Highlights
► We discuss the role of secondary meristems in the adaptation of plant growth forms. ► We highlight the integration of environmental inputs into plant growth regulation. ► Branching and secondary growth shape the plant body. ► Plant’s phenotypic plasticity can be used to dissect the evolution of growth forms.
Keywords: Phenotypic plasticity, Adaptation, Plant meristems, Branching, Cambium, Natural variation
Abstract
The developmental plasticity of organisms is a natural consequence of adaptation. Classical approaches targeting developmental processes usually focus on genetics as the essential factor underlying phenotypic differences. However, such differences are often based on the inherent plasticity of developmental programs. Due to their dependence on environmental stimuli, plants represent ideal experimental systems in which to dissect the contribution of genetic and environmental variation to phenotypic plasticity. An evident example is the vast repertoire of growth forms observed in plant shoot systems. A fundamental factor underlying the broadness of this repertoire is the activity of secondary meristems, namely the axillary meristems that give rise to side shoots, and the cambium essential for stem thickening. Differential activities of both meristem types are crucial to the tremendous variation seen in higher plant architecture. In this review, we discuss the role of secondary meristems in the adaptation of plant growth forms, and the ways in which they integrate environmental input. In particular, we explore potential approaches for dissecting the degree to which this flexibility and its consequences for plant architecture is genetically predetermined and how much it represents an adaptive value.
1. Phenotypic plasticity and evolution
Variation among organisms is manifested in the tremendous amount of growth forms found in nature. Species living in particular environments often display specific growth characteristics which in many cases, become fixed genetically as they confer an advantage in a given habitat. However, phenotypic plasticity, that is, the ability of a genotype to generate different phenotypes in response to varying environmental conditions (Pfennig et al., 2010), also plays a major role in shaping organisms (West-Eberhard, 2003). Phenotypic plasticity is a highly relevant concept in ecology and evolutionary biology because it allows organisms to react quickly to changing environmental conditions (Sommer and Ogawa, 2011; West-Eberhard, 2003). Models of population divergence often support the idea that the capacity for phenotypic plasticity relies on genetic factors (Price et al., 2003). It is assumed that high levels of plasticity normally prevent genetic selection because organisms can reach optimal fitness easily by modifying their phenotype without the need for genetic change. However, there could be a trade-off between plasticity and best performance in specific environments. Hence, the adaptive plasticity concept argues for the evolution of plasticity itself maximizing fitness, especially in variable environments (Dudley and Schmitt, 1996). For example, phenotypic plasticity allows rapid colonization of new habitats. In this case, initial success results purely from phenotypic changes. Often, this is later translated into genetic differences due to the loss of selection pressure on plasticity itself, a process known as genetic assimilation (Henry et al., 2006; Price et al., 2003). Thus, for a single species living in a range of ecosystems, individual populations might exhibit very different phenotypes optimized for each discrete environmental regime. Whether these differences are due to phenotypic plasticity or genetic variation is at first sight elusive (Henry et al., 2006).
The concept of phenotypic plasticity was first postulated based on the adaptation of Drosophila melanogaster to heat-shock treatments (Waddington, 1953), and has been illustrated in, for example, generalist herbivores locally adapted to different host plants (Funk et al., 2002; Simon et al., 2003). Although these and other examples have highlighted some aspects of phenotypic plasticity leading to the genetic fixation of phenotypes, our knowledge of the impact of phenotypic plasticity on evolution and the trade-off between phenotypic plasticity and the costs of maintaining the flexibility of developmental programs is still scarce. In this review, we describe the regulation of secondary meristems of plants and the resulting variability in plant architecture in terms of using it as an experimental system to address aspects of the role of developmental plasticity in promoting evolutionary innovation.
2. Secondary meristems of plants as a model for studying the role of phenotypic plasticity during evolution
2.1. Experimental systems for studying phenotypic plasticity
One of the major questions concerning the interplay between phenotypic plasticity and the evolution of organisms is whether genetic fixation of plastic traits is a mechanism commonly used to couple environmental input with genetic information. Sommer and Ogawa (2011), argue that a number of requirements must be fulfilled for a successful case study. First, detailed knowledge of the developmental process under study, especially at the genetic level, should be available, such that variation in molecular mechanisms controlling a particular trait can be investigated. Second, phenotypic variation among populations, or variability between closely related species, for the trait under investigation should exist. Third, knowledge of the phylogenetic framework of the individuals or populations investigated must be robust enough to support the directionality of evolutionary change.
2.2. Secondary meristems shape the plant
Plants, in particular the model plant Arabidopsis thaliana, fulfill the above mentioned requirements. A classical attempt to describe the organization of the body of higher plants, including Arabidopsis, relies on the concept of phytomers. A phytomer is a repetitive unit that consists of a leaf, a leaf attachment site including an axillary bud (nodium) and an associated piece of stem (internodium) (Gaudichaud, 1841; Gray, 1849). The variation of plant shoot morphology between most, if not all, higher plant species can be understood by considering it as the phylogenetic and ontogenetic modification of this fundamental unit.
Being sessile, plants have to be especially equipped to translate environmental cues into developmental responses. For example, this is reflected in the fact that, in contrast to animals, fully developed plant embryos consist only of the basic body plan (Leyser, 2009), providing the possibility of adjusting growth to local conditions at later growth stages. The basis for such plasticity are two stem cell niches (the primary shoot and root apical meristems) located at opposite poles of the embryo, which are connected by rudimentary stem and root tissues (Wolters and Jurgens, 2009). These meristems usually remain active during the entire life cycle and give rise to all plant organs, which are generated post-embryonically. Furthermore, as plants grow, new growth axes need to be established to extend the plant body in a manner compatible with the environment. This is achieved by establishing secondary apical meristems that can produce new axes of growth and harbor the same developmental potential as the primary meristems from which they were originally derived (De Smet et al., 2006; Leyser, 2009). In the aerial parts of higher plants, axillary meristems (AMs) are responsible for the production of new shoot axes, namely branches, from leaf axils (Leyser, 2009). Another growth process that plants use to extend and modify their body structure is lateral growth of stems and roots, which is mediated predominantly by lateral meristems, especially the vascular cambium (Elo et al., 2009). The coordinated activity of all meristems, as well as the integration of information from the environment, is crucial to the reproductive success of plants. Secondary meristems are fundamental to this success because they facilitate alteration of plant architecture and morphology at any point during the life cycle. In the next sections, we discuss the following questions: What is the genetic basis of secondary meristem regulation and how are environmental inputs integrated? Is there coordination of the regulation of different types of secondary meristems? To what extent does the differential activity of secondary meristems contribute to the establishment of different plant growth forms? Here, we summarize knowledge of the regulation of AMs and the vascular cambium in the shoot, and discuss the potential of secondary meristems to serve as a model for addressing the role of phenotypic plasticity, in this case morphological plasticity, during evolution.
3. Axillary bud formation and outgrowth
3.1. Branching shapes the plant
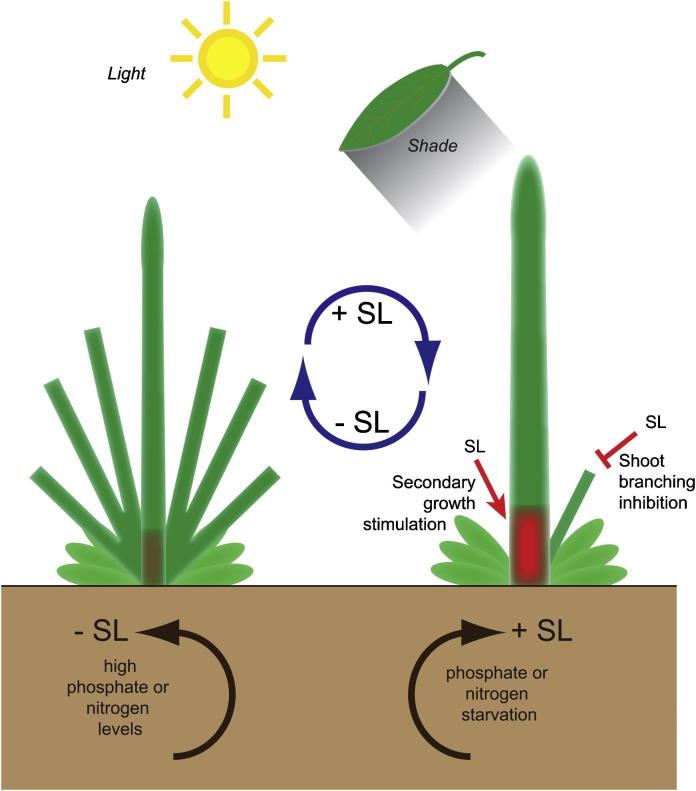
In general, AMs are formed in the axil of each leaf, and often initiate a few new leaf primordia before arresting their growth to form a dormant axillary bud (Domagalska and Leyser, 2011). This bud may either remain dormant or become activated to give rise to an axillary branch. Each branch provides new axes of growth by hosting more axillary meristems, which potentially produce higher-order branches. Under natural conditions, the environment plays a key role in regulating bud outgrowth, making it a highly plastic process that modulates plant architecture (Aguilar-Martinez et al., 2007; Doust, 2007b). For example, shading inhibits branching, while high nutrient availability promotes it (Fig. 1) (Kebrom, 2007; McSteen, 2009). This phenomenon is well illustrated in environments of high plant density, where light and nutrient competition is very strong, usually resulting in reduced branching (Doust, 2007a,b). Detailed knowledge on AM formation at the molecular level (Busch et al., 2011; Greb et al., 2003; Komatsu et al., 2001; Muller et al., 2006; Schmitz et al., 2002) allowed researchers to determine that the contribution of AM formation to phenotypic plasticity seems to be minor (Ehrenreich et al., 2007). Indeed, current opinion holds that the plasticity observed in shoot branching is mostly a consequence of differential AM activity and not formation (Leyser, 2009).
Fig. 1.
The influence of environmental cues on SL biosynthesis and shoot architecture and the role of SLs.
3.2. Auxin – An essential regulator of branching
A process fundamental to the control of axillary bud activity is apical dominance. It has long been known that the main shoot apex suppresses the outgrowth of axillary buds (Snow, 1929) and thus, apical dominance is relevant in the definition of plant architecture. Indeed, the expression of a particular growth form is linked to the degree of apical dominance. For example, some species carry a dominant primary stem, while in other species lateral branches grow similarly vigorously as the main stem and sometimes even overgrow it. These two branching habits are called excurrent and decurrent, respectively, and can easily be associated to various annual species. However, a clear distinction is not always straightforward in woody perennials, where a number of other factors like age, vigor, dormancy, nutrients availability or the presence/absence of terminal inflorescence need to be considered, as they modify the pattern of growth of individual tree branches (Brown et al., 1967).
The auxin indole 3-acetic acid (IAA) is essential for apical dominance. It is produced in the shoot apex and transported along the shoot, where it inhibits bud outgrowth (Snow, 1929; Thimann and Skoog, 1933). However, auxin does not act directly in the bud, but rather acts in the stem as, for example, it does not travel into the dormant bud (Booker et al., 2003; Prasad, 1993). Therefore, the existence of a second messenger has been postulated (Cline, 1991). Interestingly, it was demonstrated that a secondary messenger moving upwards from the root (probably in the transpiration stream) was needed for bud activation (Dun et al., 2009; Snow, 1929, 1935) but its nature has long remained elusive. Significantly in our context, plants alter auxin transport (and therefore auxin distribution) in response to environmental triggers by modifying the subcellular localization of auxin efflux-carriers of the PIN protein family. One such example is the response to shade (Keuskamp et al., 2010; Laxmi et al., 2008). Another, more prominent, example is auxin redistribution during phototropism – a response causing plants to grow towards the light by auxin-induced differential growth of two opposite stem sides (Whippo and Hangarter, 2006). Similarly, roots grow towards the gravity vector while the shoot grows against it. This response is called gravitropism and likewise involves differential growth of different root and stem sides induced by auxin redistribution (Tan et al., 2011). Collectively, these phenomena serve to demonstrate that plants are able to translate environmental input into changes in auxin transport and ultimately, into changes in auxin signaling. Whether the modulation of auxin transport is the main target of environmental cues in the context of apical dominance remains to be analyzed.
3.3. Cytokinins promote branching
Cytokinins, another class of plant hormones, promote branching (Cline, 1991). Although they can also be synthesized in specific sites of the aerial part, cytokinins are mostly synthesized in the root and transported upwards along the xylem (Chen et al., 1985). Cytokinin levels seem to be regulated negatively by auxin and increase after shoot decapitation (Shimizu-Sato et al., 2009). Interestingly, cytokinin levels are also affected by the environment, for example, nutrient availability, with nitrogen inducing cytokinin production and in turn, cytokinins regulating nitrate uptake (Krouk et al., 2011). Thus, cytokinin biosynthesis and signaling are further targets for connecting environmental input with endogenous developmental programs.
3.4. Strigolactones – the long-sought branching hormones
Recent studies have demonstrated that strigolactones (SLs) – a carotenoid-derived group of hormones – play a pivotal role in the inhibition of shoot branching, thereby closing long-existing gaps in the understanding of the regulation of branching, especially concerning the nature of the long-sought second messenger (Gomez-Roldan et al., 2008; Umehara et al., 2008). The integration of SLs into the regulatory network, however, is currently under debate. One hypothesis is that SLs act downstream of auxin in the control of shoot branching. This idea is based on the observation that direct application of SLs onto growing buds reduced their growth but did not seem to affect auxin transport from the bud (Brewer et al., 2009; Dun et al., 2009). This suggests that the effect of SLs is independent of the regulation of stem auxin content (Brewer et al., 2009). Another indication that SLs act downstream of auxin is that auxin positively regulates the expression of SL biosynthesis genes (Bainbridge et al., 2005; Foo et al., 2005; Hayward et al., 2009; Sorefan et al., 2003). However, these and other observations also support the auxin transport hypothesis (Bennett et al., 2006). According to this hypothesis, SLs negatively regulate auxin transport in the main stem, and inhibit bud outgrowth by preventing axillary buds from establishing their own polar auxin transport (PAT) stream into the stem (Bennett et al., 2006; Domagalska and Leyser, 2011). Consistently, mutants impaired in SL biosynthesis or signaling display enhanced levels of the auxin exporters PIN1 and PIN3, and SL application reduces PIN protein levels (Bennett et al., 2006; Crawford et al., 2010; Ruyter-Spira et al., 2011).
Until recently, four genes defined the SL biosynthesis and signaling pathway, namely MORE AXILLARY BRANCHES 1 (MAX1), MAX2, MAX3 and MAX4. These genes were discovered in Arabidopsis (Booker et al., 2004, 2005; Sorefan et al., 2003; Stirnberg et al., 2007), and homologues were characterized in pea (Beveridge et al., 1994), petunia (Napoli, 1996), tomato (Vogel et al., 2010) and rice (Ishikawa et al., 2005). Several lines of evidence suggest that SLs are derived from carotenoids (Matusova et al., 2005). Carotenoid biosynthesis in plants provides ß-carotenoid in all-trans configuration (DellaPenna and Pogson, 2006). A recent study revealed that 9-cis-ß-carotene is the substrate for MAX3 (CAROTENOID CLEAVAGE DIOXIGENASE 7/CCD7) and that D27, a protein first discovered in rice and already suggested to be involved in SL biosynthesis, is an isomerase that converts all-trans-ß-carotene into 9-cis-ß-carotene (Alder et al., 2012). In the same study, the authors conclude that MAX3 converts 9-cis-ß-carotene into 9-cis-ß-apo-10′-carotenal, which is used as a substrate by MAX4 (CCD8) to produce carlactone, an intermediate compound with strigolactone-like biological activities. The MAX1 gene encodes a member of the cytochrome P450 family and also plays a role in the SL biosynthesis pathway (Booker et al., 2005). So far, max1 mutants have not been described in species other than Arabidopsis. The max2 mutant is insensitive to SL application regarding apical dominance, suggesting that MAX2 functions as a receptor for SLs or an unknown downstream product (Booker et al., 2005; Gomez-Roldan et al., 2008; Umehara et al., 2008). MAX2 and its orthologs in pea (RMS4) and rice (D3), encode nuclear leucine-rich repeat F-box proteins (Domagalska and Leyser, 2011; Ishikawa et al., 2005; Johnson et al., 2006; Stirnberg et al., 2002). However, MAX2 is not specific for SL signaling, because in Arabidopsis it is also required for mediating the response to karrikins. Karrikins are compounds derived from burnt vegetation that stimulate seed germination (Nelson et al., 2011). Light on this issue was shed recently when homologs of DWARF14 (D14), an SL signaling component originally discovered in rice (Arite et al., 2009) were analyzed in Arabidopsis. The analysis resulted in the concept that two paralogous genes, AtD14 and AtD14-like (KIN2) act as SL and karrikin receptors, respectively, which bind individually to MAX2 after binding of the respective ligand and, thereby, permit the separation of karrikin and SL signaling (Waters et al., 2012). Also recently, the Petunia hybrida ABC transporter PDR1 was proposed to be an SL transporter (Kretzschmar et al., 2012). This conclusion was based on the observation that the roots of the petunia pdr1 mutant are defective in SL exudation whereas the internal concentration is not affected. Complementarily, overexpression of petunia PDR1 in Arabidopsis confers more tolerance to synthetic SL, indicating a role for PDR1 in SL exudation.
The conservation of the SL signaling pathway and its function in mediating apical dominance in a broad range of species argues for an ancient and fundamental role in plant growth regulation including mosses (Proust et al., 2011). This property, together with the very specific effects of SL deficiency in comparison to other hormones, makes the SL signaling pathway an attractive target for addressing the molecular basis of environmentally-induced adaptations in plant architecture.
3.5. Strigolactone production is affected by environmental cues
Plant morphogenesis depends on environmental inputs. Interestingly, different stresses can result into the same stress-induced morphogenic responses (SIMRs), suggesting that the same acclimation state may be reached by different, interchangeable pathways under different conditions (Potters et al., 2009). One possibility is that signaling events triggered by different stresses alter the levels of a given, or few, hormones and thereby, translate different environmental inputs into the same morphological effect. Indeed, one typical SIMR is the reduction of cell division in the main apical meristem and a stimulation of lateral organ growth (Potters et al., 2009). In these cases, different SIMRs may alter the levels of auxin or SLs. For example, nutrient availability affect the production of SLs (Fig. 1) (Xie and Yoneyama, 2010). The best studied example is the phosphate starvation-mediated upregulation of SL biosynthesis, and the accompanying change in root system architecture (Kohlen et al., 2011; Ruyter-Spira et al., 2011). In addition, nitrogen starvation can also induce SL production in roots, as observed in sorghum (Yoneyama et al., 2007). Based on such evidence, it has been proposed that the regulation of SL production in response to changes in environmental conditions could be a strategy of plants to minimize shoot branching under low nutrient conditions (Yoneyama et al., 2009). Other environmental factors may also regulate SL biosynthesis or signaling. Indeed, seedlings of the max2 mutant display enhanced hypocotyl growth, suggesting a relationship between SL signaling and shade avoidance (Fig. 1) (Shen et al., 2007).
Taken together, the SL signaling pathway is important for translating environmental input into developmental responses. This phenomenon is distributed widely and conserved among species (Dun et al., 2009; Johnson et al., 2006; Ongaro and Leyser, 2008). One interpretation of this conservation is that variation in SL biosynthesis and/or signaling is fundamental for the adaptation of plant architecture to different environments. Accordingly, different species or ecotypes may have altered their architecture during evolution by modulating the SL signaling pathway. This could lead to genetic variation within the pathway that, as a result, would shape (or at least strongly bias) the architecture in a certain way by default. Indeed, a mixed-model association mapping using polymorphisms found in 36 genes from different Arabidopsis accessions controlling different aspects of branching (from AM formation to axillary bud regulation) revealed MAX2 and MAX3 as being significantly associated with branching variation (Ehrenreich et al., 2007). In this study, the only other associated gene was SUPERSHOOT (SPS1), which is involved in axillary bud outgrowth regulation by modulating the hormonal balance within the buds (Tantikanjana et al., 2001). These observations are in agreement with the fact that signaling of the main hormones controlling branching is influenced by environmental conditions, as described above. Thus, the genetic basis for branching plasticity might be linked to variations in hormone signaling and the respective modulation of the signaling under different environmental conditions.
4. Secondary growth depends on lateral meristems
4.1. Secondary growth is essential for the establishment of large plant bodies
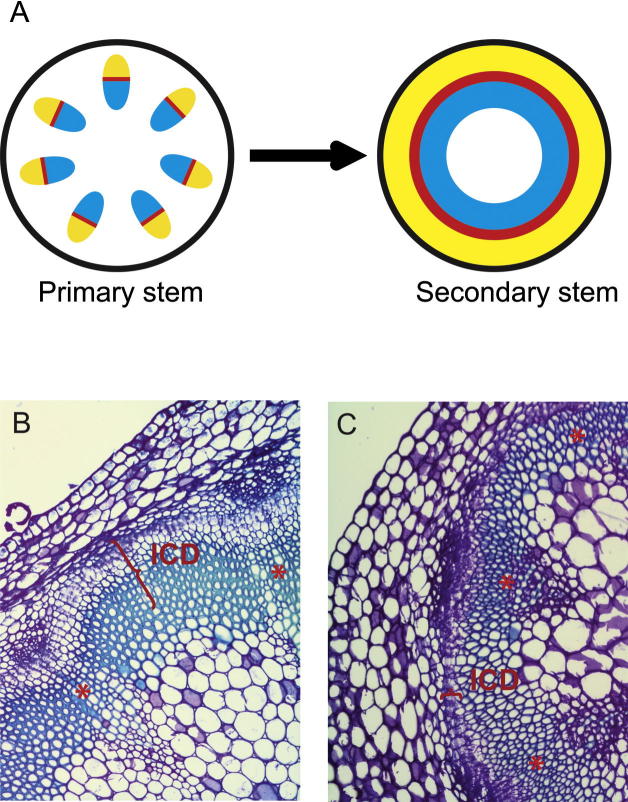
Secondary growth is the process by which most dicotyledonous plants and conifers expand their growth axes laterally. Secondary growth is a very dynamic and plastic process that, like branching, allows plants to adjust their growth to specific environmental regimes (Rowe and Speck, 2005). Secondary growth depends on the activity of the vascular cambium, a stem-cell niche that, like axillary meristems, is a clear example of postembryonic initiation of meristematic activity (Sehr et al., 2010). The cambium is found in a tube-like domain enclosing the center of growth axes (Agusti et al., 2011b), where it produces secondary phloem (bast) centrifugally and secondary xylem (wood) centripetally (Larson, 1994). These tissues are specialized for long-distance transport of water, nutrients, assimilates, and signaling molecules. Although secondary growth is most prominent in trees, it does occur in herbaceous species, including Arabidopsis (Chaffey et al., 2002). In the primary stem of Arabidopsis, the vascular tissue is organized into several isolated vascular bundles. These consist of primary phloem and primary xylem encompassing the fascicular cambium (Fig. 2). During secondary growth, the meristematic attributes of the fascicular cambium are extended into the interfascicular regions in a process that results in the formation of interfascicular cambium (IC) (Agusti et al., 2011b). The IC connects the cambial tissues of neighboring vascular bundles and, in this way, a closed cylinder of meristematic tissue is established (Fig. 2).
Fig. 2.
Secondary growth and natural variation of secondary growth in Arabidopsis thaliana. (A) Schematic illustration of the anatomy of the primary and secondary stem. Note that the primary stem shows several discrete vascular bundles composed of phloem (yellow), xylem (blue) and cambium (red), while the secondary stem carries closed cylinders of phloem (yellow), xylem (blue) and cambium (red). (B and C) Examples of natural variation in secondary growth in Arabidopsis thaliana. B shows a cross-section collected immediately above the uppermost rosette leaf of the main stem of the Pla-1 accession. (C) shows a cross-section of the main stem of the Rou-0 accession collected at the same position. Note that Pla-1 displays more IC-derived cells (ICD) than Rou-0 (indicated by red brackets). Both accessions were harvested when the height of the main stem was 20 cm. Asterisks indicate the position of primary vascular bundles.
4.2. Environmental inputs influence secondary growth
Knowledge obtained mainly from trees makes it evident that intra- and interspecies variation exists, and suggests that this variation is a result of local adaptations and depends on environmental input (Rowe and Speck, 2005; Spicer and Groover, 2010). For example, perennial woody species growing in temperate regions produce rings of xylem reflecting fluctuating conditions along seasons and specific conditions of the corresponding year (de Kort, 1993). That seasonal changes in growth conditions also influence cambium activity in herbaceous species is, again, shown by studies in Arabidopsis. Treatments that stop or delay flowering lead to enhanced secondary growth (Chaffey et al., 2002). Indeed, the late flowering double mutant suppressor of overexpression of constans (soc1) fruitful (ful) displays enhanced wood development (Melzer et al., 2008), indicating a link between flowering time and secondary growth. Along the same lines, Sibout et al., observed a tight correlation between flowering and secondary xylem production when comparing different recombinant inbred lines (RIL) and Arabidopsis accessions (Sibout et al., 2008). In addition to seasonal changes, mechanical perturbations influence cambium activity. Wind or leaning induces increased trunk diameter in trees by stimulating the production of reaction wood (tension wood in angiosperms and compression wood in gymnosperms) important for protection against mechanical damage. Reaction wood production occurs when the plant is under directional mechanical stress and thus, it usually results in asymmetric stem thickening (Du, 2007; Jacobs, 1954). It is well established that reaction wood production is mediated by the plant hormone ethylene (Du, 2003; Love et al., 2009; Nelson, 1978; Yamamoto, 1987). In Arabidopsis, it has been demonstrated that the application of weight to the inflorescence stem promotes secondary growth (Ko et al., 2004). In addition, it has been hypothesized that intra-tissue tension stimulates cambium activity in a process positively regulated by the hormone jasmonic acid (Sehr et al., 2010). Thus, like shoot branching, differential regulation of secondary growth represents a means for plants to reformulate their architecture upon environmental stimulation, representing a valuable ecological trait, and another example of phenotypic plasticity.
5. The coordination of branching and secondary growth
5.1. The regulation of branching and secondary growth is linked
It has been shown in a vast number of species that plant decapitation eliminating the main source of auxin, has a negative effect on secondary growth (Björklund et al., 2007; Ko et al., 2004; Snow, 1935). In addition, restoring auxin levels by applying IAA restores secondary growth. Thus, the regulation of secondary growth shares important features with the regulation of branching. A tight association of both processes is also suggested by the broad spectrum of the same or similar factors found to regulate apical and lateral meristems (Sanchez et al., 2012; Yaginuma et al., 2011). For example, cytokinins are very important in the control of the activity of both apical and lateral meristems, playing opposite roles in SAM and RAM. A positive regulation in the SAM is evidenced by the reduction of SAM size in cytokinin-defective mutants (Werner et al., 2003; Werner and Schmülling, 2009). On the other hand, these kinds of mutants display a larger RAM as well (Heyl et al., 2008; Mason et al., 2005; Miyawaki et al., 2006). With respect to secondary meristems, it is well established that cytokinin signaling plays positive role in the regulation of lateral growth (Matsumoto-Kitano et al., 2008; Nieminen et al., 2008) and branching (Wickson and Thymann, 1958).
Confirming a tight interaction between branching and secondary growth, a positive role for SL signaling in the regulation of cambium activity has recently been reported (Agusti et al., 2011a). All four Arabidopsis max mutants display reduced cambium activity in comparison to wild type, and direct application of the SL analog GR24 is able to induce secondary growth. Genetic and pharmacological analyses argue for a positive role of auxin-dependent stimulation of SL biosynthesis in cambium cells as the basis for the SL-dependent stimulation of cambium activity (Agusti et al., 2011a). This response seems to be conserved among species, as the pea rms1–1 mutant (impaired in SL biosynthesis) displays reduced secondary growth and GR24 treatments also induce secondary growth in Eucalyptus (Agusti et al., 2011a).
5.2. Strigolactones as central modulators of growth forms?
Taken together, both shoot branching and secondary growth are plastic processes that are highly influenced by the environment. SL signaling represses axillary bud outgrowth and induces cambium activity. Based on these observations, we have previously hypothesized that SLs are growth regulators used by plants to adjust their aerial architecture by modulating shoot branching and secondary growth in opposite directions (Agusti et al., 2011a). Depending on environmental input, plants would exhibit either a bushier form displaying a strong outgrowth of side shoots and a weak main stem, or a form in which the main shoot dominates and displays enhanced secondary growth (Fig. 1). The capacity to change between these growth forms may represent a significant advantage when growing in changing environmental conditions.
Overall, the regulation of branching and secondary growth, and the role of SLs as pivotal modulators, constitutes a potential example of developmental plasticity important for environment-dependent growth and achieving a fitness optimum. To what degree are both processes coordinated, and what role does SL signaling play in this coordination? If there is coordination, to what extent is it fixed genetically? Does it vary among populations? Is this a case of developmental flexibility driving genetic adaptation to specific environments?
5.3. The analysis of natural variation as a tool for dissecting the role of morphological plasticity
Analysis of natural variation in Arabidopsis thaliana represents a powerful tool with which to characterize variance among populations. To determine the genetic basis of adaptation, it is essential to associate loci with a variable trait (Fournier-Level et al., 2011). A very straightforward approach is the use of natural strains collected from different geographical locations to identify polymorphisms using linkage disequilibrium (LD) mapping in a genome-wide context [Genome-wide association mapping (GWAS), (Atwell et al., 2010)]. GWAS aims to link sequence polymorphisms (SNPs) to phenotypes, thereby identifying candidate genes responsible for the observed variation among populations (Trontin et al., 2011). Applications of this method have already proved to be very effective in detecting alleles responsible for fitness traits (Todesco et al., 2010). Recently, Fournier-Level et al. went a step further by linking SNPs to fitness-related loci to real-time adaptation using geographically diverse Arabidopsis accessions growing in four specific locations (Fournier-Level et al., 2011). Using GWAS, the authors observed that loci associated with fitness in natural environments display geographical and climatic signatures of adaptation.
Analyses of natural variation in morphological traits have been undertaken (Ehrenreich et al., 2007; Hilscher et al., 2009; Sibout et al., 2005; Sibout et al., 2008) but their adaptive value and their degree of plasticity in different environments has not been analyzed systematically. To evaluate the role of developmental plasticity in evolution, quantitative estimations of plasticity in different strains have to be made; the molecular basis of plasticity variations have to be determined; these differences have to be correlated with the occurrence of particular strains in various environments; and their adaptive value has to be estimated. This is certainly a challenging task as various changing variables have to be measured and integrated into such an analysis. The activity of secondary meristems represents one promising avenue in this respect as meristem activity can change constantly during plant growth and such changes can be quantified at the same time. Importantly, secondary meristems are not crucial for plant growth or fertility per se. Thus, modulation of their activity is genetically accessible and, in contrast to primary apical meristems, the existence of extreme cases can be expected. In this context, it is interesting to note that dramatic differences in SL levels and shoot architecture among different rice strains have been recently reported (Jamil et al., 2012). As soon as one appropriate environmental factor that influences the activity of several types of meristems and which can be tightly controlled under laboratory conditions has been identified, such an approach becomes feasible. Potentially, existing computational models might be exploited for visualizing the outcome of molecular variation. For example, models have described the process of bud activation and its relationship to auxin transport (Prusinkiewicz et al., 2009; Prusinkiewicz and Runions, 2012; Renton et al., 2012). To date there is no model describing lateral growth, let alone the interaction between lateral growth and branching. However, combining natural variation data from both processes in a computational model could be useful to study the benefits of coordinated developmental flexibility.
Questions that can be addressed by following such approaches are whether there is general coordination in the AM and vascular cambium activity, and to what degree this is genetically determined and evolutionarily meaningful. Current bio-statistics techniques, such as the application of GWAS, are powerful tools that can investigate the genetics of adaptation and address the evolutionary impact of trait–to-loci association. These analyses are especially powerful when applied to selfing plants like Arabidopsis thaliana, where it is possible to measure the same trait for the same genotype in distinct environmental conditions (Atwell et al., 2010).
Thus, the application of GWAS to natural variation studies on axillary bud and cambium activities of Arabidopsis under changing environmental conditions might be an ideal system, not only to unravel important aspects of the genetic basis of plant architecture regulation, but also to approach the concept of the role of phenotypic plasticity in driving such adaptation.
Acknowledgements
This work was supported by a Grant from the Austrian Science Fund (FWF, Grant number P23781-B16) to J.A. We are grateful to Arthur Korte and Stefanie Suer (both GMI, Vienna) for critically reading the manuscript.
References
- Aguilar-Martinez J.A., Poza-Carrion C., Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007;19:458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J., Herold S., Schwarz M., Sanchez P., Ljung K., Dun E.A., Brewer P.B., Beveridge C.A., Sieberer T., Sehr E.M., Greb T. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA. 2011;108:20242–20247. doi: 10.1073/pnas.1111902108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J., Lichtenberger R., Schwarz M., Nehlin L., Greb T. Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth. PLoS Genet. 2011;7:e1001312. doi: 10.1371/journal.pgen.1001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder A., Jamil M., Marzorati M., Bruno M., Vermathen M., Bigler P., Ghisla S., Bouwmeester H., Beyer P., Al-Babili S. The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335:1348–1351. doi: 10.1126/science.1218094. [DOI] [PubMed] [Google Scholar]
- Arite T., Umehara M., Ishikawa S., Hanada A., Maekawa M., Yamaguchi S., Kyozuka J. D14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009;50:1416–1424. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- Atwell S., Huang Y.S., Vilhjalmsson B.J., Willems G., Horton M., Li Y., Meng D., Platt A., Tarone A.M., Hu T.T., Jiang R., Muliyati N.W., Zhang X., Amer M.A., Baxter I., Brachi B., Chory J., Dean C., Debieu M., de Meaux J., Ecker J.R., Faure N., Kniskern J.M., Jones J.D., Michael T., Nemri A., Roux F., Salt D.E., Tang C., Todesco M., Traw M.B., Weigel D., Marjoram P., Borevitz J.O., Bergelson J., Nordborg M. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465:627–631. doi: 10.1038/nature08800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge K., Sorefan K., Ward S., Leyser O. Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J. 2005;44:569–580. doi: 10.1111/j.1365-313X.2005.02548.x. [DOI] [PubMed] [Google Scholar]
- Bennett T., Sieberer T., Willett B., Booker J., Luschnig C., Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Beveridge C.A., Ross J.J., Murfet I.C. Branching mutant rms-2 in pisum sativum (Grafting studies and endogenous indole-3-acetic acid levels) Plant Physiol. 1994;104:953–959. doi: 10.1104/pp.104.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund S., Antti H., Uddestrand I., Moritz T., Sundberg B. Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. Plant J. 2007;52:499–511. doi: 10.1111/j.1365-313X.2007.03250.x. [DOI] [PubMed] [Google Scholar]
- Booker J., Auldridge M., Wills S., McCarty D., Klee H., Leyser O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 2004;14:1232–1238. doi: 10.1016/j.cub.2004.06.061. [DOI] [PubMed] [Google Scholar]
- Booker J., Chatfield S., Leyser O. Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell. 2003;15:495–507. doi: 10.1105/tpc.007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker J., Sieberer T., Wright W., Williamson L., Willett B., Stirnberg P., Turnbull C., Srinivasan M., Goddard P., Leyser O. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell. 2005;8:443–449. doi: 10.1016/j.devcel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Brewer P.B., Dun E.A., Ferguson B.J., Rameau C., Beveridge C.A. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009;150:482–493. doi: 10.1104/pp.108.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.L., McAlpine R.G., Kormanic P.P. Apical dominance and form in woody plants: a repraisal. Am. J. Bot. 1967;54:153–162. [Google Scholar]
- Busch B.L., Schmitz G., Rossmann S., Piron F., Ding J., Bendahmane A., Theres K. Shoot branching and leaf dissection in tomato are regulated by homologous gene modules. Plant Cell. 2011 doi: 10.1105/tpc.111.087981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffey N., Cholewa E., Regan S., Sundberg B. Secondary xylem development in Arabidopsis: a model for wood formation. Physiol. Plant. 2002;114:594–600. doi: 10.1034/j.1399-3054.2002.1140413.x. [DOI] [PubMed] [Google Scholar]
- Chen C.M., Ertl J.R., Leisner S.M., Chang C.C. Localization of cytokinin biosynthetic sites in pea plants and carrot roots. Plant Physiol. 1985;78:510–513. doi: 10.1104/pp.78.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. Apical dominance. Bot. Rev. 1991;57:318–358. [Google Scholar]
- Crawford S., Shinohara N., Sieberer T., Williamson L., George G., Hepworth J., Muller D., Domagalska M.A., Leyser O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010;137:2905–2913. doi: 10.1242/dev.051987. [DOI] [PubMed] [Google Scholar]
- de Kort I. Wood production and latewood percentage of Douglas-fir from different stands and vitality. Can. J. For. Res. 1993;23:1480–1486. [Google Scholar]
- De Smet I., Vanneste S., Inze D., Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Mol. Biol. 2006;60:871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- DellaPenna D., Pogson B.J. Vitamin synthesis in plants: tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006;57:711–738. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- Domagalska M.A., Leyser O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011;12:211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- Doust A. Architectural evolution and its implications for domestication in grasses. Ann. Bot. 2007;100:941–950. doi: 10.1093/aob/mcm040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust A.N. Grass architecture: genetic and environmental control of branching. Curr. Opin. Plant Biol. 2007;10:21–25. doi: 10.1016/j.pbi.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Du s., Yamamoto F. Ethylene evolution changes in the stem of Metasequoia glyptostroboides and Aesculus turbinata seedlings in relation to gravity-induced reaction wood formation. Trees-Struct. Funct. 2003;17:522–528. [Google Scholar]
- Du s., Yamamoto F. An overview of the biology of reaction wood formation. J. Integr. Plant Biol. 2007;49:131–143. [Google Scholar]
- Dudley S.A., Schmitt J. Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis. Am. Nat. 1996;147:445–465. [Google Scholar]
- Dun E.A., Brewer P.B., Beveridge C.A. Strigolactones: discovery of the elusive shoot branching hormone. Trends Plant Sci. 2009;14:364–372. doi: 10.1016/j.tplants.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Ehrenreich I.M., Stafford P.A., Purugganan M.D. The genetic architecture of shoot branching in Arabidopsis thaliana: a comparative assessment of candidate gene associations vs. quantitative trait locus mapping. Genetics. 2007;176:1223–1236. doi: 10.1534/genetics.107.071928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo A., Immanen J., Nieminen K., Helariutta Y. Stem cell function during plant vascular development. Semin. Cell Dev. Biol. 2009;20:1097–1106. doi: 10.1016/j.semcdb.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Foo E., Bullier E., Goussot M., Foucher F., Rameau C., Beveridge C.A. The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell. 2005;17:464–474. doi: 10.1105/tpc.104.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier-Level A., Korte A., Cooper M.D., Nordborg M., Schmitt J., Wilczek A.M. A map of local adaptation in Arabidopsis thaliana. Science. 2011;334:86–89. doi: 10.1126/science.1209271. [DOI] [PubMed] [Google Scholar]
- Funk D.J., Filchak K.E., Feder J.L. Herbivorous insects: model systems for the comparative study of speciation ecology. Genetica. 2002;116:251–267. [PubMed] [Google Scholar]
- Gaudichaud C. Recherches generales sur l´organographie, la physiologie et l´organogenie des vegetaux. C. R. Accad. Sci. Paris. 1841;12:627–637. [Google Scholar]
- Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pages V., Dun E.A., Pillot J.P., Letisse F., Matusova R., Danoun S., Portais J.C., Bouwmeester H., Becard G., Beveridge C.A., Rameau C., Rochange S.F. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Gray A. On the composition of the plant by phytons, and some applications of phyllotaxis. Proc. Am. Assoc. Adv. Sci. 1849;1849:438–444. [Google Scholar]
- Greb T., Clarenz O., Schafer E., Muller D., Herrero R., Schmitz G., Theres K. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 2003;17:1175–1187. doi: 10.1101/gad.260703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A., Stirnberg P., Beveridge C., Leyser O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009;151:400–412. doi: 10.1104/pp.109.137646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L.M., Roitberg B.D., Gillespie D.R. Covariance of phenotypically plastic traits induces an adaptive shift in host selection behaviour. Proc. Biol. Sci. 2006;273:2893–2899. doi: 10.1098/rspb.2006.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyl A., Ramireddy E., Brenner W.G., Riefler M., Allemeersch J., Schmulling T. The transcriptional repressor ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis. Plant Physiol. 2008;147:1380–1395. doi: 10.1104/pp.107.115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilscher J., Schlotterer C., Hauser M.T. A single amino acid replacement in ETC2 shapes trichome patterning in natural Arabidopsis populations. Curr. Biol. 2009;19:1747–1751. doi: 10.1016/j.cub.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S., Maekawa M., Arite T., Onishi K., Takamure I., Kyozuka J. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005;46:79–86. doi: 10.1093/pcp/pci022. [DOI] [PubMed] [Google Scholar]
- Jacobs M. The effect of wind sway on the form and development of Pinus radiata D. Don. Aust. J. Bot. 1954;2:35–51. [Google Scholar]
- Jamil M., Charnikhova T., Houshyani B., van Ast A., Bouwmeester H.J. Genetic variation in strigolactone production and tillering in rice and its effect on Striga hermonthica infection. Planta. 2012;235:473–484. doi: 10.1007/s00425-011-1520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson X., Brcich T., Dun E.A., Goussot M., Haurogne K., Beveridge C.A., Rameau C. Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 2006;142:1014–1026. doi: 10.1104/pp.106.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom T.H., Brutnell T.P. The molecular analyses of the shade avoidance syndrome in the grasses has begun. J. Exp. Bot. 2007;58:3079–3089. doi: 10.1093/jxb/erm205. [DOI] [PubMed] [Google Scholar]
- Keuskamp D.H., Pollmann S., Voesenek L.A., Peeters A.J., Pierik R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl. Acad. Sci. USA. 2010;107:22740–22744. doi: 10.1073/pnas.1013457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.H., Han K.H., Park S., Yang J. Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiol. 2004;135:1069–1083. doi: 10.1104/pp.104.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlen W., Charnikhova T., Liu Q., Bours R., Domagalska M.A., Beguerie S., Verstappen F., Leyser O., Bouwmeester H.J., Ruyter-Spira C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in non-AM host Arabidopsis thaliana. Plant Physiol. 2011;155:974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Maekawa M., Shimamoto K., Kyozuka J. The LAX1 and FRIZZY PANICLE 2 genes determine the inflorescence architecture of rice by controlling rachis-branch and spikelet development. Dev. Biol. 2001;231:364–373. doi: 10.1006/dbio.2000.9988. [DOI] [PubMed] [Google Scholar]
- Kretzschmar T., Kohlen W., Sasse J., Borghi L., Schlegel M., Bachelier J.B., Reinhardt D., Bours R., Bouwmeester H.J., Martinoia E. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature. 2012;483:341–344. doi: 10.1038/nature10873. [DOI] [PubMed] [Google Scholar]
- Krouk G., Ruffel S., Gutierrez R.A., Gojon A., Crawford N.M., Coruzzi G.M., Lacombe B. A framework integrating plant growth with hormones and nutrients. Trends Plant Sci. 2011;16:178–182. doi: 10.1016/j.tplants.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Larson P.R. Springer Series in Wood Science; Springer-Verlag, Berlin: 1994. The vascular cambium: development and structure. [Google Scholar]
- Laxmi A., Pan J., Morsy M., Chen R. Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS ONE. 2008;3:e1510. doi: 10.1371/journal.pone.0001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. The control of shoot branching: an example of plant information processing. Plant, Cell Environ. 2009;32:694–703. doi: 10.1111/j.1365-3040.2009.01930.x. [DOI] [PubMed] [Google Scholar]
- Love J., Bjorklund S., Vahala J., Hertzberg M., Kangasjarvi J., Sundberg B. Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc. Natl. Acad. Sci. USA. 2009;106:5984–5989. doi: 10.1073/pnas.0811660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M.G., Mathews D.E., Argyros D.A., Maxwell B.B., Kieber J.J., Alonso J.M., Ecker J.R., Schaller G.E. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell. 2005;17:3007–3018. doi: 10.1105/tpc.105.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Kitano M., Kusumoto T., Tarkowski P., Kinoshita-Tsujimura K., Vaclavikova K., Miyawaki K., Kakimoto T. Cytokinins are central regulators of cambial activity. Proc. Natl. Acad. Sci. USA. 2008;105:20027–20031. doi: 10.1073/pnas.0805619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusova R., Rani K., Verstappen F.W., Franssen M.C., Beale M.H., Bouwmeester H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005;139:920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSteen P. Hormonal regulation of branching in grasses. Plant Physiol. 2009;149:46–55. doi: 10.1104/pp.108.129056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer S., Lens F., Gennen J., Vanneste S., Rohde A., Beeckman T. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 2008;40:1489–1492. doi: 10.1038/ng.253. [DOI] [PubMed] [Google Scholar]
- Miyawaki K., Tarkowski P., Matsumoto-Kitano M., Kato T., Sato S., Tarkowska D., Tabata S., Sandberg G., Kakimoto T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Schmitz G., Theres K. Blind homologous R2R3 Myb genes control the pattern of lateral meristem initiation in Arabidopsis. Plant Cell. 2006;18:586–597. doi: 10.1105/tpc.105.038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C. Highly branched phenotype of the petunia dad1-1 mutant is reversed by grafting. Plant Physiol. 1996;111:27–37. doi: 10.1104/pp.111.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.C., Scaffidi A., Dun E.A., Waters M.T., Flematti G.R., Dixon K.W., Beveridge C.A., Ghisalberti E.L., Smith S.M. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2011;108:8897–8902. doi: 10.1073/pnas.1100987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N.D., Hillis W.E. Ethylene and tension wood formation in Eucalyptus gomphocephala. Wood Sci. Technol. 1978;12:309–315. [Google Scholar]
- Nieminen K., Immanen J., Laxell M., Kauppinen L., Tarkowski P., Dolezal K., Tahtiharju S., Elo A., Decourteix M., Ljung K., Bhalerao R., Keinonen K., Albert V.A., Helariutta Y. Cytokinin signaling regulates cambial development in poplar. Proc. Natl. Acad. Sci. USA. 2008;105:20032–20037. doi: 10.1073/pnas.0805617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongaro V., Leyser O. Hormonal control of shoot branching. J. Exp. Bot. 2008;59:67–74. doi: 10.1093/jxb/erm134. [DOI] [PubMed] [Google Scholar]
- Pfennig D.W., Wund M.A., Snell-Rood E.C., Cruickshank T., Schlichting C.D., Moczek A.P. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. 2010;25:459–467. doi: 10.1016/j.tree.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Potters G., Pasternak T.P., Guisez Y., Jansen M.A. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant, Cell Environ. 2009;32:158–169. doi: 10.1111/j.1365-3040.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- Prasad T.K., Li X., Abdel-Rahman A.M., Hosokawa Z., Cloud N.P., LaMotte C.E., Cline M.G. Does auxin play a role in the release of apical dominance by shoot inversion in Ipomoea nil? Ann. Bot. 1993;71:223–229. [Google Scholar]
- Price T.D., Qvarnstrom A., Irwin D.E. The role of phenotypic plasticity in driving genetic evolution. Proc. Biol. Sci. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust H., Hoffmann B., Xie X., Yoneyama K., Schaefer D.G., Nogue F., Rameau C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development. 2011;138:1531–1539. doi: 10.1242/dev.058495. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P., Crawford S., Smith R.S., Ljung K., Bennett T., Ongaro V., Leyser O. Control of bud activation by an auxin transport switch. Proc. Natl. Acad. Sci. USA. 2009;106:17431–17436. doi: 10.1073/pnas.0906696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P., Runions A. Computational models of plant development and form. New Phytol. 2012;193:549–569. doi: 10.1111/j.1469-8137.2011.04009.x. [DOI] [PubMed] [Google Scholar]
- Renton M., Hanan J., Ferguson B.J., Beveridge C.A. Models of long-distance transport: how is carrier-dependent auxin transport regulated in the stem? New Phytol. 2012;194:704–715. doi: 10.1111/j.1469-8137.2012.04093.x. [DOI] [PubMed] [Google Scholar]
- Rowe N., Speck T. Plant growth forms: an ecological and evolutionary perspective. New Phytol. 2005;166:61–72. doi: 10.1111/j.1469-8137.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- Ruyter-Spira C., Kohlen W., Charnikhova T., van Zeijl A., van Bezouwen L., de Ruijter N., Cardoso C., Lopez-Raez J.A., Matusova R., Bours R., Verstappen F., Bouwmeester H. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol. 2011;155:721–734. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P., Nehlin L., Greb T. From thin to thick – major transitions during stem development. Trends Plant Sci. 2012;17:113–121. doi: 10.1016/j.tplants.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G., Tillmann E., Carriero F., Fiore C., Cellini F., Theres K. The tomato Blind gene encodes a MYB transcription factor that controls the formation of lateral meristems. Proc. Natl. Acad. Sci. USA. 2002;99:1064–1069. doi: 10.1073/pnas.022516199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehr E.M., Agusti J., Lehner R., Farmer E.E., Schwarz M., Greb T. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J. 2010;63:811–822. doi: 10.1111/j.1365-313X.2010.04283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Luong P., Huq E. The F-box protein MAX2 functions as a positive regulator of photomorphogenesis in Arabidopsis. Plant Physiol. 2007;145:1471–1483. doi: 10.1104/pp.107.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Sato S., Tanaka M., Mori H. Auxin-cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009;69:429–435. doi: 10.1007/s11103-008-9416-3. [DOI] [PubMed] [Google Scholar]
- Sibout R., Eudes A., Mouille G., Pollet B., Lapierre C., Jouanin L., Seguin A. Cinnamyl alcohol dehydrogenase-C and -D are the primary genes involved in lignin biosynthesis in the floral stem of Arabidopsis. Plant Cell. 2005;17:2059–2076. doi: 10.1105/tpc.105.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R., Plantegenet S., Hardtke C.S. Flowering as a condition for xylem expansion in Arabidopsis hypocotyl and root. Curr. Biol. 2008;18:458–463. doi: 10.1016/j.cub.2008.02.070. [DOI] [PubMed] [Google Scholar]
- Simon J.C., Carre S., Boutin M., Prunier-Leterme N., Sabater-Mun B., Latorre A., Bournoville R. Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts. Proc. Biol. Sci. 2003;270:1703–1712. doi: 10.1098/rspb.2003.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. The young leaf as the inhibiting organ. New Phytol. 1929;28:345–358. [Google Scholar]
- Snow R. Activation of cambial growth by pure hormones. New Phytol. 1935;34:347–360. [Google Scholar]
- Sommer R.J., Ogawa A. Hormone signaling and phenotypic plasticity in nematode development and evolution. Curr. Biol. 2011;21:758–766. doi: 10.1016/j.cub.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Sorefan K., Booker J., Haurogne K., Goussot M., Bainbridge K., Foo E., Chatfield S., Ward S., Beveridge C., Rameau C., Leyser O. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003;17:1469–1474. doi: 10.1101/gad.256603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer R., Groover A. Evolution of development of vascular cambia and secondary growth. New Phytol. 2010;186:577–592. doi: 10.1111/j.1469-8137.2010.03236.x. [DOI] [PubMed] [Google Scholar]
- Stirnberg P., Furner I.J., Leyser H.M.O. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2007;50:80–94. doi: 10.1111/j.1365-313X.2007.03032.x. [DOI] [PubMed] [Google Scholar]
- Stirnberg P., van De Sande K., Leyser H.M. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development. 2002;129:1131–1141. doi: 10.1242/dev.129.5.1131. [DOI] [PubMed] [Google Scholar]
- Tan C., Wang H., Zhang Y., Qi B., Xu G., Zheng H. A proteomic approach to analyzing responses of Arabidopsis thaliana root cells to different gravitational conditions using an agravitropic mutant, pin2 and its wild type. Proteome Sci. 2011;9:72. doi: 10.1186/1477-5956-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantikanjana T., Yong J.W., Letham D.S., Griffith M., Hussain M., Ljung K., Sandberg G., Sundaresan V. Control of axillary bud initiation and shoot architecture in Arabidopsis through the supershoot gene. Genes Dev. 2001;15:1577–1588. doi: 10.1101/gad.887301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K.V., Skoog F. Studies on the growth hormone of plants: III. the inhibiting action of the growth substance on bud development. Proc. Natl. Acad. Sci. USA. 1933;19:714–716. doi: 10.1073/pnas.19.7.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todesco M., Balasubramanian S., Hu T.T., Traw M.B., Horton M., Epple P., Kuhns C., Sureshkumar S., Schwartz C., Lanz C., Laitinen R.A., Huang Y., Chory J., Lipka V., Borevitz J.O., Dangl J.L., Bergelson J., Nordborg M., Weigel D. Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana. Nature. 2010;465:632–636. doi: 10.1038/nature09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trontin C., Tisne S., Bach L., Loudet O. What does Arabidopsis natural variation teach us (and does not teach us) about adaptation in plants? Curr. Opin. Plant Biol. 2011;14:225–231. doi: 10.1016/j.pbi.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., Magome H., Kamiya Y., Shirasu K., Yoneyama K., Kyozuka J., Yamaguchi S. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Vogel J.T., Walter M.H., Giavalisco P., Lytovchenko A., Kohlen W., Charnikhova T., Simkin A.J., Goulet C., Strack D., Bouwmeester H.J., Fernie A.R., Klee H.J. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 2010;61:300–311. doi: 10.1111/j.1365-313X.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- Waddington C.H. Genetic assimilation of an acquired character. Evolution. 1953;7:118–126. [Google Scholar]
- Waters M.T., Nelson D.C., Scaffidi A., Flematti G.R., Sun Y.K., Dixon K.W., Smith S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development. 2012;139:1285–1295. doi: 10.1242/dev.074567. [DOI] [PubMed] [Google Scholar]
- Werner T., Motyka V., Laucou V., Smets R., Van Onckelen H., Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T., Schmülling T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J. Oxford University Press; 2003. Developmental Plasticity and Evolution. [Google Scholar]
- Whippo C.W., Hangarter R.P. Phototropism: bending towards enlightenment. Plant Cell. 2006;18:1110–1119. doi: 10.1105/tpc.105.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickson M., Thymann K.V. The antagonism of auxin and kinetin in apical dominance. Physiol. Plant. 1958;49:304–314. [Google Scholar]
- Wolters H., Jurgens G. Survival of the flexible: hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009;10:305–317. doi: 10.1038/nrg2558. [DOI] [PubMed] [Google Scholar]
- Xie X., Yoneyama K. The strigolactone story. Annu. Rev. Phytopathol. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- Yaginuma H., Hirakawa Y., Kondo Y., Ohashi-Ito K., Fukuda H. A novel function of TDIF-related peptides: promotion of axillary bud formation. Plant Cell Physiol. 2011;52:1354–1364. doi: 10.1093/pcp/pcr081. [DOI] [PubMed] [Google Scholar]
- Yamamoto F., Kozlowski T.T. Effects of flooding, tilting of stems, and ethrel application on growth, stem anatomy and ethylene production of Pinus Densiflora seedlings. J. Exp. Bot. 1987;38:293–310. [Google Scholar]
- Yoneyama K., Xie X., Kusumoto D., Sekimoto H., Sugimoto Y., Takeuchi Y. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007;227:125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Xie X., Yoneyama K., Takeuchi Y. Strigolactones: structures and biological activities. Pest Manag. Sci. 2009;65:467–470. doi: 10.1002/ps.1726. [DOI] [PubMed] [Google Scholar]