Abstract
An ideal oral drug carrier should facilitate drug delivery to the gastrointestinal tract and its absorption into the systemic circulation. To meet these requirements, we developed a thiomer-coated liposomal delivery system composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and a maleimide-functionalized lipid, to which chitosan-thioglycolic acid (CS-TGA) was covalently coupled. In addition to conventional 77 kDa CS-TGA (CS-TGA77), we tested the 150 kDa homologue (CS-TGA150) as well as an S-protected version of this polymer (CS-TGA150-MNA), in which some of the free SH-groups are conjugated with 6-mercaptonicotinamide to protect them from oxidation. Coupling of CS-TGA to the liposomal surface led to an increase in the particle size of at least 150 nm and an increase in the zeta potential from approximately − 33 mV to a maximum of about + 36 mV, depending on the polymer. As revealed by fluorescence dequenching the formulations have a storage stability of at least two weeks without releasing any encapsulated compounds. In simulated gastric fluid, the system was shown to be stable over 24 h, while in simulated intestinal fluid, a slow, sustained release of encapsulated compounds was observed. According to our experiments, thiomer-coated liposomes did not induce immunogenic reactions after an oral administration to mice. To evaluate the permeation enhancing and efflux pump inhibiting properties of CS-TGA coated liposomes we monitored the transport of fluoresceinisothiocyanate-dextran (FD4) and rhodamine-123 (Rho-123), respectively, through rat small intestine. Permeation studies showed a 2.8-fold higher permeation of FD4 in the presence of CS-TGA77 coated liposomes and an even 4-fold higher permeation in the presence of CSA-TGA150-MNA coated liposomes. The latter also performed best when we evaluated P-glycoprotein inhibiting properties by monitoring the transport of Rho-123, revealing a 4.2-fold enhancement respective to the buffer control. Taken together, thiomer-coated liposomes were shown to protect encapsulated drugs in the stomach, slowly release them in the small intestine and enhance their absorption through the intestinal tissue by opening tight junctions and inhibiting efflux pumps.
Keywords: Liposome, Thiomer, S-protected thiomer, Permeation enhancement, Efflux pump inhibition, Immunogenicity
Graphical abstract
1. Introduction
The oral delivery of drugs is generally the most convenient route, as it allows for painless and easy administration, and therefore high patient compliance. However, many drugs cannot be administered orally due to the harsh environment and/or low absorption from gastrointestinal (GI) tract. An optimal oral delivery system should therefore (1) protect compounds from degradation and (2) improve their permeation through GI-barriers; enhancing their oral bioavailability. Different nanoparticulate systems have been developed for the protection of drugs during gastrointestinal transit — among them, liposomes. Despite several successful studies [1,2], however, liposomes have not yet reached their full potential as oral drug carriers, though in recent years several strategies have been developed to enhance the stability of liposomes and improve their properties for oral delivery — one of which is coating them with multifunctional polymers such as chitosan, Carbopol®, Eudragit® or silica [3–6]. Following this concept, we have recently generated liposomes coated with thiolated chitosan (CS-TGA) [7].
Different thiolated polymers – designated thiomers – have been previously designed, which commonly consist of SH-group-bearing agents anchored to polymeric backbones. Thiomers have also been shown to exhibit several promising properties for drug delivery, including mucoadhesion; permeation enhancement; efflux pump inhibition; and enzyme inhibition [8–11]. Despite these effects being well-established for thiomers themselves, it remained questionable as to whether thiomer-coated liposomes will still exhibit permeation enhancing and efflux pump inhibiting properties, given that the mucus layer lining the small intestine functions as a barrier refractive to access by larger particles. To address this question, liposomes were prepared by utilizing what was expected to be a more stable composition by comparison with those used in our previous study [7]. These newly designed liposomes were evaluated in the context of their storage stability, release kinetics, permeation enhancing and efflux pump inhibitory properties, as well as regarding their immunogenic behavior. To achieve even higher permeation enhancing and efflux pump inhibitory properties, liposomes were coated with ‘S-protected thiomers’, as this new type of thiomers is stable towards oxidation [12].
2. Materials and methods
2.1. Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[4-(p-maleimidomethyl) cyclohexane-carboxamide] (DPPE-MCC) were purchased from Avanti Polar Lipids (Alabaster, AL). Chitosan-thioglycolic acid of two different molecular weights (CS-TGA77; molecular weight: 77 kDa, 550 μmol SH-groups/g polymer and CS-TGA150; molecular weight: 150 kDa, 660 μmol SH-groups/g polymer) and the chitosan-thioglycolic acid 6-mercaptonicotinamide-conjugate (CS-TGA150-MNA; molecular weight: 150 kDa, 380 μmol S-protected thiol groups and 280 μmol free SH-groups/g polymer) were synthesized according to methods described previously [12,13]. Fluoresceinisothiocyanate-dextran (FD4, 4400 Da) was supplied from TdB Consultanca AB (Uppsala, Sweden). All other chemicals were of reagent grade or of the best grade available and purchased from Sigma-Aldrich (Vienna, Austria).
2.2. Preparation of liposomes
Liposomes were prepared by thin lipid film rehydration method. Briefly, DPPC and the maleimide-functionalized lipid DPPE-MCC were dissolved in methanol and mixed in a molar ratio of 3:0.3. The organic solvent was evaporated under a nitrogen stream, and the resulting lipid film was dried overnight in a vacuum chamber. A 10 mM phosphate buffer containing 150 mM NaCl, pH 7.4 (PBS) was added to the dry lipid film, which was then rehydrated for 1 h at a temperature of 50 °C with repeated vortexing. The so-formed multilamellar vesicles (final lipid concentration: 30 mg/mL) were sized by freeze and thaw and size extrusion through 200 nm polycarbonate membranes (Whatman Inc., Clifton, NJ) with a mini-extruder (Avanti Polar Lipids, Alabaster, AL).
To evaluate particle stability and release behavior, the fluorophore/quencher couple 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS)/p-xylene-bis-pyridinium bromide (DPX) was encapsulated within the liposome during the hydration step. Briefly, 5.4 mg/mL ANTS and 19 mg/mL DPX were solved in PBS and added to the lipid film. All further steps were carried out as described above. After size extrusion, free ANTS/DPX was separated from ANTS/DPX-loaded liposomes by Sephadex G75 column chromatography (Amersham Biosciences, Uppsala, Sweden).
2.3. Coupling of the polymer
Three different polymers (CS-TGA77, CS-TGA150 and CS-TGA150-MNA) were coupled to the liposomal surface by covalent bond formation between maleimide groups of the liposome and free SH-groups of the polymer, as described previously [7] (see Fig. 1). Briefly, the polymer was dissolved in deionized water in a concentration of 2 mg/mL, of which an appropriate amount was added to the preformed liposomes and incubated overnight under agitation. The molar ratio of SH-groups to maleimide groups was approximately 4:1, whereby a final lipid concentration of 1.7 mg/mL and a polymer concentration of 1.7 mg/mL were maintained throughout the study. Uncoated liposomes were diluted with deionized water to the same lipid concentration.
Fig. 1.
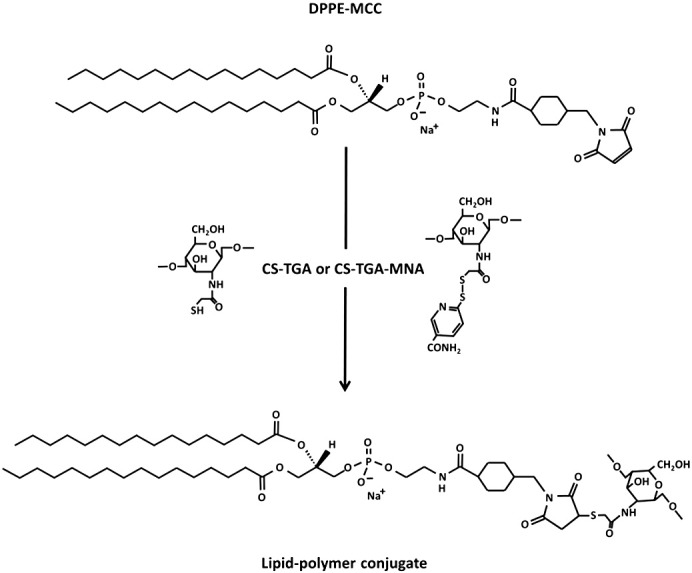
Reaction scheme for the covalent coupling of chitosan-TGA or the preactivated chitosan-TGA-MNA to a maleimide-functionalized phospholipid forming a stable thioether bond.
2.4. Particle characterization
2.4.1. Particle size
Size measurements were performed at room temperature by dynamic light scattering (DLS) using a Zetasizer 3000HS (Malvern Instruments, Herrenburg, Germany). Coated- and uncoated liposomes were measured after being diluted to a final lipid concentration of about 0.03 mg/mL with ultra-pure water (USF ELGA, High Wycombe Bucks, UK). Particle size was analyzed by calculating the auto correlation function of the detected intensity. The polydispersity index of the liposomal suspension is given by the width of the size distribution.
2.4.2. Zeta potential
Zeta potentials of coated- and uncoated liposomes were determined with a Zetasizer nano ZS (Malvern Instruments, Herrenberg, Germany) after dilution to a lipid concentration of 0.3 mg/mL with a buffer containing 10 mM Tris and 2 mM CsCl, pH 7.0. All measurements were carried out at room temperature.
2.5. Release study
Leakage and stability studies were carried out using uncoated- and CS-TGA77-coated liposomes. All stability and release measurements were carried out by measuring the fluorescence of the suspension (excitation wavelength: 360 nm; emission wavelength: 530 nm) after diluting them with PBS buffer to a final lipid concentration of approximately 50 μg/mL using a SPEX FLUOROMAX-3 fluorescence spectrometer (Jobin Yvon Horiba, Longjumeau Cedex, France). Leakage of ANTS/DPX leads to an enhanced fluorescence signal due to the greater distance between fluorophore and quencher. To determine the fluorescence intensity corresponding to 100% release of ANTS/DPX, 10 μL of 10% Triton X-100™ were added to the cuvette (2 mL) before measuring.
2.5.1. Long term storage stability
For long term storage stability, ANTS/DPX-loaded liposomes were stored at 4 °C in darkness and aliquots were measured at different time points over a time interval of 2 weeks.
2.5.2. Stability and release behavior in different simulated body fluids
To measure the release kinetics in different body fluids, freshly prepared coated- and uncoated liposomes were exposed to either simulated gastric fluid (SGF; 1 L contains 2 g sodium chloride, 3.2 g pepsin, 7 mL hydrochloric acid; pH 1.2) or simulated intestinal fluid (SIF; 1 L contains 6.8 g monobasic potassium phosphate, 10 g pancreatin, 77 mL 0.2 N sodium hydroxide; pH 6.8), which were prepared according to the U.S. Pharmacopeia. Samples were diluted 1:1 (v/v) with one of these fluids and incubated for 24 h. At fixed time points, 60 μL of these mixtures were withdrawn, mixed with 2 mL of PBS and measured fluorimetrically as described above.
2.6. Immunogenicity screening
2.6.1. Immunization of mice
Female BALB/c mice (Charles River Laboratories, Sulzfeld, Germany), aged 8 weeks, were treated according to European Community rules of animal care with the permission of the Austrian Ministry of Science (BMWF-66.009/0172-II/3b/2011). Mice (n = 8) were immunized orally with CS-TGA77-coated liposomes (100 μL /gavage) or stayed naïve. Immunizations were performed on day 7/8, 21/22, 35/36 and 49/50 and blood was drawn on day 0, 14, 28, 42, 56 and 70.
2.6.2. Detection of serum total immunoglobulins by ELISA
Antibody detection of total IgG, IgM, IgA or IgE was performed by sandwich ELISA as described previously [14] with modifications. Briefly, microtiter plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with rat anti-mouse antibodies to IgE, IgM or IgA (BD Pharmingen, Schwechat, Austria; all 500 ng/mL in Na-bicarbonate puffer, pH 8.3) or rat anti-mouse IgG (Bethyl Laboratories, Montgomery, USA; 2000 ng/mL). Mouse isotype standard IgG, IgM, IgA and IgE (BD Pharmingen, Schwechat, Austria) were used for dilution series (starting concentration 25 ng/mL for IgE and 100 ng/mL for IgM, IgA and IgG; further dilution steps 1:2 (v/v)). Mouse sera were diluted 1:20 (v/v) for IgE, 1:5000 for IgM and IgA and 1:10000 for IgG detection. Peroxidase-labeled anti-mouse antibodies (Bethyl Laboratories, Montgomery, USA) were used at a dilution of 1:10000 (v/v). Detection was performed with tetramethylbenzidine solution (BD Bioscience, Vienna, Austria) and measurement done at 450–630 nm.
2.6.3. Intradermal skin tests
Evans blue (100 μL of 5 mg/mL NaCl 0.9%; Merck, Darmstadt, Germany) was injected into the tail vein of immunized mice on day 70. Subsequently, 30 μL of coated liposomes (50 μg lipid/mL PBS); 30 μL of codfish extract (50 μg/mL PBS) as irrelevant control allergen; mast cell degranulation compound 48/80 (20 μg/mL PBS; Sigma, Steinheim, Germany) as positive control; and PBS as negative control were administered intradermally. Reactions were evaluated as previously described [14].
2.6.4. Isolation of splenocytes and evaluation of cytokines in stimulated spleen cells
Spleen cell suspensions were stimulated as described previously [14]. For stimulation, medium or irrelevant antigen codfish extract (400 μg/mL) as negative controls, Con A (5 μg/mL) as positive control, or CS-TGA77-coated liposomes (lipid concentration: 10 μg/mL) were added. Mouse IL-1α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17A, IL-21, IL-22, IL-27, IFN-γ and TNF-α were measured in supernatants of stimulated splenocytes by a multiplex immunoassay and analyzed on a flow cytometer (eBioscience, Vienna, Austria) according to the manufacturer's instructions.
2.7. Ex vivo evaluation of permeation enhancing properties
For permeation studies, 200–300 g non-fasting male Sprague–Dawley rats were sacrificed and their small intestine (jejunum, ileum) removed immediately, preserved in 0.9% NaCl solution (w/v). The intestine was cut into strips of about 1.5 cm, opened longitudinally and rinsed free of luminal contents with freshly prepared medium (138 mM NaCl, 1 mM MgSO4, 5 mM KCl, 10 mM glucose and 2 mM CaCl2 buffered with 10 mM Hepes; pH 6.8). Subsequently, the tissues were mounted in Ussing-type chambers with a permeation area of 0.64 cm2. The chambers were placed in a water bath preheated to 37 °C and immediately filled with 1 mL medium in the basolateral compartment (BL), and 900 μL liposomal suspension (lipid concentration: 1.7 mg/mL; polymer concentration in case of coated liposomes: 1.7 mg/mL) mixed with 100 μL of a 1% FD4 solution in the apical compartment (AP). To determine the permeation in absence of any formulation, an FD4 solution in a final concentration of 0.1% (v/v) was used as control. Over an incubation period of 3 h, 100 μL aliquots were withdrawn every 30 min from the basolateral chamber and replaced by 100 μL of preheated medium. The amounts of permeated marker were analyzed by fluorescence spectroscopy at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a plate reader (Tecan Austria GmbH, Austria). Cumulative corrections were made for previously removed samples. Apparent permeability coefficients (Papp; cm/s) for FD4 were calculated according to the following equation:
where Q is the amount of marker permeated within 3 h (μg), A is the diffusion area of the Ussing-type chamber (0.64 cm2), c is the initial concentration of marker in the apical compartment (μg/cm3) and t is the time of the experiment (10,800 s). Transport enhancement ratios (ER) were calculated by:
2.7.1. Determination of the transepithelial electric resistance (TEER)
EVOM® (World Precision Instruments, Germany) connected to a pair of adjacent electrodes was used to monitor the TEER of the intestinal tissues throughout the experiment. Measurements were performed at the beginning of the study to guarantee the integrity of intestinal tissue, and after 60, 120 and 180 min to observe the effect of the different liposomal formulations. The TEER measured prior to each experiment was set as 100%, and all other values were calculated in relation to this value.
2.8. Ex vivo evaluation of P-glycoprotein inhibitory properties
To study the permeation of rhodamine-123 (Rho-123), the distal part of rat small intestine was used, since more efflux pumps are present in this area [15,16]. Rho-123 is a well-known P-gp substrate used for testing efflux pump activity [12,17,18]. Identical to permeation enhancement studies, freshly excised tissues were mounted on Ussing-type chambers. To confirm the presence of P-gp on the intestinal tissues, chambers containing a 0.001% Rho-123 solution in the apical compartment and 1 mL medium (138 mM NaCl, 1 mM MgSO4, 5 mM KCl, 10 mM glucose and 2 mM CaCl2 buffered with 10 mM Hepes; pH 6.8) in the basolateral compartment were placed in the incubator (37 °C) and the refrigerator (4 °C). As efflux pumps are energy-dependent active transporters, their activity is lower at 4 °C [19,20] and an increased permeation of the P-gp substrate at 4 °C indicates the expression of P-gp. Positively evaluated, the tissues could be used to determine efflux pump inhibitory properties.
Then we investigated the transport of Rho-123 in presence of different liposomal suspensions (uncoated liposomes, CS-TGA-coated liposomes of 77 and 150 kDa, and CS-TGA-MNA-coated liposomes). 1 mL medium was added to the basolateral compartment, and 900 μL samples mixed with 100 μL of a 0.01% Rho-123 solution were added to the apical compartment. Over a period of 3 h, 100 μL aliquots were withdrawn at different time points from the basolateral compartment and replaced immediately by the same volume of preheated medium. The amounts of transported Rho-123 were analyzed by fluorescence spectroscopy (λex = 488 nm and λem = 525 nm) and Papp values for Rho-123 as well as transport enhancement ratios were calculated according to the equations described for the permeation of FD4.
In a second approach we aimed to compare the absorptive (AP to BL) with the secretory (BL to AP) transport of Rho-123 with and without thiomer-coated liposomes. For this reason we monitored the permeation of Rho-123 alone, in presence of verapamil as a potent inhibitor of P-gp [21] (100 μM apical), in presence of CS-TGA150-MNA-coated liposomes (apical; in the same concentration as for the studies mentioned above) and in presence of both, verapamil and CS-TGA150-MNA-coated liposomes. The efflux ratio was calculated by dividing the secretory Papp by the absorptive Papp. Additionally, the TEER was monitored for absorptive transport studies, as described for permeation enhancement studies.
2.9. Statistical analysis
All values are expressed as means ± standard deviation (SD). Statistical data analyses were performed using the Student's t-test with p ≤ 0.05 as the minimal level of significance, p ≤ 0.01 for very significant and p ≤ 0.001 for highly significant. All tests were performed using the statistical and process management software MINITAB 13.0.
3. Results and discussions
3.1. Characterization of thiolated liposomes
When thiomers are coupled to functionalized liposomes, covalent bonds are formed between free SH-groups of the polymer and maleimide groups on the surface of liposomes. A molar ratio of approximately 4:1 (SH-groups:maleimide groups) was chosen for the coupling reaction, since at this ratio the whole surface of liposomes is covered with polymer [7].
After coupling, the particles showed an increase in size and a wider size distribution, most probably due to a crosslinking of liposomes by single polymer chains and an entanglement of the latter [7,22]. Accordingly, liposomes coated with the higher molecular mass CS-TGA150 or CS-TGA150-MNA were bigger and more heterogeneous than CS-TGA77-coated ones (the corresponding values are listed in Table 1). However, oral delivery is not restricted according to particle size. Similar to particle size, the zeta potential of liposomes was also increased after coupling (see Table 1). Uncoated liposomes display a negative zeta potential due to the negatively-charged phosphate group of the functionalized lipid. Thiolated chitosans themselves show a positive zeta potential and thus the overall surface charge of thiomer-coated liposomes becomes positive. An increase in zeta potential was also reported for the electrostatic binding of positively ionized chitosan to the surface of negatively charged liposomes [23–25]. In our study there was not much difference between CS-TGA77-coated- and CS-TGA150-coated liposomes, whereas liposomes coated with pre-activated CS-TGA (CS-TGA150-MNA) were less positively charged due to the lesser amount of free SH-groups in the sample.
Table 1.
Composition of all liposomal suspensions used within this study and characterization concerning their size, polydispersity and zeta potential after coupling the polymer to the liposome (means ± SD; n ≥ 3).
| Sample description | Lipid composition a | Added polymer b | Size [nm] | Polydispersity index | Zeta potential [mV] |
|---|---|---|---|---|---|
| Uncoated liposomes | DPPC/DPPE-MCC | – | 201.3 ± 1.5 | 0.091 ± 0.023 | − 32.68 ± 1.99 |
| CS-TGA77-coated liposomes | DPPC/DPPE-MCC | CS-TGA, 77kD | 354.5 ± 3.4 | 0.213 ± 0.036 | 28.90 ± 1.45 |
| CS-TGA150-coated liposomes | DPPC/DPPE-MCC | CS-TGA, 150kD | 590.2 ± 18.9 | 0.629 ± 0.155 | 35.85 ± 3.19 |
| CS-TGA150-MNA-coated liposomes | DPPC/DPPE-MCC | CS-TGA-MNA, 150 kDa | 702.6 ± 138 | 0.738 ± 0.264 | 8.62 ± 1.36 |
The molar ratio of DPPC to DPPE-MCC was in all cases 3:0.3.
A polymer to lipid weight ratio of 1:1 was used for all formulations.
3.2. Stability testing and release behavior
In order to study the stability and release profiles of uncoated- and CS-TGA77-coated liposomes, the ANTS/DPX-model system was used, consisting of the anionic fluorophore ANTS and the cationic quencher DPX. This test system was originally used to investigate the influence of different agents on membrane stability [26], and was shown to be a useful tool also for monitoring stability and drug release from liposomes [7,27].
In principle, both the fluorophore ANTS and the quencher molecule DPX are encapsulated in liposomes during the hydration step. The molecules are in close proximity to each other, and the short distance between quencher and fluorophore leads to a quenching of the latter. Consequently, the monitored fluorescence intensity is low, though upon leakage and release of ANTS/DPX, the fluorescence intensity increases in a concentration-dependent manner. With this technique, the storage stability of coated- and uncoated liposomes as well as release profiles in simulated gastric- and simulated intestinal fluid were monitored. Released ANTS/DPX was measured at fixed time points without separating the released fluorophore.
Our liposome formulation is composed of saturated phospholipids being in the gel phase at room temperature. In general, such formulations containing high phase transition temperature lipids exhibit a lower membrane permeability, and are less leaky than those formed by unsaturated lipids [28]. Thus, the formulation was assumed to be stable in PBS at 4 °C. Indeed, over a period of 14 days, neither the uncoated nor the coated liposomes released more than 2% of the encapsulated compounds, and within this period, the liquid formulation could for example be converted into a solid dosage form without additional loss of encapsulated compounds. Uncoated liposomes were stable also for the following 4 weeks, whereas a slow continuous release of ANTS/DPX from coated liposomes was recorded (data not shown), leading to a 37% decrease of encapsulated compounds by the end of this period.
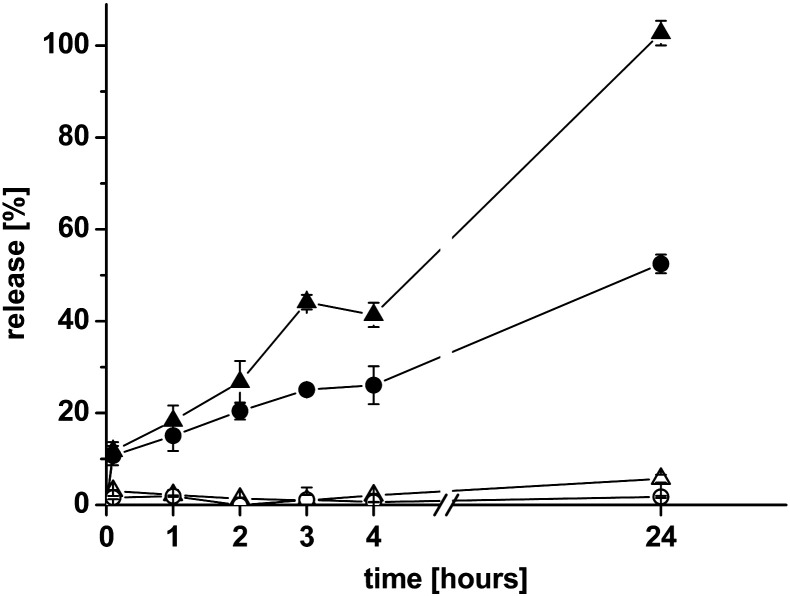
To test the stability in different body fluids, CS-TGA77-coated or uncoated liposomes were mixed with either SGF or SIF and the leakage measured by an increase in fluorescence intensity was estimated over 24 h, as seen in Fig. 2. The stability of both liposomal suspensions in the gastric environment was very high, and the release was in no case more than 6%. Both formulations can therefore be considered stable in the gastric environment. When mixing the particles with SIF containing pancreatin as an enzyme mixture simulating the environment in the small intestine, a slow release from coated and uncoated liposomes could be monitored over 24 h. Nevertheless, the release in the intestinal environment was slower for thiomer-coated liposomes compared to uncoated ones. As it was shown for membrane bound aminopeptidase [11], thiomers exhibit enzyme-inhibitory properties. Accordingly they might also inhibit lipid digesting enzymes in SIF, leading to a better performance of thiomer-protected liposomes compared with uncoated ones.
Fig. 2.
Release of ANTS/DPX at predetermined time points from uncoated DPPC/DPPE-MCC liposomes (△,▲) and DPPC/DPPE-MCC liposomes coated with CS-TGA77 (4:1 molar ratio of SH-groups to maleimide groups) (○,●) in simulated gastric fluid (open symbols) and simulated intestinal fluid (filled symbols). Indicated values are the means ± SD of at least three experiments.
Even though the delivery system should release encapsulated drugs once bound to the mucus, it has to be stable until there to prevent encapsulated drugs from degradation by gastrointestinal enzymes. This is even more important if liposomes are loaded with sensitive drugs like peptides or DNA-based drugs.
3.3. Immunogenicity screening
As liposomal formulations may induce immunogenic reactions [29], we evaluated potential immunostimulatory effects of orally delivered thiochitosan-coated liposomes by investigating their potency to affect total antibody levels in mice in a sandwich ELISA assay. No effects on total antibody levels of IgA, IgM, IgG and IgE could be detected in sera of treated or naïve mice. These results already suggested that none of the anaphylactogenic antibody isotypes in mice (IgE, IgG1) were formed. Still, to rule out the residual likelihood for specific sensitization as a side effect of oral gavages, type I skin tests were performed. The results showed that mice immunized with thiochitosan-coated liposomes or naïve mice showed no type I skin reaction to any of the test substances and reacted singularly to the positive control 48/80. Therefore, oral gavages of the CS-TGA77-liposomes did not affect Th2 immunity or induce specific sensitization in this mouse model. To capture any other potential immunomodulatory effects of thiochitosan-coated liposomes, a broad panel of cytokines derived from stimulated splenocytes of the two groups of mice were analyzed. Isolated splenocytes of mice immunized with CS-TGA77-coated liposomes or of naïve mice were stimulated with medium, CS-TGA77-liposomes, irrelevant antigen codfish extract or positive control Con A. Analysis of cytokines in multiplex analysis in FACS showed that levels of cytokines were not elevated with CS-TGA77-liposome-stimulation above medium or codfish control in splenocytes of mice immunized with CS-TGA77-liposomes or in naïve mice. Collectively, no increase in total antibody levels of IgM, IgA, IgE or IgG in sera of these mice were observed, nor was a reaction in intradermal skin tests or an induction of cytokines (Th1/Th2/Th17/Th22). Therefore, from the viewpoint of the immunological parameters, we suggest that thiomer-coated liposomes are suitable carriers for the oral application.
3.4. Ex vivo evaluation of permeation enhancing properties
The potential of CS-TGA77-coated liposomes as permeation enhancers was investigated by monitoring the transport of FD4 through rat small intestine. We consider the test system advantageous over the Caco-2 cell model, as the mucus layer is still present on the luminal side of the tissue. Caco-2 cells, which are vastly used for testing the intestinal absorption, do not secrete any mucus — a fact which might influence the rate of diffusion from the apical to the basolateral compartment. Accordingly, freshly excised small intestine is much closer to the situation in vivo than the well-established Caco-2 monolayer.
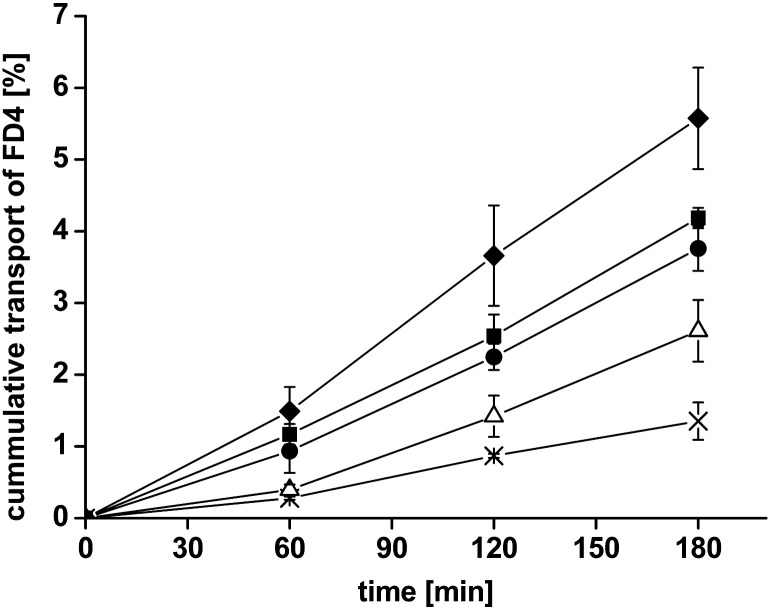
The absorptive transport of FD4 was investigated over a time period of 3 h in the presence or absence of uncoated liposomes, or CS-TGA77-coated liposomes. FD4 was selected as a marker molecule to mimic the paracellular transport of hydrophilic macromolecules. All results displaying the cumulative transport of FD4 are illustrated in Fig. 3. The resulting Papp values and enhancement ratios are shown in Table 2. In the presence of uncoated liposomes, the permeation of FD4 was slightly higher than observed for the model drug itself, as reflected by an enhancement ratio of 1.9. This could be due to the presence of free maleimide groups on the liposomal surface, which may interact with SH-groups within the mucosa and thereby promote permeation of the marker. However, using CS-TGA77-coated liposomes, the permeation enhancing effect was increased to a ratio of 2.8.
Fig. 3.
Absorptive permeation studies of FD4 across rat intestinal mucosa. Effect of uncoated liposomes (△) and liposomes coated with the thiolated chitosans CS-TGA77 (●), CS-TGA150 (■), the S-protected chitosan CS-TGA150-MNA (♦) in comparison with the FD4 control (×). Indicated values are the means ± SD of at least three experiments.
Table 2.
Comparison of Papp values of uncoated liposomes, chitosan-TGA77-coated liposomes, chitosan-TGA150-coated liposomes and chitosan-TGA150-MNA-coated liposomes. Enhancement ratios result from the comparison of each test solution with the corresponding control solution. Indicated values represent the means ± SD of at least three experiments (*p < 0.05, **p < 0.01 and ***p < 0.001 compared with each buffer control). Additionally the effect of named test compounds was tested on the TEER. Table shows the decrease in TEER and the merit index, which is defined as the ratio of the increase in Papp to the decrease in the TEER.
| Substrate | Test compound | Papp × 10− 6 [cm/s] | Fold increase in Papp | Fold decrease in TEER | Merit index |
|---|---|---|---|---|---|
| FD4 | Buffer 37 °C | 1.98 ± 0.36 | – | – | – |
| Uncoated liposomes | 3.79 ± 0.64 | 1.9* | 1.2 | 1.5 | |
| CS-TGA77-coated liposomes | 5.45 ± 0.47 | 2.8** | 1.5 | 1.9 | |
| CS-TGA150-coated liposomes | 6.06 ± 0.20 | 3.1*** | 1.5 | 2.1 | |
| CS-TGA150-MNA-coated liposomes | 8.07 ± 0.73 | 4.1*** | 1.6 | 2.6 | |
| Rho-123 | Buffer 37 °C | 1.89 ± 0.06 | – | – | – |
| Buffer 4 °C | 2.65 ± 0.03 | 1.4 | – | – | |
| Uncoated liposomes | 2.95 ± 0.22 | 1.6* | – | – | |
| CS-TGA77-coated liposomes | 5.29 ± 0.77 | 2.8* | – | – | |
| CS-TGA150-coated liposomes | 6.42 ± 0.82 | 3.4* | – | – | |
| CS-TGA150-MNA-coated liposomes | 7.94 ± 0.95 | 4.2* | – | – |
The mechanism of permeation enhancing caused by thiomers was described previously [30]. Crucial for this effect is the protein tyrosine phosphatase (PTP), which is responsible for the dephosphorylation of tyrosine subunits on tight junctions, resulting in a closing of the latter. Consequently, an inhibition of PTP leads to a phosphorylated subunit and therefore an opening of tight junctions. As PTP shows a cysteine moiety as active site being responsible for its activation, reduced glutathione (GSH) is capable of inhibiting the activity of PTP by a disulfide bond formation [31]. However, GSH is rapidly oxidized on the cell surface and so loses its ability to inhibit the activity of PTP [32]. Thiomers, like thiochitosan, are able to reduce oxidized glutathione (GSSG) to GSH, as highlighted in previous studies [9,33]. To explain the permeation enhancing properties of thiomer-coated liposomes the following situation is expected to happen in vivo: Thiomer-coated liposomes are bound to the mucosa of the small intestine due to their high mucoadhesive properties [7], and therefore their concentration increases sufficiently to ‘shift’ the balance between GSSG and GSH in the direction of GSH, leading to an opening of tight junctions and therefore an enhanced permeation of associated compounds. To investigate if the permeation enhancement ratio of 2.8 measured for TGA-77-coated liposomes could be further improved, the suitability of other thiolated chitosans as coating materials was also tested, utilizing CS-TGA150 — the same polymer as CS-TGA77, only with a molecular weight of 150 kDa compared to 77 kDa to allow for examination of potential molecular weight influences. As the problem of SH-group oxidation was already mentioned, we sought to also test the S-protected thiomer CS-TGA150-MNA in which some of the free SH-groups are protected from oxidation by linking them to mercaptonicotinamide. We observed only a small difference in the permeation enhancement between CS-TGA77 and CS-TGA150; indicating that the permeation enhancing properties do not strictly depend on the molecular weight of the polymeric backbone. On the contrary, liposomes coated with CS-TGA150-MNA resulted in a 4.1-fold increase in the permeation of model substance FD4, representing a 1.3-fold increase in permeation enhancement when compared with liposomes coated with the corresponding conventional thiomer (CS-TGA150), and a 2.2-fold increase when compared with uncoated liposomes. Interestingly, this value was even higher than the one reported for the same thiomer in solution [12]. Within this study, the polymer concentration in the apical compartment was 0.5%, whereas in our studies a concentration of 0.15% has been used. The permeation enhancement ratio for FD4 published by Duennhaupt et al. was 3.3 in comparison with the buffer control, whereas a 4-fold enhancement was achieved with three times less polymer being immobilized on liposomes. One reason for improved permeation enhancement properties of liposomes coated with S-protected thiochitosan compared to those coated with conventional thiochitosan might be the protection of SH-groups from oxidation. Accordingly, after binding to the mucus, more SH-groups are available for reducing GSSG. In vivo, the higher mucoadhesive effect of S-protected thiomers [12] also contributes to an increase in permeation enhancing properties. More clearly, if more polymers are bound to the mucus for an enhanced period of time, more SH-groups will be available to open tight junctions.
3.4.1. TEER-measurements
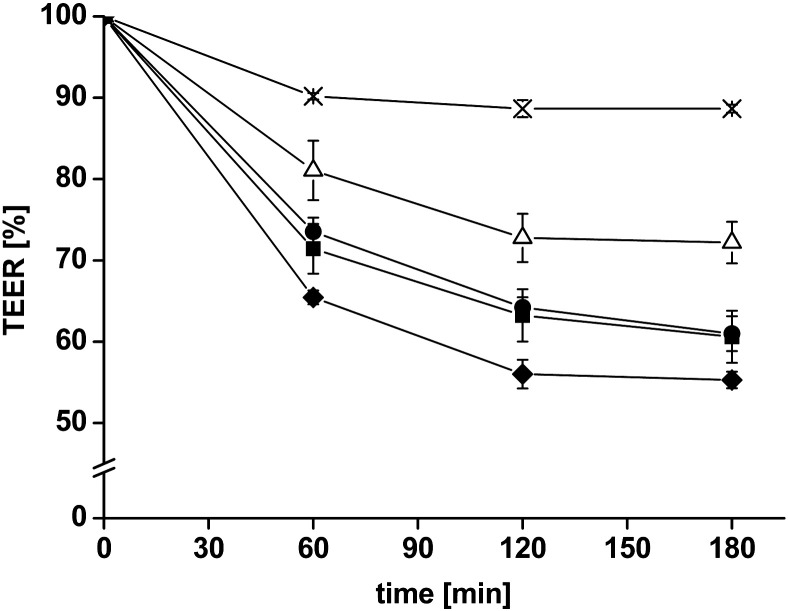
Measuring the TEER provides information concerning the integrity of the tissue and the opening/closing of tight junctions. In parallel to the permeation studies, TEER was measured before starting the experiment to guarantee that no tissues were damaged by mounting them on the Ussing-type chambers. To monitor the effect of the different formulations, measurements were repeated every hour and after finishing the study (Fig. 4). In the absence of test compounds, only a slight decrease in the TEER was measured over 3 h. The addition of uncoated liposomes, CS-TGA77-coated liposomes, CS-TGA150-coated liposomes and CS-TGA150-MNA-coated liposomes resulted in a reduction of the TEER, whereby the slightest decrease was detected for uncoated liposomes and the highest decrease was measured for liposomes coated with S-protected thiochitosan. Hence, all tested liposomal formulations increased the permeation of FD4 and decreased the TEER; indicating that the integrity of the tissue was affected. Song et al. [34] discuss in their work using different permeation enhancers the relationship between an enhanced permeability and a reduced TEER, and argue that an increased permeation enhancement is often related to a decrease in TEER, but the proportionality is not always the same. Permeation enhancers with high proportionality indices (called “merit indices”, which are defined as the ratio between the fold increase in Papp over the fold decrease in TEER) preferably increase the permeability and have less tendency to reduce the membrane integrity, thus would likely serve as efficient and safe absorption enhancers in the body. The merit indices of all three thiomer-coated liposomes are very high compared with their investigated permeation enhancers [34], and could therefore be considered highly suitable for in vivo application.
Fig. 4.
Decrease of the transepithelial electrical resistance (TEER) after adding uncoated liposomes* (△) and liposomes coated with the thiolated chitosans CS-TGA77** (●), CS-TGA150** (■) and the S-protected chitosan CS-TGA150-MNA** (♦) in comparison with the FD4 control (×). Indicated values are the means ± SD of at least three experiments (*p < 0.05, **p < 0.01 compared with the FD4 control solution).
3.5. Ex vivo evaluation of P-glycoprotein inhibitory properties
P-gp is one of the most widely studied and most important efflux pumps controlling the disposition of drugs. Many drugs are substrates for P-gp, and after binding to the carrier, they are transported back out of the cell. In order to increase the bioavailability of these kinds of drugs, P-gp inhibiting compounds, like thiomers, have been developed.
To test the P-gp inhibitory properties of thiomer-coated liposomes the absorptive transport of 0.001% (m/v) Rho-123 was determined using Ussing type chambers. A classical indicator for efflux pump mediated transport is the permeation rate of substrates from the AP to BL and BL to AP direction. In a previous study, it was found that the Rho-123 efflux ratio (secretory Papp/absorptive Papp) was about 2.8 — clearly identifying Rho-123 as a substrate for efflux pumps [10]. Within this study, the influence of different coated- and uncoated liposomes on the absorptive transport of the P-gp substrate Rho-123 was investigated over a time period of 3 h. The most promising formulation was then used for bidirectional transport studies of Rho-123 in presence and absence of the P-gp inhibitor verapamil.
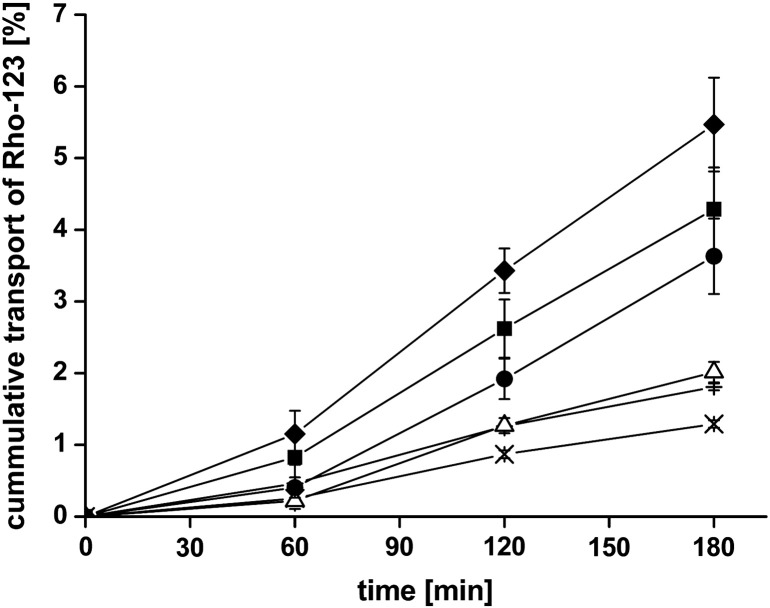
As an orientating experiment, the transport of Rho-123 across freshly excised rat intestine was determined at 4 °C and 37 °C. At 37 °C, the transport of Rho-123 is a combination of an active, efflux pump mediated transport and passive diffusion [21] whereas at 4 °C, the activity of ATP hydrolysis is low and therefore the mechanism of drug efflux is stopped [19,20]. To this end, the permeation of Rho-123 at 4 °C is not influenced by efflux pumps, but is only the product of passive diffusion, leading to a higher permeation of Rho-123 at 4 °C than at 37 °C. All results obtained from these studies are summarized in Table 2 and Fig. 5. In the presence of uncoated liposomes, the transport of Rho-123 through the intestinal tissue was only slightly enhanced, as it was also seen by permeation studies with FD4. Liposomes coated with CS-TGA77 or CS-TGA150 showed a pronounced enhancement ratio of the Papp of 2.8 and 3.4 fold, respectively. Unlike many other efflux pump inhibiting substrates, which inhibit P-gp based on a competitive inhibition or ATP-depletion [35], thiomers and therefore also thiomer-coated liposomes are not taken up from the gut due to their high molecular weight [33]. The postulated mechanism for thiomer-mediated P-gp inhibition is on the contrary based on the interaction of thiol groups with the channel-forming transmembrane domain of the efflux pump. As two of the twelve transmembrane domains of P-gp also contain a cysteine residue, thiomers were expected to form disulfide bridges with one or both of these subunits, thereby blocking the allosteric change of the pump essential for drug transport [36]. However, based on our new data, the above mentioned mechanism has to be seen with caution. Thiomer-coated liposomes are between 354.5 ± 3.4 and 702.6 ± 138 nm in their size, depending on the polymer used (see Table 1). Due to this size, it is highly unlikely that the particles are able to interact with cysteine residues within the channel. It can therefore be concluded that the mechanisms leading to an enhanced efflux pump inhibition are not yet fully understood and need to be further investigated. Nevertheless, the results clearly indicate an efflux pump inhibitory effect of thiomer-coated liposomes. Even though CS-TGA77- and CS-TGA150-coated liposomes demonstrated good permeation enhancing properties, the best results were obtained for CS-TGA150-MNA-coated liposomes, leading to an enhancement ratio of 4.2. Some of the thiol groups in S-protected thiomers are coupled to thiolated pyridyl substructures, leading to a higher reactive form of thiol groups compared to alkyl thiols, whose thiol groups are not in the reactive state at physiological conditions given that the pKa of alkyl thiols is in the range of 8–10 [37]. Consequently, thiomers do not reach their full delivery potential in cases where the pH is lower. In contrast to that, S-protected thiolated chitosan reacts independently from the surrounding pH. For this reason, CS-TGA150-MNA-coated liposomes performed better in this study than CS-TGA77 or CS-TGA150-coated liposomes, where the SH-groups were not protected.
Fig. 5.
Efflux pump inhibition studies of Rho-123 across rat intestinal mucosa. Effect of uncoated liposomes (△) and liposomes coated with the thiolated chitosans CS-TGA77 (●), CS-TGA150 (■), the S-protected chitosan CS-TGA150-MNA (♦) in comparison with the Rho-123 control at 4 °C (+) and 37 °C (×). Indicated values are the means ± SD of at least three experiments.
As thiomer-coated liposomes were shown to enhance the paracellular route of absorption, it is likely that the permeation of Rho-123 is also improved via this pathway. In order to evaluate if this enhanced permeation is exclusively due to an opening of tight junctions, or the combination of an opening of tight junction with an inhibition of P-gp – the latter being what we propose – bidirectional transport studies were performed using CS-TGA150-MNA coated liposomes. These liposomes were chosen on the basis of their performance in our previous investigations, and were compared with the known P-gp inhibitor verapamil. All results are summarized in Table 3. We first monitored the absorptive Rho-123 transport with and without verapamil, which revealed a reduced permeation in presence of verapamil; thus validating the presence of efflux pumps on the tissue used for this study. This was further confirmed by showing that the transport of Rho-123, without any further additives, from the basolateral to the apical side of the tissue was higher than from the apical to the basolateral side (efflux ratio: 3.3). When the same bidirectional experiment was performed in presence of verapamil, the efflux ratio decreased to 1.6 — again validating the P-gp inhibitory effect of the drug, and the reliability of the test system. Although no complete inhibition was achieved under these conditions, which would be indicated by an efflux ratio of 1, the effect of verapamil was still very prominent.
Table 3.
Comparison of the absorptive and secretory apparent permeability coefficients (Papp) of Rho-123 and resulting efflux ratios in the presence of different test compounds. Indicated values represent the means ± SD of at least three experiments. Additionally the effect of named test compounds was tested on the TEER (AP to BL direction).
| Substrate | Test compound | Absorptive (AP to BL) Papp × 10− 6 [cm/s] | Secretory (BL to AP) Papp × 10− 6 [cm/s] | Efflux ratio (secretory Papp/absorptive Papp) | Fold decrease in TEER |
|---|---|---|---|---|---|
| Rho-123 | Buffer | 1.86 ± 0.78 | 6.22 ± 2.07 | 3.3 | – |
| Verapamil 100 μM (apical) | 3.49 ± 0.86 | 5.47 ± 0.99 | 1.6 | 1.1 | |
| CS-TGA150-MNA-coated liposomes (apical) | 2.77 ± 0.24 | 5.01 ± 0.74 | 1.8 | 1.5 | |
| CS-TGA150-MNA coated liposomes plus verapamil 100 μM (apical) | 3.54 ± 0.51 | 3.58 ± 1.66 | 1.0 | 1.6 |
When CS-TGA150-MNA-coated liposomes were added to the apical compartment, the efflux ratio decreased to a value of 1.8, being in the range of verapamil. If the enhanced permeation of Rho-123 was exclusively due to an enhanced paracellular transport, the permeation from BL to AP should be much higher than from AP to BL, reflected by an efflux ratio in the range of 3 to 4, which was not the case. Even though it is well known that different transporters are present on the basolateral and apical side of the tissue of the small intestine aside from P-gp – and therefore the findings from all experiments investigating bidirectional transport through the entire cross section of the small intestine have to be viewed in light of this – our results strongly support the hypothesis that thiomer-coated liposomes do exhibit P-gp inhibitory properties. This data indeed corroborates the findings of our study monitoring the effect of different thiomer-coated liposomes only in the absorptive direction, and show that the enhanced permeation of Rho-123 is most probably due to a combination of permeation enhancing and efflux pump inhibitory properties.
4. Conclusion
Within this study, a delivery system was developed to demonstrate high stability in the gastrointestinal environment and to provide a sustainable release of encapsulated compounds in the small intestinal milieu. These thiomer-coated liposomes did not cause immunogenic reactions in mice, and the thiomers, which were immobilized at the surface of liposomes, showed strong permeation enhancing- and efflux pump inhibitory properties.
Taken together, our results indicate a high potential of thiomer-coated liposomes for the oral delivery of unstable and poorly absorbed drug compounds within the harsh GI-environment.
Acknowledgment
For financial support of this study we thank the Austrian Research Promotion Agency FFG (project Nano Health 819721) and the Austrian Science Fund FWF (SFB F4606-B19; CCHD APW01205FW). We thank Anna Willensdorfer, Caroline Stremnitzer, Anna Lukschal and Susanne Diesner for their excellent technical assistance during immunogenicity studies. Further we thank Miss Grace Hatton from the Department of Pharmaceutics, UCL School of Pharmacy, London, for carefully reading the manuscript.
Contributor Information
K. Gradauer, Email: kerstin.gradauer@oeaw.ac.at.
S. Dünnhaupt, Email: sarah.duennhaupt@uibk.ac.at.
C. Vonach, Email: caroline.vonach@oeaw.ac.at.
H. Szöllösi, Email: helen_sz@gmx.at.
I. Pali-Schöll, Email: isabella.pali@meduniwien.ac.at.
H. Mangge, Email: harald.mangge@medunigraz.at.
E. Jensen-Jarolim, Email: erika.jensen-jarolim@meduniwien.ac.at.
A. Bernkop-Schnürch, Email: andreas.bernkop@uibk.ac.at.
R. Prassl, Email: ruth.prassl@oeaw.ac.at.
References
- 1.Patel H.M., Stevenson R.W., Parsons J.A., Ryman B.E. Use of liposomes to aid intestinal absorption of entrapped insulin in normal and diabetic dogs. Biochim. Biophys. Acta. 1982;716:188–193. doi: 10.1016/0304-4165(82)90267-7. [DOI] [PubMed] [Google Scholar]
- 2.Chiang C.M., Weiner N. Gastrointestinal uptake of liposomes. II. In vivo studies. Int. J. Pharm. 1987;40:143–150. [Google Scholar]
- 3.Parmentier J., Hartmann F.J., Fricker G. In vitro evaluation of liposomes containing bio-enhancers for the oral delivery of macromolecules. Eur. J. Pharm. Biopharm. 2010;76:394–403. doi: 10.1016/j.ejpb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi H., Matsui Y., Yamamoto H., Kawashima Y. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J. Control. Release. 2003;86:235–242. doi: 10.1016/s0168-3659(02)00411-x. [DOI] [PubMed] [Google Scholar]
- 5.Karn P.R., Vanic Z., Pepic I., Skalko-Basnet N. Mucoadhesive liposomal delivery systems: the choice of coating material. Drug Dev. Ind. Pharm. 2011;37:482–488. doi: 10.3109/03639045.2010.523425. [DOI] [PubMed] [Google Scholar]
- 6.Dwivedi N., Arunagirinathan M.A., Sharma S., Bellare J. Silica-coated liposomes for insulin delivery. J. Nanomater. 2010;2010:1–8. [Google Scholar]
- 7.Gradauer K., Vonach C., Leitinger G., Kolb D., Frohlich E., Roblegg E., Bernkop-Schnürch A., Prassl R. Chemical coupling of thiolated chitosan to preformed liposomes improves mucoadhesive properties. Int. J. Nanomedicine. 2012;7:2523–2534. doi: 10.2147/IJN.S29980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitner V.M., Marschutz M.K., Bernkop-Schnürch A. Mucoadhesive and cohesive properties of poly(acrylic acid)-cysteine conjugates with regard to their molecular mass. Eur. J. Pharm. Sci. 2003;18:89–96. doi: 10.1016/s0928-0987(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 9.Bernkop-Schnürch A., Kast C.E., Guggi D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems. J. Control. Release. 2003;93:95–103. doi: 10.1016/j.jconrel.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Werle M., Hoffer M. Glutathione and thiolated chitosan inhibit multidrug resistance P-glycoprotein activity in excised small intestine. J. Control. Release. 2006;111:41–46. doi: 10.1016/j.jconrel.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Bernkop-Schnürch A., Zarti H., Walker G.F. Thiolation of polycarbophil enhances its inhibition of intestinal brush border membrane bound aminopeptidase N. J. Pharm. Sci. 2001;90:1907–1914. doi: 10.1002/jps.1140. [DOI] [PubMed] [Google Scholar]
- 12.Dünnhaupt S., Barthelmes J., Rahmat D., Leithner K., Thurner C.C., Friedl H., Bernkop-Schnürch A. S-protected thiolated chitosan for oral delivery of hydrophilic macromolecules: evaluation of permeation enhancing and efflux pump inhibitory properties. Mol. Pharm. 2012;9:1331–1341. doi: 10.1021/mp200598j. [DOI] [PubMed] [Google Scholar]
- 13.Kast C.E., Bernkop-Schnürch A. Thiolated polymers-thiomers: development and in vitro evaluation of chitosan-thioglycolic acid conjugates. Biomaterials. 2001;22:2345–2352. doi: 10.1016/s0142-9612(00)00421-x. [DOI] [PubMed] [Google Scholar]
- 14.Pali-Scholl I., Herzog R., Wallmann J., Szalai K., Brunner R., Lukschal A., Karagiannis P., Diesner S.C., Jensen-Jarolim E. Antacids and dietary supplements with an influence on the gastric pH increase the risk for food sensitization. Clin. Exp. Allergy. 2010;40:1091–1098. doi: 10.1111/j.1365-2222.2010.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouly S., Paine M.F. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm. Res. 2003;20:1595–1599. doi: 10.1023/a:1026183200740. [DOI] [PubMed] [Google Scholar]
- 16.Lacombe O., Woodley J., Solleux C., Delbos J.M., Boursier-Neyret C., Houin G. Localisation of drug permeability along the rat small intestine, using markers of the paracellular, transcellular and some transporter routes. Eur. J. Pharm. Sci. 2004;23:385–391. doi: 10.1016/j.ejps.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Troutman M.D., Thakker D.R. Rhodamine 123 requires carrier-mediated influx for its activity as a P-glycoprotein substrate in Caco-2 cells. Pharm. Res. 2003;20:1192–1199. doi: 10.1023/a:1025096930604. [DOI] [PubMed] [Google Scholar]
- 18.Fortuna A., Alves G., Falcao A., Soares-da-Silva P. Evaluation of the permeability and P-glycoprotein efflux of carbamazepine and several derivatives across mouse small intestine by the Ussing chamber technique. Epilepsia. 2012;53:529–538. doi: 10.1111/j.1528-1167.2012.03409.x. [DOI] [PubMed] [Google Scholar]
- 19.Profit L., Eagling V.A., Back D.J. Modulation of P-glycoprotein function in human lymphocytes and Caco-2 cell monolayers by HIV-1 protease inhibitors. AIDS. 1999;13:1623–1627. doi: 10.1097/00002030-199909100-00004. [DOI] [PubMed] [Google Scholar]
- 20.Saha P., Yang J.J., Lee V.H. Existence of a p-glycoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest. Ophthalmol. Vis. Sci. 1998;39:1221–1226. [PubMed] [Google Scholar]
- 21.Varma M.V., Ashokraj Y., Dey C.S., Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol. Res. 2003;48:347–359. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 22.Mertins O., Dimova R. Binding of chitosan to phospholipid vesicles studied with isothermal titration calorimetry. Langmuir. 2011;27:5506–5515. doi: 10.1021/la200553t. [DOI] [PubMed] [Google Scholar]
- 23.Mertins O., Schneider P.H., Pohlmann A.R., da Silveira N.P. Interaction between phospholipids bilayer and chitosan in liposomes investigated by 31P NMR spectroscopy. Colloids Surf. B Biointerfaces. 2010;75:294–299. doi: 10.1016/j.colsurfb.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang J., Ping Q., Song Y., Qi J., Cui Z. Effects of chitosan coating on physical properties and pharmacokinetic behavior of mitoxantrone liposomes. Int. J. Nanomedicine. 2010;5:407–416. doi: 10.2147/ijn.s10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriksen I., Smistad G., Karlsen J. Interactions between liposomes and chitosan. Int. J. Pharm. 1994;101:227–236. [Google Scholar]
- 26.Ellens H., Bentz J., Szoka F.C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry. 1984;23:1532–1538. doi: 10.1021/bi00302a029. [DOI] [PubMed] [Google Scholar]
- 27.Nayar R., Schroit A.J. Generation of pH-sensitive liposomes: use of large unilamellar vesicles containing N-succinyldioleoylphosphatidylethanolamine. Biochemistry. 1985;24:5967–5971. doi: 10.1021/bi00342a042. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A., Sharma U.S. Liposomes in drug delivery: progress and limitations. Int. J. Pharm. 1997;154:123–140. [Google Scholar]
- 29.Ma Y., Yang Q., Wang L., Zhou X., Zhao Y., Deng Y. Repeated injections of PEGylated liposomal topotecan induces accelerated blood clearance phenomenon in rats. Eur. J. Pharm. Sci. 2012;45:539–545. doi: 10.1016/j.ejps.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Clausen A.E., Bernkop-Schnürch A. Thiolated carboxymethylcellulose: in vitro evaluation of its permeation enhancing effect on peptide drugs. Eur. J. Pharm. Biopharm. 2001;51:25–32. doi: 10.1016/s0939-6411(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 31.Barrett W.C., DeGnore J.P., Konig S., Fales H.M., Keng Y.F., Zhang Z.Y., Yim M.B., Chock P.B. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999;38:6699–6705. doi: 10.1021/bi990240v. [DOI] [PubMed] [Google Scholar]
- 32.Grafstroem R., Stead A.H., Orrenius S. Metabolism of extracellular glutathione in rat small-intestinal mucosa. Eur. J. Biochem. 1980;106:571–577. doi: 10.1111/j.1432-1033.1980.tb04605.x. [DOI] [PubMed] [Google Scholar]
- 33.Clausen A.E., Kast C.E., Bernkop-Schnürch A. The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm. Res. 2002;19:602–608. doi: 10.1023/a:1015345827091. [DOI] [PubMed] [Google Scholar]
- 34.Song K.H., Chung S.J., Shim C.K. Enhanced intestinal absorption of salmon calcitonin (sCT) from proliposomes containing bile salts. J. Control. Release. 2005;106:298–308. doi: 10.1016/j.jconrel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Lo Y.L., Huang J.D. Effects of sodium deoxycholate and sodium caprate on the transport of epirubicin in human intestinal epithelial Caco-2 cell layers and everted gut sacs of rats. Biochem. Pharmacol. 2000;59:665–672. doi: 10.1016/s0006-2952(99)00377-9. [DOI] [PubMed] [Google Scholar]
- 36.Gottesman M.M., Pastan I. The multidrug transporter, a double-edged sword. J. Biol. Chem. 1988;263:12163–12166. [PubMed] [Google Scholar]
- 37.Bernkop-Schnürch A., Hornof M., Zoidl T. Thiolated polymers-thiomers: synthesis and in vitro evaluation of chitosan-2-iminothiolane conjugates. Int. J. Pharm. 2003;260:229–237. doi: 10.1016/s0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]