Abstract
Transforming growth factor β (TGF-β) is a cytokine which has been shown to suppress the antimycobacterial immune responses of humans and experimental animals. In this study, the contributions of TGF-β to cytokine production in vivo were investigated by using the established guinea pig model of tuberculous pleurisy. Mycobacterium bovis BCG-vaccinated guinea pigs were injected intrapleurally with heat-killed virulent Mycobacterium tuberculosis. Eight days following induction of an antigen-specific pleural effusion, guinea pigs were injected intrapleurally with anti-TGF-β1 or isotype control antibody. The following day, pleural exudates were removed, and the fluid volume and characteristics of the infiltrating cells were determined. Pleural fluid was analyzed for total interferon (IFN) and tumor necrosis factor (TNF) protein levels by using appropriate bioassays. RNA from pleural effusion cells was examined to determine TGF-β1, TNF-α, IFN-γ, and interleukin-8 mRNA levels by using real-time PCR. Proliferative responses of pleural effusion lymphocytes were examined in response to concanavalin A and purified protein derivative (PPD) in vitro. Treatment with anti-TGF-β1 resulted in decreased pleural fluid volume and decreased cell numbers in the pleural space along with an increased percentage of lymphocytes and a decreased percentage of neutrophils. The bioactive TNF protein levels in pleural fluid were increased in guinea pigs treated with anti-TGF-β1, while the bioactive IFN protein concentrations were not altered. Expression of TGF-β1 and TNF-α mRNA was significantly increased following TGF-β1 neutralization. Finally, PPD-induced proliferative responses of pleural cells from anti-TGF-β1-treated animals were significantly enhanced. Thus, TGF-β1 may be involved in the resolution of this local, mycobacterial antigen-specific inflammatory response.
Mycobacterium tuberculosis is the leading cause of death from an infectious disease worldwide, with the exception of human immunodeficiency virus. It is estimated that approximately one-third of the world's population is infected with this organism (4). Tuberculous pleurisy is the predominant extrapulmonary manifestation of tuberculosis and is caused by a severe delayed-type hypersensitivity response to mycobacteria or mycobacterial antigens that have escaped from lung granulomas and invaded the pleural space (13). A guinea pig model that mimics tuberculous pleurisy was developed in our lab (3, 30) and has been useful for examining the cell-mediated immune response to mycobacteria in the context of an antigen-specific inflammatory exudate. This model allows components of the immune system to be manipulated in vivo in order to examine their roles in this delayed-type hypersensitivity response. One cytokine likely to be involved is transforming growth factor β (TGF-β), due to its known contributions to processes such as fibrosis and resolution of inflammatory responses (37). Tuberculous pleurisy is a manifestation of tuberculosis that is self-healing (31), which implies that the local immune response results in successful control of the mycobacteria. Increased levels of TGF-β1 have been observed in the pleural fluid of tuberculous pleurisy patients compared with the levels in pleural exudates with other etiologies (27). Furthermore, elevated levels of TGF-β are also observed in the plasma of tuberculous pleuritis patients (29). In patients with recurrent pleural effusions, typically due to cancer, pleurodesis is performed, in which the pleural space is obliterated by the induction of pleural fibrosis (21). TGF-β has been used to induce pleurodesis in animal models and appears to be a good candidate for use in humans (20, 22, 23, 25).
TGF-β levels have also been examined in patients with pulmonary tuberculosis. Elevated levels of TGF-β were found in plasma of pulmonary tuberculosis patients compared with the levels in healthy contacts and patients who had undergone successful treatment for tuberculosis (29). Additionally, purified protein derivative (PPD) stimulation of peripheral blood mononuclear cells (PBMC) from tuberculosis patients on chemotherapy for less than 1 month resulted in significantly increased TGF-β protein production compared with the production in cells from patients on chemotherapy for more than 1 month and cells from healthy controls (16). Unstimulated PBMC from tuberculosis patients also produced significantly larger amounts of TGF-β than PBMC from healthy tuberculin-positive individuals produced, and granulomas from tuberculosis patients stained positive for TGF-β (33). Increased TGF-β levels have been associated with increased severity of tuberculosis. PBMC from patients with advanced pulmonary tuberculosis produced significantly higher levels of TGF-β in the absence or presence of whole sonicated antigen from M. tuberculosis than PBMC from patients with mild to moderate disease and PBMC from healthy controls produced (11).
In a previous study, it was shown that the TGF-β1 concentrations in the pleural exudate of guinea pigs with experimental tuberculous pleurisy were relatively low during the first 2 days postinduction and increased throughout the experiment, peaking between days 7 and 9 postinduction (3). In this study, TGF-β1 levels in the pleural fluid were neutralized 8 days postinduction by intrapleural injection of anti-TGF-β1 antibody, and the effect on the cellular nature and cytokine content of the pleural effusion was examined 1 day later.
MATERIALS AND METHODS
Reagents.
Chicken anti-human TGF-β1, the human TGF-β1 DuoSet enzyme-linked immunosorbent assay (ELISA) development system, and control chicken immunoglobulin Y were obtained from R&D Systems (Minneapolis, Minn.). RPMI 1640 medium supplemented with HEPES buffer, 10% Hartley guinea pig serum (Harlan Laboratories, Indianapolis, Ind.), 2-mercaptoethanol (10 μM), l-glutamine (2 μM), penicillin (100 U/ml), and streptomycin (100 μg/ml) was used in all experiments. Concanavalin A (ConA) (Sigma, St. Louis, Mo.) and M. tuberculosis PPD (Statens Seruminstitut, Copenhagen, Denmark) were used to stimulate lymphoproliferation.
Animals and vaccination.
Outbred Hartley strain guinea pigs from Charles River Breeding Laboratories, Inc. (Wilmington, Mass.) were used in this study. Guinea pigs were vaccinated via intradermal injection of 0.1 ml (103 CFU) of a viable Mycobacterium bovis BCG (Danish 1331 strain; Statens Seruminstitut) culture. All protocols were approved by the Texas A&M University Laboratory Animal Care Committee.
Preparation of mycobacteria and pleurisy induction.
A vial of live M. tuberculosis H37Rv (ATCC 27294; American Type Culture Collection, Manassas, Va.) was removed from storage at −80°C and rapidly thawed. The bacterial suspension was vortexed and sonicated with an Ultrasonics sonicator (Heat Systems-Ultrasonics, Inc., Plainview, N.Y.) for 45 to 60 s at an output setting of 8.0 to disrupt bacterial clumps. The bacteria were then heat killed by incubation in an 80°C water bath for 2 h and diluted in a sterile 0.9% sodium chloride solution to a final concentration of 1.8 × 107 CFU/ml. Five weeks after vaccination (day 0), guinea pigs were anesthetized by using a previously described protocol (30), and pleurisy was induced by injection of 1 ml of heat-killed M. tuberculosis H37Rv bilaterally into the pleural cavity. On day 8 postinduction, guinea pigs were anesthetized, and 12.5 μg of anti-TGF-β1 or the same concentration of control chicken immunoglobulin Y was injected bilaterally into the pleural cavity. The animals were euthanized 24 h later.
Necropsy and cell isolation.
Guinea pigs were euthanized by intraperitoneal injection of an overdose (100 mg/kg) of sodium pentobarbital (Sleepaway; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). Fluid in the pleural cavity was collected, and the area was washed two times with ice-cold phosphate-buffered saline to remove accumulated inflammatory cells. The pleural fluid was centrifuged at 200 × g for 15 min to remove the cells. The fluid was then transferred to a new conical tube and centrifuged at 1,400 × g for 20 min to remove all cellular debris, and it was stored at −80°C until it was needed. All cells from the pleural space were combined, and the erythrocytes were lysed with ACK lysing buffer (0.15 M NH4Cl, 0.01 M KHCO3, 0.1 mM Na2EDTA), followed by two washes with phosphate-buffered saline.
Differential cell counts.
Cells obtained from the pleural cavity were characterized to determine the proportion of major leukocyte types. Cells were centrifuged onto silanated glass slides (CSA-100; PGC Scientifics, Gaithersburg, Md.) at 140 × g for 5 min (Cytospin 2; Shandon Southern Instrument, Inc., Sewickley, Pa.). Cells were stained with Diff-Quik (Dade Behring Inc., Newark, Del.) and viewed under a light microscope in order to determine the relative immune cell composition by cell morphology.
Histopathology.
Diaphragms and mediastinae were taken from pleuritic guinea pigs and fixed in 10% buffered formalin for examination of the pleural lining. Tissues were embedded in paraffin and sectioned to obtain 5-μm sections, and the sections were stained with hematoxylin and eosin for histopathological examination.
Measurement of cytokine proteins in pleural fluid.
In order to determine TGF-β1 protein concentrations, pleural fluid was analyzed by an ELISA (human TGF-β1 DuoSet ELISA development system; R&D Systems). Prior to the assay, pleural fluid samples were activated by using the manufacturer's protocol for activation of serum samples. Use of human TGF-β1 reagents in guinea pigs is justified because the mature guinea pig TGF-β1 protein (GenBank accession no. AF191297) exhibits 98% sequence homology with mature human TGF-β1, and we and several other investigators have successfully used human TGF-β1 reagents, including antibody, for guinea pig studies (8, 9, 17, 32, 42).
The guinea pig fibroblast cell line 104C1 (ATCC CRL-1405; American Type Culture Collection) and the challenge virus encephalomyocarditis virus (ATCC VR-129B) were used to determine the concentrations of bioactive interferon (IFN) in the pleural fluid as previously described (41). Briefly, 104C1 cells were seeded onto 96-well plates at a concentration of 3 × 104 cells/well and incubated at 37°C in the presence of 5% CO2 for 24 h. Human IFN-α (400 U/ml) and pleural fluid samples were twofold serially diluted, added (50%, vol/vol) to the cells, and incubated for 20 to 24 h. Encephalomyocarditis virus was then added to the plate at a concentration of 900 PFU/well, and the plate was incubated for 24 to 30 h. WST-1/1-methoxy PMS (Dojindo, Kumamoto, Japan) was added to the cells and incubated for 2 to 4 h at 37°C. Color development was stopped with 1 N H2SO4. The optical density at 450 nm was measured with a microtiter plate reader with a 630-nm reference filter.
The mouse fibroblast cell line L929 (ATCC CCL-1; American Type Culture Collection) was used to determine the concentrations of total bioactive tumor necrosis factor (TNF) in the pleural fluid by the method of Espevik et al. (15), with some modifications. Briefly, L929 cells were seeded onto 96-well plates (4 × 104 cells/well) and grown to confluence overnight at 37°C in the presence of 5% CO2. Human TNF-α and pleural fluid samples were twofold serially diluted and added (25%, vol/vol) to the cells. Actinomycin D (8 μg/ml) was added to each well, and then the plates were incubated for approximately 20 h. Cell viability was determined as described above for the IFN bioassay.
Total RNA isolation and real-time PCR.
Total RNA was isolated from cells by using RNeasy columns, and contaminating DNA was removed by on-column treatment with RNase-free DNase (QIAGEN, Valencia, Calif.). Reverse transcription, performed by using TaqMan reverse transcription reagents, and real-time PCR, performed by using SYBR Green I double-stranded DNA binding dye (Applied Biosystems, Foster City, Calif.), were carried out by using the previously described protocol (3, 26). The real-time primers used for guinea pig TGF-β1, IFN-γ, TNF-α, interleukin 8 (IL-8), and 18S RNA were the primers described previously (3). The level of induction of mRNA was determined from the threshold cycle values normalized for 18S RNA expression and then normalized to the value derived from healthy, pleuritis-free animals.
Lymphoproliferation.
Cells from the pleural effusion were analyzed to determine their ability to proliferate in response to ConA (10 μg/ml) and PPD (12.5 and 25 μg/ml) in vitro. Pleural cells were seeded onto 96-well plates (2 × 105 cells/well) in medium alone or in the presence of ConA or PPD and cultured by using our standard procedure (7). [3H]thymidine was added at a concentration of 1 μCi/well for the final 6 h of incubation. Cells were harvested onto glass fiber filters, and proliferative activity was quantified by determining the number of counts per minute with a liquid scintillation counter (LS8000; Beckman Instruments, Inc., Fullerton, Calif.). Stimulation indices were calculated by dividing the number of counts per minute from stimulated cells by the number of counts per minute from unstimulated cells.
Statistical analysis.
A t test was used to examine statistical differences between anti-TGF-β1-treated and control guinea pigs at the 95% confidence interval. The statistical tests were performed with SAS software (release 8.01; SAS Institute, Inc., Cary, N.C.).
RESULTS
Cell and fluid accumulation in control and anti-TGF-β1-treated guinea pigs.
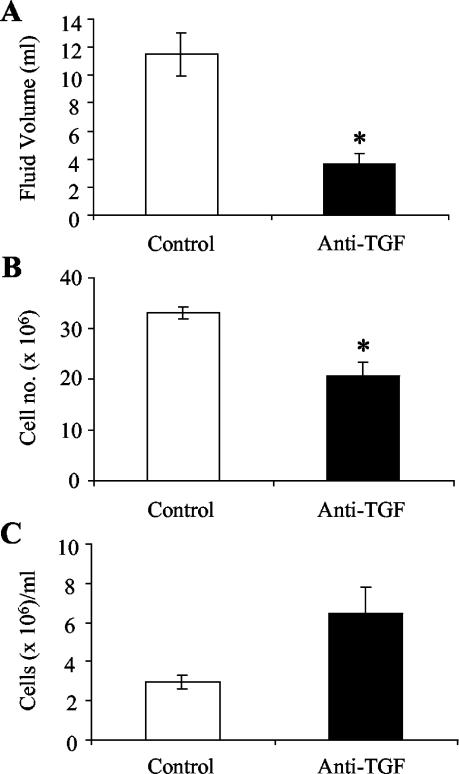
Eight days after pleurisy induction, guinea pigs were injected with anti-TGF-β1 or control antibody. One day later, the guinea pigs were euthanized, and accumulated fluid and cells were removed from the pleural cavity. Figure 1 shows the total volumes of fluid and the numbers of cells that accumulated in the pleural space of control and treated guinea pigs. Large amounts of fluid were present in the pleural cavities of control guinea pigs, while there was a dramatic decrease in the fluid volume in the pleural spaces of anti-TGF-β1-treated guinea pigs (Fig. 1A) (P < 0.01). Similar to the increased fluid volume seen in control animals, the total numbers of recoverable cells were higher in the control guinea pigs than in the anti-TGF-β1-treated animals (Fig. 1B). Injection of anti-TGF-β1 resulted in significantly decreased total cell numbers in the pleural effusions of treated guinea pigs (P = 0.01). The concentration of cells in the pleural fluid was actually higher in the anti-TGF-β1-treated guinea pigs than in the controls (Fig. 1C); however, the difference was not significant.
FIG. 1.
Accumulation of fluid and cells in pleural exudates of guinea pigs with tuberculous pleurisy. Fluid accumulation (A), cell accumulation (B), and cell concentration (C) were measured in control pleuritic guinea pigs and guinea pigs receiving experimental injections of anti-TGF-β1. Cells were stained with trypan blue and counted with a hemocytometer. The bars and error bars indicate averages and standard errors of the means for three of four animals. An asterisk indicates that there is significant difference between the number of cells or fluid volume and the number of cells or fluid volume in control guinea pigs.
Differential cell counts and histopathology of pleural tissues.
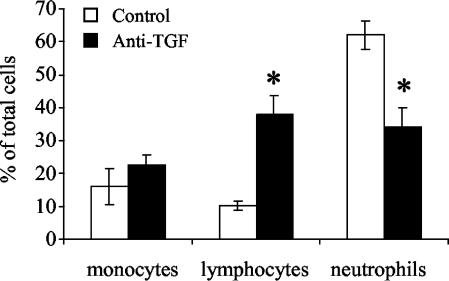
A portion of the pleural exudate cells was centrifuged onto glass slides and stained with Diff-Quik to determine the percentages of monocytes, lymphocytes, and neutrophils. The resident cells in the pleural cavities of two healthy guinea pigs were found to consist of 63.6 and 65% large monocytes, 2% lymphocytes, and 0 and 0.3% neutrophils. The remaining cells were eosinophils (34.7 and 32.7%). Figure 2 shows that in control antibody-treated guinea pigs with experimental tuberculous pleurisy, neutrophils were by far the predominant cell type present in the pleural fluid, while much lower numbers of monocytes and lymphocytes were present. On the other hand, in pleuritic guinea pigs treated with anti-TGF-β1, lymphocytes and neutrophils were present at roughly equal proportions, and there were fewer monocytes. Compared with controls, treatment with anti-TGF-β1 resulted in a statistically significant increase in the proportion of lymphocytes and a statistically significant decrease in the proportion of neutrophils present in the pleural space (P < 0.05). The remaining cell types found in the pleural cavities of guinea pigs were eosinophils (4.57% ± 1% in controls and 2.38% ± 0.86% in anti-TGF-β1-treated animals) and Kurloff cells (6.95% ± 0.68% in controls and 3.09% ± 0.96% in anti-TGF-β1-treated animals). Anti-TGF-β1 treatment did not significantly affect the proportions of these cells found in the pleural space.
FIG. 2.
Differential counts of pleural exudate cells from pleuritic control and anti-TGF-β1-treated guinea pigs. Cells were stained with Diff-Quik as described in Materials and Methods, and cell morphology was used to determine which cell types were present in the pleural fluid. Two counts (300 cells each) were performed, and the number of each cell type was divided by the total number of cells to obtain the percentage. The bars indicate the percentages of the total cell population recovered from the pleural space for three or four animals, and the error bars indicate the standard errors of the means. An asterisk indicates that there is a significant difference between the percentage of a cell type and the percentage in the controls.
Hematoxylin- and eosin-stained paraffin sections of pleural tissues taken from control and anti-TGF-β1-treated guinea pigs were evaluated histopathologically. Guinea pigs in both groups developed well-formed granulomas with extensive fibrosis in the diaphragmatic and mediastinal pleurae. No differences were observed between the groups with respect to the percentage of the tissue affected, the number of intralesional organisms, the level of necrosis, the inflammatory cell types, or the formation of organized granulomas.
Cytokine profiles in pleural fluid of guinea pigs.
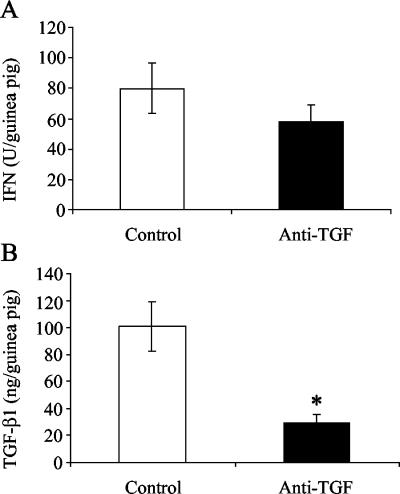
During the course of experimental tuberculous pleurisy in guinea pigs, the TGF-β1 protein concentrations in the pleural fluid were highest between days 7 and 9 (3). In order to assess whether neutralization of TGF-β1 during this time affected the production of other cytokines, pleural fluid was analyzed for total IFN and TNF protein concentrations. Figure 3 shows the total levels of proteins in pleural exudates of guinea pigs rather than the concentrations as the amount of pleural fluid in control antibody-treated guinea pigs was significantly greater than the amount found in anti-TGF-β1-treated guinea pigs. The total bioactive IFN protein levels in the pleural fluid were lower, but not significantly lower, as a result of anti-TGF-β1 treatment (Fig. 3A). The bioactive TNF protein levels were below the level of detection in pleural fluid from the control guinea pigs. However, bioactive TNF was detected in two of four animals in the anti-TGF-β1-treated group (mean, 2,366 pg/guinea pig), suggesting that removal of TGF-β1 enhanced TNF protein production during experimental tuberculous pleurisy.
FIG. 3.
Cytokine protein levels in pleural fluids of control and anti-TGF-β1-treated guinea pigs. Total bioactive IFN (A) and TGF-β1 (B) protein levels in pleural exudates of guinea pigs were measured. Total IFN protein levels were analyzed by a bioassay, as described in Materials and Methods, and are expressed in units per total volume of fluid in the pleural space of each guinea pig. TGF-β1 protein levels were analyzed by an ELISA and are expressed in nanograms per total volume of fluid in the pleural space of each guinea pig. The bars indicate the means for three or four animals per day, and the error bars indicate the standard errors of the means. An asterisk indicates that there is a significant difference between the protein levels and the levels in the control animals.
In order to ensure that treatment of guinea pigs with anti-TGF-β1 actually removed TGF-β1 from the pleural space, TGF-β1 protein levels in the pleural fluids of control and treated guinea pigs were measured. As shown in Fig. 3B, injection of anti-TGF-β1 into the pleural cavity of guinea pigs on day 8 significantly lowered the TGF-β1 levels in the pleural fluid on day 9 (P < 0.01).
Effect of neutralizing TGF-β1 on in vivo expression of mRNA of several cytokines.
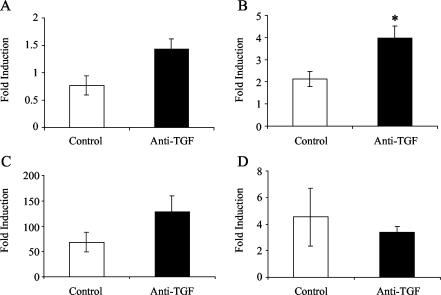
To examine the effect of removal of TGF-β1 on the cytokine response of pleural exudate cells, total RNA was isolated from cells obtained from the pleural space of pleuritic guinea pigs and was analyzed for expression of TGF-β1, TNF-α, IFN-γ, and IL-8 mRNA by real-time PCR. As shown in Fig. 4A, the levels of TGF-β1 mRNA in anti-TGF-β1-treated guinea pigs were greater than the levels in control animals (P = 0.056). Likewise, Fig. 4B shows that the levels of TNF-α mRNA in anti-TGF-β1-treated guinea pigs were significantly greater than the levels in control animals (P < 0.05). The levels of IFN-γ mRNA expression are shown in Fig. 4C. Treatment of guinea pigs with anti-TGF-β1 resulted in an increase in IFN-γ mRNA expression compared with the expression in the controls. However, due to large differences in IFN-γ expression between animals in each group, the difference between groups was not significant. Finally, as Fig. 4D shows, IL-8 mRNA expression was not affected by treatment of guinea pigs with anti-TGF-β1.
FIG. 4.
Cytokine mRNA expression in pleural exudate cells from guinea pigs with tuberculous pleurisy. Expression of TGF-β1 mRNA (A), expression of TNF-α mRNA (B), expression of IFN-γ mRNA (C), and expression of IL-8 mRNA (D) were measured in pleural fluid cells obtained from control and anti-TGF-β1-treated guinea pigs with experimental tuberculous pleurisy. The level of induction was determined from the threshold cycle values normalized for 18S expression and then normalized to the values derived from resident pleural cells of healthy, pleuritis-free animals. The bars indicate the means for three or four animals, and the error bars indicate the standard errors of the means. An asterisk indicates that there is a significant difference between mRNA expression in cells from treated animals and mRNA expression in cells from control animals.
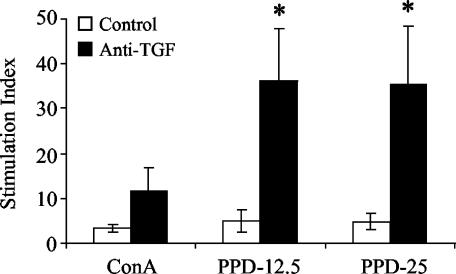
Pleural cell proliferation is enhanced in the absence of in vivo TGF-β1.
Cells from the pleural space were collected and stimulated in vitro with ConA or with two concentrations of PPD (12.5 and 25 μg/ml) for 4 days to determine the proliferative responses of antigen-specific T lymphocytes in the exudate. Figure 5 shows that anti-TGF-β1 treatment resulted in enhanced proliferative responses of pleural effusion lymphocytes to ConA and both doses of PPD. While there was an obvious increase in proliferation by cells from anti-TGF-β1-treated guinea pigs upon ConA stimulation, only the responses to both PPD concentrations were significantly greater than the responses of pleural cells from control animals (P < 0.05).
FIG. 5.
Proliferation of pleural exudate cells from pleuritic guinea pigs in control and anti-TGF-β1-treated groups. Pleural exudate cells from guinea pigs with experimental tuberculous pleurisy were cultured for 96 h in the presence of 10 μg of ConA per ml, 12.5 μg of PPD per ml (PPD-12.5), or 25 μg of PPD per ml (PPD-25). Proliferative responses are expressed as the stimulation index (counts per minute for stimulated cells/counts per minute for unstimulated cells) for individual animals. The bars indicate the means for three or four animals, and the error bars indicate the standard errors of the means. An asterisk indicates that the proliferative response is significantly greater than that of cells from control guinea pigs.
DISCUSSION
Tuberculous pleurisy is an antigen-specific delayed-type hypersensitivity response that occurs in a confined, accessible anatomical site and thus is ideal for studying the role of cytokines in the immune response against mycobacterial antigens. One regulatory cytokine that is of particular interest in this context is TGF-β. TGF-β is a pluripotent cytokine that is known to participate in both pro- and anti-inflammatory processes (37). Some proinflammatory activities of TGF-β1 include chemotactic activity for monocytes (39), neutrophils (5), and lymphocytes (1), upregulation of cell surface integrin expression (38), and enhancement of expression of mRNA of proinflammatory cytokines, including IL-1 (39), TNF, and IL-6 (35), in resting monocytes. Perhaps more well-known are the immunosuppressive properties of TGF-β1, including downregulation of IL-1 receptor (12) and simultaneous induction of IL-1 receptor antagonist expression (36), suppression of proinflammatory cytokine production by various cell types (6, 14), macrophage deactivation (10, 34), and suppression of T lymphocyte proliferation in response to mitogenic stimulation (18).
In a recent study, TGF-β1 protein concentrations in pleural fluid during the first 9 days of experimentally induced tuberculous pleurisy in guinea pigs were examined (3). It was found that the TGF-β1 concentrations were approximately 3.2 to 4.8 ng/ml during the first 3 days and then significantly increased to between 10 and 11.5 ng/ml on days 7 through 9 of the inflammatory response. The high levels of TGF-β1 detected on days 7 through 9 prompted us to neutralize TGF-β1 during this period and examine the resulting effect on the cellular and cytokine responses in the pleural space of guinea pigs. On the one hand, the results presented here suggest that TGF-β1 acts in a proinflammatory manner. Neutralization of TGF-β1 resulted in reduced fluid and cell accumulation in the pleural space compared with the accumulation in the controls (Fig. 1). One explanation for the increased numbers of cells in control guinea pigs with tuberculous pleurisy may be that TGF-β1 continued to induce chemotaxis of cells, particularly neutrophils, since the number of these cells was significantly greater in control guinea pigs than in anti-TGF-β1-treated animals (Fig. 2). Alternatively, cell death may have been responsible for the lower numbers of cells in anti-TGF-β1-treated guinea pigs. Previous research has demonstrated that TGF-β1 enhances survival of human neutrophils (19), suggesting a possible explanation for the decreased number of neutrophils in the anti-TGF-β1 treated guinea pigs.
On the other hand, TGF-β1 appears to have an anti-inflammatory effect in the guinea pig tuberculous pleuritis model. For instance, the concentration of lymphocytes in the pleural cavity was significantly decreased in the absence of TGF-β1 (Fig. 2). Additionally, the proliferative responses of these cells to PPD stimulation in vitro when TGF-β1 levels were reduced by antibody treatment were dramatically stronger than the responses of pleural cells from control guinea pigs (Fig. 5), possibly indicating that TGF-β1 plays a role in the resolution of the adaptive immune response against mycobacterial antigens. Bioactive TNF protein levels and TNF-α mRNA expression (Fig. 4) were also increased in guinea pigs treated with anti-TGF-β1, suggesting that TGF-β1 normally may limit the production of TNF to promote the resolution of the inflammatory response. Finally, while IFN protein concentrations were slightly lower in the anti-TGF-β1-treated guinea pigs, expression of IFN-γ mRNA in pleural cells was somewhat increased in the absence of TGF-β1 (Fig. 3 and 4). Neither of these differences was significant, suggesting that TGF-β1 did not have a significant effect on the presence of IFN-γ in tuberculous pleurisy.
Previous studies have demonstrated that TGF-β is present in the pleural exudates of patients with pleuritis having various etiologies, including tuberculosis (27, 29). In addition, investigators have examined the ability of TGF-β2 to act as a pleurodesing agent to stop the recurrence of pleuritis (22, 25). However, to our knowledge, no one has altered the levels of TGF-β in pleural exudates to examine the role of this cytokine in the inflammatory response that occurs during pleuritis. We attempted to define the role of TGF-β1 in the mycobacterium-specific delayed-type hypersensitivity response as a predominantly proinflammatory or immunosuppressive response. Instead, we confirmed the dichotomous nature of this cytokine.
McCartney-Francis and Wahl (28) suggested that low levels of TGF-β at the beginning of an inflammatory response initiate recruitment to and activation of cells at the site of injury. TGF-β levels continually increase at the site, and as lymphocytes and macrophages differentiate, they become suppressed by this cytokine, leading to resolution of the inflammatory response and decreasing levels of TGF-β. Based on previous studies of induction of pleuritis in response to mycobacteria or their products in guinea pigs (2, 24, 30, 40), we believed that the pleuritis might resolve itself by day 9. It is possible, however, that the pleuritis might persist for several more days. Thus, during this period of the antigen-specific delayed-type hypersensitivity reaction, TGF-β may both sustain the inflammatory response and act to resolve it. It may induce chemotaxis of neutrophils and enhance their survival, while it suppresses new expression of TNF-α and IFN-γ mRNA by differentiated macrophages and lymphocytes, respectively. Finally, it may further suppress lymphocytes by inhibiting their antigen-specific proliferative response to PPD. Therefore, while TGF-β1 may be involved in proinflammatory activities, we believe that this cytokine plays an essential role in the resolution of tuberculous pleurisy in guinea pigs.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 AI 15495 to D.N.M.
We are very grateful to Toshiko Yamamoto for her assistance with the IFN bioassay. We also thank Susan Phalen for her expertise and assistance with induction of tuberculous pleurisy in the guinea pigs.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Adams, D. H., M. Hathaway, J. Shaw, D. Burnett, E. Elias, and A. J. Strain. 1991. Transforming growth factor-β induces human T lymphocyte migration in vitro. J. Immunol. 147:609-612. [PubMed] [Google Scholar]
- 2.Allen, J. C., and M. A. Apicella. 1968. Experimental pleural effusion as a manifestation of delayed hypersensitivity to tuberculin PPD. J. Immunol. 101:481-487. [PubMed] [Google Scholar]
- 3.Allen, S. S., and D. N. McMurray. 2003. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect. Immun. 71:4271-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom, B. R., and C. J. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 5.Brandes, M. E., U. E. Mai, K. Ohura, and S. M. Wahl. 1991. Type I transforming growth factor-β receptors on neutrophils mediate chemotaxis to transforming growth factor-β. J. Immunol. 147:1600-1606. [PubMed] [Google Scholar]
- 6.Chen, C. C., and A. M. Manning. 1996. TGF-β1, IL-10 and IL-4 differentially modulate the cytokine-induced expression of IL-6 and IL-8 in human endothelial cells. Cytokine 8:58-65. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, M. K., R. A. Bartow, C. L. Mintzer, and D. N. McMurray. 1987. Effects of diet and genetics on Mycobacterium bovis BCG vaccine efficacy in inbred guinea pigs. Infect. Immun. 55:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai, G., and D. N. McMurray. 1999. Effects of modulating TGF-β1 on immune responses to mycobacterial infection in guinea pigs. Tuber. Lung Dis. 79:207-214. [DOI] [PubMed] [Google Scholar]
- 9.De Ceuninck, F., P. Pastoureau, F. Bouet, J. Bonnet, and P. M. Vanhoutte. 1998. Purification of guinea pig YKL40 and modulation of its secretion by cultured articular chondrocytes. J. Cell Biochem. 69:414-424. [DOI] [PubMed] [Google Scholar]
- 10.Ding, A., C. F. Nathan, J. Graycar, R. Derynck, D. J. Stuehr, and S. Srimal. 1990. Macrophage deactivating factor and transforming growth factors-β1 -β2 and -β3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-γ. J. Immunol. 145:940-944. [PubMed] [Google Scholar]
- 11.Dlugovitzky, D., M. L. Bay, L. Rateni, L. Urizar, C. F. Rondelli, C. Largacha, M. A. Farroni, O. Molteni, and O. A. Bottasso. 1999. In vitro synthesis of interferon-γ, interleukin-4, transforming growth factor-β and interleukin-1β by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand. J. Immunol. 49:210-217. [DOI] [PubMed] [Google Scholar]
- 12.Dubois, C. M., F. W. Ruscetti, E. W. Palaszynski, L. A. Falk, J. J. Oppenheim, and J. R. Keller. 1990. Transforming growth factor β is a potent inhibitor of interleukin 1 (IL-1) receptor expression: proposed mechanism of inhibition of IL-1 action. J. Exp. Med. 172:737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellner, J. J., P. F. Barnes, R. S. Wallis, and R. L. Modlin. 1988. The immunology of tuberculous pleurisy. Semin. Respir. Infect. 3:335-342. [PubMed] [Google Scholar]
- 14.Espevik, T., I. S. Figari, M. R. Shalaby, G. A. Lackides, G. D. Lewis, H. M. Shepard, and M. A. Palladino, Jr. 1987. Inhibition of cytokine production by cyclosporin A and transforming growth factor β. J. Exp. Med. 166:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espevik, T., and J. Nissen-Meyer. 1986. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J. Immunol. Methods 95:99-105. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch, C. S., R. Hussain, Z. Toossi, G. Dawood, F. Shahid, and J. J. Ellner. 1996. Cross-modulation by transforming growth factor β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon γ production. Proc. Natl. Acad. Sci. 93:3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James, N. H., and R. A. Roberts. 1996. Species differences in response to peroxisome proliferators correlate in vitro with induction of DNA synthesis rather than suppression of apoptosis. Carcinogenesis 17:1623-1632. [DOI] [PubMed] [Google Scholar]
- 18.Kehrl, J. H., L. M. Wakefield, A. B. Roberts, S. Jakowlew, M. Alvarez-Mon, R. Derynck, M. B. Sporn, and A. S. Fauci. 1986. Production of transforming growth factor β by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 163:1037-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagraoui, M., and L. Gagnon. 1997. Enhancement of human neutrophil survival and activation by TGF-β1. Cell. Mol. Biol. 43:313-318. [PubMed] [Google Scholar]
- 20.Lee, Y. C., C. J. Devin, L. R. Teixeira, J. T. Rogers, P. J. Thompson, K. B. Lane, and R. W. Light. 2001. Transforming growth factor β2 induced pleurodesis is not inhibited by corticosteroids. Thorax 56:643-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, Y. C., and K. B. Lane. 2001. The many faces of transforming growth factor-β in pleural diseases. Curr. Opin. Pulm. Med. 7:173-179. [DOI] [PubMed] [Google Scholar]
- 22.Lee, Y. C., K. B. Lane, R. E. Parker, D. S. Ayo, J. T. Rogers, R. W. Diters, P. J. Thompson, and R. W. Light. 2000. Transforming growth factor β(2) (TGF β(2)) produces effective pleurodesis in sheep with no systemic complications. Thorax 55:1058-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, Y. C., J. R. Yasay, J. E. Johnson, R. E. Parker, P. J. Thompson, K. B. Lane, and R. W. Light. 2002. Comparing transforming growth factor-β2, talc and bleomycin as pleurodesing agents in sheep. Respirology 7:209-216. [DOI] [PubMed] [Google Scholar]
- 24.Leibowitz, S., L. Kennedy, and M. H. Lessof. 1973. The tuberculin reaction in the pleural cavity and its suppression by antilymphocyte serum. Br. J. Exp. Pathol. 54:152-162. [PMC free article] [PubMed] [Google Scholar]
- 25.Light, R. W., D. S. Cheng, Y. C. Lee, J. Rogers, J. Davidson, and K. B. Lane. 2000. A single intrapleural injection of transforming growth factor-β(2) produces an excellent pleurodesis in rabbits. Am. J. Respir. Crit. Care Med. 162:98-104. [DOI] [PubMed] [Google Scholar]
- 26.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2002. Mycobacterium bovis BCG vaccination augments interleukin-8 mRNA expression and protein production in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 70:5471-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda, J., N. Ueki, T. Ohkawa, N. Iwahashi, T. Nakano, T. Hada, and K. Higashino. 1993. Local production and localization of transforming growth factor-β in tuberculous pleurisy. Clin. Exp. Immunol. 92:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCartney-Francis, N. L., and S. M. Wahl. 1994. Transforming growth factor β: a matter of life and death. J. Leukoc. Biol. 55:401-409. [DOI] [PubMed] [Google Scholar]
- 29.Olobo, J. O., M. Geletu, A. Demissie, T. Eguale, K. Hiwot, G. Aderaye, and S. Britton. 2001. Circulating TNF-α, TGF-β, and IL-10 in tuberculosis patients and healthy contacts. Scand. J. Immunol. 53:85-91. [DOI] [PubMed] [Google Scholar]
- 30.Phalen, S. W., and D. N. McMurray. 1993. T-lymphocyte response in a guinea pig model of tuberculous pleuritis. Infect. Immun. 61:142-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roper, W. H., and J. J. Waring. 1955. Primary serofibrinous pleural effusion in military personnel. Am. Rev. Tuberc. Pulm. Dis. 71:616-634. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, Y., T. Tanigaki, D. Heimer, W. Wang, W. G. Ross, G. A. Murphy, A. Sakai, H. H. Sussman, T. H. Vu, and T. A. Raffin. 1994. TGF-beta 1 causes increased endothelial ICAM-1 expression and lung injury. J. Appl. Physiol. 77:1281-1287. [DOI] [PubMed] [Google Scholar]
- 33.Toossi, Z., P. Gogate, H. Shiratsuchi, T. Young, and J. J. Ellner. 1995. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J. Immunol. 154:465-473. [PubMed] [Google Scholar]
- 34.Tsunawaki, S., M. Sporn, A. Ding, and C. Nathan. 1988. Deactivation of macrophages by transforming growth factor-β. Nature 334:260-262. [DOI] [PubMed] [Google Scholar]
- 35.Turner, M., D. Chantry, and M. Feldmann. 1990. Transforming growth factor β induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine 2:211-216. [DOI] [PubMed] [Google Scholar]
- 36.Turner, M., D. Chantry, P. Katsikis, A. Berger, F. M. Brennan, and M. Feldmann. 1991. Induction of the interleukin 1 receptor antagonist protein by transforming growth factor-β. Eur. J. Immunol. 21:1635-1639. [DOI] [PubMed] [Google Scholar]
- 37.Wahl, S. M. 1992. Transforming growth factor β (TGF-β) in inflammation: a cause and a cure. J. Clin. Immunol. 12:61-74. [DOI] [PubMed] [Google Scholar]
- 38.Wahl, S. M., J. B. Allen, B. S. Weeks, H. L. Wong, and P. E. Klotman. 1993. Transforming growth factor β enhances integrin expression and type IV collagenase secretion in human monocytes. Proc. Natl. Acad. Sci. 90:4577-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wahl, S. M., D. A. Hunt, L. M. Wakefield, N. McCartney-Francis, L. M. Wahl, A. B. Roberts, and M. B. Sporn. 1987. Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. 84:5788-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widstrom, O., and B. S. Nilsson. 1982. Pleurisy induced by intrapleural BCG in immunized guinea pigs. Eur. J. Respir. Dis. 63:425-434. [PubMed] [Google Scholar]
- 41.Yamamoto, T., A. Jeevan, K. Ohishi, Y. Nojima, K. Umemori, S. Yamamoto, and D. N. McMurray. 2002. A new assay system for guinea pig interferon biological activity. J. Interferon Cytokine Res. 22:793-797. [DOI] [PubMed] [Google Scholar]
- 42.Yanaka, A., H. Muto, H. Fukutomi, S. Ito, and W. Silen. 1996. Role of transforming growth factor-beta in the restitution of injured guinea pig gastric mucosa in vitro. Am. J. Physiol. 271:G75-G85. [DOI] [PubMed] [Google Scholar]