Abstract
Genomic O island 122 (OI-122) of the verocytotoxin-producing Escherichia coli (VTEC) strain EDL933 contains four putative virulence genes, Z4321, Z4326, Z4332, and Z4333. However, strain CL3 (serotype O113:H21) contains only Z4321, not the other three genes. To determine whether Z4321 is part of a different genomic island in CL3, a region of 27,293 bp up- and downstream of Z4321 was sequenced and found to contain elements of two different EDL933 genomic islands (OI-48 and OI-122) and a Yersinia pestis-like hemolysin/adhesin gene cluster. The region contained OI-48 genes Z1635, Z1636, and Z1637 at the left terminus and Z1641, Z1642, Z1643, and Z1644 at the right. The middle portion consisted of OI-48 gene Z1640, which was separated into three fragments by genomic segments including the Y. pestis cluster and EDL933 OI-122 genes Z4322, Z4321, and Z4318. In a PCR investigation of 36 VTEC strains of different serotypes, intact Z1640 was present in strains of serotypes O157:H7, O26:H11, O103:H2, O111:NM, and O145:NM, which are associated with hemolytic uremic syndrome and outbreaks. In contrast, fragmented Z1640 was seen in strains of nonepidemic serotypes, such as O91:H21 and O113:H21, and in animal serotypes that have not been associated with human disease, indicating that Z1640 might be a virulence gene.
Verocytotoxin-producing Escherichia coli (VTEC) strains (24), also referred to as Shiga toxin-producing E. coli (3), are major causes of zoonotic food- and water-borne infection that may be associated with massive outbreaks and with the serious complication of hemolytic uremic syndrome (HUS) (10, 20, 22, 30). Over 200 VTEC serotypes have been associated with human illness (44). Some VTEC serotypes (e.g., O26:H11, O103:H2, O111:H−, and O145:H−) are associated with outbreaks and HUS, but less commonly than serotype O157:H7 (10, 16, 30). Other serotypes such as O91:H21 and O113:H21 are associated with sporadic cases of HUS but not with outbreaks (10, 16, 30). Yet others, isolated from patients with diarrhea, have not been associated with HUS or outbreaks (44). Also, a large group of serotypes isolated from animals have never been associated with human disease (43, 44).
The scientific basis for the apparent differences in virulence between different serotypes is not known. Growing evidence suggests that major differences in virulence between groups of strains in bacteria such as E. coli, Salmonella enteritidis, and Helicobacter pylori may be related to the presence of specific pathogenicity islands (PAIs) (11, 19). One example of such a PAI is the locus of enterocyte effacement (LEE) which encodes the structural, accessory, effector, and regulatory molecules necessary for the development of the characteristic attaching and effacing cytopathology on enterocytes by some VTEC strains (27, 30). However, LEE cannot alone explain virulence differences between VTEC serotypes because the LEE-positive serotype O157:H7 is associated with outbreaks and HUS much more commonly than other LEE-positive serotypes such as O26:H11, O103:H2, and O111:NM (10, 30), and some LEE-positive serotypes from bovines have never been associated with human disease (43, 44). Furthermore, LEE-negative serotypes such as O113:H21 are also associated with HUS (10, 16, 30). These observations suggest that other hitherto unknown factors, perhaps on other PAIs, are also involved in modulating the virulence of VTEC.
The genome sequences of VTEC O157:H7 strains EDL933 (35) and Sakai (13) contain several additional putative PAIs (35), including the 87-kb O island 48 (OI-48) and the 23-kb OI-122 (35). OI-48 is duplicated (as OI-43) in the EDL933 genome (35), whereas it is present as a single copy (SpLE1) in the Sakai genome (13). OI-122 comprises 26 open reading frames (ORFs), including four putative virulence genes, Z4321, Z4326, Z4332, and Z333, that encode proteins with significant homology to the Salmonella enterica serovar Typhimurium PagC (pagC) (36) (Z4321), Shigella flexneri enterotoxin (senA) (31) (Z4326), and the enterohemorrhagic E. coli factor for adherence (efa1) (32) (Z4332 and Z4333).
During a study to investigate the distribution of these OI-122 genes in different VTEC serotypes, we observed that VTEC strain CL3 (serotype O113:H21) was positive for gene Z4321 but negative for Z4326, Z4332, and Z4333 (21). The objective of the present study was to investigate whether Z4321 is part of an incomplete OI-122 or of a novel genomic structure.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Genomic studies were conducted on CL3, a clinical VTEC isolate of serotype O113:H21 that was isolated from a child with HUS (22). E. coli K-12 strain MG1655 (ATCC 700926) was used as a negative control, and EDL933 (serotype O157:H7) was the positive control. The distribution of selected novel genes identified in the CL3 genome was investigated in 35 additional VTEC strains of 22 different serotypes (Table 3), which were grouped into three categories as follows: serotypes that are known to be associated with HUS and outbreaks (10, 16, 30, 44), serotypes that are associated with sporadic HUS but not outbreaks (10, 16, 30, 44), and serotypes that have been isolated from ruminants but have not been associated with human disease (43, 44).
TABLE 3.
Distribution of Z1640 and S1 in different VTEC serotypes
| Serotype group | Strain | Source | Z1640 | S1 | Z1640::S1c |
|---|---|---|---|---|---|
| Strains associated with HUS and outbreaks | |||||
| O157:H7a | 93111 | T. Whittam | + | − | − |
| 279F1 | LFZd | + | − | − | |
| 157F1 | LFZ | + | − | − | |
| O157:NMa | ER63-94 | F. Jamieson | + | − | − |
| O111:NMa | R82F2 | LFZ | + | − | − |
| C69F1 | LFZ | + | − | − | |
| CL101 | LFZ | + | − | − | |
| O103:H2a | N01-2454 | J. Isaac-Renton | + | − | − |
| N01-7015 | J. Isaac Renton | + | − | − | |
| N02-1626 | J. Isaac Renton | − | − | − | |
| O26:H11a | CL1 | LFZ | + | − | − |
| CL4 | LFZ | + | − | − | |
| CL9 | LFZ | + | − | − | |
| O145:NMa | N01-2051 | J. Isaac-Renton | + | − | − |
| N00-6496 | J. Isaac-Renton | + | − | − | |
| N02-5149 | J. Isaac-Renton | + | − | − | |
| Strains associated with HUS but not with outbreaks | |||||
| O113:H21b | N89-0541 | J. Preiksaitis | − | + | + |
| N90-6057 | P. VanCaeseele | − | + | + | |
| N88-0578 | J. Isaac-Renton | − | + | + | |
| O91:H21b | EC7-181 | LFZ | − | + | + |
| EC6-990 | S. Aleksic | − | + | + | |
| Strains not associated with human disease | |||||
| O8:H19b | EC6-448 | LFZ | − | + | + |
| O39:H49b | EC2-293 | LFZ | − | + | + |
| O46:H38b | EC7-451 | LFZ | − | + | + |
| O88:H25b | EC4-453 | LFZ | − | + | + |
| O156:NMb | EC2-020 | LFZ | − | + | + |
| O163:NMb | EC2-459 | LFZ | − | + | + |
| O6:H34b | EC6-626 | J. Preiksaitis | − | − | − |
| O76:H7a | EC9-333 | P. Desmarchellier | − | − | − |
| O84:NMa | EC2-044 | LFZ | − | − | − |
| O98:H25a | EC3-377 | LFZ | − | − | − |
| O113:NMb | EC2-211 | LFZ | − | − | − |
| O136:NMb | EC2-258 | LFZ | − | − | − |
| O136:H12b | EC3-208 | LFZ | − | − | − |
| O153:H31b | EC2-104 | LFZ | − | − | − |
Strain positive for the eae gene (marker of LEE).
Strain negative for the eae gene.
S1 and Z1640 junction.
Laboratory for Foodborne Zoonoses.
Strains were grown routinely at 37°C in Luria-Bertani (LB) broth (1% tryptone, 0.5% yeast extract, 171 mM NaCl) (2).
DNA techniques.
Standard techniques were used for DNA extraction, purification, analysis, and PCR (2, 38). Genomic DNA was isolated with the Genomic-tip 100/G (Qiagen).
Genome walking.
Adjacent DNA sequences upstream and downstream of Z4321 in the CL3 genome were investigated with the Clontech Universal GenomeWalker kit (Clontech Laboratories, Inc.). Details of methodology are accessible from the user manual at http://www.clontech.com/. Briefly, CL3 genomic DNA was digested with one of four different blunt-end restriction enzymes (provided by the manufacturer). The purified restricted DNA fragments were ligated to an adaptor (supplied by the manufacturer) and became part of four genome-walking libraries generated by the four different restriction enzymes. Gene-specific primers were designed and used in combination with adaptor-specific primers to amplify large genomic segment adjacent to genes of interest by long-distance PCR with the Expand System kit (Roche Diagnostics, Mannheim, Germany) in the PCR Express thermal cycler (ThermoHybaid, Middlesex, United Kingdom). PCR products were gel purified with the Qiagen QIAquick gel extraction kit for sequencing. The terminal portions of the sequenced DNA regions were used to design primers for subsequent sequential genome-walking steps either upstream or downstream.
DNA sequencing and open reading frame (ORF) prediction and annotation.
DNA sequencing was performed by the Laboratory Service Division, University of Guelph, on an Applied Biosystems ABI Prism 377 DNA sequencer. DNA sequence data were aligned, edited, and assembled into contiguous sequence with the Omiga program (Oxford Molecular Ltd.). Putative ORFs larger than 150 bp were identified with GeneMark (26), Lasergene (DNAStar Inc.), and Glimmer 2.02 (6) applications. ORFs homologous to sequences in the EDL933 genome were annotated according to the EDL933 sequence (35). Amino acid homology with other ORFs in the public databases were sought with the BlastN, BlastP and BlastX programs (1) at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov).
Test of contiguity of adjacent genome-walking sequences by Southern hybridization.
To ensure contiguity of the sequences from the genome-walking library, Southern blotting was applied to BsiWI and EcoRI restriction fragments of the CL3 genome to determine if the hybridized fragments on the CL3 genome matched the sizes of fragments predicted from the genome-walking sequences. In practice, two samples of CL3 genomic DNA (2.5 g) were digested with BsiWI and EcoRI, respectively, and separated by agarose gel electrophoresis. DNA fragments were transferred overnight by capillary transfer onto positively charged nylon membranes (Roche Diagnostics) (38). Digoxigenin-labeled probes were synthesized with a PCR digoxigenin probe synthesis kit (Roche Diagnostics) according to the manufacturer's instructions. The digoxigenin nucleic acid detection kit (Roche Diagnostics) was used to detect hybridized bands.
Probe B1 (555 bp), generated with primers B1F (5′GTAAAACCCGGTGATGTGAAC3′) and B1R (5′CGTACTCTGTGTGACGCCCTG3′), specific for the region spanning the second BsiWI restriction site (Fig. 1), was used to hybridize the BsiWI blot. Probes E1 (521 bp) and E2 (494 bp) were used to probe the EcoRI blot separately. Probe E1 was generated with primers E1F (5′-CTGAAACCGTTTGTGGCTGG-3′) and E1R (5′-GACATACAGAAAGCGGACGAG-3′), specific for the region spanning the second EcoRI restriction digestion site (Fig. 1). Probe E2 was generated with primers B2F (5′-GAATCTACGGCGATGCTG-3′) and B2R (5′-GGTTTCTTCCACGGTACG-3′), specific for the region following the first EcoRI site.
FIG. 1.
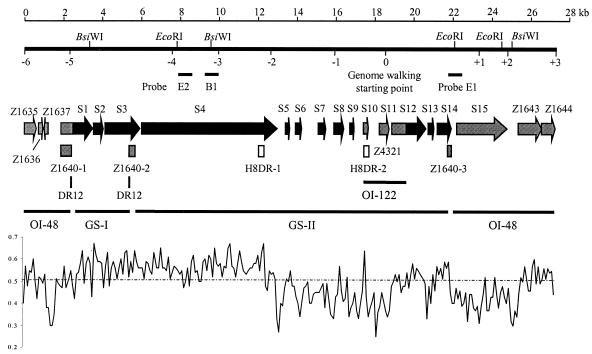
Genetic and physical map of the sequenced region of the genomic island of E. coli O113:H21 strain CL3. BsiWI and EcoRI restriction sites and the sizes and locations of DNA fragments amplified by genome walking PCR are marked. Southern hybridization probes B1, E1, and E2 are indicated. EDL933 genes are indicated by grey shading, and new genes are indicated by black shading. Arrows indicate the size, location, and transcription orientation of ORFs present in the region. Grey boxes indicate the position of Z1640 gene fragments, which are separated by genomic segments. White boxes indicate the position of the 190-bp direct repeat H8 (H8DR). The peak diagram is the G+C content of the region determined with a 101-nucleotide window. The line at 51% represents the average G+C content of the E. coli K-12 chromosome. GS-I, genomic segment I; GS-II, genomic segment II; OI-122, the fragment from OI-122; H8DR, H8-homologous direct repeat; DR12, the 12-bp direct repeat GCAGGGGCTGGA.
Reverse transcription PCR.
Expression of predicted genes was investigated by reverse transcription PCR (2, 38). RNA was isolated from bacteria harvested during the logarithmic growth phase (OD600 = 0.3 after 4 h of incubation in LB broth with shaking), with the Qiagen RNeasy mini kit according to the manufacturer's instruction (Qiagen, Berlin, Germany). Omniscript reverse transcriptase (Qiagen) was used for reverse transcription. A 140-ng aliquot of purified total RNA was added to a 20-μl reverse transcription mixture, and gene-specific primers (Table 2) were used for first-strand synthesis. To amplify the cDNA sequences specific to the predicted genes, 3 μl of the reverse transcription reaction was used as a template in a 50-μl PCR with gene-specific primers (Table 2). PCR without prior reverse transcription was used as a control to confirm that the reverse transcription PCR products were attributable to RNA.
TABLE 2.
Primers used in RT-PCR for detecting gene expression
| Gene | Use of primer | Primer sequence | Size (bp) |
|---|---|---|---|
| Z1635 | 1st strand | 5′GGTACTTTGCGGGTTGTTC3′ | 473 |
| Forward | 5′CTGTCTTCGCCAGCAGTG3′ | ||
| Reverse | 5′TCTCAGCCTCAGAACATCAGC3′ | ||
| Z1636 | 1st strand | 5′CTCATCCCCGGAGACAAG3′ | 162 |
| Forward | 5′GGAACATGAACTGAATGAGTGG3′ | ||
| Reverse | 5′CACTCCGGGTGTTCACTGTA3′ | ||
| Z1637 | 1st strand | 5′CTGTTTCTTCCCGAGGTTC3′ | 153 |
| Forward | 5′GTCAGAAGATGTTCCATTACCG3′ | ||
| Reverse | 5′CCCAGAAAGTTGTACATCGG3′ | ||
| S1 | 1st strand | 5′CGTCATAATCCGTACTGAACAG3′ | 334 |
| Forward | 5′GATAGGGAAATCCAGGTGC3′ | ||
| Reverse | 5′CAAAACCACGAACCGTCC3′ | ||
| S2 | 1st strand | 5′CTCTTCACGACTGACCTGTG3′ | 277 |
| Forward | 5′GTATGTGGTGGCAACAGAGC3′ | ||
| Reverse | 5′GAAATTCATCACCCGTAACCC3′ | ||
| S3 | 1st strand | 5′CAGAACCACTGTTATGCAGC3′ | 430 |
| Forward | 5′CTCAGATGAAAAACGGCAGTC3′ | ||
| Reverse | 5′GTTGTCGCCAGATGCACA3′ | ||
| S4 | 1st strand | 5′CCAATCTCACCAATGAGTTG3′ | 384 |
| Forward | 5′GTCACCACCGGACAGCATAC3′ | ||
| Reverse | 5′CTGTTGCTCTTTTTCCCTGTCA3′ | ||
| S5 | 1st strand | 5′CAAGTAACCACATAGCTTGGG3′ | 153 |
| Forward | 5′GCAGTGAATGGCTCCAGAC3′ | ||
| Reverse | 5′CTCCCTGGTAACATCCACCT3′ | ||
| S6 | 1st strand | 5′CAAATAACCCCATTCTCCG3′ | 277 |
| Forward | 5′GAGAGGCTATGCCTGCAC3′ | ||
| Reverse | 5′CAATAATCAGCCAGTTCCATTTC3′ | ||
| S7 | 1st strand | 5′CAGTCACGTAACTGTTTGCTCC3′ | 311 |
| Forward | 5′CGTTACTGGACAAAATGTACGC3′ | ||
| Reverse | 5′CTCAGGATGACGTTTGTGTG3′ | ||
| S8 | 1st strand | 5′CGAGATAACAGGCAAGAGC3′ | 353 |
| Forward | 5′CACAGCAATTCTTTCAGAGC3′ | ||
| Reverse | 5′CAGGCATCATCCATAGACG3′ | ||
| S9 | 1st strand | 5′GTGCAAGTTGTTCATCAACC3′ | 203 |
| Forward | 5′GTTTGTCGAGTATGTGGTGC3′ | ||
| Reverse | 5′GACCAGTTTTGTGGTTCCG3′ | ||
| S10 | 1st strand | 5′CTATCATCCCCACCACCTC3′ | 141 |
| Forward | 5′GAATGCTTTACAGAGAGTGGC3′ | ||
| Reverse | 5′GCGTTCTTACCCTGTGCC3′ | ||
| S11 | 1st strand | 5′CTCCAACAGTAAATCCATTGAC3′ | 391 |
| Forward | 5′GGTTATGCTCAGAGTAAGGTACAG3′ | ||
| Reverse | 5′CCCACGGATTAATAATAACACC3′ | ||
| S12 | 1st strand | 5′CACATACGGGAACTCCAGC3′ | 476 |
| Forward | 5′CCGTTCACTGCTCCTGATG3′ | ||
| Reverse | 5′GCACCACATCCTTACGTCG3′ | ||
| S13 | 1st strand | 5′CAGGAAAGGTATTCCACTGC3′ | 257 |
| Forward | 5′CCCTGTTATTTCACGATATTCAG3′ | ||
| Reverse | 5′GATTGCCGGTGTCACATCC3′ | ||
| S14 | 1st strand | 5′CAGGTAAAATGCTGCTCCTC3′ | 363 |
| Forward | 5′GAAGGCACTGAGTGACATACTG3′ | ||
| Reverse | 5′GATGGCTGTGTCAGAAACTGC3′ | ||
| S15 | 1st strand | 5′CAGTCGTGCCACCACATATC3′ | 438 |
| Forward | 5′GCCTTGACTTTCAACAGTGTTC3′ | ||
| Reverse | 5′CCAGTTGCTGTTTGTCCATC3′ | ||
| Z1643 | 1st strand | 5′GGTCATTCTCCTGTTCATCC3′ | 344 |
| Forward | 5′CTGTGCCATCTACAATCCTC3′ | ||
| Reverse | 5′GATACCAGCGCAGGTAGAC3′ | ||
| Z1644 | 1st strand | 5′GGCTGAAATCCTCTTCCTG3′ | 446 |
| Forward | 5′GAGCCTGTGGACTGTGTG3′ | ||
| Reverse | 5′CCGCTAATCTGTTTCAGCC3′ |
Detection of intact Z1640 and fragmented Z1640 containing putative insertions.
The intact Z1640 gene and the S1 gene associated with Z1640 (Z1640::S1) were investigated in 35 VTEC strains of different serotypes by PCR with specific primers. Primers Z1640F (5′-GACATGTGCGTCTGACACC-3′, complementary to fragment Z1640-1) (Fig. 1) and Z1640R (5′-CACAGACACCAGCCACAAAC-3′, complementary to fragment Z1640-3) were used to amplify Z1640 DNA. Primers S1-F (5′-CACATTACGGGGGTAAGG-3′) and S1-R (5′-GTGTAAAGGGCAGATTAAAGG-3′) were used to amplify S1 DNA. Primers Z1640F and S1-R were used to amplify DNA of the Z1640::S1 junction.
G+C content analysis.
A plot of G+C content along the 27,297-bp sequence was generated by the sliding 101-nucleotide window method (29).
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been deposited in the GenBank database (accession number AY275838).
RESULTS
Genome walking.
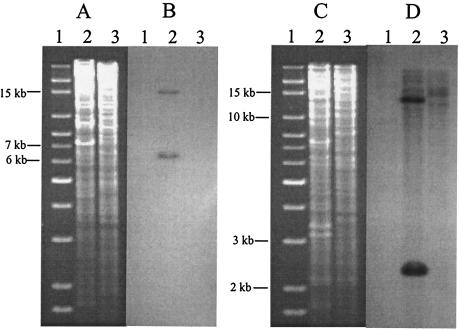
Genome walking was used to sequence 27,297 bp in the regions upstream and downstream of Z4321 on the CL3 genome. To determine whether the contiguity of sequences in the CL3 genome matched that predicted by the genome-walking sequence, Southern hybridization was performed on BsiWI and EcoRI digests of the CL3 genome separately. There are three BsiWI cleavage sites in the physical map, at 3,323 bp, 9,553 bp, and 24,994 bp (Fig. 1). When probe B1, which spans the second BsiWI site (Fig. 1), is used in the hybridization, the blot is predicted to show two DNA bands with sizes of 6,230 bp and 15,441. Hybridization results did show two bands of the predicted sizes (6.2 kb and 15.4 kb) (Fig. 2A and B).
FIG. 2.
Ethidium bromide-stained agarose gel (A) and corresponding Southern hybridization analysis (B) of BsiWI-digested genomic DNA probed with digoxigenin-labeled probe B1, and agarose gel (C) and corresponding Southern hybridization analysis (D) of EcoRI-digested genomic DNA probed with digoxigenin-labeled probe E1. The probes hybridized with E. coli strain CL3 in both blots, but not with K-12. Lane 1, DNA size markers (Invitrogen); lane 2, CL3; lane3, K-12.
EcoRI also has three cleavage sites in the physical map at 7,806 bp, 22,072 bp, and 24,486 bp. If probe E1, which spans the second EcoRI site, is used in the hybridization, two DNA fragments of 14,266 bp and 2,414 bp (Fig. 1) are expected on the blot. Hybridization with the E1 probe also showed two fragments of the predicted sizes (14.3 kb and 2.4 kb) (Fig. 2C and D). The EcoRI blot was also hybridized with probe E2, which is located near the first EcoRI site on the physical map (Fig. 1). E2 hybridized with a band of the predicted 14.3-kb size (data not shown). Hybridization of all three probes with restriction fragments of the predicted sizes is consistent with the contiguity of adjacent genome-walking fragments in the sequenced region.
Features of the sequenced region.
Analysis of the sequenced region of the CL3 genome indicates that it is part of a hybrid genomic island that contains segments of two EDL933 genomic islands, OI-48 and OI-122, as well as sequences that show homology to Yersinia pestis genes (Fig. 1). The average G+C content of the whole sequenced region is 49.58%. Its left terminus contains ORFs that show homology to EDL933 OI-48 genes Z1635, Z1636, and Z1637. The right terminal end of the region consists of ORFs that show homology to EDL933 OI-48 genes Z1641, Z1642, Z1643, and Z1644 (Fig. 1). Z1641 and Z1642 are contained in a single, putative helicase gene (S15). Central to the region of this CL3 genomic island is gene Z1640, which is separated into three fragments by two genomic segments (GS-I and GS-II) (Fig. 1). The three Z1640 fragments, Z1640-1, Z1640-2, and Z1640-3, have sizes of 539 bp, 355 bp, and 168 bp, respectively. The region between Z1640-1 and Z-1640-2 consists of a 2,973-bp genomic segment (GS-I), which comprises ORFs S1, S2, and S3. Z1640-1 is part of S1 and Z1640-2 is part of S3 (Fig. 1). There is a 12-bp direct repeat (GCAGGGGCTGGA, part of the Z1640 sequence, referred to as DR12 in Fig. 1) at both ends of GS-I (bp 2427 to 2438 and bp 5412 to 5423).
The region between Z1640-2 and Z1640-3 contains a 19,353-bp genomic segment (GS-II). In GS-II, there are two 190-bp-long direct repeats located at bp 12070 to 12259 (in S4) and at 17447 to 17636 (in S10). These repeats are 90% identical. Each has a 100-bp sequence that shows strong homology (91% identity for the left and 95% identity for the right) to the E. coli reference collection (ECOR3) random amplified polymorphic DNA fragment H8 (GenBank accession number AF127011) (15). They are thus referred to as H8DR (H8-homologous direct repeat) in Fig. 1. The region between the two H8DRs comprises the five ORFs S5 to S9, two of which (S6 and S7) have homology to transposases, suggesting that the region is an insertion sequence-associated element. Adjacent to the latter is the OI-122 region comprising part of Z4322, the complete Z4321, and part of Z4318.
Genes in the sequenced region. (i) EDL933 OI-48 ORFs.
The CL3 predicted Z1635, Z1636, Z1637, Z1643, and Z1644 gene products are 97%, 96%, 95%, 97%, and 98% identical, respectively, to their EDL933 counterparts at full length (Table 1). The functions of all these genes are unknown. Z1638 and Z1639 are absent in the CL3 genome. The sequences of the two EDL933 genes Z1641 and Z1642 are contained in one gene, S15, in CL3 (Table 1), which turns out to encode a UvrD/Rep-helicase (Conserved Domain database, abbreviated CD, pfam00580) (Table 1).
TABLE 1.
Amino acid homologies of the putative ORFs identified in the sequenced region
| ORF and location (bp) | Size (no. of amino acids) | Conserved domain | Similar protein | % Identity/ no. of aaa | Accession number of homologous protein |
|---|---|---|---|---|---|
| Z1635 (28-651) | 207 | None | Z1635, unknown protein, E. coli EDL933 (35) | 97/207 | AAG55750.1 |
| Z1636 (747-980) | 77 | None | Z1636, unknown protein, E. coli EDL933 (35) | 96/77 | AAG55751.1 |
| Z1637 (1,033-1,224) | 63 | None | Z1637, unknown protein, E. coli EDL933 (35) | 95/63 | AAG55752.1 |
| S1 (1,900-3,552) | 550 | HlyB | YPO2491, putative hemolysin activator, Y. pestis CO92 (33) | 64/508 | NP_406025.1.1 |
| S2 (3,562-4,089) | 175 | HlyC | RS02573, putative hemolysin activating-like protein, R. solanacearum (37) | 57/167 | CAD18225.1 |
| S3 (4,156-6,006) | 616 | None | YPO2490, putative hemolysin | 53/527 | NP_406024.1 |
| YPO0599, putative adhesin, Y. pestis CO92 (33) | 50/557 | NP_404241.1 | |||
| S4 (6,027-13,058) | 2,343 | None | YPO2490, putative hemolysin | 41/2085 | NP_406024.1 |
| YPO0599, putative adhesin, Y. pestis CO92 (33) | 39/2123 | NP_404241.1 | |||
| S5 (13,446-13,670) | 74 | None | YPO0599, putative adhesin, Y. pestis (33) | 83/53 | NP_404241.1 |
| S6 (13,960-14,289) | 109 | None | Y2435, putative transposase, Y. pestis KIM (7) | 38/79 | AAM85993.1 |
| S7 (15,128-15,526) | 132 | rve | TnpA, transposase, P. syringae (12) | 60/130 | AAB81642.1 |
| S8 (15,958-16,485) | 175 | None | FN0835, hypothetical protein, F. nucleatum ATCC 25586 (17) | 27/115 | AAL95031.1 |
| S9 (16,739-17,014) | 91 | None | YozI, unknown protein, B. subtilis (25) | 32/68 | CAB13779.1 |
| S10 (17,428-17,670) | 80 | None | Z4322, unknown protein, E. coli EDL933 (35) | 94/51 | AAG58106.1 |
| Z4321 (S11) (18,256-18,789) | 177 | None | Z4321, unknown protein, E. coli EDL933 (35) | 98/177 | AAG58105.1 |
| S12 (18,898-20,712) | 604 | UvrD-helicase | Orf1, similarity with helicase, S. enterica (42) | 40/591 | CAA68055.1 |
| S13 (20,778-21,083) | 101 | None | ST0071, hypothetical esterase Sulfolobus tokodaii (23) | 30/69 | NP_375919.1 |
| S14 (21,242-21,949) | 235 | None | Y2679, hypothetical protein, Y. pestis KIM (7) | 39/217 | AAM86232.1 |
| Z1640, unknown protein, E. coli EDL933 (35) | 90/55 | NP_287143.1 | |||
| S15 (22,200-24,881) | 893 | UvrD-helicase | Z1641, unknown protein, E. coli EDL933 (35) | 96/164 | AAG55756.1 |
| Z1642, unknown protein, E. coli EDL933 (35) | 99/711 | AAG55757.1 | |||
| Z1643 (25,381-26,613) | 410 | PAPS-reductaseb | Z1643, unknown protein, E. coli EDL933 (35) | 97/410 | AAG55758.1 |
| Z1644 (26,598-27,236) | 212 | ParBc | Z1644, unknown protein, E. coli EDL933 (35) | 98/212 | AAG55759.1 |
Number of amino acids in a contiguous stretch from which the identity was calculated.
PAPS, phosphoadenosine phosphosulfate.
(ii) Hemolysin/adhesin cluster.
S1 comprises the first fragment of Z1640 and part of GS-I (Fig. 1). The first 179 amino acid residues of its predicted product are 97% identical to the Z1640 product in EDL933, and 508 amino acid residues out of 550 are 64% identical to the putative hemolysin activator encoded by gene YPO2491 in Y. pestis (Table 1) (33). Its protein structure shows 70.5% identity to the conserved domain of HlyB (CD pfam03865). The first 167 of 175 amino acid residues of the S2 product are 57% identical to the putative hemolysin activating-like protein encoded by gene RS02573 in Ralstonia solanacearum (37), and the protein shows 91.1% identity to the conserved domain of HlyC (CD pfam02794).
Both predicted products of S3 and S4 are homologous to the putative hemolysin (encoded by gene YPO2490) as well as the putative adhesin (encoded by gene YPO0599) in Y. pestis (33). The YPO2490 and YPO0599 products are large proteins, with 2,535 and 3,295 amino acids, respectively. The first 527 of 616 amino acids of S3 are 53% identical to the putative Y. pestis hemolysin, and the first 557 amino acids are 50% identical to the putative Y. pestis adhesin. S4 is a large gene encoding a protein with 2,343 amino acid residues, of which 2,085 amino acid residues are 40% identical to the YPO2490 product, and 2,123 amino acids are 39% identical to the YPO0599 product. No conserved domain match was found for either the S3 or S4 protein. S5 is a small gene encoding a protein with 74 amino acids in which a 55-amino-acid sequence is identical to the YPO0599 product.
(iii) Putative transposase genes.
The S6 product has 79 of 109 amino acids with 38% homology to a putative transposase encoded by gene Y2435 in Y. pestis KIM (7), but no conserved domain is found for this protein (Table 1). The predicted product of S7 is 44.4% aligned with the integrase core domain rve (CD pfam00665), and shows 60% and 55% identity to the carboxyl-terminal region of the TnpA transposase of Pseudomonas syringae (12) and Y. pestis (33), respectively. A region upstream of S7 (from bp 14499 to 14975) encodes the amino-terminal region of the TnpA transposase. A 1-bp insertion or a −1 translational frameshift before the stop codon of the amino-terminal region would make the region from 14,499 to 15,526 bp (S7 and the region upstream of S7) into a complete coding region for a transposase with 100% alignment with the integrase core domain. However, the coding region for the amino-terminal region is not predicted to be an active gene (Fig. 1, Table 1).
(iv) OI-122 homologous ORFs.
S10, S11, and S12 are EDL933 OI-122 homologues (Table 1). S10 contains the H8DR-2 sequence and part of the OI-122 segment. Its amino acids 30 to 80 (out of 80) are 94% identical to Z4322 in EDL933. The S11 protein shows 98% homology to the Z4321 product of EDL933 (35). S12 comprises part of the OI-122 segment and its downstream sequence, and the product is 96% identical to Z3218 in 1 to 240 (out of 604) amino acids. But overall, the S12 protein appears to belong to the family of UvrD/Rep-helicases (CD pfam00580), which catalyze ATP-dependent unwinding of double-stranded DNA to single-stranded DNA.
(v) Other ORFs.
The S8 product has 117 of 175 amino acids with 27% homology to the FN0835 product of Fusobacterium nucleatum (17). The S9 product has 68 of 91 amino acids 32% like the unknown Bacillus subtilis protein YozI (25). Amino acids 25 to 93 of 101 of the S13 protein are 30% identical to the ST0071 esterase in Sulfolobus tokodaii (23). The first 217 amino acids of 235 of the S14 protein are 39% homologous to the unknown protein encoded by Y2679 in Y. pestis KIM (7).
Expression of genes in the sequenced region.
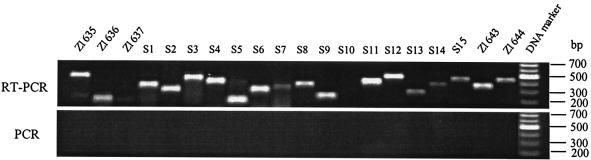
Expression of the predicted genes after CL3 growth in LB broth at 37°C with shaking was determined by reverse transcription PCR. The results showed PCR bands of the predicted sizes (Table 2) for all genes (Fig. 3). Although not visible in Fig. 3, very faint bands of the predicted sizes for Z1637 and S10 were seen. This indicates that the genes are transcribed in the direction predicted and are active, at least at the transcriptional level. This supports our gene predictions for this genomic region.
FIG. 3.
Ethidium bromide-stained agarose gel of reverse transcription PCR products of RNA isolated from E. coli strain CL3. Reverse transcription was carried out with the primer specific to each gene, followed by PCR with specific primers and with the reverse transcription product as the template. PCR without prior reverse transcription was used as a control to confirm that the reverse transcription PCR products were attributable to RNA. Gene designations are indicated above each lane.
Distribution of the CL3 hybrid genomic island in VTEC strains of different serotypes.
Intact Z1640 was present in strains of serotypes that are associated with hemolytic uremic syndrome and epidemic disease but was absent in strains of serotypes that are not associated with epidemic disease and in strains of serotypes that are not associated with human disease (Table 3). In contrast, S1 was not present in strains of serotypes associated with hemolytic uremic syndrome and epidemic disease. But it was present in association with Z1640 in all three strains of serotype O113:H21, in both strains of serotype O91:H21, and in 5 of 11 strains that have only been isolated from animals (Table 3).
DISCUSSION
The initial objective of this study was to determine whether Z4321, a pagC-like gene located in OI-122 of the EDL933 genome, is part of an OI-122-like genomic island in the CL3 genome or part of another genetic structure. Our findings show that in the CL3 genome, Z4321 is not part of an OI-122-like structure, but rather a part of a unique hybrid genomic island that contains segments of two EDL933 genomic O islands (OI-122 and OI-48) and other elements that show homology to putative virulence genes from Y. pestis. The sequenced 27,297-bp region does not show homology to any genomic region of the K-12 strain MG1655 and is thus consistent with a horizontally acquired genomic island (11), whose complete size will be larger than that sequenced to date. Given the presence of putative virulence genes and mobile genetic elements, the sequenced genomic region is consistent with a PAI (11). Because this is the first PAI found in the CL3 genome, we designated it PAI ICL3. Further sequencing may reveal that, like other PAIs (11, 18), PAI ICL3 is inserted close to a tRNA gene.
The unique character of PAI ICL3 appears to be due to genomic events related to OI-48 genes. The genes at both ends of the sequenced PAI ICL3 region resemble OI-48 genes Z1635 to Z1644, which, in CL3, are highly conserved and present in the same order as in EDL933. Of particular interest is the observation that one of the OI-48 genes, Z1640, is separated into three fragments, the spaces between which are occupied by genomic segments, GS-I and GS-II, which include a Y. pestis-like hemolysin/adhesin gene cluster and an EDL933 OI-122 segment (Fig. 1).
The initial event in the generation of PAIs is probably the preferential integration of plasmids, phages, conjugative transposons, or cointegrates of these into the chromosome (11, 18). Further evolution of the PAI can occur by a number of processes, including recombination events between insertion sequence elements, direct repeats, and other homologous sequences, leading to deletion or acquisition of DNA and generation of mosaic structures (11, 18, 39). In some cases, whole PAIs can be deleted. For example, mutants exhibiting spontaneous deletion of PAI IC5 on sheep blood agar have been observed in E. coli strain C5 that was isolated from a patient with neonatal meningitis (14). While such processes tend to make PAIs unstable, mutations in genes associated with recombination events, such as insertion sequence elements and direct repeats, or in those associated with mobility, such as transposases and integrases, leading to their inactivation, can serve to stabilize PAIs (11).
One possibility for the development of PAI ICL3 is that it evolved in a stepwise manner through the insertion of different genetic elements. In favor of this is the observation that the GS-I and H8DR-bordered regions are bracketed by direct repeat sequences (Fig. 1), a feature typical of transposon insertions (5), which provides evidence that these elements were acquired as insertions. Furthermore, there is evidence that this region may have become stabilized through the inactivation of a transposase enzyme. Between bp 14499 and 14975 in the PAI ICL3 region, there is a sequence (S7 and its preceding region) that probably encoded an active transposase which is postulated to have facilitated the insertion of the H8DR-bordered segment (Fig. 1) into the region. However, following the acquisition of the segment, deletion of one base pair leading to the creation of a stop codon in the middle of the sequence resulted in truncation of the transposase gene.
An alternative explanation for the aberrant sequence is that it facilitates the regulation of an active transposase through the process of translational frameshifting (4, 40). This would require a −1 frameshift before the stop codon of the amino-terminal region to translate an active transposase enzyme (4). Translational frameshifting occurs with the expression of some transposase genes. However, in CL3 the two parts of the transposase coding region do not overlap, which is a requirement for a −1 frameshift translation (4). Moreover, the coding region for the amino-terminal region is not predicted to be an active gene.
The hemolysin/adhesion cluster on PAI ICL3 represents potential virulence genes for CL3. On the other hand, S1, used as a marker of this cluster, was absent in the most virulent VTEC serotypes that are associated with outbreaks and HUS (Table 3), but was present in less virulent serotypes (O113:H21 and O91:H21) and in about half the cattle strains that have not been associated with human disease (Table 3). Thus, the hemolysin/adhesin virulence genes may have no role in human disease. On the other hand, the fact that the Y. pestis-like adhesin genes are present only in LEE-negative serotypes (Table 3) suggests that they could provide an alternative mechanism to the LEE-mediated attaching and effacting lesion. For example, it could account for the mechanism of adherence of a strain of the LEE-negative serotype O113:H21 strain, which has been shown to adhere to cultured cells in vitro and to stimulate a signal transduction response in a manner that is different from that of serotype O157:H7 strains (9). However, other candidate adhesins have also been proposed for VTEC serotype O113:H21 strains. These include Saa, a plasmid-mediated adhesin (34), and a chromosomal region homologous to EDL933 OI-154 which encodes long polar fimbriae (8).
In contrast to the hemolysin/adhesin gene cluster that is found in strains that appear to be less virulent than E. coli O157:H7 strains, intact Z1640 was present exclusively in O157:H7 strains and other serotypes that are associated with HUS and epidemic disease (Table 3). Thus, Z1640 could, theoretically, be a virulence gene and thus warrants experimental investigation.
We have argued for a stepwise evolution of PAI ICL3 through acquisition of insertions because of the presence of several genetic elements bracketed by direct repeat sequences. However, it is also possible that PAI ICL3 more closely resembles the ancestral, and less virulent, state and that the EDL933 OI-48 arose as part of the more pathogenic serotype O157:H7 through complete or stepwise deletions of the GS-I and GS-II segments, leading to the formation of the putative virulence gene Z1640. Such a scenario could also explain the UvrD helicase-like gene (S15) as being the ancestral state and the two EDL933 ORFs Z1641 and Z1642 as a derived state resulting from the decay of the original full-length coding region.
In conclusion, the CL3 genomic region reported here is a part of a hybrid putative PAI (PAI ICL3) that contains segments of EDL933 OI-48 and OI-122 and other putative virulence factors, including a Y. pestis-like hemolysin/adhesin cluster. OI-122 elements have also been reported to be part of mosaic structures with the LEE PAI in enteropathogenic rabbit E. coli strains 83/89 and RDEC-1 of serotype O15:H− (41, 45) and in some LEE-positive human enteropathogenic and verocytotoxin-producing E. coli strains (28). These studies and ours provide evidence for considerable plasticity in EDL933 genomic islands, which may contribute to the on-going evolution of VTEC.
Acknowledgments
This work was supported by Health Canada's postdoctoral fellowship awarded to Songhai Shen and an award from the Health Canada Genomics R&D Fund.
We thank T. Whittam, F. Jamieson, J. Isaac-Renton, J. Preiksaitis, P. VanCaeseele, S. Aleksic, and P. Desmarchellier for generously providing the VTEC strains used in this study.
Editor: B. B. Finlay
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, and J. A. Smith. 1991. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 3.Calderwood, S., D. W. K. Acheson, G. T. Keusch, T. J. Barrett, P. M. Griffin, N. A. Strockbine, B. Swaminathan, J. B. Kaper, M. M. Levine, B. S. Kaplan, H. Karch, A. D. O'Brien, T. G. Obrig, Y. Takeda, P. I. Tarr, and I. K. Wachsmuth. 1996. Proposed new nomenclature for SLT (VT) family. ASM News 62:118-119. [Google Scholar]
- 4.Chandler, M., and O. Fayet. 1993. Translational frameshifting in the control of transposition in bacteria. Mol. Microbiol. 7:497-503. [DOI] [PubMed] [Google Scholar]
- 5.Dale, J. W. 1998. Molecular genetics of bacteria, p. 1-310. John Wiley and Sons, Chichester, England.
- 6.Delcher, A. L., D. Harmon, S. Kasif, O. White, and S. L. Salzberg. 1999. Improved microbial gene identification with glimmer. Nucleic Acids Res. 27:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doughty, S., J. Sloan, V. Bennett-Wood, M. Robertson, R. M. Robins-Browne, and E. L. Hartland. 2002. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect. Immun. 70:6761-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dytoc, M. T., A. Ismaili, D. J. Philpott, R. Soni, J. L. Brunton, and P. M. Sherman. 1994. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect. Immun. 62:3494-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin, P. M. 1995. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli, p. 739-761. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract. Raven Press, Ltd., New York, N.Y.
- 11.Hacker, J., and J. B. Kaper. 1999. The concept of pathogenicity islands, p. 1-11. In J. B. Kaper and J. Hacker (ed.), Pathogenicity islands and other mobile virulence elements. ASM Press, Washington, D.C.
- 12.Hanekamp, T., D. Kobayashi, S. Hayes, and M. M. Stayton. 1997. Avirulence gene D of Pseudomonas syringae pv. tomato may have undergone horizontal gene transfer. FEBS Lett. 415:40-44. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 14.Houdouin, V., S. Bonacorsi, N. Brahimi, O. Clermont, X. Nassif, and E. Bingen. 2002. A uropathogenicity island contributes to the pathogenicity of Escherichia coli strains that cause neonatal meningitis. Infect. Immun. 70:5865-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurtado, A., and F. Rodriguez-Valera. 1999. Accessory DNA in the genomes of representatives of the Escherichia coli reference collection. J. Bacteriol. 181:2548-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, R., R. C. Clarke, J. B. Wilson, S. C. Read, K. Rahn, S. A. Renwick, K. A. Sandhu, D. Alves, M. A. Karmali, H. Lior, S. A. McEwen, J. S. Spika, and C. L. Gyles. 1996. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J. Food Prot. 59:1112-1122. [DOI] [PubMed] [Google Scholar]
- 17.Kapatral, V., I. Anderson, N. Ivanova, G. Reznik, T. Los, A. Lykidis, A. Bhattacharyya, A. Bartman, W. Gardner, G. Grechkin, L. Zhu, O. Vasieva, L. Chu, Y. Kogan, O. Chaga, E. Goltsman, A. Bernal, N. Larsen, M. D'Souza, T. Walunas, G. Pusch, R. Haselkorn, M. Fonstein, N. Kyrpides, and R. Overbeek. 2002. Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J. Bacteriol. 184:2005-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaper, J. B., and J. Hacker (ed.). 1999. Pathogenicity islands and other mobile virulence elements. ASM Press, Washington, D.C.
- 19.Kaper, J. B., J. L. Mellies, and J. Nataro. 1999. Pathogenicity islands and other mobile genetic elements of diarrheagenic Escherichia coli, p. 33-58. In J. B. Kaper and J. Hacker (ed.), Pathogenicity islands and other mobile virulence elements. ASM Press, Washington, D.C.
- 20.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmali, M. A., M. Mascarenhas, S. Shen, K. Ziebell, S. Johnson, R. Reid-Smith, J. Isaac-Renton, C. Clark, K. Rahn, and J. B. Kaper. 2003. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41:4930-4940. [DOI] [PMC free article] [PubMed]
- 22.Karmali, M. A., M. Petric, C. Lim, P. C. Fleming, G. S. Arbus, and H. Lior. 1985. The association between hemolytic uremic syndrome and infection by Verotoxin-producing Escherichia coli. J. Infect. Dis. 151:775-782. [DOI] [PubMed] [Google Scholar]
- 23.Kawarabayasi, Y., Y. Hino, H. Horikawa, K. Jin-no, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Kato, T. Yoshizawa, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki, S. Masuda, M. Yanagii, M. Nishimura, A. Yamagishi, T. Oshima, and H. Kikuchi. 2001. Complete genome sequence of an aerobic thermoacidophilic crenarchaeon, Sulfolobus tokodaii strain7. DNA Res. 8:123-140. [DOI] [PubMed] [Google Scholar]
- 24.Konowalchuk, J., J. I. Speirs, and S. Stavric. 1977. Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 26.Lukashin, A. V., and M. Borodovsky. 1998. GeneMark. hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morabito, S., R. Tozzoli, E. Oswald, and A. Caprioli. 2003. A mosaic pathogenicity island made up of the locus of enterocyte effacement and a pathogenicity island of Escherichia coli O157:H7 is frequently present in attaching and effacing E. coli. Infect. Immun. 71:3343-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss, J. E., T. J. Cardozo, A. Zychlinsky, and E. A. Groisman. 1999. The selC-associated SHI-2 pathogenicity island of Shigella flexneri. Mol. Microbiol. 33:74-83. [DOI] [PubMed] [Google Scholar]
- 30.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nataro, J. P., J. Seriwatana, A. Fasano, D. R. Maneval, L. D. Guers, F. Noriega, F. Dubovsky, M. M. Levine, and J. G. Morris. 1995. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect. Immun. 63:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2001. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 33.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 34.Paton, A. W., P. Srimanote, M. C. Woodrow, and J. C. Paton. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 69:6999-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. Kirkpatrick, G. Posfal, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 36.Pulkkinen, W. S., and S. I. Miller. 1991. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J. Bacteriol. 173:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salanoubat, M., S. Genin, F. Artiguenave, J. Gouzy, S. Mangenot, M. Arlat, A. Billault, P. Brottier, J. C. Camus, L. Cattolico, M. Chandler, N. Choisne, C. Claudel-Renard, S. Cunnac, N. Demange, C. Gaspin, M. Lavie, A. Moisan, C. Robert, W. Saurin, T. Schiex, P. Siguier, P. Thebault, M. Whalen, P. Wincker, M. Levy, J. Weissenbach, and C. A. Boucher. 2002. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 415:497-502. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Schmidt, H., W. L. Zhang, U. Hemmrich, S. Jelacic, W. Brunder, P. I. Tarr, U. Dobrindt, J. Hacker, and H. Karch. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekine, J., and E. Ohtsubo. 1989. Frameshifting is required for production of transposase encoded by insertion sequence 1. Proc. Natl. Acad. Sci. 86:4609-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tauschek, M., R. A. Strugnell, and R. Robins-Brown. 2002. Characterization and evidence of mobilization of the LEE pathogenicity island of rabbit-specific strains of enteropathogenic Escherichia coli. Mol. Microbiol. 44:1533-1550. [DOI] [PubMed] [Google Scholar]
- 42.Titheradge, A. J., D. Ternent, and N. E. Murray. 1996. A third family of allelic hsd genes in Salmonella enterica: sequence comparisons with related proteins identify conserved regions implicated in restriction of DNA. Mol. Microbiol. 22:437-447. [PubMed] [Google Scholar]
- 43.Wilson, J. B., R. C. Clarke, S. Renwick, K. Rahn, R. P. Johnson, M. A. Karmali, H. Lior, D. Alves, C. Gyles, K. S. Sandhu, S. A. McEwen, and J. Spika. 1996. Verocytotoxigenic Escherichia coli infection in dairy farm families. J. Infect. Dis. 174:1021-1027. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 1999. Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC). Report of a W.H.O. Scientific Working Group Meeting, Berlin, Germany, 22-23 June 1998, p. 1-30. World Health Organization. Geneva, Switzerland.
- 45.Zhu, C., T. S. Agin, S. Elliot, L. A. Johnson, T. Thate, J. B. Kaper, and E. C. Bodeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]