Abstract
Infections caused by the opportunistic pathogen Pseudomonas aeruginosa involve the interplay of several bacterial virulence factors. It has recently been established that the delivery of toxic effector proteins by the type III secretion system is an important virulence mechanism in several animal models. Furthermore, the expression of the type III secretion system and its effectors has been correlated with a poor clinical outcome during human infections. A novel cyclic AMP (cAMP) regulatory network that controls the expression of virulence factors, including the type III secretion system, was examined to determine its contribution to P. aeruginosa colonization and dissemination in a mouse pneumonia model. Mutants lacking the two genome-encoded adenylate cyclases, CyaA and CyaB, and the cAMP-dependent regulator Vfr were examined. Based on the enumeration of bacteria in lungs, livers, and spleens, as well as the assessment of mouse lung pathology, mutations in the cyaB and vfr genes resulted in a more significantly attenuated phenotype than mutations in cyaA. Moreover, in this model, expression of the type III secretion system was essential for lung colonization and pathology. Strains with mutations in the exsA gene, which encodes a type III regulatory protein, or pscC, which encodes an essential component of the secretion apparatus, were also significantly attenuated. Finally, we demonstrate that virulence can be restored in an adenylate cyclase mutant by the overexpression of exsA, which specifically restores expression of the type III secretion system in the absence of a functional cAMP-dependent regulatory network.
Pseudomonas aeruginosa has become one of the most common causes of nosocomial pneumonia and is emerging as a major cause of community-acquired pneumonia in severely ill patients (10). Individuals with significant immune defects such as those associated with human immunodeficiency virus infections, cancer, and mechanical ventilation are particularly vulnerable to P. aeruginosa pneumonia. The morbidity and mortality associated with P. aeruginosa pneumonia are considerable. In particular, patients with ventilator-associated pneumonia caused by P. aeruginosa have an alarming mortality rate of approximately 70 to 80%, which is significantly higher than that associated with pneumonia caused by other bacteria (4). In addition, the majority of cystic fibrosis patients develop chronic P. aeruginosa respiratory infections, resulting in persistent inflammation and tissue damage. Many of these individuals become infected with multiantibiotic-resistant strains of P. aeruginosa, and despite aggressive therapy, eradication of this bacterium is difficult.
The severity of P. aeruginosa respiratory infections is due in part to the production and secretion of multiple factors that contribution to disease progression and pathogenesis. In particular, P. aeruginosa utilizes a type III secretion system (TTSS) to deliver a number of toxic effector proteins directly into host cells, where they target host signal transduction pathways. The TTSS is a widely distributed protein-targeting mechanism found in many gram-negative human and plant pathogens, including Yersinia, Salmonella, Chlamydia, Shigella, and others (15). The P. aeruginosa TTSS directs the secretion of four known effector proteins, namely ExoS, ExoU, ExoT, and ExoY, although ExoU and ExoS are rarely expressed by the same bacterium (8, 29-31). Studies utilizing multiple P. aeruginosa infection models have suggested that the TTSS contributes significantly to infection outcomes (13, 16, 18, 20). Furthermore, immunization of mice with PcrV, a component of the TTSS, protected mice from P. aeruginosa-induced lung injury (22). Several studies have drawn a direct link between the TTSS and the severity of P. aeruginosa human infections. The expression of the TTSS was correlated with increased morbidity and mortality in patients with lower respiratory tract infections and bacteremia and with disease severity in patients with ventilator-associated pneumonia (11, 21).
The induction of the TTSS and the secretion of effectors are regulated by environmental signals such as contact with eukaryotic cells and calcium limitation (19, 25). The transcriptional regulation of the TTSS and its effectors is under the control of an AraC-like transcriptional regulator, ExsA, and its repressor, ExsD (14, 17). More recently, it was shown that the expression of the TTSS is part of a global regulatory network involving 3′,5′-cyclic AMP (cAMP). These studies demonstrated that the generation of cAMP in P. aeruginosa is due primarily to CyaB, a class III membrane-associated adenylate cyclase, and to a lesser extent, to CyaA, which is a member of the cytoplasmic class I adenylate cyclase family (27). The cAMP second messenger subsequently acts as a cofactor for the transcriptional regulator Vfr, a member of the cAMP receptor protein family. Vfr positively regulates the expression of over a hundred genes, including those encoding the TTSS and components of type IV pili, and negatively regulates the expression of flagellar biogenesis genes (3, 7, 27). Additional studies have also shown that Vfr is important for the regulation of exotoxin A and protease, which is due in part to its influence on the expression of the LasR/LasI quorum-sensing system (1, 26). Therefore, the adenylate cyclase CyaB, its product cAMP, and the cAMP-dependent transcriptional regulator Vfr constitute a signaling network that acts as a master regulator of virulence gene expression in P. aeruginosa.
For this study, we evaluated the pathogenesis of P. aeruginosa in a mouse model of acute pneumonia and assessed the role that this cAMP signaling pathway has on virulence. We demonstrate here that defects in this pathway result in a significant reduction in bacterial colonization, lung destruction, and dissemination. Consistent with in vitro studies, we confirm that the CyaB adenylate cyclase plays a pivotal role in controlling virulence. The expression of the TTSS in attenuated mutants that are defective in cAMP production and signaling can restore wild-type virulence, emphasizing the critical importance of this secretion mechanism for disease. Furthermore, our experiments indicate that the expression of the TTSS early in the infection process plays a critical role in bacterial dissemination.
MATERIALS AND METHODS
Bacterial strains.
P. aeruginosa strain PAK and a series of isogenic nonpolar deletion mutants were used for these studies (27). Deletions were made in cyaA, which encodes a class I adenylate cyclase; cyaB, which encodes a class III adenylate cyclase; vfr, which encodes a cAMP binding transcriptional regulator; exsA, which encodes a TTSS regulator; and pscC, which encodes an essential component of the type III secretion apparatus.
Mouse infections.
Cultures were grown in tryptic soy broth (Difco) overnight and then subcultured into fresh medium and grown at 37°C with aeration to an optical density at 660 nm (OD660) of 2.0. Bacteria were centrifuged, and the concentration was adjusted to give approximately 2.5 × 109 CFU/ml in 1% proteose peptone in phosphate-buffered saline (PBS). The exact number of bacteria in each inoculum was determined by plating serial dilutions on Luria-Bertani agar (L-agar) plates. Female BALB/c mice (Charles River) (approximately 50 days old) were housed in accordance with National Institutes of Health guidelines. Mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (65 mg/kg of body weight) and xylazine (13 mg/kg). Anesthetized animals were intranasally infected with 10 μl of culture in each nostril, giving a total infection volume of 20 μl. At 16 h postinfection, mice were sacrificed, and lungs, spleens, and 300 mg of liver were removed and homogenized in 1 ml of 1% peptone-PBS. The number of bacteria in each tissue was determined by growing serial dilutions of the homogenate on L-agar plates. Data are expressed as numbers of CFU obtained in the entire spleens or lungs and those found in 300 mg of liver.
Histology.
At 16 h postinfection with wild-type or mutant P. aeruginosa strains, mouse lungs were removed and fixed with 10% buffered formalin. Fixed tissues were dehydrated in grades of alcohol, embedded in paraffin, and cut into approximately 5-μm-thick sections. Deparaffinized tissue sections were stained with either hematoxylin and eosin, Gram's stain, or an anti-lipopolysaccharide (LPS) antibody. Antibody staining was performed as follows. Tissue sections were incubated for 1 h at room temperature with 5% normal goat serum diluted in PBS with 0.1% bovine serum albumin. Sections were then incubated overnight at 4°C with an anti-P. aeruginosa LPS serotype 6 rabbit polyclonal antibody (a gift from G. Pier at Harvard Medical School). Antibody binding was detected by incubation of samples for 1 h at room temperature with a phosphatase-conjugated goat anti-rabbit immunoglobulin antibody (KPL, Gaithersburg, Md.) diluted 1:500 in PBS-0.1% bovine serum albumin. Binding was visualized with a 3,3-diaminobenzidine substrate (Vector Labs) per the manufacturer's protocol. The 3,3-diaminobenzidine reagent reacts with the phosphatase to form a brown precipitate. Cells were counterstained with hematoxylin. Images were taken with an Olympus microscope.
Electron microscopy.
Lung samples were collected at 16 h postinfection from mice infected with either wild-type PAK or a strain with a deletion of both cyaA and cyaB (cyaAB mutant). Samples were fixed for 2 h with 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer (pH 7.4) and then were stored in cacodylate buffer overnight at 4°C. Samples were incubated for 1 h with 1% osmium tetroxide and 1.5% potassium ferrocyanide, washed with water, and stained with 1% aqueous uranyl acetate for 30 min. Tissues were dehydrated in grades of alcohol and then were infiltrated and embedded in TAAB Epon (Marivac Canada Inc., St. Laurent, Canada). Approximately 60-nm-thick sections were cut on a Richert Ultracut-S microtome and then were placed on copper grids. Tissues were stained with uranyl acetate and lead citrate and were examined in a JEOL 1200EX transmission electron microscope. The lungs from one mouse of each infection group were examined. At least two tissue samples from different sites in each lung were processed, and several sections were cut and examined. Approximately 10 to 20 fields from each section were examined.
Overexpression of ExsA in vivo.
The complete exsA open reading frame was cloned into pMMB67EH to create pPa-exsA as previously described (9, 27). The pPa-exsA vector carries the lacIq repressor, and thus the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) allows for the controlled expression of exsA from the tac promoter. This plasmid was conjugated into the cyaAB or pscC mutant strain. These strains were grown in tryptic soy broth with carbenicillin (150 μg/ml) overnight and then were subcultured into fresh medium with antibiotic. Bacteria that were induced in vitro were grown to an OD660 of 1.5, at which point 500 μM IPTG was added to the cultures. When cultures reached an OD660 of 2.0, they were centrifuged and resuspended in 1% peptone-PBS at a concentration of approximately 109 CFU/ml. Mouse infections were done as described above. For in vivo induction of exsA expression, mice were given drinking water containing 12 mM IPTG 24 h prior to infection. This method of IPTG delivery has been shown to be sufficient to regulate lac promoter expression in most tissues (28). At 16 h postinfection, the lungs, spleens, and livers (300 mg) were removed and homogenized in 1 ml of 1% peptone-PBS. The number of bacteria in each tissue was determined by growing serial dilutions of homogenates on L-agar with or without 150-μg/ml carbenicillin. The RNA was extracted from infected lung tissues with Trizol (Invitrogen) per the manufacturer's procedures. Reverse transcription (RT)-PCR was used to evaluate the expression of exsA or Pseudomonas 16S rRNA. Samples were standardized to approximately equal amounts of 16S rRNA and were visualized on an ethidium bromide-stained agarose gel.
RESULTS
CyaB and the cAMP binding protein Vfr are essential for P. aeruginosa lung colonization and dissemination.
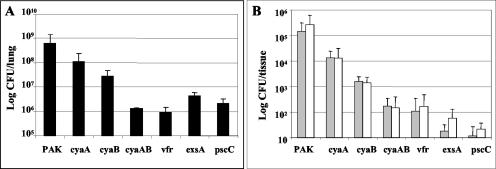
Previous studies have demonstrated that the deletion of the type III secretion genes exsA or pscC significantly attenuates P. aeruginosa virulence in animal models (12, 13). By use of various in vitro assays, it was also shown that the expression of the TTSS and other virulence determinants was dependent on the adenylate cyclases CyaA and CyaB; however, the role that these factors play during P. aeruginosa infections was undetermined (27). Prior studies have demonstrated the efficacy of using an adult mouse model of acute pneumonia to identify and study various P. aeruginosa virulence factors (2, 5). Therefore, we utilized this model to evaluate the role that adenylate cyclases play during P. aeruginosa infections. When we infected mice via intranasal inoculation with 5 × 107 CFU of wild-type strain PAK, there was a 10-fold increase in the number of bacteria recovered from the lungs of these animals at 16 h postinfection (Fig. 1A). An examination of the livers (300 mg) and spleens of infected animals revealed that significant numbers of bacteria could be isolated from these organs (Fig. 1B). These results demonstrate that wild-type P. aeruginosa not only is able to colonize and proliferate in the lungs of infected mice, but also disseminates to establish secondary systemic infections. When strains containing mutations in either exsA or pscC, both of which are defective for type III secretion, were used to infect mice, the numbers of bacteria isolated from the lungs, livers, and spleens were substantially lower than with wild-type infections. The numbers of bacteria that could be isolated from the lungs of these mice at 16 h postinfection were >10-fold less than the initial inocula, and <100 bacteria could be detected in their livers and spleens (Fig. 1).
FIG. 1.
Strains of P. aeruginosa containing deletions in cyaB, vfr, or type III secretion genes have attenuated virulence during acute lung infections in mice. Mice were infected with 5 × 107 CFU of wild-type PAK or various strains that contained deletions in one or more genes. At 16 h postinfection, mice were euthanized and organs were removed. The numbers of bacteria in the lungs (A) or in the spleen (gray bars) and 300 mg of liver (white bars) (B) were determined by growing serial dilutions of homogenates on L-agar plates. Error bars indicate standard deviations. A minimum of five mice were included in each group.
For determination of how the expression of cAMP by either one or both of the chromosomally encoded adenylate cyclases affects the virulence of P. aeruginosa, nonpolar deletion mutants cyaA and cyaB were tested in the acute pneumonia model. When mice were infected with a cyaA mutant, there was only a minor but reproducible decrease in the number of bacteria isolated from the lungs of these animals compared to that of wild-type PAK infections. Despite this slight decrease, the numbers of recovered bacteria were still larger than the initial inoculum size (Fig. 1A). There was also only a small reduction in the number of bacteria found in the spleens or livers of these animals compared to wild-type infections (Fig. 1B). These data indicate that the deletion of cyaA has little effect on the colonization and dissemination of P. aeruginosa during acute pneumonia infections. Conversely, when mice were infected with a cyaB mutant, there was a 23-fold decrease in the number of bacteria isolated from the lungs and a 90- to 200-fold decrease in the number of bacteria isolated from the spleens and livers compared to the numbers obtained with wild-type infections (Fig. 1). Infections with a strain with deletions of both the cyaA and cyaB adenylate cyclase genes or vfr resulted in an even more attenuated phenotype, with over a 400-fold decrease in the number of bacteria isolated from the lungs of infected animals compared to the wild type (Fig. 1A). These values are over 30-fold lower than the initial inoculum size. A limited dissemination of the bacteria into spleens and livers was also observed, with <200 bacteria being recovered from each of these organs (Fig. 1B). Since none of the mutants had growth defects when grown in either rich (L-broth or tryptic soy broth) or minimal (M63) medium, these results demonstrate that the low level of bacterial recovery is very likely due to the activity of the host defense mechanisms in the infected mice. The numbers of bacteria obtained from infections with the cyaAB and vfr mutants were similar to those found with the exsA and pscC TTSS mutants, suggesting that defects in cAMP signaling could be accounted for by a lack of TTSS expression.
Characterization of infections induced by P. aeruginosa strains containing mutations in adenylate cyclases or type III secretion genes.
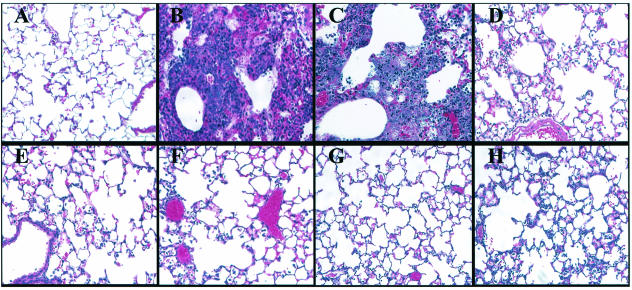
Lungs from mice at 16 h postinfection with wild-type PAK had significant neutrophil infiltration, edema, and tissue damage (Fig. 2B). Many of the airways in the lungs of these mice were completely occluded with cellular infiltrate. Infections with the cyaA mutant induced similar inflammatory characteristics as those found with wild-type PAK (Fig. 2C). The extent of inflammation caused by the cyaA mutant correlated with the ability of this strain to persist and proliferate in the lungs of infected animals. Infections with the cyaB mutant showed significantly reduced inflammation, with substantially fewer neutrophils occurring in the alveolar spaces, compared to infections with wild-type PAK or the cyaA mutant (Fig. 2D). Infections with the cyaAB mutant, which was unable to express either of the two adenylate cyclases, or the vfr mutant showed minimal infiltration of neutrophils into the lungs, and the pathology was similar to infections with TTSS mutants (Fig. 2E to H).
FIG. 2.
Differential inflammatory cell migration and lung pathology were observed when mice were infected with various PAK mutants. Mice were infected with either no bacteria (PBS) (A) or 5 × 107 CFU of wild-type PAK (B) or a strain containing a deletion of cyaA (C), cyaB (D), cyaAB (E), vfr (F), exsA (G), or pscC (H). At 16 h postinfection, lungs from infected mice were removed, fixed, sectioned, and stained with hematoxylin and eosin. Images were taken with a 20× objective.
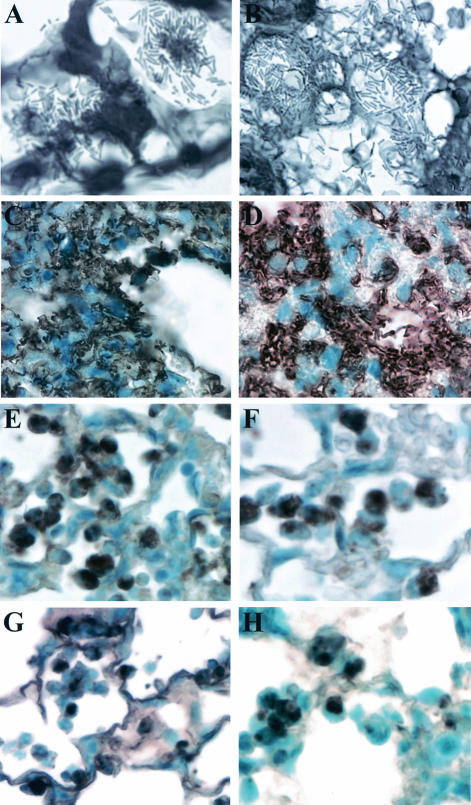
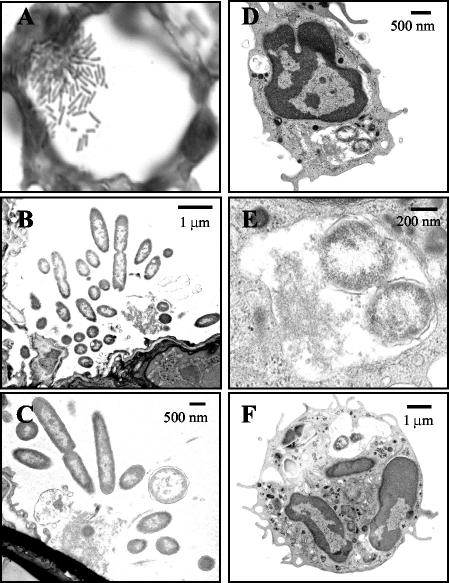
When lung sections were stained with Gram's stain and examined for bacterial localization, it was observed that after 16 h wild-type P. aeruginosa was spread throughout the lung. The majority of stained bacteria appeared to be associated with epithelial and inflammatory cell surfaces in the alveolar airways. These bacteria also appeared to be growing in clusters or microcolonies (Fig. 3A and 4A). This presence of bacterial clusters in the lungs of PAK-infected mice was confirmed when lung sections were stained and examined by electron microscopy (FIG. 4B and C). When lung sections from mice that were infected for only 5 h with wild-type PAK were examined, only single isolated bacteria could be observed (data not shown). This indicates that the observed clusters of bacteria occurred during their growth in the lungs of infected animals and were not an artifact from aggregated bacteria in the inoculating culture. Gram staining of lung sections from mice infected with the cyaA mutant showed similar characteristics to those with PAK infections. These bacteria could also be detected throughout the lungs of infected animals (Fig. 3B). In contrast, when lung sections from mice infected with the cyaB, cyaAB, vfr, or exsA mutant were examined, only a few bacteria could be visualized (data not shown). For further evaluation of the localization of bacteria in the lungs of infected mice, lung sections were stained with an anti-LPS antibody. As demonstrated in Gram-stained sections, lungs from mice infected with wild-type PAK or the cyaA mutant had substantial amounts of bacteria throughout (Fig. 3C and D). Interestingly, although an examination of lung sections from mice infected with the cyaB, cyaAB, vfr, or exsA mutant showed very little LPS staining of intact bacteria, as was seen with Gram-stained sections, there were significant amounts of staining associated with the infiltrating cells (Fig. 3E to H). When lung sections from mice infected with the cyaAB mutant were examined by electron microscopy, bacteria in the airways were seen infrequently; however, those that were detected were often found to be engulfed by what appeared to be neutrophils (Fig. 4D to F). This suggests that the pattern of LPS staining seen with the cyaB, cyaAB, vfr, and esxA mutants may reflect phagocytosed bacteria or ingested bacterial membrane components.
FIG. 3.
Localization and growth of bacteria in the lungs of infected mice. Mice were infected with a wild-type PAK (A and C), cyaA deletion (B and D), cyaB deletion (E), cyaAB deletion (F), vfr deletion (G), or exsA deletion (H) strain, and at 16 h postinfection, lungs were removed and fixed and sections were stained with either Gram's stain (A and B) or an anti-LPS polyclonal antibody (C to H). LPS staining is indicated by a brown precipitate, and cells were stained with hematoxylin.
FIG. 4.
Electron microscopy of lungs infected with wild-type P. aeruginosa or a cyaAB mutant. Mice were infected with wild-type PAK (A to C) or the cyaAB mutant (D to F). Infected lungs were removed and fixed in glutaraldehyde. (A) A 0.5-μm-thick section was cut, and the bacteria were stained with Gram's stain. The image was taken with a 100× objective. (B to F) Approximately 60-nm-thick sections were cut and stained with uranyl acetate and lead citrate. Samples were examined in an electron microscope. (E) The image is a higher magnification of panel D and shows the intracellular bacteria.
Expression of exsA in a cyaAB mutant induces wild-type levels of inflammation and dissemination.
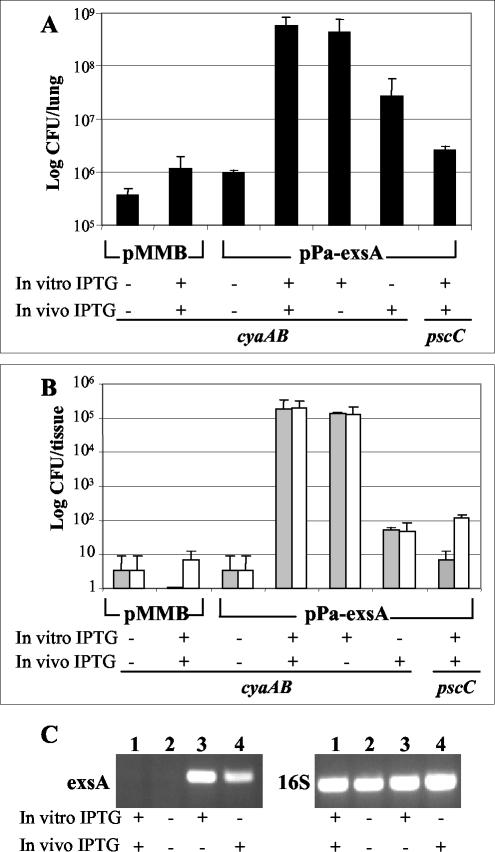
We have previously demonstrated that Vfr and its coactivator cAMP regulate the expression of the TTSS and that overexpression of exsA bypasses this regulatory pathway and restores the expression and function of the TTSS. Moreover, we have also shown that cAMP, with Vfr, controls the expression of over a hundred P. aeruginosa genes (27). For determination of whether the attenuated virulence associated with mutations in the cAMP signaling pathway is due to their regulatory effects on the TTSS or to the activation of another gene(s), exsA was overexpressed in the cyaAB mutant. The expression plasmid pPa-exsA, in which the exsA gene is regulated by the IPTG-inducible tac promoter, was conjugated into the cyaAB mutant, and this strain was used to infect mice. Bacteria in homogenates of lungs, spleens, and livers from infected mice were enumerated by plating on L-agar or L-agar with 150 μg of carbenicillin/ml, which allowed us to determine if the pMMB67EH (vector control) or pPa-exsA plasmid was maintained during infection in the absence of antibiotic selection. In all experiments, there were no considerable differences in the numbers of bacteria obtained on plates with or without the addition of carbenicillin (data not shown). When mice were infected with the cyaAB mutant strain containing the pMMB67EH vector control, the number of bacteria observed in the lungs of infected animals was similar to that with the cyaAB mutant without the plasmid (Fig. 5A). These numbers did not change dramatically when this strain was grown in IPTG-containing medium prior to inoculation into mice given IPTG in their drinking water. Similarly, omitting the IPTG inducer during the growth of the cyaAB mutant containing the pPa-exsA expression plasmid and from the water of infected mice resulted in equally low numbers of bacteria obtained from the lungs, livers, or spleens of infected mice (Fig. 5A and B). However, when the expression of the tac-regulated exsA gene was induced by first growing the bacteria in the presence of IPTG and including IPTG in the drinking water of mice, the number of bacteria observed in the lungs of these animals was over 5 × 108 CFU, a level equal to that found with wild-type PAK infections (Fig. 5A). These mice also had disseminating infections, with high numbers of bacteria being isolated from their livers and spleens (Fig. 5B). Interestingly, when exsA was induced by the addition of IPTG to bacterial cultures (in vitro) and mice were infected without providing IPTG in the drinking water, the numbers of bacteria found in the lungs, livers, and spleens were equal to those obtained with wild-type infections (Fig. 5A and B). This indicates that the continued induction of exsA is not essential once the bacteria have established an infection. Additionally, when mice were given IPTG in their drinking water and then infected with uninduced cultures, the number of bacteria in the lungs of these animals was only slightly reduced from that found with fully induced strains. However, when livers and spleens from these mice were examined, there was a substantial decrease in the number of bacteria that could be detected (Fig. 5B). These data indicate that although the delay in the activation of exsA and thus type III secretion was not substantial enough to significantly alter the lung infection, there was a considerable effect found on the ability of the bacteria to disseminate and induce systemic infections. For evaluation of whether the addition of IPTG in vitro to cultures or the feeding of IPTG to mice did indeed regulate exsA expression during infection, exsA transcript levels in lung tissues from infected mice were examined by RT-PCR. Lungs from mice infected with the cyaAB mutant containing the pMMB vector control or pPa-exsA without the addition of IPTG showed no detectable transcript for exsA, although there were abundant levels of transcript for the Pseudomonas 16S ribosome. However, when IPTG was added to cultures prior to infection or was fed to mice, exsA was readily detected (Fig. 5C). These data demonstrate that the addition of IPTG regulated the expression of exsA and thus the induction of the TTSS during infections. As a control, the pPa-exsA expression plasmid was also conjugated into a pscC mutant. Because PscC is a structural component of the TTSS, its deletion prevents the formation of the secretion apparatus, regardless of exsA overexpression. When exsA was induced in this strain with IPTG and IPTG-fed mice were infected with the resultant strain, the number of bacteria that could be isolated from the lungs, spleens, or livers of these animals was similar to those from infections with uninduced cultures (Fig. 5A and B). This demonstrates that the overexpression of exsA does not have secondary effects on the bacteria independent of the TTSS.
FIG. 5.
Overexpression of exsA restores the wild-type phenotype in the cyaAB mutant. Mice were infected with the mutant cyaAB strain containing either the pMMB67EH vector or the exsA expression plasmid pPa-exsA. As a control, the pPa-exsA plasmid was also expressed in the pscC mutant. For in vitro induction, cultures were induced with 500 μM IPTG prior to mouse infections. For in vivo induction, mice were given water that contained IPTG. At 16 h postinfection, mice were sacrificed and the numbers of bacteria in their lungs (A) or spleens (gray bars) and 300 mg of liver (white bars) (B) were determined by growing dilutions of homogenates on L-agar plates and L-agar plates with 150-μg/ml carbenicillin. Error bars represent standard deviations. Each group consisted of three to five mice. (C) Expression of exsA and P. aeruginosa 16S rRNA as evaluated by RT-PCR with infected lungs. RNAs were extracted from the lungs of mice infected with the cyaAB mutant containing the pMMB67EH vector (lanes 1) or pPa-exsA (lanes 2 to 4). PCR samples were resolved on ethidium bromide-stained agarose gels.
DISCUSSION
P. aeruginosa respiratory infections are becoming more prevalent in patients with compromised immune defense mechanisms. Several studies have demonstrated that expression of the TTSS is correlated with the severity of Pseudomonas infections in humans (11, 21). Additionally, by using various models of infection, a number of reports have highlighted the role that the TTSS plays in the virulence of P. aeruginosa. We have recently described a signaling pathway in P. aeruginosa that utilizes cAMP and the cAMP binding protein Vfr for the regulation of multiple genes, including those encoding the TTSS (27). Remarkably, the P. aeruginosa genome encodes two adenylate cyclases (CyaA and CyaB), both of which are expressed under most laboratory conditions. By utilizing a mouse model of acute pneumonia infection, we examined the consequences of cyaA, cyaB, and vfr gene deletions on the colonization of the lung and systemic dissemination. In this study, we demonstrated that the cyaB, cyaAB, and vfr mutants are strongly attenuated during lung infections, a phenomenon comparable to that observed with strains lacking expression of the TTSS due to mutations in exsA or pscC (Fig. 1). Although cyaA and cyaB mutants were demonstrably attenuated, the loss of cyaB resulted in significantly lower numbers of bacteria in the lungs than the modest effect observed with the cyaA mutant. The bacterial load in the lungs of mice infected with the cyaA or cyaB mutant also correlated with the severity of lung pathology associated with the infection (Fig. 2C and D). Similar differences between these two strains were found upon dissemination into the liver and spleen after infection (Fig. 1). Although the effects of a cyaA deletion on P. aeruginosa virulence were modest compared to those of a cyaB deletion, there was a synergistic response with the deletion of both adenylate cyclases. We therefore conclude that during the course of infection both adenylate cyclases are expressed, but CyaB is responsible for the production of the majority of the cAMP, which is essential for the activation of the transcriptional pathway leading to the expression of the TTSS. An examination of the cAMP levels produced in vitro by the cyaA, cyaB, and cyaAB mutants demonstrated the predominance of CyaB for the production of cAMP in P. aeruginosa, with the cyaAB mutant producing only background levels of cAMP (27). Therefore, the virulence phenotype of the various cya mutants in the mouse acute pneumonia model accurately reflects the availability of cAMP, a cofactor for the transcriptional regulator Vfr.
This work and our previous studies point to a significant difference in the utilization of cAMP signaling pathways among different bacteria. Escherichia coli expresses a single adenylate cyclase, a close homologue of P. aeruginosa CyaA. The production of cAMP in E. coli is important for the activation of the global regulator CRP, which is homologous to Vfr in P. aeruginosa (26). Although there is significant homology between these two systems, they appear to have evolved to regulate distinctly different bacterial functions. In E. coli and other enteric bacteria, the adenylate cyclase and the production of cAMP are activated by the detection of an environmental signal such as a carbon source; however, this does not seem to be the case for P. aeruginosa (24). The cAMP signaling system in P. aeruginosa seems to respond to environmental calcium concentrations and potentially host cell contact (27). A further difference between these systems is highlighted by the fact that cAMP signaling in P. aeruginosa depends primarily on CyaB, a class III adenylate cyclase, rather than the E. coli homologue CyaA. CyaB contains multiple putative transmembrane domains, which suggests that it is localized to the inner membrane. It is therefore conceivable that environmental or cell contact signals activate the P. aeruginosa adenylate cyclases.
The induction of the TTSS system via the overexpression of exsA in an adenylate cyclase mutant (cyaAB) was sufficient to restore wild-type levels of infection and dissemination (Fig. 5), thus demonstrating that the expression of the TTSS in our model was sufficient for virulence. Although the activation of the TTSS by the cAMP pathway played a predominant role in acute infections, we cannot exclude the possibility that this pathway plays a broader role in regulating the virulence traits of this organism in other infection models or during human infections. Transcriptional profiling using cyaAB and vfr mutants has shown that the expression of 163 genes is controlled by this regulatory mechanism (27). We also demonstrated that the controlled regulation of exsA and thus the TTSS in the cyaAB mutant greatly altered the outcome of P. aeruginosa infections. The activation of exsA prior to infection was sufficient to induce wild-type levels of bacterial infection in the lungs, livers, and spleens of mice, even in the absence of additional in vivo activation. However, when exsA expression was turned on only after intranasal infection in mice, colonization of the lungs was not greatly altered from that of wild-type infections, but reduced bacterial dissemination was observed (Fig. 5). These data suggest that the induction of the TTSS during infection, which may occur only after adherence to a cell surface or detection of an environmental signal, is sufficient for colonization and thus allows P. aeruginosa to establish an environmental niche, induce tissue damage, and disseminate. However, if expression of the TTSS is delayed, the host may have a selective advantage and thus prevent significant lung pathology and bacterial dissemination.
Bacterial LPS staining of lung sections from mice infected with wild-type P. aeruginosa demonstrated that the bacteria were in intimate contact with both epithelial and inflammatory cell surfaces, thus implicating the role of cell contact in bacterial activation. Observations of lung sections revealed that wild-type P. aeruginosa formed microcolonies, while no such bacterial communities were visible in the cyaB, cyaAB, vfr, and exsA mutants, all of which lack expression of the TTSS (Fig. 3 and 4). However, when mutant bacteria were observed in the lung, they were often found engulfed by what appeared to be polymorphonuclear cells. It has been proposed that P. aeruginosa grows as a biofilm during infections and that this mode of growth is instrumental in bacterial resistance to antibiotics and host immunity (6, 23). The observed microcolonies of wild-type P. aeruginosa may represent a distinct phase in biofilm formation. The absence of such structures from the various TTSS mutants may further highlight the interplay of various bacterial factors in the establishment of infections in a biological system, as opposed to in vitro models. In a well-defended organ, such as the respiratory tract, inflammatory responses play an important role in bacterial clearance. It would be particularly informative to compare an acute infection model to a model that more closely resembles chronic infections in order to define the full range of the activities of the cAMP regulatory network. It is conceivable that cAMP produced in P. aeruginosa by any one of the adenylate cyclases during chronic infections may regulate some of the same functions observed during acute infections, such as biofilm formation and resistance to host defense mechanisms, while coordinating the expression of novel genes involved in prolonged persistence in the infected host.
Acknowledgments
The work in Stephen Lory's laboratory was supported by grants from the Cystic Fibrosis Foundation. R.S.S. and M.C.W. were Cystic Fibrosis Foundation Postdoctoral Fellows.
Editor: V. J. DiRita
REFERENCES
- 1.Albus, A. M., E. C. Pesci, L. J. Runyen-Janecky, S. E. West, and B. H. Iglewski. 1997. Vfr controls quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3928-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatson, S. A., C. B. Whitchurch, J. L. Sargent, R. C. Levesque, and J. S. Mattick. 2002. Differential regulation of twitching motility and elastase production by Vfr in Pseudomonas aeruginosa. J. Bacteriol. 184:3605-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chastre, J., and J. Y. Fagon. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867-903. [DOI] [PubMed] [Google Scholar]
- 5.Comolli, J. C., A. R. Hauser, L. Waite, C. B. Whitchurch, J. S. Mattick, and J. N. Engel. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 67:3625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta, N., E. P. Ferrell, K. J. Kanack, S. E. West, and R. Ramphal. 2002. fleQ, the gene encoding the major flagellar regulator of Pseudomonas aeruginosa, is sigma70 dependent and is downregulated by Vfr, a homolog of Escherichia coli cyclic AMP receptor protein. J. Bacteriol. 184:5240-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 9.Furste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 10.Garau, J., and L. Gomez. 2003. Pseudomonas aeruginosa pneumonia. Curr. Opin. Infect. Dis. 16:135-143. [DOI] [PubMed] [Google Scholar]
- 11.Hauser, A. R., E. Cobb, M. Bodi, D. Mariscal, J. Valles, J. N. Engel, and J. Rello. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30:521-528. [DOI] [PubMed] [Google Scholar]
- 12.Hauser, A. R., P. J. Kang, and J. N. Engel. 1998. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807-818. [DOI] [PubMed] [Google Scholar]
- 13.Holder, I. A., A. N. Neely, and D. W. Frank. 2001. Type III secretion/intoxication system important in virulence of Pseudomonas aeruginosa infections in burns. Burns 27:129-130. [DOI] [PubMed] [Google Scholar]
- 14.Hovey, A. K., and D. W. Frank. 1995. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 177:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurahashi, K., O. Kajikawa, T. Sawa, M. Ohara, M. A. Gropper, D. W. Frank, T. R. Martin, and J. P. Wiener-Kronish. 1999. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 104:743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCaw, M. L., G. L. Lykken, P. K. Singh, and T. L. Yahr. 2002. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol. Microbiol. 46:1123-1133. [DOI] [PubMed] [Google Scholar]
- 18.Miyata, S., M. Casey, D. W. Frank, F. M. Ausubel, and E. Drenkard. 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 71:2404-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 20.Pukatzki, S., R. H. Kessin, and J. J. Mekalanos. 2002. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 99:3159-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 22.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 23.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 24.Suh, S. J., L. J. Runyen-Janecky, T. C. Maleniak, P. Hager, C. H. MacGregor, N. A. Zielinski-Mozny, P. V. Phibbs, Jr., and S. E. West. 2002. Effect of vfr mutation on global gene expression and catabolite repression control of Pseudomonas aeruginosa. Microbiology 148:1561-1569. [DOI] [PubMed] [Google Scholar]
- 25.Vallis, A. J., T. L. Yahr, J. T. Barbieri, and D. W. Frank. 1999. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect. Immun. 67:914-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West, S. E., A. K. Sample, and L. J. Runyen-Janecky. 1994. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J. Bacteriol. 176:7532-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfgang, M. C., V. T. Lee, M. E. Gilmore, and S. Lory. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4:253-263. [DOI] [PubMed] [Google Scholar]
- 28.Wu, J. D., H. C. Hsueh, W. T. Huang, H. S. Liu, H. W. Leung, Y. R. Ho, M. T. Lin, and M. D. Lai. 1997. The inducible lactose operator-repressor system is functional in the whole animal. DNA Cell Biol. 16:17-22. [DOI] [PubMed] [Google Scholar]
- 29.Yahr, T. L., J. T. Barbieri, and D. W. Frank. 1996. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J. Bacteriol. 178:1412-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1997. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]