes) were analyzed as specified by the manufacturer. The analysis was done in duplicate with samples from two different experiments for a total of four replicates. Cytokine concentrations were calculated using cytometric bead array analysis software v 1.2 (BD Biosciences).
Statistical analysis.
LD50s were determined by the methods of Reed and Muench (32). All statistical analyses were performed using GraphPad Prism v.3.03 (Graphpad Software, San Diego, Calif.). Survival curves were constructed using the Kaplan-Meier method and compared using the Mantel-Haenszel log-rank test (1). Nonparametric analysis of variance (ANOVA) using the Kruskal-Wallis test and Dunns post test were used to compare the means of experimental groups. A P value of <0.05 was considered statistically significant.
RESULTS
Survival of C57BL/6j, BALB/cj, and 129X1/Svj mice following oral administration of Y. enterocolitica.
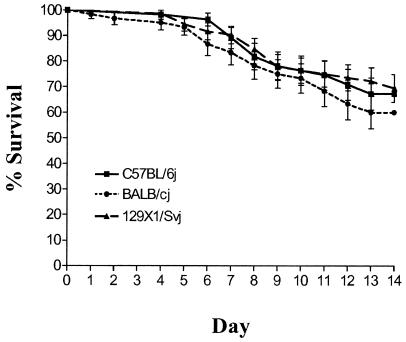
Previous studies have reported a mouse strain-dependent difference in resistance to i.v. administered Y. enterocolitica (C57BL/6 mice are 40- to 667-fold more resistant than BALB/c) (2, 26). This phenotype was confirmed using our wild-type laboratory strain of Y. enterocolitica (JB580v) in C57BL/6j and BALB/cj mice. Following i.v. administration, the LD50 in C57BL/6j mice was 118-fold greater than in BALB/cj mice (LD50, 5.9 × 104 for C57BL/6j and 5 × 102 for BALB/cj). To determine if a similar phenotype was observable following oral administration of Y. enterocolitica, an LD50 analysis was done using C57BL/6j, BALB/cj, and 129X1/Svj mouse strains. There was little difference between the LD50s for C57BL/6j mice and 129X1/Svj mice (Table 1). There was a 5.1-fold difference between the LD50s for BALB/cj mice and C57BL/6j mice and a similar (2.3-fold) difference between those for BALB/cj mice and 129X1/Svj. This correlates with previous reports in the literature that BALB/c mice are more susceptible to i.v.-administered Y. enterocolitica. However, this difference was much smaller and was not statistically significant following duplicate LD50 analysis (P = 0.2636).
TABLE 1.
Duplicate LD50 analysis for each mouse straina
| Mouse strain | First LD50 (CFU) | Second LD50 (CFU) | Avg LD50 (CFU) | Fold change vs. BALB/Cj | Hazard ratio (CI95%)b |
|---|---|---|---|---|---|
| 129X1/Svj | 2.0 × 107 | 4.0 × 106 | 1.2 × 107 | 2.3 | 1.398 (0.785-2.569) |
| C57BL/6j | 3.7 × 107 | 1.8 × 107 | 2.7 × 107 | 5.1 | 1.306 (0.711-2.442) |
| BALB/cj | 7.8 × 106 | 2.8 × 106 | 5.3 × 106 |
Survival curves using combined data sets from both LD50 analyses were constructed and compared by using the Mantel-Haenszel log-rank test (Fig. 1). There was no statistically significant difference between the survival curves of each mouse strain (BALB/cj versus C57BL/6j, P = 0.3812; BALB/cj versus 129X1/Svj, P = 0.2465; C57BL/6j versus 129X1/Svj, P = 0.8100). Furthermore, the mice did not die at significantly different rates, as indicated by their hazard ratios (Table 1).
FIG. 1.
LD50 analysis. Survival curves for three strains of mice over a 14-day period are shown. Survival percentages were calculated using the Kaplan-Meier method (1). Six groups of five or six mice from each genetic background were infected orally with successive 10-fold dilutions of the bacterial suspension (104 to 109 bacteria) and monitored twice daily for 14 days. The analysis was done in duplicate in two independent experiments.
Colonization of 129X1/Svj, C57BL/6j, and BALB/cj mouse tissues following oral infection with Y. enterocolitica.
Kinetic analysis was done to determine levels of bacterial colonization in the PP, MLN, and spleens from each mouse strain. Hancock et al. previously showed decreased colonization of C57BL/6 PP tissue compared with infected BALB/cBy mouse tissue following oral infection with Y. enterocolitica (26). In this study, tissues were analyzed for their bacterial titers (CFU per gram of tissue) on days 1, 3, and 7 postinfection (Fig. 2). All 84 mice used in the study had detectable bacterial colonies in the PP as early as day 1 postinfection. Bacterial levels in the PP were consistently high (mean CFU per gram, 6.2 × 107 to 7.4 × 109) throughout the course of infection for all mouse strains (Fig. 2A). Furthermore, there was little variability with the data obtained from PP tissue compared to MLN and spleens (standard deviations [SD] from log-transformed data: PP = 0.50 to 1.02; MLN = 0.69 to 3.03; spleens = 1.27 to 4.72). There were no statistically significant differences (P < 0.05) between the mean daily levels of colonization in the PP between the mouse strains.
FIG. 2.
Kinetic analysis. CFU per gram of tissue in the PP (A), MLN (B), and spleens (C) of mice infected for 1, 3, or 7 days are shown. Results from two independent experiments were combined. Analysis was performed in duplicate for each mouse strain (C57BL/6j, dose 1 = 8 × 108 and dose 2 dose = 1 × 108; BALB/cj, dose 1 = 1 × 108 and dose 2 = 5 × 108); 129X1/Svj, dose 1 = 5 × 108 and dose 2 = 5 × 108). The limit of detection for each tissue is indicated by a dashed line. Nonparametric ANOVA using the Kruskal-Wallis statistic and Dunns post-test was used to compare mean CFU/gram of tissue for each day. Symbols at the limit of detection represent mice that survived but did not have detectable numbers of bacteria. Two C57BL/6j mice died by day 7.
Many of the MLN lacked colonization until day 3 postinfection (Fig. 2B). Consistent colonization was observable by day 3 and remained high through day 7, when a majority of the lymph nodes were colonized to very high levels (69% of MLN colonized on day 1, 96% on days 3 and 7). C57BL/6j MLN had significantly lower levels of colonization than did BALB/cj MLN (P < 0.05) on day 3 or 129X1/Svj MLN (P < 0.001) on days 3 and 7 postinfection (C57BL/6j versus 129X1/Svj, P < 0.001). Colonization of C57BL/6j MLN was also more variable than the BALB/cj and 129X1/Svj data (SD of log-transformed data: day 3, C57BL/6j = 2.39, BALB/cj = 1.20, 129X1/Svj = 0.69; day 7, C57BL/6j = 3.03, BALB/cj = 1.96, 129X1/Svj = 0.42).
Spleens, similar to the MLN, had increasingly consistent bacterial colonization throughout the 7-day course of infection (42% of spleens colonized on day 1, 86% on day 3, and 89% on day 7 postinfection) (Fig. 2C). There were no statistically significant differences (P < 0.05) in the mean bacterial colonization between mouse strains on each day. Variation increased in data gathered from spleens as infection progressed (SD of log-transformed data: day 1, 1.27 to 2.01; day 3, 1.74 to 2.06, day 7, 2.93 to 4.72). A majority of the 129X1/Svj mouse spleens were not colonized until day 7 of infection, in contrast to the spleens of C57BL/6j and BALB/cj mice, which were colonized by day 3 (day 3 spleen colonization: C57BL/6j, 100%; BALB/cj, 90%; 129X1/Svj, 63%). Although more of the 129X1/Svj spleens become colonized by day 7, this colonization is limited to a small number of bacteria compared to the levels in C57BL/6j and BALB/cj mice.
Increases in tissue weights were used as a further indicator of disease severity and progression. A previous study indicated that following an i.v. infection with Y. enterocolitica, C57BL/6 mice developed splenomegaly 7 days postinfection while BALB/c mice did not (2). In this study, all tissues from all three mouse strains increased in weight over the 7-day period of infection (data not shown). This increase was equivalent among the three strains of mice for the PP and the MLN. However, the 129X1/Svj mice showed a more significant increase in spleen weight on day 7 postinfection than did C57BL/6j (P = 0.0066) and BALB/cj (P = 0.0070) mice (difference in spleen weight between days 1 and 7: C57BL/6j, 0.0313 g; BALB/cj, 0.0204 g; 129X1/Svj, 0.1985 g).
Histological analysis revealed inflammatory pathologies previously described for Y. enterocolitica-infected tissues (4, 6, 31). Significant differences in pathology among mouse strains were not apparent in all tissues analyzed. No inflammation was apparent in uninfected mouse tissues; however, bacterial colonization always correlated with pathology. Inflammation and tissue destruction was noticeable in PP of mice as early as day 1 postinfection (Fig. 3). This early response was restricted to the area just beneath the dome of the PP and was almost always associated with bacteria. By day 3 postinfection, PP were frequently completely ablated, consisting of dead cells, bacterial colonies, granulolcytes, and macrophages (Fig. 3). The severity of this pathology was similar to what was seen on day 7 postinfection (data not shown).
FIG. 3.
Histological analysis of PP following oral infection with Y. enterocolitica. Peyer's patches were removed from uninfected mice (day 0) and infected mice on days 1 and 3 postinfection. Tissues were fixed in either 10% neutral buffered formalin or Bouin's fixative and embedded in paraffin for sectioning. Sections (5 μm) were stained with hematoxylin and eosin (A to I) or with Y. enterocolitica-specific Ab (J to R), indicated by the brown staining. Arrows indicate regions of inflammation seen on day 1 postinfection. Arrowheads indicate Ab stained bacterial colonies and corresponding inflammation.
Noticeable pathology was not present in the MLN until day 3 postinfection (Fig 4). The pathology in the MLN differed significantly from that in the PP and consisted primarily of granuloma-like lesions. These lesions were the most common pathological phenotype and consisted of a ring of inflammatory cells surrounding all visible bacterial colonization. These granuloma-like lesions were never observed in infected PP.
FIG. 4.
Histological analysis of MLN following oral infection with Y. enterocolitica. MLN were removed from uninfected and infected mice on day 3 postinfection. Tissues were fixed in either 10% neutral buffered formalin or Bouin's fixative and embedded in paraffin for sectioning. Sections (5 μm) were stained with hematoxylin and eosin (A, B, D, E, G, and H) or with Y. enterocolitica-specific Ab (C, E, and I), indicated by the brown staining.
A similar granuloma-like lesion was observed in the spleens of all three mouse strains (data not shown). This pathology was rarely evident on day 3 but increased in frequency in the spleens of mice infected for 7 days.
Levels of representative Th1 and Th2 cytokines produced by cell suspensions from C57BL/6j, BALB/cj, and 129X1/Svj PP, MLN, and spleens.
Th-cell phenotypes are characterized into Th1 or Th2 subsets on the basis of the cytokines they produce (29). Both subsets provide assistance to innate immune cells in killing microbial pathogens and polarizing subsequent adaptive cellular immune responses. Th1 cells which characteristically produce IFN-γ can activate macrophages, helping them to kill extracellular and intracellular pathogens which resist killing after being engulfed. Th2 cells predominantly produce IL-4, IL-5, and IL-13 and can initiate the humoral immune response by activating naive antigen-specific B cells to produce immunoglobulin M (IgM) and subsequently different isotypes of Ig. Previous studies using the i.v. model of Y. enterocolitica infection have demonstrated that in a mouse, the IFN-γ producing Th1 phenotype predominates during Y. enterocolitica infection (2, 5, 9-11, 27). However, a comprehensive study of Th1 and Th2 cytokine production in different tissues following oral infection has yet to be done. Therefore, the levels of several characteristic Th1 (IFN-γ and IL-2) and Th2 (IL-5 and IL-4) cytokines and the proinflammatory cytokine TNF-α were measured from cell populations isolated from uninfected and infected mice on days 1, 3, and 7 postinfection. Cytokine production was measured by coculturing single-cell suspensions from infected PP, MLN, and spleens exposed to HKY-loaded antigen-presenting cells.
TNF-α levels in all tissues do not increase until after day 1 postinfection (Fig. 5A to C). The PP of BALB/cj mice produced increasing levels of TNF-α throughout the 7-day period of infection, while levels in C57BL/6j and 129X1/Svj mice increased on day 3 but had receded by day 7. Levels of TNF-α increased from 5.3- to 13.6-fold between days 1 and 3 in the MLN of all three strains of mice (Fig. 5B). The levels in the spleens also increased between days 1 and 3 by approximately 6.0- to 10.6-fold (Fig. 5C). However, on day 7, the spleens of 129X1/Svj mice contained higher TNF-α levels than did those of both C57BL/6j and BALB/cj mice.
FIG. 5.
Cytokine production by cell suspensions from C57BL/6j (▪), BALB/cj (•), and 129X1/Svj (▴) mouse tissue suspensions cocultured with HKY-stimulated macrophages. Levels of TNF-α (A to C) and IFN-γ (D to F) measured following 48 h of incubation are shown. Cells were isolated from PP, MLN, and spleens of uninfected mice (day 0) and infected mice on days 1, 3, and 7 postinfection.
Basal levels of IFN-γ were equivalent among mouse strains for all tissues analyzed (Fig. 5D to F). Increased IFN-γ production was not detected until day 3 postinfection. IFN-γ levels in the isolated cell suspensions from PP of each mouse strain reached a plateau between days 3 and 7 postinfection, with overall levels being lower in the PP of C57BL/6j mice (Fig. 5D). IFN-γ levels were several log units higher in the MLN and spleens than in the PP. There was no difference in the levels of IFN-γ in the MLN in all three strains of mice throughout the time course of infection (Fig. 5E). IFN-γ levels in the spleens of all three mouse strains increased on day 3 post-infection. However, the levels on day 7 increased dramatically in 129X1/Svj mice while receding in C57BL/6j and BALB/cj mice (Fig. 5F).
IL-2 levels were below the limit of detection (20 pg ml−1) in all tissues in each mouse strain at each time point analyzed, except in the spleens of 129X1/Svj mice (data not shown). On day 7, splenocytes from 129X1/Svj mice produced detectable levels of IL-2 (24.2 pg ml−1) while those of C57BL/6j and BALB/cj mice did not.
Overall Th2 cytokine (IL-4 and IL-5) levels were below the limit of detection (20 pg ml−1) in the tissues of all three strains of mice at each time point analyzed (data not shown). Collectively, the combined cytokine profile in tissues from all three mouse strains is proinflammatory (increasing levels of TNF-α), with a Th1 phenotype (increasing IFN-γ level, no increase in IL-4 or IL-5 levels).
FACS analysis of infected and uninfected tissues of C57BL/6j, BALB/cj, and 129X1/Svj mice.
Fluorescence-activated cell sorter (FACS) analysis for cell types involved in the innate and adaptive immune response was performed to characterize the different types of immune cells present in different tissues before and after the initiation of an oral Y. enterocolitica infection. Single-cell suspensions from the PP, MLN, and spleens of all three mouse strains were analyzed for uninfected and infected mice on days 1, 3, and 7 postinfection.
The quantities of granulocytes (Gr-1+) and macrophages (BM-8+) were measured to partially characterize the innate immune compartment of each tissue following oral Y. enterocolitica infection (Fig. 6). Gr-1+ cell numbers in the PP increased rapidly between days 1 and 3 in C57BL/6j (sevenfold), 129X1/Svj (fourfold), and BALB/cj (fourfold) mice, with high levels in all three mouse strains by day 7 postinfection (C57BL/6j, 41%; BALB/cj, 56%; 129X1/Svj, 41%). Overall, there were smaller numbers of Gr-1+ cells in the MLN and spleen of each mouse strain than in the PP. There was little difference between mouse strains in the levels or in the increase in the numbers of Gr-1+ cells in the MLN. However, C57BL/6j mice had a threefold increase in the numbers of Gr-1+ cells in their spleens compared to the relatively small change observed in either the BALB/cj or 129X1/Svj mice.
FIG. 6.
Immunophenotyping. FACS analysis was performed on cell suspensions from C57BL/6j (▪), BALB/cj (•), and 129X1/Svj (▴) mouse tissues following oral infection with Y. enterocolitica. Values on the y axis indicate the percentage of cells from a gate encompassing all live cells (Gr-1, granulocytes; BM-8, macrophages).
Recruitment of macrophages during infection as determined by BM-8 staining showed recruitment to the PP, MLN, and spleens of all three mouse strains (Fig. 6). Variation in BM-8+ recruitment among mouse strains was evident in the MLN, with the level in BALB/cj mice increasing eightfold, on contrast to the levels in C57BL/6j (only twofold) and 129X1/Svj (threefold) mice over the 7-day course of infection. There was a similar yet smaller increase in the number of BM-8+ cells in the PP and spleens of BALB/cj mice compared to those in C57BL/6j and 129X1/Svj mice (PP: C57BL/6j = threefold, BALB/cj = fourfold, 129X1/Svj = twofold; spleen: C57BL/6j = twofold, BALB/cj = threefold, 129X1/Svj = twofold). Although 129X1/Svj mice started with similar numbers of BM-8+ cells in their PP and MLN, the level this cell type did not increase as extensively throughout infection as it did in C57BL/6j and BALB/cj PP and MLN (Fig. 6D and E).
The levels of natural killer (NK) cells (Ly49g+), and dendritic cells (CD11c+) were also analyzed and found to consistently be less than 1 to 3% of the cell population and to change little during the time course analyzed (data not shown).
The adaptive immune compartment was assessed by quantifying the levels of B cells (CD19+), and several subsets of T cells including αβ T cells (βTCR+), γδ T cells (γδTCR+), CD8+ T cells (βTCR+-CD8+), CD4+ T cells (βTCR+-CD4+), regulatory T cells (CD4+-CD25+), and activated T cells (CD3+-CD62L−) (Fig. 7).
FIG. 7.
Immunophenotyping. T-cell populations and B cells from C57BL/6j (▪), BALB/cj (•), and 129X1/Svj (▴) tissues following oral infection with Y. enterocolitica are shown. Values on y axis indicate the percentage of cells from the lymphocyte gate (αβTCR, mature T-cells; CD3+-CD62L−, activated T cells; CD-19, B cells) or ratios of these values (CD4, Th cells; CD8, cytotoxic T cells).
129X1/Svj PP contained more cells expressing the αβ-TCR both before and after oral infection with Y. enterocolitica than did PP of both C57BL/6j and BALB/cj mouse strains (Fig. 7A). The levels of αβ-TCR-expressing cells also remained fairly constant in the C57BL/6j and BALB/cj mouse strains throughout the 7-day course of infection. This contrasts with what was observed in the MLN of all three mouse strains. During infection, there was a 25% average decrease in the αβ-TCR-expressing cells in all mouse strains during the 7-day course of infection (Fig. 7B). The cell population expressing the αβ-TCR in the spleen fluctuated between 25 and 50%; however, over the 7-day course of infection, there was no difference in the increase or decrease of these cells, except that BALB/cj mice had increased numbers before and throughout the course of infection compared with C57BL/6j and 129X1/Svj mice (Fig. 7C). The levels of γδ T cells and regulatory T cells (CD4+ CD25+) were less than 1 to 3% in all tissues and mouse strains throughout infection (data not shown).
CD-19+ were the predominant cell type identified in the PP of all three strains of mice. CD19+-expressing B cells remained at constant levels in the PP of each mouse strain over the 7-day course of infection (Fig. 7G to I). In contrast, the levels of CD19+ B cells steadily increased by 3.5 to 5.0-fold in the MLN and 2-fold in the spleens of each mouse strain (Fig. 7H).
Both BALB/cj and 129X1/Svj mice had higher CD4+-to-CD8+ ratios (more CD4+ Th cells than CD8+ cytotoxic T cells) than did C57BL/6j mice, which had more CD8+ T cells in all tissues analyzed (Fig. 7D to F). The ratio difference is due to C57BL/6j mice having both more CD8+ and fewer CD4+ cells in all tissues and at all time points. This difference remains constant in the PP, MLN, and spleens throughout the duration of the Y. enterocolitica infection.
CD62L (L-selectin) is a T-cell outer membrane protein which can be used to monitor T-cell activation. Resting naive T cells express L-selectin, which they use for homing to peripheral lymph nodes (34). On T-cell activation, L-selectin expression is lost. We monitored the quantity of CD3+-CD62L+ versus CD3+-CD62L− cells as a marker of T-cell activation. Of the CD3+ T cells remaining as infection progressed, CD3+ cells gradually reduce CD62L expression in each tissue analyzed from each mouse strain (Fig. 7J to L). The only exception to this was the MLN of 129X1/Svj mice, which have similar numbers of CD3+-CD62L− cells at on day 7 postinfection to those in uninfected mice (Fig. 7K). This decrease was more immediate in the PP and spleens than in the MLN. The number of CD3+-CD62L− T cells was greater in the spleens of C57BL/6j and BALB/cj mice than in spleens of 129X1/Svj mice (Fig. 7L).
DISCUSSION
Studies initiated by Autenrieth and followed up on by Bohn and Autenrieth have demonstrated several potential mechanisms to explain the increased resistance of C57BL/6 mice to BALB/c mice in an i.v.-administered Y. enterocolitica infection model (2, 3, 9-12, 27). Their work demonstrates that as an i.v.-administered infection progresses, C57BL/6 mouse spleens mount a more pronounced inflammatory response than do BALB/c mouse spleens. This tissue-specific response is characterized by splenomegaly, bacterial clearance, and more rapid activation of both CD4+ and CD8+ T cells (2). Splenic T cells transferred from C57BL/6 mice to naive mice 7 days after infection provided protective immunity (2). Similar protective immunity for BALB/c mice was demonstrated but required T cells isolated 21 days postinfection. Splenocytes isolated from i.v.-infected C57BL/6 mice were also able to proliferate and produced IFN-γ more rapidly in response to stimulation with HKY and IL-12 than did splenocytes from BALB/c mice (9). Furthermore, treatment of BALB/c mice with IFN-γ, IL-12, or anti-IL-4 rendered them more resistant but had little to no effect on Y. enterocolitica infection in C57BL/6 mice (2). Taken together, these studies suggest that BALB/c mice have delayed splenic T-cell activation correlating with levels in Th1 cytokines, resulting in a state of increased susceptibility compared with C57BL/6 mice.
Although the above studies have elucidated a number of molecular and cellular mechanisms involved in Y. enterocolitica pathogenesis, many of them have been out of the context of the typical route of infection. i.v. administration of bacteria immediately exposes the host to a systemic infection with the primary sites of infection, i.e., those that filter blood, such as the spleen and liver. However, Y. enterocolitica is usually naturally acquired via an oral route (e.g., ingestion of contaminated food), which exposes the bacteria to the mucosal immune environment of the small intestine prior to dissemination to deeper tissues such as the spleen, lungs, and liver.
In this study, we present data that suggest when Y. enterocolitica is administered via the oral route, host resistance, as determined by LD50 and survival curve analysis, is highly similar among C57BL/6j, BALB/cj, and 129X1/Svj mouse strains. This contrasts with results of i.v. infection studies completed in our and other laboratories, in which C57BL/6j mice are more resistant than BALB/c mice (2, 25, 26). Furthermore, the bacteria were capable of colonizing the PP of the small intestine and subsequently spreading into the MLN and spleens of each mouse strain tested. Although consistently high levels of bacteria were detected in the PP of orally infected mice, differences in the levels of bacterial colonization increased between mouse strains as the bacteria disseminated into the MLN and spleen of each mouse strain. This was particularly evident in the MLN of C57BL/6j mice, which had lower levels of bacteria than did the MLN of both BALB/cj and 129X1/Svj mice on day 3 postinfection. This may be reflective of previous studies using the i.v.-infection model, in which C57BL/6 mice are generally able to control bacterial colonization better than BALB/c mice. Although there were quantitative differences in the levels of bacterial colonization in these tissues between mouse strains, the strains exhibited a similar pattern of tissue colonization and corresponding tissue pathology as determined by a detailed histological analysis. Therefore, each mouse strain could be useful in studies concerning the infectious processes occurring in the PP and MLN of mice orally infected with Y. enterocolitica.
Colonization of C57BL/6j and BALB/cj mouse spleens became increasingly varied as infection progressed. Levels of bacterial colonization in these strains varied over a 6- to 8-log-unit range on days 3 and 7 postinfection. This increased variation was not apparent in the spleens of 129X1/Svj mice throughout the course of infection. The ability of 129X1/Svj mice to maintain consistently low levels of bacteria in their spleen may be due to the increased production of TNF-α and IFN-γ compared with the C57BL/6j and BALB/cj mouse strains (see below).
The increased variation of splenic colonization raises specific challenges in analyzing the host response in the spleens following oral infection with Y. enterocolitica. Not only will there be different responses in individual spleens due to quantitative differences in bacterial colonization, but also there could potentially be variation due to the variation in the frequency of colonization. This effect is most prominent early during infection, when many spleens lack any detectable colonization, and becomes less severe on day 7, when colonization is more frequent. Variation in the frequency of colonization will result in two populations of spleens: one which is responding to both colonization of itself and to colonization at other sites in the animal such as the PP and MLN, and one which is not colonized and is responding only to events occurring elsewhere in the animal. There are two basic approaches which can be taken to deal with this phenomenon. For this study, we combined the spleens of many mice in an attempt to screen for global immunological responses occurring during infection. This may have potentially masked responses differing between the two populations of spleens. An alternative approach would be to analyze individual spleens of mice for bacterial colonization and cellular and molecular immune responses simultaneously. This approach may prove to be more useful when asking questions concerning a specific cellular or molecular event involving oral Y. enterocolitica infection.
Along with demonstrating a difference in LD50 of i.v.-administered Y. enterocolitica infection between mouse strains Hancock et al. report that BALB/cBy mice restricted the infection to the small intestine following oral infection. The MLN of BALB/cBy mice never became colonized with Y. enterocolitica, while those of C57BL/6 mice did (26). BALB/cBy and BALB/cj are substrains that were separated from the original BALB/c mouse strain in 1935 (21). Very little is known about the differences between the two substrains other than the variation existing at the nonpolymorphic major histocompatibility complex class I-like antigen locus, Qa-2 (30). BALB/cBy have a large genomic deletion resulting in a Qa-2 phenotype, while BALB/cj are Qa-2+; this may be important when considering pathogens which colonize areas of the small intestine. The presence of the unique subset of intestinal intraepithelial lymphocytes, the CD8α/α T cells, is dependent on the presence of the Qa-2 locus (18). Therefore, CD8α/α T cells are not present in BALB/cBy mice but are present in BALB/cj mice. Little is known about the specific role of CD8α/α T cells in the host response to bacterial pathogens. However, it is hypothesized that they are a resident population that does not participate in antigen-specific immune responses, but may play a role in localized immune regulation (24). It is interesting to speculate that CD8α/α T cells may play a role in dissemination from the PP to the MLN or in other host responses restricting Y. enterocolitica to the small intestine.
The data presented here may also differ from previous reports due to differences in mouse source and bacterial strain (2, 9-12, 26, 27). The C57BL/6 and BALB/c mice used in the present study were acquired from The Jackson Laboratory in Bar Harbor, Maine. Autenrieth et al. and Bohn et al. acquired C57BL/6 and BALB/c mice for their studies from Charles River Wiga in Sulzfeld, Germany. Both of these mouse strains have been continuously inbred from the same original breeding stocks; however, it is possible that subtle differences due to environmental conditions could affect experimental outcomes. Furthermore, while we used Y. enterocolitica strain JB580v, the other groups used strain WA; both strains are serotype O8 and are considered to be highly virulent. To be certain that both the mouse and bacterial strains used in the present study exhibited similar phenotypes previously described in other laboratories, we completed an LD50 analysis using mice obtained from The Jackson Laboratory and i.v.-administered Y. enterocolitica JB580v. Similar to previously reported data, the C57BL/6j mouse strain was more resistant to i.v.-administered Y. enterocolitica strain JB580v than was the BALB/cj strain. Therefore, both the mouse and Y. enterocolitica strains used in this study produced a similar response to that reported in previous studies. Data acquired comparing the differences in the mouse strains infected orally with Y. enterocolitica strain JB580v versus mice infected i.v. should be valid and useful.
In view of the differences between our oral infection model and previous studies in which an i.v. infection model was used, we were interested in determining whether cellular and molecular immune phenotypes recognized as being important during the host response to Y. enterocolitica also differed. In addition, we were interested in characterizing and quantifying critical molecular and cellular components of the immune response following an oral Y. enterocolitica infection in tissues commonly colonized following an oral infection. The results from our Th1-Th2 cytokine analysis confirm observations made by other researchers that a Th1 environment, with high levels of IFN-γ and low levels of IL-4 and IL-5, predominates during a Y. enterocolitica infection (10, 12, 27). We found this to be true in PP, MLN, and spleens of all mouse strains.
TNF-α production also increased as infection progressed. Beuscher et al. have demonstrated that there is no TNF-α protein production in BALB/c PP 6 days after oral infection with Y. enterocolitica as determined by immunostaining and in ex vivo tissue and cell culture assays (8). The reduction in TNF-α protein production is due to the YopB protein, encoded by the virulence plasmid of Y. enterocolitica. In addition, both YopP for Y. enterocolitica and YopJ for Y. pseudotuberculosis have been assigned roles for decreasing the amount of TNF-α produced in macrophage cell culture (17). Similarly, the virulence-associated V antigen (LcrV) can decrease TNF-α production in stimulated macrophages through the induction of IL-10 (33). Therefore, there are at least three mechanisms by which Y. enterocolitica can actively suppress TNF-α levels. The experiments presented here do not address whether any TNF-α suppression is occurring due to bacterial or host factors. Therefore, although we see increased levels of TNF-α throughout the 7-day course of infection, it is impossible to detect the extent of TNF-α suppression occurring in the tissues analyzed.
There were few major differences in tissue cytokine levels between the mouse strains. PP of BALB/cj mice had increased TNF-α production compared to C57BL/6j and 129X1/Svj mice. Since these tissues were equally colonized with bacteria at this stage in infection, this was probably a result of differences between mouse strains. TNF-α levels in the MLN were decreased between days 3 and 7 postinfection in both C57BL/6j and BALB/cj mice; this differed from TNF-α production in 129X1/Svj mice, which produced a smaller but more consistent amount during this time. TNF-α production increased in the spleens of 129X1/Svj mice compared to that in the spleens of C57BL/6j and BALB/cj mice. Along with increased TNF-α production, there was an increase in IFN-γ production and a slight increase in IL-2 production. The heightened cytokine response in the spleens of 129X1/Svj mice may explain the decreased bacterial colonization observed throughout infection compared to that in C57BL/6j and BALB/cj mice.
Previous studies have shown that T cells isolated from spleens of i.v.-infected C57BL/6 mice produce more IFN-γ on exposure to HKY than do BALB/c splenic T cells (2). Increased IFN-γ production was seen as early as day 2 postinfection and increased steadily through day 10, eventually decreasing to levels comparable to those in BALB/c mice by day 20. Our data demonstrate that following oral infection, there was no difference in IFN-γ production by C57BL/6j and BALB/cj mouse splenocytes stimulated with HKY. However, a difference was seen between 129X1/Svj and both C57BL/6j and BALB/cj mouse strains. PP from the BALB/cj mouse strain were better IFN-γ producers than those from both the C57BL/6j and 129X1/Svj mouse strains. Although there appears to be some level of difference between these mouse strains, the difference also appears to be dependent on the route of infection and the tissue being analyzed.
To further characterize the oral-infection model, it was useful to perform FACS analysis on several immune cell types involved in both the innate and adaptive immune responses. This was done not only to corroborate findings found using other routes of infection and to assess differences between mouse strains but also to determine the levels of important immune cell types in a tissue-specific manner following oral infection. Quantitative differences in cell type were seen between each mouse strain and also between the different lymphoid tissues. However, cell types typically recruited during an inflammatory response to Y. enterocolitica and to bacteria in general were found in all mouse strains and all tissues during our analysis.
Taken together, our data emphasize that when using a mouse model to study disease pathology and immunological response, it is important to take into consideration not only the mouse strain but also the way in which the bacteria are administered. Although some of our data correspond to results of previous studies which utilized an i.v. infection model (e.g., Th1 bias and recruitment of neutrophils and macrophages), overall pathological conditions such as differences between mouse strains in LD50, rate of death, and bacterial colonization of specific tissues do not agree when using an oral-infection model. Both infection models have proven useful in the discovery of many molecular and cellular mechanisms involved in the pathogenesis of Y. enterocolitica. Future comparative studies using both model systems will be useful for elucidating new mechanisms in which the mammalian host recognizes and defends against bacteria by using the mucosal and the systemic immune responses.
Acknowledgments
This study was supported by National Institutes of Health grants AI27342 and AI52167 awarded to V.L.M. and 1 F32DK59700-02 awarded to P.H.D. Histological sections were prepared by the Washington University Digestive Diseases Research Core Facility funded by grant P30-DK52574 from the National Institutes of Health.
Editor: J. T. Barbieri
REFERENCES
- 1.Altman, D. G. 1991. Practical statistics for medical research. Chapman & Hall, London, United Kingdom.
- 2.Autenrieth, I. B., M. Beer, E. Bohn, S. H. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., M. Beer, P. Hantschmann, S. Preger, U. Vogel, B. Heymer, and J. Heesemann. 1993. The cellular immune response against Yersinia enterocolitica in different inbred strains of mice: evidence for an important role of T lymphocytes. Zenbl. Bakteriol. 278:383-395. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth, I. B., P. Hantschmann, B. Heymer, and J. Heesemann. 1993. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology 187:1-16. [DOI] [PubMed] [Google Scholar]
- 5.Autenrieth, I. B., and J. Heesemann. 1992. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med. Microbiol. Immunol. 181:333-338. [DOI] [PubMed] [Google Scholar]
- 6.Autenrieth, I. B., V. Kempf, T. Sprinz, S. Preger, and A. Schnell. 1996. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autenrieth, I. B., U. Vogel, S. Preger, B. Heymer, and J. Heesemann. 1993. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 61:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beuscher, H. U., F. Rodel, A. Forsberg, and M. Rollinghoff. 1995. Bacterial evasion of host immune defense: Yersinia enterocolitica encodes a suppressor for tumor necrosis factor alpha expression. Infect. Immun. 63:1270-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohn, E., and I. B. Autenrieth. 1996. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J. Immunol. 156:1458-1468. [PubMed] [Google Scholar]
- 10.Bohn, E., J. Heesemann, S. Ehlers, and I. B. Autenrieth. 1994. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect. Immun. 62:3027-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohn, E., E. Schmitt, C. Bielfeldt, A. Noll, R. Schulte, and I. B. Autenrieth. 1998. Ambiguous role of interleukin-12 in Yersinia enterocolitica infection in susceptible and resistant mouse strains. Infect. Immun. 66:2213-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 13.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter, P. B. 1975. Animal model of human disease. Yersinia enteritis. Animal model: oral Yersinia enterocolitica infection of mice. Am. J. Pathol. 81:703-706. [PMC free article] [PubMed] [Google Scholar]
- 15.Carter, P. B. 1975. Pathogenecity of Yersinia enterocolitica for mice. Infect. Immun. 11:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis, G. 1987. Yersinia enterocolitica, an excellent model for the molecular study of the pathogenicity of invasive bacteria. Bull. Mem. Acad. R. Med. Belg. 142:126-136. [PubMed] [Google Scholar]
- 17.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das, G., D. S. Gould, M. M. Augustine, G. Fragoso, E. Sciutto, I. Stroynowski, L. Van Kaer, D. J. Schust, H. Ploegh, C. A. Janeway, Jr., and E. Scitto. 2000. Qa-2-dependent selection of CD8alpha/alpha T cell receptor alpha/beta(+) cells in murine intestinal intraepithelial lymphocytes. J. Exp. Med. 192:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Koning-Ward, T. F., A. C. Ward, E. L. Hartland, and R. M. Robins-Browne. 1995. The urease complex gene of Yersinia enterocolitica and its role in virulence. Contrib. Microbiol. Immunol. 13:262-263. [PubMed] [Google Scholar]
- 20.Dube, P. H., S. A. Handley, P. A. Revell, and V. L. Miller. 2003. The rovA mutant of Yersinia enterocolitica displays differential degrees of virulence depending on the route of infection. Infect. Immun. 71:3512-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green M. C., and B. A. Witham. 1991. Handbook on genetically standardized JAX mice, 4th ed. The Jackson Laboratory, Bar Harbor, Maine.
- 22.Gripenberg-Lerche, C., L. Zhang, P. Ahtonen, P. Toivanen, and M. Skurnik. 2000. Construction of urease-negative mutants of Yersinia enterocolitica serotypes O:3 and O:8: role of urease in virulence and arthritogenicity. Infect. Immun. 68:942-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grutzkau, A., C. Hanski, H. Hahn, and E. O. Riecken. 1990. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut 31:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy-Grand, D., O. Azogui, S. Celli, S. Darche, M. C. Nussenzweig, P. Kourilsky, and P. Vassalli. 2003. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J. Exp. Med. 197:333-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hancock, G. E., R. W. Schaedler, and T. T. MacDonald. 1988. Multigenic control of resistance to Yersinia enterocolitica in inbred strains of mice. Infect. Immun. 56:532-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock, G. E., R. W. Schaedler, and T. T. MacDonald. 1986. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect. Immun. 53:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hein, J., V. A. Kempf, J. Diebold, N. Bucheler, S. Preger, I. Horak, A. Sing, U. Kramer, and I. B. Autenrieth. 2000. Interferon consensus sequence binding protein confers resistance against Yersinia enterocolitica. Infect. Immun. 68:1408-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R−M+ mutant. Gene 136:271-275. [DOI] [PubMed] [Google Scholar]
- 29.McGuirk, P., and K. H. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 30.Mellor, A. L., J. Antoniou, and P. J. Robinson. 1985. Structure and expression of genes encoding murine Qa-2 class I antigens. Proc. Natl. Acad. Sci. USA 82:5920-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepe, J. C., M. R. Wachtel, E. Wagar, and V. L. Miller. 1995. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect. Immun. 63:4837-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 33.Sing, A., A. Roggenkamp, A. M. Gieger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10- deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 34.Tamatani, T., F. Kitamura, K. Kuida, M. Shirao, M. Mochizuki, M. Suematsu, G. W. Schmid-Schonbein, K. Watanabe, S. Tsurufuji, and M. Miyasaka. 1993. Characterization of rat LECAM-1 (L-selectin) by the use of monoclonal antibodies and evidence for the presence of soluble LECAM-1 in rat sera. Eur. J. Immunol. 23:2181-2188. [DOI] [PubMed] [Google Scholar]
- 35.West, A. 1997. Pathology of infectious diseases. Appleton & Lange, Stamford, Conn.