Abstract
The lipopeptide FSL-1 [S-(2,3-bispalmitoyloxypropyl)-Cys-Gly-Asp-Pro-Lys-His-Pro-Lys-Ser-Phe, Pam2CGDPKHPKSF] synthesized on the basis of the N-terminal structure of a Mycoplasma salivarium lipoprotein capable of activating normal human gingival fibroblasts to induce the cell surface expression of ICAM-1 revealed an activity to induce production of monocyte chemoattractant protein 1, interleukin-6 (IL-6), and IL-8. FSL-1 also activated macrophages to produce tumor necrosis factor alpha as the Mycoplasma fermentans-derived lipopeptide MALP-2 (Pam2CGNNDESNISFKEK), a potent macrophage-activating lipopeptide, did. The level of the activity of FSL-1 was higher than that of MALP-2. This result suggests that the difference in the amino acid sequence of the peptide portion affects the activity because the framework structure other than the amino acid sequence of the former is the same as that of the latter. To determine minimal structural requirements for the activity of FSL-1, the diacylglyceryl Cys and the peptide portions were examined for this activity. Both portions did not reveal the activity. A single amino acid substitution from Phe to Arg and a fatty acid substitution from palmitic acid to stearic acid drastically reduced the activity. Similar results were obtained in measuring the NF-κB reporter activity of FSL-1 to human embryonic kidney 293 cells transfected with Toll-like receptor 2 and 6, together with a NF-κB-dependent luciferase reporter plasmid. These results suggest that both the diacylglyceryl and the peptide portions of FSL-1 are indispensable for the expression of biological activities and for the recognition by Toll-like receptors 2 and 6 and that the recognition of FSL-1 by Toll-like receptors 2 and 6 appears to be hydrophobic.
Various bacterial cell wall components such as lipopolysaccharides (LPS), lipoteichoic acid, peptidoglycans, and lipoproteins (LP) have been shown to activate macrophages, fibroblasts, or lymphocytes to induce production of cytokines (16). Escherichia coli LP were first characterized and sequenced by Braun (9), and they have been demonstrated to be biologically active (5-8, 20). The part of LP responsible for biological activity is the N-terminal lipopeptide moiety, the structure of which is S-(2,3-bispalmitoyloxypropyl)-N-palmitoyl-Cys-Ser-Ser-Asp-Ala- (Pam3CSNNA-) (7).
Mycoplasmas, wall-less microorganisms, also possess LP capable of activating macrophages or fibroblasts (11, 27, 28, 31, 32). Mühlradt et al. (27, 28) recently identified a 2-kDa lipopeptide, MALP-2, from Mycoplasma fermentans that is capable of activating monocytes/macrophages, and these authors determined the structure to be S-(2,3-bispalmitoyloxypropyl)Cys-Gly-Asn-Asn-Asp-Glu-Ser-Asn-Ile-Ser-Phe-Lys-Glu-Lys(Pam2CGNNDESNISFKEK). We have also found that Mycoplasma salivarium LP activate normal human gingival fibroblasts (HGF) to induce production of inflammatory cytokines and surface expression of ICAM-1 and have purified a 44-kDa LP (LP44) responsible for the activity (32). The structure of the N-terminal lipopeptide moiety of LP44 has been determined to be Pam2CGDPKHPKSFTGWVA- (32). The lipopeptide Pam2CGDPKHPKSF (FSL-1) synthesized on the basis of the N-terminal structure of LP44 showed the same activity as LP44 (32). The framework structure of FSL-1 is the same as that of MALP-2, but they differ in the amino acid sequence and length of the peptide portion. Mycoplasmal lipopeptides such as FSL-1 and MALP-2 contain two ester-linked fatty acids bound to glyceryl Cys and a free N terminus of the peptide portion. It is of great interest to know the structural requirements of these lipopeptides for the expression of their biological activities.
A key element in the initiation of an innate immune response against pathogens is the recognition of components commonly found on the pathogen that are not normally found in the host. These components have been referred to as pathogen-associated molecular patterns (24).
Toll-like receptors (TLRs) have recently been identified and implicated as receptors for pathogen-associated molecular patterns such as LPS, peptidoglycan, and LP (1, 2, 19, 23, 39). It has already been demonstrated that TLR2 functions as a receptor for microbial LP and lipopeptides (2-4, 10, 13, 17, 18, 22, 23, 29, 36, 38) and that signaling by MALP-2 is mediated by TLR2 (10, 17, 18, 38). More recently, it has been demonstrated that TLR2 requires TLR6 as a coreceptor for recognition of diacylated lipopeptides (38).
In the present study, therefore, experiments were carried out to further clarify the structure-function relationship of FSL-1 and the structural requirements of diacylated lipopeptides for recognition by the receptor consisting of TLR2 and TLR6.
MATERIALS AND METHODS
Antibodies, reagents, and cells.
Dulbecco modified Eagle medium (DMEM), RPMI 1640 medium, penicillin G, streptomycin, and trypsin-EDTA were obtained from Gibco-BRL (Rockville, Md.). Fluorescein isothiocyanate (FITC)- and peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) and FITC-conjugated anti-rabbit IgG antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, Pa.). All of the other chemicals were obtained from commercial sources and were of analytical or reagent grade.
HGF prepared and used in our previous study (11) were cultured in DMEM containing 10% (vol/vol) fetal bovine serum (FBS; Gibco), penicillin G (100 U/ml), and streptomycin (100 μg/ml) in plastic culture dishes. In the present study, HGF between passages 6 and 8 were used.
A human acute monocytic leukemia cell line, THP-1 (40), was obtained from Health Science Research Resources Bank (Osaka, Japan). These cell lines were grown at 37°C in a humidified atmosphere of 5% CO2 in RPMI 1640 medium supplemented with 10% (vol/vol) FBS, penicillin G (100 U/ml), and streptomycin (100 μg/ml).
Human embryonic kidney 293 (HEK293) cells obtained from ATCC (CRL-1573) were maintained in DMEM containing 10% FBS, penicillin G (100 U/ml), and streptomycin (100 μg/ml).
Synthesis of lipopeptides.
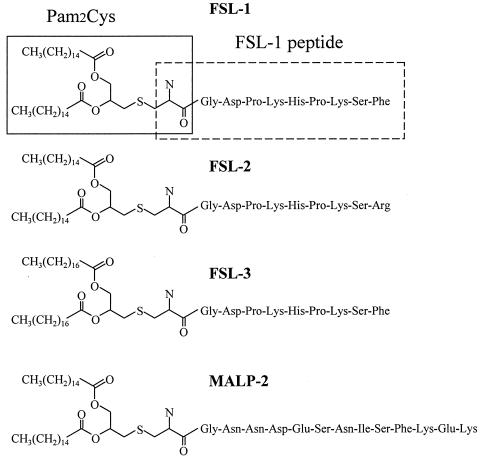
FSL-1 and its derivatives (FSL-2 and FSL-3) and MALP-2, the structures of which are given in Fig. 1, were synthesized as follows. The side chain-protected GDPKHSPKSF, GDPKHSPKSR, or GNNDESNISFKEK was built up with an automated peptide synthesizer, model 433 (Applied Biosystems, Foster City, Calif.). Fmoc (9-fluorenylmethoxy carbonyl)-S-(2,3-bis-palmitoyloxypropyl)-cysteine and Fmoc-S-(2,3-bisstearyloxypropyl)-cysteine(Novabiochem, Laeufelfingen, Switzerland) were manually coupled to the peptide-resin by using a solvent system of 1-hydroxy-7-azabenzotriazole-1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide/CH2Cl2-N,N′-dimethylformamide. The Fmoc and resin were removed from the lipopeptide by trifluoroacetic acid. The lipopeptides were purified by preparative high-pressure liquid chromatography with a reversed-phase C18 column (30 by 250 mm). The purities of these lipopeptides were confirmed by analytical high-pressure liquid chromatography with a reversed-phase C18 column (4.6 by 150 mm) to be >90%. These lipopeptides were used without separation of the S and R stereoisomers. These lipopeptides were originally dissolved in phosphate-buffered saline (PBS) containing 10 mM n-octyl-β-glucopyranoside in PBS and diluted with PBS to reduce the n-octyl-β-glucopyranoside concentration to <0.5 mM when used for stimulation.
FIG. 1.
Structures of FSL-1, Pam2Cys, FSL-1 peptide, and derivatives of FSL-1.
Cytokine production.
HGF (104 cells) were seeded in a 96-well flat-bottom microplate. After the HGF had reached confluency, the cells were stimulated at 37°C for 15 h with lipopeptides in a humidified atmosphere of 5% CO2 in DMEM supplemented with 0.1% (vol/vol) human serum. The culture supernatant was collected and examined for production of IL-6 and IL-8 by using an HU IL-6 Cytoset (Biosource International, Inc., Camarillo, Calif.). Monocyte chemoattractant protein 1 (MCP-1) was measured by using an MCP-1 immunoassay (R&D Systems, Minneapolis, Minn.).
THP-1 cells were incubated at 37°C for 3 days in the presence of 100 nM vitamin D3. After a 0.2-ml volume of cell suspension of THP-1 (5 × 105 cells) in each well of a 96-well tissue culture plate had been incubated at 37°C for 15 h with FSL-1 in the culture medium supplemented with 0.1% (vol/vol) human serum, the culture supernatant was collected by centrifugation at 400 × g for 10 min. TNF-α in the supernatant was determined by using an HU TNF-α Cytoset (Biosource).
Cloning of human TLR2, TLR6, and dominant-negative TLR6.
The cDNAs of human TLR2 and TLR6 were obtained by reverse transcriptase PCR (RT-PCR) of RNA isolated from THP-1 cells. The cDNAs of TLR2 and TLR6 were cloned into a pEF6/V5-His TOPO vector (Invitrogen Co., Carlsbad, Calif.), and the constructs were referred to as TLR2- and TLR6-TOPO, respectively. The DNA sequences were confirmed by the dideoxy chain termination method by using ABI Prism 3100 genetic analyzer (Foster City, Calif.). The dominant-negative gene of TLR6 with a substitution of Pro residue at a position of 680 to His (TLR6Pro680His) was produced by using a QuickChange XL site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions with a TLR6-TOPO construct.
NF-κB reporter assay.
Activation of NF-κB was measured as described previously (26). Briefly, HEK293 cells were plated at 105 cells per well in 24-well plates on the day before transfection. The cells were transiently transfected by Fugene 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, Ind.) with 30 ng of an NF-κB luciferase reporter plasmid (pNF-κB-Luc; Stratagene) and 3.5 ng of a construct-directing expression of Renilla luciferase under the control of the constitutively active thymidine kinase promoter (pRL-TK; Promega Co., Madison, Wis.), together with 166.5 ng of each transfectant gene of TLR2, TLR6, and TLR6Pro680His. At 24 h after transfection, the cells were stimulated for 6 h with FSL-1, FSL-2, FSL-3, or MALP-2 in the absence of FBS, and luciferase activity was measured by using the dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions.
Generation of polyclonal antibody to human TLR2.
An extracellular domain of TLR2 protein (Met1-Arg508) was subcloned into the pGEX-2T (Amersham Pharmacia Biotech, Tokyo, Japan) vector by the PCR method to generate a glutathione S-transferase-TLR2 fusion protein and transformed into E. coli DH5α. The fusion protein formed inclusion bodies in the cells. Therefore, these inclusion bodies were collected from cells lysed in 20 mM Tris-HCl (pH 8.0) containing 0.5% NP-40 after treatment with lyzozyme by centrifugation at 8,000 × g for 30 min. The purified inclusion bodies were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and the band corresponding to the glutathione S-transferase-TLR2 fusion protein was excised from the SDS-polyacrylamide gel electrophoresis gels and then homogenized. The homogenate was suspended in PBS and used for immunogen after being mixed at a ratio of 1:1 with Freund complete adjuvant. The polyclonal anti-TLR2 antibody was generated by immunizing Japanese White rabbits with the mixture. On days 1, 14, and 28, rabbits were injected into five subcutaneous and intramuscular sites. Sera were drawn 1 week after the final immunization and used as polyclonal anti-TLR2 antibody.
Confocal laser microscopic analysis of the expression of TLR2 on the cell surface of HEK293 cells.
HEK293 cells were grown on poly-l-lysine-coated cover glasses on the day before transfection. The cells were transiently transfected by Fugene 6 transfection reagent with a TLR2 gene. After a 24-h of incubation, the medium was removed, and the cells were incubated with serum-free DMEM containing rhodamine-conjugated concanavalin A (5 μg/ml) (Molecular Probes, Eugene, Oreg.), followed by methanol fixation (4 min at −20°C). The cells were washed with PBS and then 1 μg of rabbit polyclonal anti-TLR2 antibody generated in our laboratory/ml, diluted with PBS, and further incubated with Alexa anti-rabbit IgG antibody (Molecular Probe) at room temperature for 45 min. The cells were finally washed three times with PBS, sealed in the presence of 90% glycerol, and observed by using a laser microscope (LSM510; Carl Zeiss, Tokyo, Japan). Digital images were acquired and processed by using Adobe Photoshop (version 5.0; Mountain View, Calif.).
Surface expression of TLR2 in THP-1 cells as determined by flow cytometry and expression of mRNAs of TLR2 and TLR6 as determined by RT-PCR.
In order to assess the surface expression of TLR2 on THP-1 cells by flow cytometry, a single cell suspensions of THP-1 (n = 106) cells were incubated at 4°C for 1 h with or without anti-TLR2 (TL2.1) and then with FITC-conjugated anti-mouse IgG. The surface expression was measured by using the flow cytometer EPICS (Beckman Coulter). Anti-TLR2 monoclonal antibody (TL2.1) was generated as described previously (12).
The RNAs were prepared from THP-1 cells by using an RNeasy kit (Qiagen, Inc., Chatsworth, Calif.) according to the manufacturer's instructions and were dissolved in 50 μl of RNase-free water. By using a RT-PCR kit (Takara Shuzo Co., Ltd., Shiga, Japan), the RNAs (0.1 μg) were transcribed with avian myeloblastosis virus RT by using the antisense primer of β-actin (21) and those of human TLR2 and TLR6 (35). The specificities of the primers for TLR2 and TLR6 were confirmed by Southern hybridization with a probe coding the internal sequence. The RT reaction was performed in an automated DNA thermal cycler according to the manufacturer's instructions. Briefly, a 1-μl volume (0.1 μg) of RNA was adjusted to a total volume of 20 μl in 10 mM Tris-HCl (pH 8.3) containing 1 μM concentrations of dATP, dCTP, dTTP, and dGTP; a 1 μM concentration of the antisense primer for the cytokine; 5 mM MgCl2; 50 mM KCl; 20 U of an RNase inhibitor; and 5 U of avian myeloblastosis virus RT. The RNA was transcribed at 55°C for 30 min after incubation at 30°C for 10 min and was then denatured at 99°C for 5 min and cooled at 5°C for 5 min. The resulting mixture containing cDNA was added to 80 μl of a mixture containing 10 mM Tris-HCl (pH 8.3), 0.25 μM concentrations of each of the sense primers for β-actin and TLR2, 1.8 mM MgCl2, and 1 U of Taq polymerase (Takara) and was then amplified by PCR as follows. After 2 min of denaturation at 94°C, 28 amplification cycles (30 s of denaturation at 94°C, 30 s of annealing at 60°C, and 1.5 min of extension at 72°C) were performed. The PCR products were separated on a 2% gel of NuSieve 3:1 agarose (FMC, Rockland, Maine) in 0.5× TBE buffer containing ethidium bromide (5 μg/ml).
Western blotting.
The transfected HEK293 cells grown in a six-well plate were washed twice with ice-cold PBS and then lysed by 62.5 mM Tris-HCl (pH 6.8) containing 2% SDS, 10% glycerol, and 50 mM dithiothreitol (an SDS sample buffer) in the presence of inhibitor cocktails of proteases (Sigma) and boiled for 10 min. The lysates were centrifuged at 14,000 rpm for 10 min, and the resulting supernatants containing cytosolic and membrane proteins were collected. Proteins in the supernatant were separated by electrophoresis on SDS-10% polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was incubated at 4°C overnight with polyclonal anti-TLR2 antibody as described above and then with peroxidase-conjugated anti-rabbit IgG antibody. Immunoreactive proteins were detected by using ECL detection reagents (Amersham Pharmacia).
RESULTS
Activation of HGF by FSL-1.
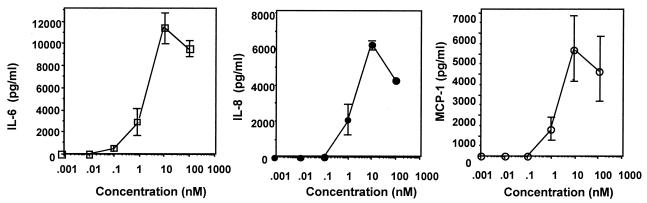
It has been demonstrated that FSL-1 synthesized on the basis of the N-terminal strucuture of LP44 is capable of activating HGF to induce the cell surface expression of ICAM-1 (32). First, experiments were carried out to determine whether FSL-1 induced the production of IL-6 and IL-8 by HGF because M. salivarium LP, including LP44, can induce the production of these cytokines by HGF (32, 33). FSL-1 possessed the activity to induce the production of IL-6, IL-8, and MCP-1 by HGF (Fig. 2). The activity increased with increase in the concentration up to 10 nM and then decreased (Fig. 2). Thus, in addition to previous findings (32), this finding also suggests that FSL-1 is the N-terminal lipopeptide moiety of LP44 and is its active site.
FIG. 2.
Production of IL-6, IL-8 and MCP-1 by HGF induced by FSL-1. HGF were cultured until confluency and then stimulated at 37°C for 15 h with various concentrations of FSL-1 in DMEM containing 0.1% human serum. The culture supernatants were collected and examined for amounts of IL-6 (□), IL-8 (•), and MCP-1 (○), which were determined by using enzyme immunoassay (EIA) kits. Data, expressed as means ± standard deviations from triplicate wells, are representative of three separate experiments.
Activation of a monocytic cell line, THP-1 cells, by FSL-1.
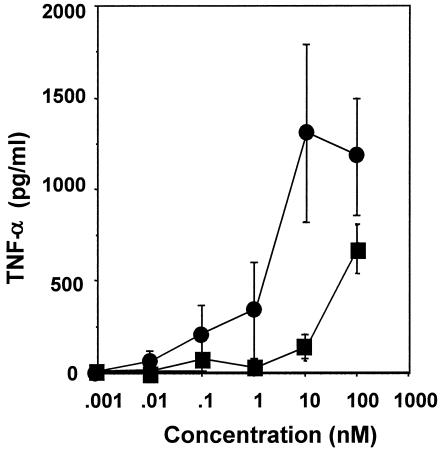
An experiment was carried out to determine whether FSL-1 is capable of activating THP-1 cells because MALP-2, which is similar to FSL-1 in framework structure (Fig. 1), is a potent macrophage-activating lipopeptide (27, 28). FSL-1 also activated the cells to produce TNF-α at concentrations ranging from 1 to 100 nM (Fig. 3). The level of the activity of FSL-1 was higher than that of MALP-2 (Fig. 3), suggesting that the difference in the amino acid sequence of diacylated lipopeptides affects the activity.
FIG. 3.
Production of TNF-α by THP-1 cells induced by FSL-1 and MALP-2. THP-1 cells were incubated at 37°C for 3 days in the presence of 100 nM vitamin D3 and then stimulated at 37°C for 15 h with various concentrations of FSL-1 (•) and MALP-2 (▪) in RPMI 1640 medium containing 0.1% human serum. The culture supernatants were collected and examined for the amount of TNF-α by using an EIA kit. Data, expressed as means ± standard deviations from triplicate wells, are representative of three separate experiments.
Structure-function relationship of FSL-1.
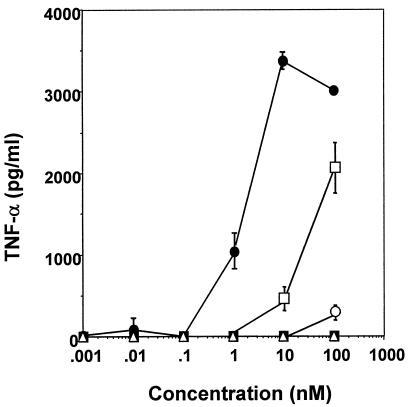
In order to further clarify the roles of the diacylglycerol and peptide portions of FSL-1 in its TNF-α production-inducing activity to THP-1 cells, the diacylglyceryl Cys (Pam2Cys) and the peptide (CGDPKHSPKSF) portions of FSL-1 (Fig. 1) were examined for this activity. Neither Pam2Cys nor the peptide showed activity at concentrations of up to 100 nM (Fig. 4), suggesting that the lipopeptide structure of FSL-1 is essential for expression of the activity.
FIG. 4.
Production of TNF-α by THP-1 cells induced by FSL-1, Pam2Cys, FSL-1 peptide, and derivatives of FSL-1. THP-1 cells were incubated at 37°C for 3 days in the presence of 100 nM vitamin D3 and then stimulated at 37°C for 15 h with various concentrations of FSL-1 (•), Pam2Cys (▪), FSL-1 peptide (▵), FSL-2 (○), and FSL-3 (□) in RPMI 1640 medium containing 0.1% human serum. The culture supernatants were collected and examined for the amount of TNF-α by using an EIA kit. Data, expressed as means ± standard deviations from triplicate wells, are representative of three separate experiments.
To determine more precisely minimal molecular requirements for the activity to THP-1 cells, Pam2CGDPKHSPKSR (FSL-2) in which the hydrophobic Phe residue at the C terminus of the peptide portion of FSL-1 has been converted to a hydrophilic Arg residue, and S-(2,3-bisstearyloxypropyl)-CGDPKHSPKSF (FSL-3), in which palmitic acid (C16:0) has been converted to stearic acid (C18:0), were synthesized (Fig. 1). These lipopeptides were examined for the activity to induce TNF-α production by THP-1 cells. Three lipopeptides showed such activities, but the levels of activity varied (Fig. 4). That is, a single amino acid substitution from Phe to Arg in the C terminus drastically reduced the activity, and a fatty acid substitution from palmitic acid to stearic acid also significantly reduced, although to a lesser extent, this activity (Fig. 4).
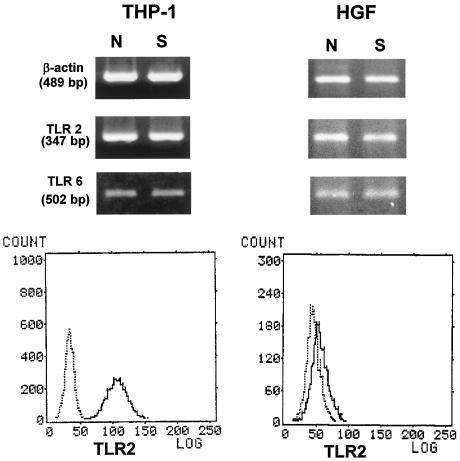
Expression of mRNAs of TLR2 and TLR6 in THP-1 cells and HGF.
Takeuchi et al. (38) suggest that TLR2 requires TLR6 as a coreceptor for recognition of diacylated lipopeptides such as mycoplasmal lipopeptides. Therefore, experiments were carried out to determine the expression of TLR2 and TLR6 in THP-1 cells and HGF. RT-PCR analysis indicated that mRNAs of TLR2 and TLR6 were expressed in both types of cells and that the expression levels were not upregulated by stimulation with FSL-1 (Fig. 5). The surface expression of TLR2 on THP-1 cells and HGF was also investigated by flow cytometry. TLR2 was expressed in the cell surface of both THP-1 cells and HGF, but the expression level in the former is much higher than that in the latter (Fig. 5).
FIG. 5.
Expressions of mRNA of TLR2 and TLR6 and surface expression of TLR2 molecule in THP-1 cells and HGF. THP-1 cells and HGF were stimulated with 100 ng of FSL-1 (S)/ml or not stimulated (N). THP-1 cells incubated at 37°C for 3 days in the presence of 100 nM vitamin D3 and HGF cultured until confluency were stimulated with 100 ng of FSL-1 (S)/ml or not stimulated (N). The expressions of mRNAs of TLR2 and TLR6 were assessed by RT-PCR. The cells were stained with mouse IgG2a (dotted line) and anti-TLR2 (TL2.1) (solid line) for flow cytometric analysis. See the text for details.
Recognition of mycoplasmal lipopeptides by TLR2 and/or TLR6.
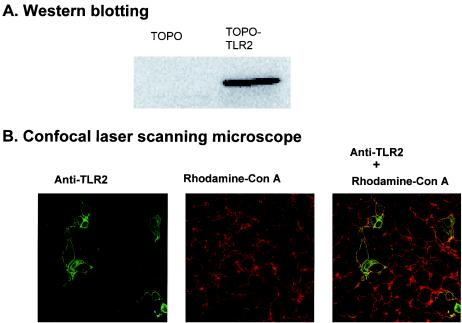
In order to confirm whether FSL-1, FSL-2, FSL-3, and MALP-2 are recognized by TLR2 and TLR6, HEK293 cells were transiently transfected with TLR2 and/or TLR6 (referred to hereafter as 293TLR2, 293TLR6, and 293TLR2/6 cells), together with an NF-κB luciferase reporter plasmid, and then examined for the NF-κB reporter activity after stimulation with these lipopeptides. First, we attempted to define the cellular localization of the TLR2 protein in transiently trasfected HEK293 cells by using a laser scanning confocal microscope (Fig. 6). TLR2 protein was detected in the transfectant and was localized in the cell membrane indicated by colocalization with concanavalin A used as an established marker for cell surface glycoproteins and also in the cytosol (Fig. 6).
FIG. 6.
Expression of the TLR2 molecule in HEK293 transient transfectants by Western blotting and confocal microscopic analysis. (A) HEK293 cells were transiently transfected with or without (TOPO, an empty vector) a TLR2 gene and lysed in the SDS sample buffer. The lysates were centrifuged, and the resulting supernatants containing cytosolic and membrane proteins were collected. Proteins in the supernatant were separated by electrophoresis on SDS-10% polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was incubated at 4°C overnight with polyclonal anti-TLR2 antibody and then with peroxidase-conjugated anti-rabbit IgG antibody. (B) HEK293 cells were transiently transfected with a TLR2 gene were incubated with rhodamine-conjugated concanavalin A. The cells were fixed and immunostained with a polyclonal rabbit anti-TLR2 antibody and then with FITC-anti-rabbit IgG monoclonal antibody. See the text for details.
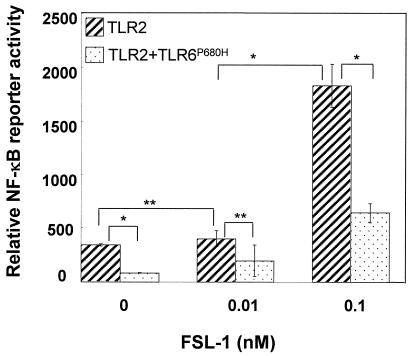
FSL-1 stimulated the NF-κB reporter activity in 293TLR2 and 293TLR2/6, but not 293TLR6 cells, in a dose-dependent manner (13). In addition, the activity is higher in 293TLR2/6 cells than in 293TLR2 cells, supporting the finding of Takeuchi et al. (38) as described above. The finding that FSL-1 did not stimulate the activity in 293TLR6 suggests that endogenous TLR2 is not functional. The reason why FSL-1 stimulated the activity in 293TLR2 cells not transfected with TLR6 may be explained by the idea that endogenous TLR6 functions as a coreceptor in 293TLR2 cells. Indeed, FSL-1 stimulated NF-κB reporter activity in HEK293 cells transfected with a dominant-negative gene of TLR6, with conversion of a Pro residue at position 680 to a His, but the level of activity was drastically reduced compared to that observed in 293TLR2 (Fig. 7).
FIG. 7.
NF-κB activation in HEK293 cells transiently transfected with TLR2 and/or TLR6P680H by FSL-1. HEK293 cells (n = 105) were plated in 24-well plates and transfected transiently with TLR2wt-TOPO and TLR6P680H-TOPO (TLR2/6), together with an NF-κB reporter plasmid and Renilla luciferase control reporter plasmid. Cells were stimulated at 37°C for 6 h with 0.1 or 1.0 nM FSL-1. Results, expressed as the means ± the standard deviation of triplicate wells, are representative of three separate experiments. See the text for details. Statistical significance was analyzed by Student t test. ✽, P < 0.01; ✽✽, P < 0.05.
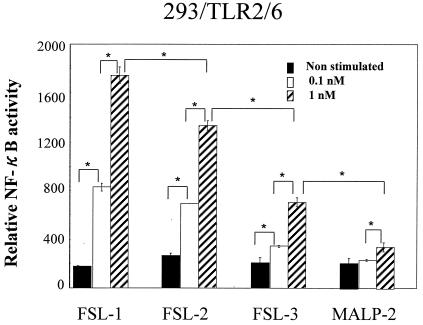
As described above, the level of activity of FSL-1 to THP-1 cells was much higher than that of MALP-2, FSL-2, and FSL-3 (Fig. 3 and 4). Therefore, experiments were carried out to reproduce these results in HEK293 transfected with TLR2 and/or TLR6. FSL-1, FSL-2, FSL-3, and MALP-2 showed NF-κB reporter activity in 293TLR2/6 cells, but the level of the activity decreased in that order (Fig. 8). The same result was obtained in the case of 293TLR2 cells, although the level of NF-κB reporter activity was lower (data not shown). That is, substitutions of an amino acid at C terminus and the fatty acid and the difference in the amino acid sequence of the peptide portion affected the activity, suggesting that both diacyl and peptide portions are involved in the recognition by TLR2 and/or 6.
FIG. 8.
NF-κB activation in HEK293 cells transiently transfected with TLR2 and TLR6 by FSL-1, FSL-2, FSL-3, and MALP-2. HEK293 cells (105) were plated in 24-well plates and transfected transiently with TLR2wt-TOPO and TLR6-TOPO (TLR2/6), together with an NF-κB reporter plasmid and Renilla luciferase control reporter plasmid. Cells were stimulated at 37°C for 6 h with 0.1 or 1.0 nM FSL-1, FSL-2, FSL-3, and MALP-2. Results, expressed as the means ± the standard deviation of triplicate wells, are representative of three separate experiments. See the text for details. Statistical significance was analyzed by Student t test. ✽, P < 0.01; ✽✽, P < 0.05.
DISCUSSION
Multicellular organisms have developed various defense mechanisms that have the capacity to protect the host by destroying invading microbes and neutralizing their virulence factors. The immediate response to microbial pathogens, which is coordinated by the innate immune system, is characterized by the de novo production of mediators that either kill the pathogens directly or induce phagocytic cells to ingest and kill them. LPS, a glycolipid of the outer cell membrane of gram-negative bacteria, is one of the most potent known stimulators of immune responses, including cytokine production by macrophages/monocytes (16). Bacterial LP have been shown to possess LPS-like biologic activities (16). Although LP and LPS are recognized by TLR2 and TLR4, respectively (1-4, 10, 17, 19, 21-23, 30, 36-39), they share many characteristics, including a biologically active lipid modification, cell types that are responsive, and types of responses that are included. Braun (9) first isolated LP (murein LP) from the cell wall of E. coli and determined its structure. The murein LP has potent mitogenic activity toward B lymphocytes, the activity resides in the N-terminal lipopeptide moiety, and the delipidated LP was not biologically active (5-8, 20). A well-defined series of lipopeptide analogs has been synthesized on the basis of the structure of the N-terminal lipopeptide of murein LP, and the relationship between their structures and biological activities has been investigated (5). The lipopeptides carrying two to five amino acids exhibit strong stimulatory activity comparable to that of native murein LP (5). In contrast, the lipopeptides containing only one amino acid are only marginally active, suggesting that the presence of a dipeptide structure is necessary for the expression of full biological activity (5). Lipopeptides containing two ester-bonded palmitoyl residues exhibit more potent mitogenic activity toward murine splenocytes than did a lipopeptide containing one ester-bonded palmitoyl residue (34). Rhodopseudomonas viridis lipopeptides containing two ester-bonded palmitoyl residues and a free N terminus exhibit more potent activity toward murine splenocytes than do lipopeptides containing three palmitoyl residues or N-terminally elongated lipopeptides (25). Thus, many aspects of the relationship between the structures and biological activities of bacterial triacylated lipopeptides have been elucidated.
We focused here on the relationships between the structures and biological activities of mycoplasmal diacylated lipopeptides. The M. fermentans-derived lipopeptide MALP-2 has been shown to be a potent macrophage activator (27, 28). We also found that the lipopeptide FSL-1 synthesized on the basis of the structure of the N-terminal lipopeptide moiety of M. salivarium LP44 (32) is able to activate HGF to induce the cell surface expression of ICAM-1. In addition, the present study demonstrated that FSL-1 possesses activities to induce the production of inflammatory cytokines by macrophages, as well as HGF. To determine the minimal molecular requirements for the activity of FSL-1 toward macrophages, Pam2Cys and the peptide portions were examined for their activities. It was found that both portions failed to activate macrophages, suggesting that the whole structure of the lipopeptide is required for the expression of the macrophage-stimulating activity. This speculation is supported by the results of a previous study showing that nonlipidated MALP-2 and Pam3Cys failed to activate monocytes/macrophages (15).
FSL-2, FSL-3, and MALP-2 were less active toward THP-1 cells and TLR-transfected HEK293 cells than was FSL-1 (Fig. 4 and 8), suggesting that both the fatty acid of the diacylglycerol portion and the amino acid sequence of the peptide portion are indispensable for TLR2/6-mediated signaling. The finding that a single amino acid substitution from Phe (FSL-1) to Arg (FSL-2) reduced the activity also suggests that the hydrophobic interaction plays an important role in recognition of the peptide portion of mycoplasmal lipopeptides by the receptor. Furthermore, this is supported by the present finding that FSL-1 was more active toward macrophages and TLR2/6-transfected HEK293 cells than was MALP-2 (Fig. 4 and 8) containing a hydrophilic Lys residue at the C terminus (Fig. 1). The finding that FSL-2 was more active toward HEK293 cells transfected with TLR2/6 than toward cells transfected with MALP-2 suggests that the size, as well as the hydrophobicity, of the peptide portion of the lipopeptide affects the recognition of the lipopeptide by TLR2/6. Furthermore, there is a possibility that the difference in the activities of these synthetic lipopeptides is attributed to the difference in ratios of R and S isomers of these lipopeptides, because the R stereoisomer of MALP-2 is known to be more active to macrophages than the S stereoisomer (36). Although the TNF-α production-inducing activity of FSL-2 to THP-1 cells was lower than that of FSL-3 (Fig. 4), the former was higher than the latter in the NF-κB reporter activity to HEK293 transfectants (Fig. 8). This discrepancy may be explained by the difference in the assay system. Namely, the TNF-α production-inducing activity was measured by using a monocytic cell line, THP-1 cells, whereas the NF-κB reporter activity was measured by using HEK293 cells transiently transfected with cloned human TLR2 and/or TLR6 genes. In addition, the sensitivity of the former assay system appears to be much lower than that of the latter, judging from the concentrations of FSL-1 used for these experiments (Fig. 4 and 8).
LP are membrane-bound proteins with a diacyl group of the N-terminal lipid moiety by which it is anchored into the membrane and has been found extensively in gram-positive and gram-negative bacteria, spirochetes such as Treponema pallidam and Borrelia burgdoferi, and Mycoplasma species. Many scientists have believed the prevailing view that endotoxin is only LPS. However, Galanos et al. have recently proposed that the mycoplasmal lipopeptide MALP-2 is an endotoxin because it possesses many classical endotoxic properties such as the cytokine production-inducing activity toward macrophages, mitogenic activities toward B lymphocytes, pyrogenicity, and lethal toxicity (14). However, we think that LP rather than lipopeptides is an important microbial endotoxin, because lipopeptide is only the N-terminal part of LP.
Studies are in progress to compare the biological activities of LP with those of LPS in order to characterize the endotoxic and pathogenic properties of microbial LP.
Acknowledgments
This study was partially supported by Grants-in-Aid for Scientific Research (C)(2)(1367891) and (B)(2)(15390549) and for Encouragement of Young Scientists (A)(14770999), which were provided by the Ministry of Education, Science, and Culture of Japan.
Editor: B. B. Finlay
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis, A. O., D. Weiss, J. D. Radolf, and A. Zychlinsky. 2001. Release of Toll-like receptor-2-activating bacterial lipoproteins in Shigella flexneri culture supernatants. Infect. Immun. 69:6248-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliprantis, A. O., R.-B. Yang, M. R. Mark., S. Sugett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor 2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 5.Bessler, W. G., M. Cox, M. Lex, B. Suhr, K. H. Wiesmüller, and G. Jung. 1985. Synthetic lipopeptide analogs of bacterial lipoproteins are potent polyclonal activators for murine B lymphocytes. J. Immunol. 135:1900-1905. [PubMed] [Google Scholar]
- 6.Bessler, W. G., M. Cox, K. H. Wiesmüller, and G. Jung. 1984. The mitogenic principle of Escherichia coli lipoprotein: B-lymphocyte mitogenicity of the synthetic analogue palmitoyl-tetrapeptide (Pam-Ser-Ser-Asn-Ala). Biochem. Biophys. Res. Commun. 121:55-61. [DOI] [PubMed] [Google Scholar]
- 7.Bessler, W. G., R. B. Johnson, K. H. Wiesmüller, and G. Jung. 1977. Induction of lymphocyte proliferation and membrane changes by lipopeptide derivatives of the lipoprotein from the outer membrane of Escherichia coli. Z. Immun.-Forsch. 153:11-22. [PubMed] [Google Scholar]
- 8.Bessler, W. G., and R. B. Johnson. 1982. B-lymphocyte mitogenicity in vitro of a synthetic lipopeptide fragment derived from bacterial lipoprotein. Hoppe-Seyler's Z. Physiol. Chem. 363:767-770. [PubMed] [Google Scholar]
- 9.Braun, V. 1975. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim. Biophys. Acta 415:335-337. [DOI] [PubMed] [Google Scholar]
- 10.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 11.Dong, L., K.-I. Shibata, Y. Sawa, A. Hasebe, Y. Yamaoka, S. Yoshida, and T. Watanabe. 1999. Transcriptional activation of mRNA of intercellular adhesion molecule 1 and induction of its cell surface expression in normal human gingival fibroblasts by Mycoplasma salivarium and Mycoplasma fermentans. Infect. Immun. 67:3061-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flo, T. H., Ø. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human Toll-like receptors mediate monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, M., T. Into, M. Yasuda, T. Okusawa, S. Hamahira, Y. Kuroki, A. Eto, T. Nisizawa, M. Morita, and K. I. Shibata. 2003. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and Staphylococcus aureus peptidoglycans. J. Immunol. 171:3675-3683. [DOI] [PubMed] [Google Scholar]
- 14.Galanos, C., M. Gumenscheimer, P. F. Mühlradt, E. Jirillo, and M. A. Freudenberg. 2001. MALP-2, a Mycoplasma lipopeptide with classical endotoxic properties: end of an era of LPS monopoly. J. Endotoxin Res. 6:471-476. [PubMed] [Google Scholar]
- 15.Garcia, J., B. Lemerecier, S. Roman-Roman, and G. Rawadi. 1998. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-κB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273:34391-34398. [DOI] [PubMed] [Google Scholar]
- 16.Henderson, B., S. Poole, and M. Wilson. 1996. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbial Rev. 60:316-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 18.Hertz. C. J., S. M. Kiertscher, P. J. Godowski, D. A. Bouis, M. V. Norgard, M. D. Roth, and R. L. Modlin. 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166:2444-2450. [DOI] [PubMed] [Google Scholar]
- 19.Janeway, C. A., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, R. B., S. Kohl, K. Weismüller, G. Jung, and W. G. Bessler. 1983. Synthetic analogues of the N-terminal lipid part of bacterial lipoprotein are B-lymphocyte mitogens in vitro and in vivo. Immunobiology 165:27-35. [DOI] [PubMed] [Google Scholar]
- 21.Kirk, A. D., R. R. Bollinger, and O. J. Finn. 1995. Rapid, comprehensive analysis of human cytokine mRNA and its application to the study of acute renal allograft rejection. Hum. Immunol. 43:113-128. [DOI] [PubMed] [Google Scholar]
- 22.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadii, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 23.Means, T. K., D. T. Golenbock, and M. J. Fenton. 2000. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 11:219-232. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate Immunity: the virtues of a non-clonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 25.Metzger, J. W., A. G. Beck-Sickingeer, M. Loleit, M. Eckert, G. G. Bessler, and G. Jung. 1996. Synthetic S-(2,3-dihydroxypropyl)-cysteinyl peptides derived from the N terminus of the cytochrome subunit of the photoreaction center of Rhodopseudomonas viridis enhance murine splenocyte proliferation. J. Peptide Sci. 3:184-190. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuzawa, H., I. Wada, H. Sano, D. Iwaki, S. Murakami, T. Himi, N. N. Matsushima, and Y. Kuroki. 2001. Extracellular Toll-like receptor 2 region containing Ser-Ile but not Cys-Ser is critical for the recognition of Staphylococcus aureus peptide glycan. J. Biol. Chem. 276:41350-41356. [DOI] [PubMed] [Google Scholar]
- 27.Mühlradt, P. F., M. Keiss, H. Meyer, R. Süssmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentrations. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mühlradt, P. F., H. Meyer, and R. Jansen. 1996. Identification of S-(2,3-dihydroxypropyl)cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans. Biochemistry 35:7781-7786. [DOI] [PubMed] [Google Scholar]
- 29.Noss, E. H., R. K. Pai, T. J. Sellati, J. D. Radolf, J. Belisle, D. T. Golenbock, W. H. Boom., and C. V. Harding. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by a 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 30.Poltrak, A., P. Ricciardi-Castagnoli, S. Citterio, and B. Beutler. 2000. Physical contact between lipopolysaccharide and Toll-like receptor4 revealed by genetic complementation. Proc. Natl. Acad. Sci. USA 96:2163-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata, K.-I., A. Hasebe, T. Into, M. Yamada, and T. Watanabe. 2000. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J. Immunol. 165:6538-6544. [DOI] [PubMed] [Google Scholar]
- 33.Shibata, K.-I., A. Hasebe, T. Sasaki, and T. Watanabe. 1998. Mycoplasma salivarium induces interleukin-6 and interleukin-8 in human gingival fibroblasts. FEMS Immunol. Med. Microbiol. 19:275-283. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu, T., Y. Iwamoto, Y. Yanagihara, M. Kurimura, and K. Achiwa. 1994. Mitogenic activity and the induction of tumor necrosis factor by lipopeptide analogs of the N-terminal part of lipoprotein in the outer membrane of Escherichia coli. Biol. Pharm. Bull. 17:980-982. [DOI] [PubMed] [Google Scholar]
- 35.Takeshi, I., M. Fujita, T. Okusawa, A. Hasebe, M. Morita, and K. Shibata. 2002. Synergic effects of mycoplasmal lipopeptides and extracellular ATP on activation of macrophages. Infect. Immun. 70:3586-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Mühlradt, and S. Akira. 2000. Preferentially the R-stereoisomer of the mycoplsmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164:554-557. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterila cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi, O., T. Kawai, P. F. Mühlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 39.Takeda, K., and S. Akira. 2001. Roles of Toll-like receptors in innate immune responses. Genes Cells 6:733-742. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Can. 26:171-176. [DOI] [PubMed] [Google Scholar]